Abstract
Background:
Graves ophthalmopathy (GO) is one of the remaining enigmas in thyroidology. Glucocorticoids (GCs) are strongly recommended but their effects are not completely satisfactory and adverse reactions can occur. Tripterygium glycosides (TG) is a promising component extracted from Tripterygium wilfordii Hook F (TwHF), and numerous patients with GO have benefited from it. However, its practical application value is still unclear. The aim of this systematic review and meta-analysis was to investigate the efficacy and safety of TG for patients with GO.
Methods:
By retrieving the PubMed, Embase, the Cochrane Library, CNKI, VIP, CBM, and WanFang Databases, the open published randomized controlled trials (RCTs) related to TG in the treatment of GO were collected. And inclusion and exclusion criteria were established. The Cochrane bias risk assessment tool conducts the evaluation of included studies, and meta-analysis was performed using Revman 5.3 software.
Trial registration number:
PROSPERO CRD42019131915.
Results:
A total of 19 trials (involving 1517 GO patients) were included in this review with generally acceptable validity of included RCTs. TG therapy brought about a significantly higher efficacy rate compared with non-TG treatments (RR: 1.40; 95% CI: 1.31–1.49). Subgroup meta-analysis showed that TG with or without immunosuppressive therapies were all better than controls: with GC (RR: 1.36; 95% CI: 1.27–1.46), with multiple intensification of immunosuppressive therapies (RR: 1.91; 95% CI: 1.37–2.67), with no immunosuppressive therapies (RR: 1.39; 95% CI:1.21–1.59); the dosage of TG for 15–60 mg/d (RR: 1.41; 95% CI: 1.30–1.53) were better compared with for ≥90 mg/d (RR: 1.47; 95% CI: 1.29–1.68); the course of treatment for ≤3 months (RR: 1.43; 95% CI: 1.33–1.52) was better than controls, but when >3 months (RR: 1.15; 95% CI: 0.94–1.41) there was no significant differences. After treatment, the degree of exophthalmus (SMD: −2.55; 95% CI: −2.93 to 2.17), the recurrence rate of 1 year (RR: 0.45; 95% CI: 0.27–0.74), and adverse reactions rate (RR: 0.32; 95% CI: 0.20–0.53) were all lower, while the CAS was no obvious gap in 2 groups (SMD: 0.08; 95% CI: −0.60 to 0.75).
Conclusions:
This review found that TG has some advantages in treating GO, especially in improving clinical efficacy and reducing adverse reactions. Nevertheless, large sample, multi-center, reasonable design, and high quality clinical studies are still needed for further verification.
Keywords: Graves ophthalmopathy, meta-analysis, randomized control trial, systematic review, tripterygium glycosides
1. Introduction
Graves ophthalmopathy (GO) is the main extrathyroidal manifestation of Graves disease (GD), characterized by eyelid retraction, proptosis, swelling and erythema of conjunctiva and periocular tissues, and altered ocular motility.[1] It is one of the commonest adult orbital diseases, which usually occurs in 25–50% of GD patients.[2] If not being treated promptly and properly, it can lead to either irreversible visual impairment or even blindness. GO impairs the quality of life (QOL) significantly, and causes great indirect and direct costs for public health systems.[3,4] Although the precise pathogenesis of GO remains completely unclear, autoimmune mechanisms obviously play an important role.[5] At present, a effective and radical treatment of GO is still being explored. The therapies recommended in the guidelines for the management of GO comprising glucocorticoids (GCs), orbital radiotherapy, cyclosporine, andrituximab, however, are an ongoing matter of concern due to their adverse effects and contraindications. In addition, rehabilitative surgery is the last choice of treatment when GO reached an advanced stage.[6] Therefore, a better therapy is desperately required.
Tripterygium glycosides (TG) is an effective component extracted from the root bark of Tripterygium wilfordii Hook F (TwHF), which is a vital Chinese herb belonging to the Celastraceae family.[7] Functions like anti-inflammation, antianaphylaxis, and immunosuppression of TG have been well proved, which enables a wide usage in treatments of various autoimmune diseases, including rheumatoid arthritis (RA),[8] chronic urticaria,[9] henoch schonlein purpura nephritis (HSPN),[10] systemic lupus erythematosus (SLE),[11] chronic kidney disease (CKD),[12] diabetic nephropathy (DN),[13] and ankylosing spondylitis (AS).[14] Currently, many reports have indicated that the effect of TG for GO sufferers is satisfactory. Nevertheless, it lacks scientific evidence regarding the efficacy and safety of TG for GO patients. Hence, we undertook this systematic review of all relevant published literature relating to randomized controlled trials (RCTs) of TG to further examine the efficacy and safety of the TG treatment for GO.
2. Methods
2.1. Inclusion criteria
The criteria for inclusion were as follows:
-
1.
Types of studies design: RCTs, regardless of the methods of blinding, reported in either English or Chinese;
-
2.
Types of participants: patients with definite diagnosed with GO,[6] irrespective of the cause or presence of other diseases;
-
3.
RCTs: regardless of gender and race, comprising patients ranging from 16 to 70 years of age;
-
4.
Interventions: treatment groups used TG as the main intervention measure in the treatment of GO, without restriction of its doses and therapeutic periods, and control groups were treated with GCs or other immunosuppressive therapies.
2.2. Exclusion criteria
The criteria for exclusion were as follows:
-
1.
non-RCTs, including animal experiments, experience summary, systematic review, case report, and self-controlled trials;
-
2.
non-English and Chinese studies;
-
3.
can not meet the above mentioned GO diagnostic criteria;
-
4.
failing to strictly follow the doctor's advice during the process, or losing to follow-up midway, or accepting other special treatments that affect the observation indicators of this study;
-
5.
repeated publication of articles and incomplete information, which makes it impossible to extract and merge data.
2.3. Outcomes
Primary outcome measures were the efficacy rate, the degree of exophthalmos, and the clinical activity score (CAS). Secondary outcome measures included the recurrence rate of 1 year and the adverse reaction rate.
2.4. Search strategy
2.4.1. Electronic searches
Our study protocol was registered in the PROSPERO database before the start of the review process (CRD42019131915). We searched 3 English language electronic databases and 4 Chinese language electronic databases: PubMed, Embase, the Cochrane Library, China Network Knowledge Infrastructure (CNKI), Chinese Scientific Journal Database (VIP), Chinese Biomedicine (CBM), Wan Fang Database. All the databases were searched from their date of inception to March, 2019. Languages included English and Chinese, and to collect a sufficient number of trials, the references cited by the retrieved articles were tracked. We utilized the medical subject headings “Graves Ophthalmopathy”, “Thyroid Associated Ophthalmopathy”, “Tripterygium”, “Tripterygium wilfordii”, and “Tripterygium glycosides” in both English and Chinese databases. The independent work was conducted by 2 researchers to ensure a comprehensive scope of the search results
2.4.2. Manual searches
Retrievals were conducted manually in Liaoning University of Chinese Medicine library collection (2015–2019): International Eye Science, Chinese Journal of Experimental Ophthalmology, Chinese Journal of Practical Ophthalmology, China Journal of Traditional Chinese Medicine and Pharmacy, Liaoning Journal of Chinese Medicine, Journal of Liaoning University of Chinese Medicine. Searched part of clinical trials, and the reference lists from included studies as a supplement.
2.5. Studies screening and data extraction
Studies screening and date extraction were undertaken by 2 researchers independently, and then cross-checked. In case of disagreement, resolved by the third person. The following information is extracted and entered into a database: title, sample size, baseline characteristics of participants, inclusion/exclusion criteria, interventions, treatment duration, outcome measures, follow-up, and adverse events.
2.6. Quality assessment
Two researchers independently evaluated the methodological quality of eligible trials with the Cochrane Collaboration Risk of Bias tool.[15] Each term was assessed as 3 grades — high risk, low risk, or unclear—based on 7 aspects including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. The risk of bias graphs were generated by RevMan5.3 software.
2.7. Statistical analysis
Meta-analysis was conducted with RevMan5.3 software provided by the Cochrane Collaboration. The dichotomous data was expressed in terms of risk ratio (RR), and the continuous data was expressed in terms of standard mean difference (SMD) with a 95% confidence interval (CI). Heterogeneity was tested using I2 statistics. If there was homogeneity(P > .1, I2 < 50%)in the result, we used the fixed effect model. Otherwise, we used the random effect model or descriptive analysis. A funnel plot was used to estimate publication bias. If the scatter distribution was both side symmetrical, it was considered that there was no publication bias. Otherwise, there may be publication bias.
2.8. Ethics
This is a systematic review and meta-analysis and ethical approval was not necessary.
3. Results
3.1. Procedure for study inclusion
We initially identified 237 relevant studies from 7 databases. One hundred thirty six duplicate studies were removed; an additional 104 studies were excluded after reading titles and abstracts. After the full-text reading of the resulting 32 studies, 19 RCT studies[16–34] met our inclusion criteria and were included in the meta-analysis. The screening procedure is illustrated in Figure 1.
Figure 1.
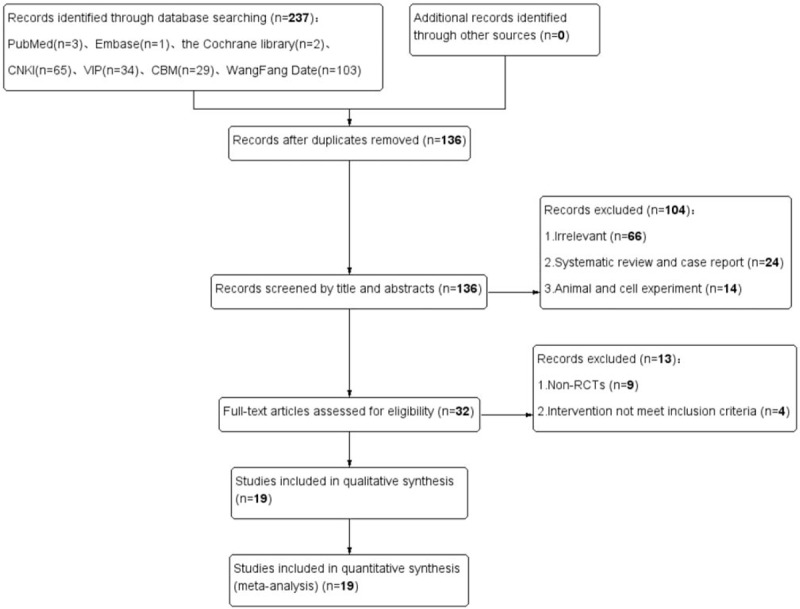
Literature search and study selection flowchart.
3.2. Characteristics of included studies
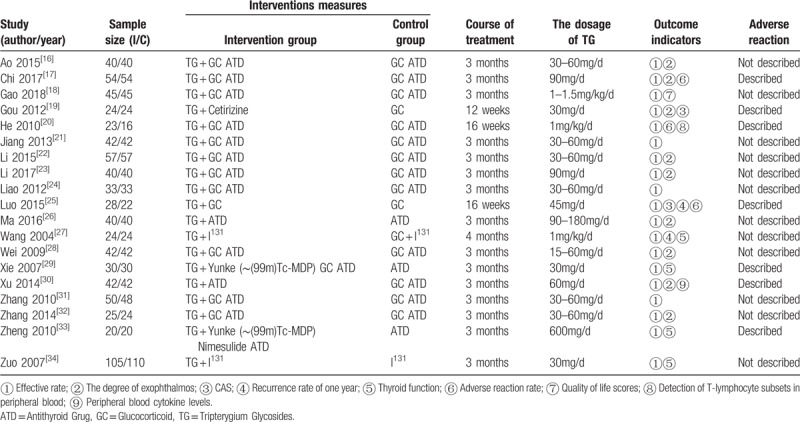
The 19 studies included in this review were all conducted and published in Chinese from 2004 to 2018. Together, these studies included 1517 GO patients consisting of 764 patients in the intervention groups and 753 patients in the control groups.
In intervention groups, 12 trials[16–18,20–25,28,31,32] are experimented with TG with GC, 2 trials[29,33] used TG with multiple intensification of immunosuppressive therapies, and 5 trials[19,26,27,30,34] did not use any immunosuppressive therapies. In addition, 15 trials[16–18,20–24,26,28–33] used an antithyroid drug (ATD) to control thyroid function while 2 trials[27,34] used I131, and the thyroid function of the patients who from the rest of 2 trials[19,25] were under control.
Regarding the dosage of TG and the course of treatment. In 12 trials,[16,19,21,22,24,25,28–32,34] the TG was given at 15 to 60 mg/d; in 4 trials,[17,23,26,33] it was given at ≥90 mg/d. Course of treatment in 16 trials[16–19,21–24,26,28–34] was ≤3months and the other 3 trials[20,25,27] was >3months. The basic characteristics of the included studies are shown in Table 1.
Table 1.
Characteristics of included trials.
3.3. Methodological quality
In this review, we employed a quality standard of RCTs evidence recommended by the Cochrane Collaboration to assess the risk of bias in the included studies. The studies included were all assessed, overall, its methodological quality was low (Figs. 2 and 3). Among them, 4 studies[17,25,29,33] used a random number table for random sequence generation, and 2 studies[16,21] used random allocation based on therapies and 1 studies[22] according to the order of admission were assessed as “high risk”. The other studies only mentioned the word “randomization”, thus they were considered to be “unclear”. Two studies[19–20] applied double-blind and single-blind methods, respectively. There is no patient of the included studies withdrew from the trial, and thus they were assessed as “low risk” for “incomplete outcome data”. All studies were considered to be “low risk” for “selective reporting” because they reported the outcomes prespecified in the trial. All studies were assessed as “low risk” for “other bias”, which refers to the inappropriate influence of cofounders.
Figure 2.
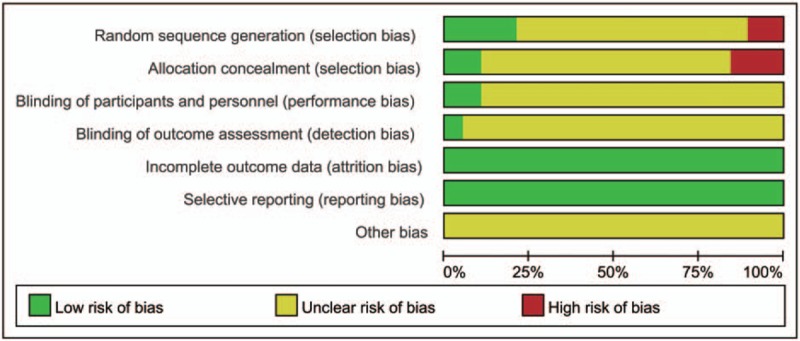
Risk of bias graph.
Figure 3.
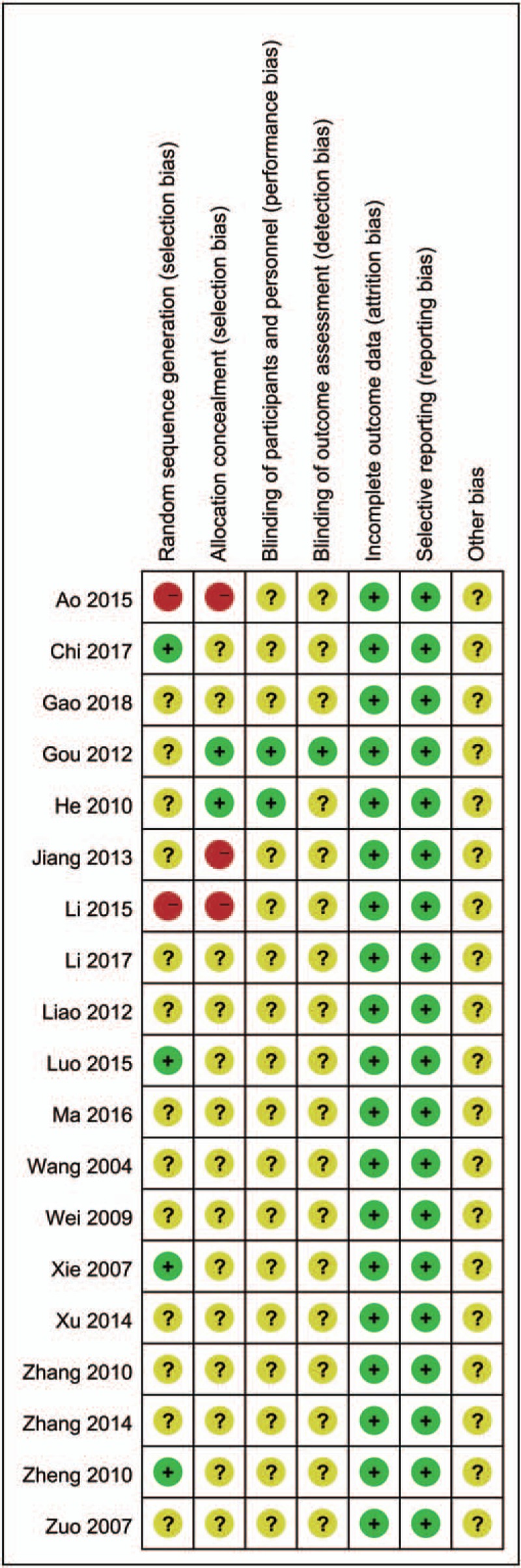
Risk of bias summary.
Scatters in the funnel plot were almost symmetrical visually (Fig. 4), which implies that the publication bias for this studies were controlled passably.
Figure 4.
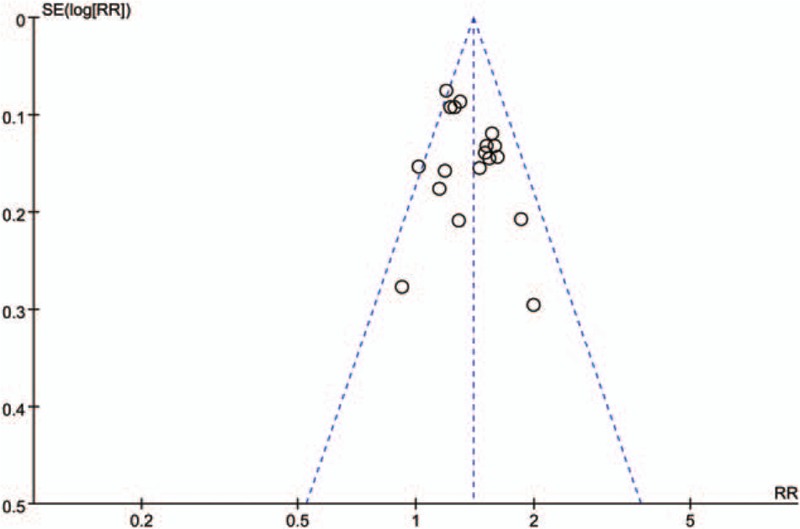
Funnel plot graph.
3.4. Efficacy of TG
3.4.1. Efficacy rate
Effective rate was all evaluated in intervention and control groups in 19 studies. According to the variety of intervention measures, all trails were divided into 3 groups to perform subgroup analysis (Fig. 5). Total meta-analysis showed that groups with TG presented a higher efficacy rate than controls (RR:1.40; 95% CI:1.31–1.49; P < .00001; fixed model; I2 = 28%; n = 1517), subgroup meta-analysis showed that TG in combination with other immunosuppressive therapies or not were all better than controls: for TG with GC (RR:1.36; 95% CI:1.27–1.46; P < .00001; fixed model; I2 = 22%; 12 trials; n = 942), TG with multiple intensification of immunosuppressive therapies (RR:1.91; 95% CI:1.37–2.67; P = .0002 < .05; fixed model; I2 = 0%; 2 trials; n = 100), TG with no immunosuppressive therapies (RR: 1.39; 95% CI:1.21–1.59; P < .00001; fixed model; I2 = 31%; 5 trials; n = 475).
Figure 5.
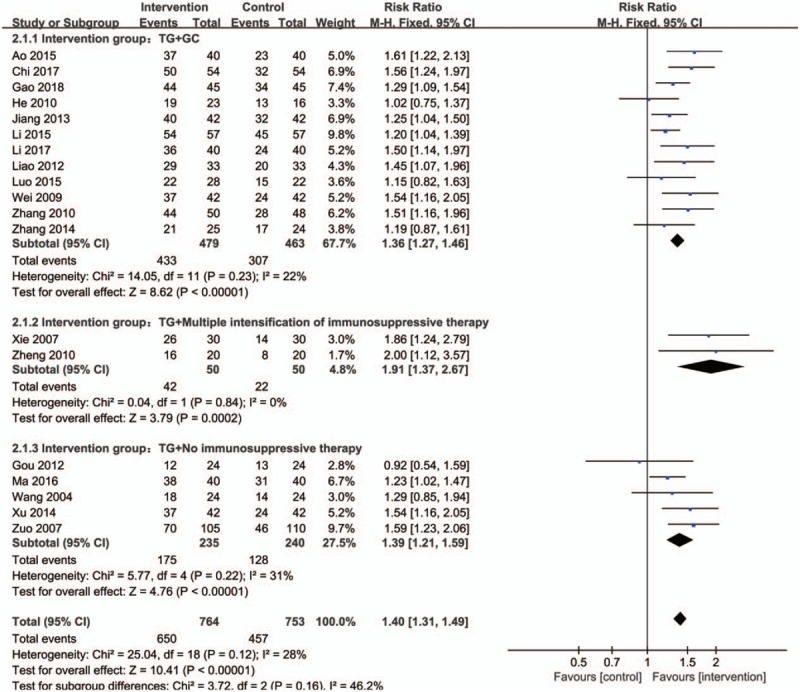
Forest plot of total efficacy rate and subgroup meta-analysis based on intervention measures. CI indicates confidence interval.
Subgroup meta-analysis based on dosage also revealed that TG achieved a better performance than controls on the treatment of GO for all doses (Fig. 6): for 15 to 60 mg/d (RR: 1.41; 95% CI:1.30–1.53; P < .00001; fixed model; I2 = 30%; 12 trials; n = 1032), ≥90 mg/d (RR: 1.47; 95% CI:1.29–1.68; P < .00001; fixed model; I2 = 43%; 4 trials; n = 308). When the dose is 15 to 60 mg/d, the effect is better in comparison.
Figure 6.
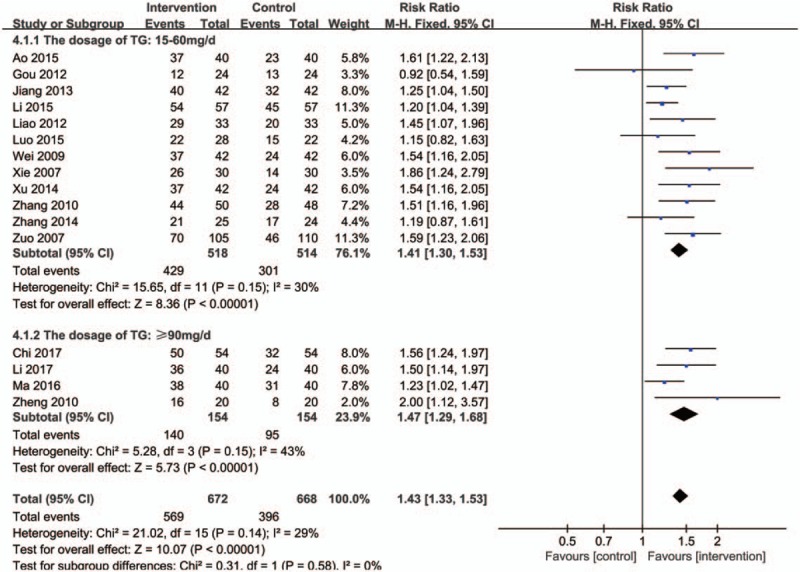
Forest plot of subgroup meta-analysis based on dosage of TG. CI indicates confidence interval.
As for the course of treatment (Fig. 7), duration ≤3 months (RR: 1.43; 95% CI:1.33–1.52; P < .00001; fixed model; I2 = 28%; 16 trials; n = 1380) showed its greater effect than controls, nevertheless, duration >3 months (RR: 1.15; 95% CI:0.94–1.41; P = .19>.05; fixed model; I2 = 0%; 3 trials; n = 137) had no significant differences.
Figure 7.
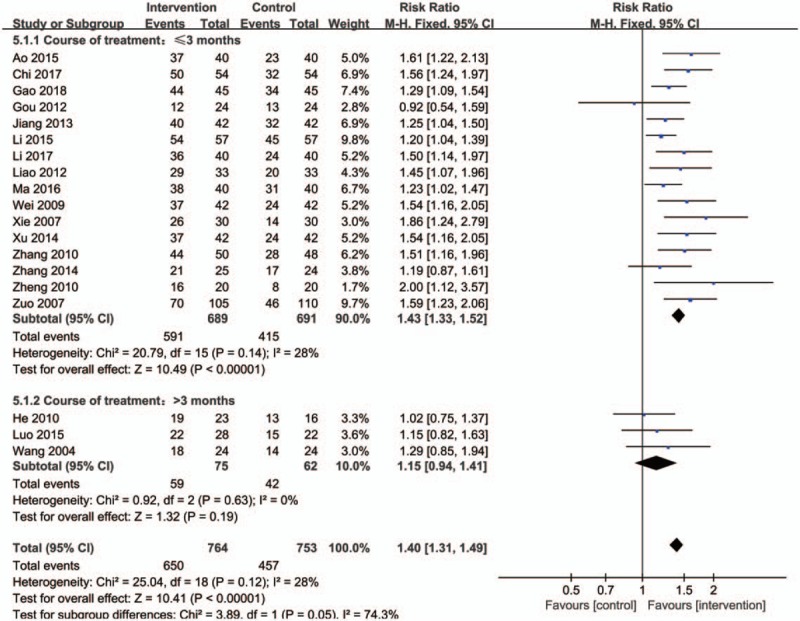
Forest plot of subgroup meta-analysis based on course of treatment. CI indicates confidence interval.
3.4.2. The degree of exophthalmus
Nine trials measured the degree of exophthalmus as an outcome. One study[19] showed the deviation in mean difference was quite great. Results of the remaining studies showed a obviously descending trend in intervention groups, when compared with controls (Fig. 8) (SMD: −2.55; 95% CI:−2.93 to 2.17; P < .00001; random model; I2 = 70%; 16 trials; n = 679).
Figure 8.
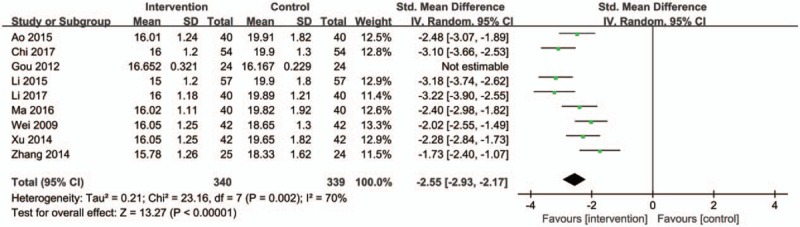
Forest plot of the degree of exophthalmus. CI indicates confidence interval.
3.4.3. CAS
Two trials provided information on CAS and this meta-analysis revealed that there is no significant difference existing between the 2 groups (Fig. 9) (SMD: 0.08; 95% CI:−0.60–0.75; P = .83 > .05; random model; I2 = 65%; 2 trials; n = 98). However, due to the heterogeneity among 2 studies, the random effect model was selected, and greater bias is more likely to arise, as there were few studies were included.
Figure 9.
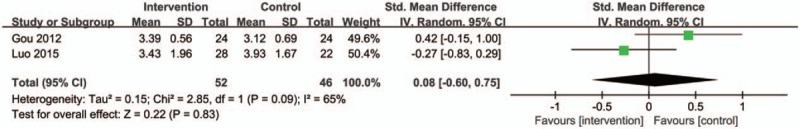
Forest plot of the CAS score. CI indicates confidence interval.
3.4.4. Recurrence rate of 1 year
Two trials reported on the recurrence rate of 1 year. Meta-analysis indicated that the groups of taken TG as the main intervention measure in the treatment of GO demonstrated a significantly higher recurrence rate than controls (Fig. 10) (RR: 0.45; 95% CI:0.27–0.74; P = .002; fixed model; I2 = 0%; 2 trials; n = 69).
Figure 10.
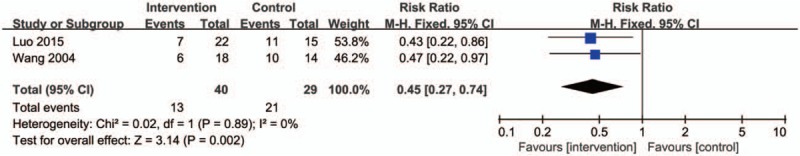
Forest plot of the recurrence rate of one year. CI indicates confidence interval.
3.5. Adverse events
None of the included studies reported severe adverse events of TG. Among the 19 studies, 12 of them did not report the safety indices. In the other 7 studies, 4 of them did not provided clearly proportions, the remaining studies reported drug-induced symptoms (such as nausea, vomiting, and gain weight), and the respondents had fewer of these symptoms in the intervention groups compared with the controls (Fig. 11) (RR: 0.32; 95% CI: 0.20–0.53; P < .00001; fixed model; I2 = 0%; 3 trials; n = 197). Furthermore, Xie[29] and Zheng[33] found that a small number of patients with mild discomfort symptoms at Yunke (∼(99m)Tc-MDP) infusion site disappeared after treatment. The liver and kidney functions of the intervention group and the control group were both normal throughout the whole treatment period, and no adverse reactions caused by TG were observed. Gou[19] did not see any adverse reactions caused by TG, but adverse reactions appeared such as centripetal obesity and acne in the control group causing by GC. Xu[30] found that the serum glutamic pyruvate transaminase (SGPT) of participants slightly increased and menstrual quantity decreased in the intervention group, while weight gain, osteoporosis, and peptic ulcer occurred in the control group. Above all, all of the aforementioned studies indicate that the occurrence rates of reported adverse effects in TG groups were fewer than controls.
Figure 11.
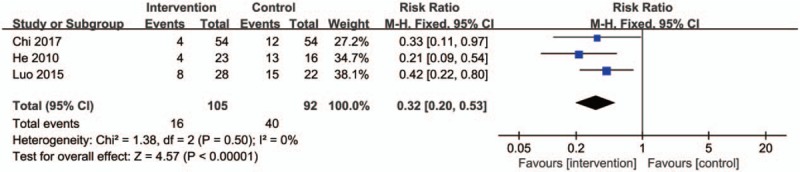
Forest plot of the adverse reactions rate. CI indicates confidence interval.
4. Discussion
This systematic review, for the first time, focused on evaluating the efficacy and safety of TG in the treatment of GO. The results of the current study manifested the effectiveness of TG in treating GO patients when contrasted non-TG groups. More importantly, the result of current analysis found that TG is safe, causing no more drug adverse reactions than controls. So our findings suggest that TG plays an important role in treating GO due to its effectiveness and safety, offering GO patients a potentially effective and safe alternative therapy.
The inflammatory phase of GO is characterized by T cells infiltration, often accompanied by mast cells, B cells, and macrophages.[35] T cells infiltrates in GO orbital tissues are predominantly CD4+, with some studies suggesting presence of both CD4+ and CD8+ T cells.[36–39] A study[20] we included, also suggested that the imbalance of T cell subsets may be an important factor leading to GO by detecting peripheral blood T cell subsets before and after treatment.
Furthermore, Th1 cell subsets profile predominates in GO retrobulbar tissue.[39] Th1 cell subsets expression profile consisting of Interleukin (IL)-1β, IL-2, interferon γ(IFNγ), and tumor necrosis factor α(TNFα), are responsible for cellular immunity, whereas Th2 cell subsets produce IL-4, IL-5, and IL-10, which participated in humoral immune response.[40] Aniszewski et al found that Th1 cells may dominate in early (<2 years) GO, shifting towards Th2 cells may in the later stages.[41] A great deal of pharmacological studies have demonstrated that TG can effectively inhibit the activation of T cells, induce apoptosis, restore the balance of Th1/Th2 cell, and regulate cellular immunity,[42–45] which may be the important underlying mechanisms of TG in the treatment of GO. Another study[30] we included also confirmed that, TG therapy can remarkably reduce the level of TNFα, IL-2, IFNγ, and increase the level of IL-10. In addition, Th17 is attracting greater attention currently, which is a new type CD4+ T cell subset that may be associated with GO,[46] and the IL-23/Th17 axis may extends to thyroid autoimmunity. The therapeutic mechanism of TG may be related to its effect on T cell subset, but still needs to be further explored.
Meanwhile, adverse reactions have always been a focus of concern. This review implied that the TG therapy induced lower adverse effects than controls, which was consistent with the results of TG in the treatment of CKD,[12] HSPN,[47] AS,[8] etc. Currently, gastrointestinal complaints, menstrual disorders in females, abnormal liver, or renal function induced by TG were reported in some studies,[48] but majority of them were mild and tolerable, and after reducing the dosage of TG or stopping it completely, it disappeared spontaneously. In the studies inclusive in our research, few patients had gastrointestinal discomforts, menstrual quantity reduction and slightly elevated SGPT.
TG exerts its therapeutic functions mainly through the same mechanisms underlying its toxic functions, have a narrow safe and effective therapeutic window, and the occurrence of adverse reactions is dose-dependent.[49–51] In most studies, when the dose of TG was 15 to 60 mg/d and the course of treatment was ≤3 months, the effect is better and there are few side effects, and no better efficacy was found in larger doses and longer courses of treatment. So we speculate that such treatment plan may be beneficial to some patients in clinical. However, these results are only based on current research, and we still know that there are much more researches to be done in the future.
In this meta-analysis, we comprises a profound and extensive literature search, presents data of sufficient quality, and computes outcome measures independent of the studies’ risk of bias. However, there are several potential limitations:
-
1.
The general quality of the included studies were not very high and multi-center/national RCTs were not found. Most of the studies simply mentioned “randomization” and only 2 studies adopted the blinding method;
-
2.
The sample size of each study included in the meta-analysis was insufficient to reach a robust conclusion;
-
3.
The follow-up periods were short. Only 2 studies followed patients for 1 year, which may influence our evaluation of efficacy and adverse reactions to TG;
-
4.
The number of some outcome events was very low. Several analyses were based on only 2 or 3 studies, which can affect the interpretation of results;
-
5.
Some unpublished studies that were inevitably missed.
5. Conclusion
Our meta-analysis, for the first time, summarized the application of TG therapy in GO. We found that TG has some advantages in treating GO. In addition, although adverse events should always be noticed, the incidence rate of adverse reactions is lower in the patients with TG therapy. Nevertheless, despite our rigorous methodology, the inherent limitations of the included studies prevent us from reaching a definitive conclusion. More adequately powered, randomized, double-blind, double-dummy trials with multi-center, and longer follow-up duration will be expected to perform in the future to further verify the finding of this analysis.
Author contributions
Conceptualization: Xiaowei Liu, Tianshu Gao.
Date curation: Xiaowei Liu, Chenghan Gao.
Formal analysis: Xiaowei Liu.
Funding acquisition: Tianshu Gao.
Investigation: Xiaowei Liu, Xiaolin Liu.
Software: Xiaowei Liu, Chenghan Gao.
Supervision:Tianshu Gao.
Writing – original draft: Xiaowei Liu.
Writing – review & editing: Xiaowei Liu, Tianshu Gao.
Footnotes
Abbreviations: ATD = antithyroid drug, CAS = clinical activity score, CBM = Chinese biomedicine, CI = confidence interval, CNKI = China Network Knowledge Infrastructure, GCs = glucocorticoids, GO = Graves ophthalmopathy, RR = risk ratio, SMD = standard mean difference, TG = Tripterygium glycosides, TwHF = Tripterygium wilfordii Hook F, VIP = Chinese Scientific Journal Database.
How to cite this article: Liu X, Gao C, Liu X, Gao T. Efficacy and safety of tripterygium glycosides for graves’ ophthalmopathy: A systematic review and meta-analysis. Medicine. 2019;98:50(e18242).
This project was supported by the National Natural Science Foundation of China (No. 81874441).
The authors have no conflicts of interest to disclose.
References
- [1].Bahn RS. Graves’ ophthalmopathy. N Engl J Med 2010;362:726–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tanda ML, Piantanida E, Liparulo L, et al. Prevalence and natural history of Graves’ orbitopathy in a large series of patients with newly diagnosed Graves’ ohyperthyroidism seen at a single center. J Clin Endocrinol Metab 2013;98:1443–9. [DOI] [PubMed] [Google Scholar]
- [3].Gerding MN, Terwee CB, Dekker FW, et al. Quality of life in patients with Graves’ ophthalmopathy is markedly decreased: measurement by the medical outcomes study instrument. Thyroid 1997;7:885–9. [DOI] [PubMed] [Google Scholar]
- [4].Ponto KA, Merkesdal S, Hommel G, et al. Public health relevance of Graves’ orbitopathy. J Clin Endocrinol Metab 2013;98:145–52. [DOI] [PubMed] [Google Scholar]
- [5].Wiersinga WM. Advances in treatment of active, moderate-to-severe Graves’ ophthalmopathy. Lancet Diabetes Endocrinol 2017;5:134–42. [DOI] [PubMed] [Google Scholar]
- [6].Bartalena L, Baldeschi L, Boboridis K, et al. The 2016 European Thyroid Association/European Group on Graves’ Orbitopathy guidelines for the Management of Graves’ Orbitopathy. Eur Thyroid J 2016;5:9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li CX, Li TS, Zhu Z, et al. Advance in studies on anti-inflammatory and immunoregulatory monomers of Tripterygium wilfordii. China J Chin Materia Medica 2014;39:4159–64. [PubMed] [Google Scholar]
- [8].Wang HL, Jiang Q, Feng XH, et al. Tripterygium wilfordii Hook F versus conventional synthetic disease-modifying anti-rheumatic drugs as monotherapy for rheumatoid arthritis: a systematic review and network meta-analysis. BMC Complement Altern Med 2016;16:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu L, Zhao H, Sun X, et al. Efficacy and safety of Tripterygium wilfordii hook F for chronic urticaria: a systematic review and meta-analysis. BMC Complement Altern Med 2018;18:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang JW, Zou XR, Wang CJ, et al. Tripterygium glycosides in treatment of henoch-schonle in purpura nephritis: a systematic review of randomized controlled trials. China J Chin Materia Medica 2018;43:2806–16. [DOI] [PubMed] [Google Scholar]
- [11].Liu W, Yan L, Zhu Q, et al. Therapeutic effect of Tripterygium glycosides plus prednisone on moderate active systemic lupus erythematosus. J Chin Pract Diag Ther 2014;28:1234–5. [Google Scholar]
- [12].Wang D, Zhao XH, Cui Y, et al. Efficacy and safety of Tripterygium wilfordii Hook F for CKD in Mainland China: a systematic review and meta-analysis. Phytother Res 2018;32:436–51. [DOI] [PubMed] [Google Scholar]
- [13].Wu WH, Wang H, Zhang MP, et al. Effects of Tripterygium on diabetic nephropathy: a systematic review. Chin J Evid-Based Med 2010;10:693–9. [Google Scholar]
- [14].Li H, Guo F, Luo YC, et al. Efficacy of tripterygium glycosides tablet in treating ankylosing spondylitis: a systematic review and meta-analysis of randomized controlled trials. Clin Rheumatol 2015;34:1831–8. [DOI] [PubMed] [Google Scholar]
- [15]. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org. [Google Scholar]
- [16].Ao W. Observation on the curative effect of tabazole combined with prednisone and tripterygium wilfordii polyglucoside in the treatment of patients with exophthalmia of hyperthyroidism. Med J Chin People's Health 2015;27:95–6. [Google Scholar]
- [17].Chi PW. Clinical analysis of tripterygium wilfordii polyglycosides combined with methimazole and prednisone in the treatment of hyperthyroid exophthalmos. J North Pharm 2017;14:34–5. [Google Scholar]
- [18].Gao X. Clinical efficacy evaluation of tripterygium wilfordii polyglycosides combined with tabazole and prednisone on hyperthyroidism exophthalmia. Guide China Med 2018;16:187–8. [Google Scholar]
- [19].Gou XY, Cheng G. Clinical study on certirizine combined tripterygium glycosides for treating 24 cases of thyroid associated ophthalmopathy. China Pharmaceut 2012;21:85–6. [Google Scholar]
- [20].He XH, Kong DM. Clinical observation on the curative effect of tripterygium wilfordii polyglycosides combined with low-dose prednisone in the treatment of 23 cases of Graves ophthalmopathy. J of New Chin Med 2010;24:65–6. [Google Scholar]
- [21].Jiang WH. Clinical observation on the curative effect of tripterygium wilfordii polyglycosides combined with tabazole and prednisone in the treatment of hyperthyroidism exophthalmos. World Health Digest 2013;22:178–9. [Google Scholar]
- [22].Li XH. Clinical analysis of tripterygium wilfordii polyglycosides combined with tabazole and prednisone in the treatment of hyperthyroidism exophthalmos. Clin Res 2015;23:60–1. [Google Scholar]
- [23].Li XL, Ma T. Clinical effect of tripterygium glycosides combined with tazobactam and prednisone on patients with hyperthyroid exophthalmos. Clin Res Pract 2017;2:75–6. [Google Scholar]
- [24].Liao XD. Observation on the curative effect of tabazole, prednisone combined with tripterygium wilfordii polyglycosides in the treatment of hyperthyroidism exophthalmos. China Health Care Nutr 2012;22:1514–5. [Google Scholar]
- [25].Luo J, Huang J, Ye M. The observation of curative effect of glucocorticoids combined with Glucosida Tripterygii TOTA in the treatment of Graves’ ophthalmopathy. Pract J Clin Med 2015;5:174–6. [Google Scholar]
- [26].Ma CF, Wang YS. Clinical effect of tripterygium glycosides combined with methimazole in the treatment of hyperthyroidism exophthalmos. World Latest Med Inf 2016;16:104. [Google Scholar]
- [27].Wang W, Yang B, Sun HJ, et al. Clinical study of 131I and glucoside tripterygium total tablets on Graves’ ophthalmopathy. Chin J Nucl Med 2004;24:166–7. [Google Scholar]
- [28].Wei XB. Analysis on the curative effect of tripterygium wilfordii polyglycosides in the treatment of hyperthyroidism exophthalmia. China Modern Doctor 2009;47:97–101. [Google Scholar]
- [29].Xie H, Sun L, Shu XC, et al. Combination of technetium [99Tc] methylenediphosphonate, dexamasone and tripterygium glucosides in treatment of Graves ophthalmopathy. Chin J New Drugs Clin Rem 2007;26:905–8. [Google Scholar]
- [30].Xu JP, Xu C, Chen J, et al. Peripheral blood cell factors of Graves ophthalmopathy and effect of intervention with tripterygium glycosides. China J Chin Materia Medica 2014;39:544–7. [PubMed] [Google Scholar]
- [31].Zhang ZY, Wang JH. Tripterygium wilfordii polyglycosides combined with tabazole and prednisone in the treatment of hyperthyroidism exophthalmia. J Med Forum 2010;31:110–1. [Google Scholar]
- [32].Zhang JF, Kong YZ, Pan HZ. Clinical observation on the curative effect of tripterygium wilfordii polyglycosides in the treatment of hyperthyroidism exophthalmos. Chin Rural Health Serv Admin 2014;34:761–2. [Google Scholar]
- [33].Zheng ST, Zhang B, Mei F. Multiple enhanced immunosuppression for hyperthyroidism exophthalmia--yunke, niemesuride and tripterygium wilfordii polyglucoside tablets for Graves’ ophthalmopathy. World Health Digest 2010;7:65–6. [Google Scholar]
- [34].Zuo LJ, Yang JS. Efficacy of 131I combined with tripterygium wilfordii polyglycosides in the treatment of Graves’ ophthalmopathy. Chinese Health Care 2007;15:51–2. [Google Scholar]
- [35].Khong JJ, McNab AA, Ebeling PR, et al. Pathogenesis of thyroid eye disease: review and update on molecular mechanisms. Br J Ophthalmol 2016;100:142–50. [DOI] [PubMed] [Google Scholar]
- [36].Yang D, Hiromatsu Y, Hoshino T, et al. Dominant infiltration of T(H)1-type CD4+ Tcells at the retrobulbar space of patients with thyroid-associated ophthalmopathy. Thyroid 1999;9:305–10. [DOI] [PubMed] [Google Scholar]
- [37].Förster G, Otto E, Hansen C, et al. Analysis of orbital T cells in thyroid-associated ophthalmopathy. Clin Exp Immunol 1998;112:427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pappa A, Calder V, Ajjan R, et al. Analysis of extraocular muscle-infiltrating T cells in thyroid-associated ophthalmopathy (TAO). Clin Exp Immunol 1997;109:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].de Carli M, D’Elios MM, Mariotti S, et al. Cytolytic T cells with Th1-like cytokine profile predominate in retroorbital lymphocytic infiltrates of Graves’ ophthalmopathy. J Clin Endocrinol Metab 1993;77:1120–4. [DOI] [PubMed] [Google Scholar]
- [40].Hiromatsu Y, Yang D, Bednarczuk T, et al. Cytokine profiles in eye muscle tissue and orbital fat tissue from patients with thyroid-associated ophthalmopathy. J Clin Endocrinol Metab 2000;85:1194–9. [DOI] [PubMed] [Google Scholar]
- [41].Aniszewski JP, Valyasevi RW, Bahn RS. Relationship between disease duration and predominant orbital T cell subset in Graves’ ophthalmopathy. J Clin Endocrinol Metab 2000;85:776–80. [DOI] [PubMed] [Google Scholar]
- [42].Hu DJ, Peng ZY, He DC. Advances in pharmacological effects of Tripterygium wilfordii. Herald Med 2018;37:586–92. [Google Scholar]
- [43].Wu MF, Li B. Research progress on pharmacological action and treatment of immune dermatosis of Tripterygium Wilfordii. Chinese J Int Trad Western Med 2018;38:1271–6. [Google Scholar]
- [44].Yang Y, Liu Z, Tolosa E, et al. Triptolide induces apoptotic death of T lymphocyte. Immunopharmacology 1998;40:139–49. [DOI] [PubMed] [Google Scholar]
- [45].Kusunoki N, Yamazaki R, Kitasato H, et al. Triptolide, an active compound identified in a traditional Chinese herb, induces apoptosis of rheumatoid synovial fibroblasts. BMC Pharmacol 2004;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Huber AK, Jacobson EM, Jazdzewski K, et al. Interleukin(IL)-23 receptor is a major susceptibility gene for Graves’ ophthalmopathy: the IL-23/T-helper 17 axis extends to thyroid autoimmunity. J Clin Endocrinol Metab 2008;93:1077–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wu YQ, Zhao JY, Chen Q, et al. Tripterygium for henoch-schonlein purpura nephritis in Children: systematic review and meta analysis. Chin Arch Trad Chin Med 2016;34:1497–503. [Google Scholar]
- [48].Yang DM, Liu J. Study progress on clinical application and adverse reactions of tripterygium glycosides. Chin J Hospital Pharm 2018;38:2185–90. [Google Scholar]
- [49]. Dong YZ, Zhang B, Lin ZJ, Zhang XM. Pharmacodynamic effect and virulent effect of Tripterygium wilfordii based on network pharmacology. China Journal of Chinese Materia Medica. [DOI] [PubMed] [Google Scholar]
- [50].Li XJ, Jiang ZZ, Zhang LY. Triptolide: progress on research in pharmacodynamics and toxicology. J Ethnopharmacol 2014;155:67–9. [DOI] [PubMed] [Google Scholar]
- [51].Zhao QH, Li XY, Feng Q, et al. Evaluation on dosage-based efficacy-toxicity correlation of Tripterygium wilfordii against immune in mice. China J Chin Materia Medica 2015;40:1139–43. [PubMed] [Google Scholar]


