Supplemental Digital Content is available in the text
Keywords: GNAS, IPMN, McCune Albright syndrome, secretin-test
Abstract
Rationale:
Intraductal papillary and mucinous neoplasms of the pancreas (IPMN) are preneoplastic lesions diagnosed with an increasing incidence. Recently, several groups have described, in up to 70% of IPMN, activating mutations of the G-protein alpha stimulatory sub-unit (Gsα subunit) gene (GNAS). GNAS-activating somatic, post-zygotic, mutations are also associated with McCune-Albright syndrome (MCAS) characterized by fibrous dysplasia, precocious puberty, and café-au-lait spots.
Patient concerns:
We herein report a patient with McCune Albright Syndrome that presented with malignant IPMN and underwent pancreatic resection.
Diagnoses and interventions:
Leucocyte and duodenum juice DNA analysis, endoscopically collected from secretin-stimulated pancreatic juice revealed the same (GNAS) activating mutation also found in the invasive pancreatic colloid adenocarcinoma arising from intestinal subtype IPMN.
Outcomes:
Thirty months after surgery, the patient was alive with recurrence (bone only metastasis).
Lessons:
In this observation, we show that MCAS should be view as a new genetic predisposition to IPMN associated pancreatic cancer, and consequently a targeted screening in this high-risk population might be proposed.
1. Introduction
McCune-Albright syndrome (MCAS) is a rare disorder characterized by polycystic fibrous dysplasia, precocious puberty, and café au lait spots. It is caused by somatic, post-zygotic, with mosaic distribution, GNAS activating mutations.[1] In addition to other hepatobiliary neoplasms,[2,3] IPMN have been associated with MCAS.
Somatic activating mutations of the G-protein alpha stimulatory subunit (Gsα subunit) encoded by the GNAS gene (GNAS) have been reported in up to 70% of pancreatic intraductal papillary mucinous neoplasms (IPMN),[4–7] that is a precursor of pancreatic adenocarcinoma. In this setting, GNAS mutations, known to lead to elevated intracellular cAMP levels and activation of downstream dependent pathways,[1] open new clinical insights on IPMN. As an example, IPMN intestinal pattern of differentiation is associated with GNAS mutation[8] underlining the functional consequences of GNAS activating mutation. Once symptomatic, pancreatic adenocarcinoma is associated with a dismal prognosis. Identifying individuals at risk and detecting early lesions are crucial to improve patient's outcome. Several conditions have been found to be associated with an increased risk of pancreatic adenocarcinoma, and targeted screening of high-risk individuals is important.
The aim of the present study is to examine the mutation status of GNAS in a patient with McCune Albright Syndrome and IPMN who underwent pancreatic resection.
2. Case report
A 50-year old woman, 144 cm for 58 kg, initially presented with abdominal pain. Patient has provided informed consent for publication of the case. She was previously diagnosed with McCune Albright Syndrome (MCAS) with severe fibrous dysplasia and precocious puberty. She had a past medical history of total thyroidectomy, and multiple surgery for fractures. Cross sectional imaging (Fig. 1) and endoscopic ultrasonography reveled a global main pancreatic duct dilatation over 10 mm associated with a cephalic 25-mm enhanced mural nodule with portal vein lateral abutment, without distant metastasis. Fine-needle aspiration pathology confirmed an IPMN related colloid pancreatic adenocarcinoma. Leucocyte and duodenum juice deoxyribose nucleic acid (DNA) analysis[9] (endoscopically collected from secretin-stimulated pancreatic juice; Supplemental Video) revealed the same G-protein alpha stimulatory sub-unit (Gsα subunit) gene (GNAS) (NM_000516) activating mutation c.601C>T (p.Arg201Cys) (Fig. 2). The same mutation was also detected in plasma circulating DNA. The patient underwent pancreaticoduodenectomy after 3 months of FOLFIRINOX neoadjuvant chemotherapy. Pathological examination revealed an invasive pancreatic colloid adenocarcinoma (Supplemental Fig.) (ypT2 N1 R0) arising from intestinal subtype IPMN (ie, MUC1-, MUC2+, MUC5AC+ immunohistochemistry) with a major (over 90%) response to chemotherapy. Genetic analysis of the IPMN revealed a GNAS (NM_000516) activating mutation c.601C>T (p.Arg201Cys), which was not detected at a 2% variant allele frequency threshold in the adjacent normal pancreas. No KRAS or other driver mutation was detected in the IPMN associated cancer with a targeted 50 genes NGS panel. Imaging and medical work-up, plasma circulating tumor DNA, Formalin-Fixed Paraffin Embedded and pancreatic juice somatic mutation analysis technics are available in Supplemental Material. Thirty months after surgery, the patient is alive with recurrence (bone only metastasis).
Figure 1.
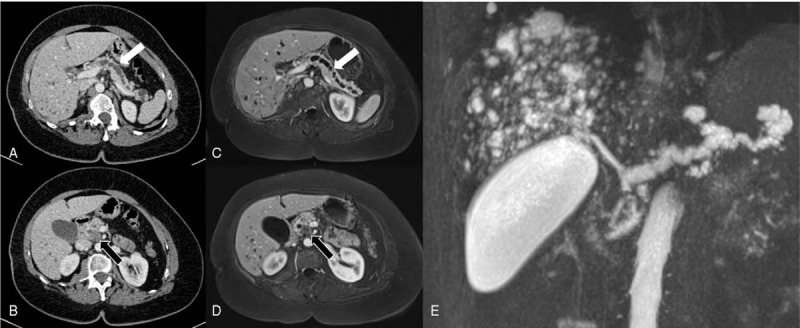
Cross sectional imaging. A: Portal phase enhanced CT scan showing main pancreatic duct dilatation (white arrow). B: Portal phase enhanced CT scan showing pancreatic head adenocarcinoma (black arrow). C: T1 Gadolinium enhanced MRI showing main pancreatic duct dilatation (white arrow). D: Gadolinium enhanced MRI showing pancreatic head adenocarcinoma (black arrow). E: CP-MRI showing main pancreatic duct dilatation.
Figure 2.
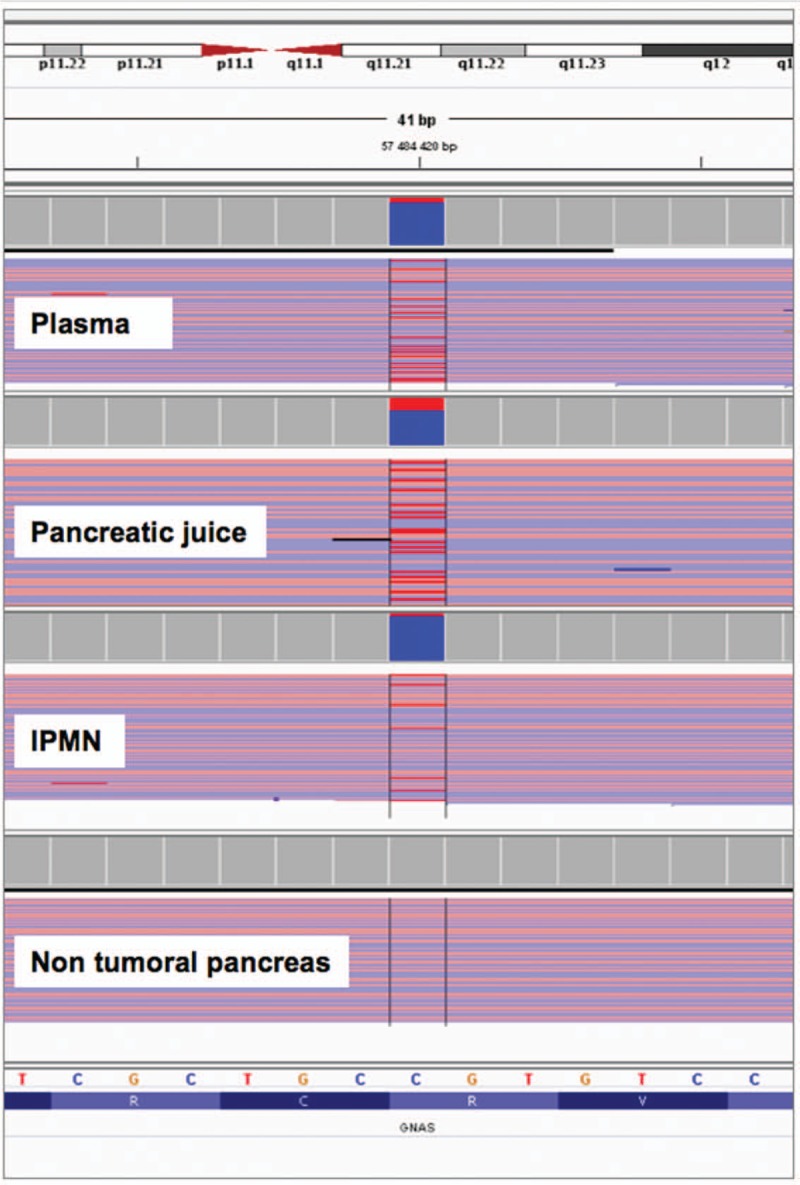
GNAS sequencing reads: GNAS (NM_000516): c.601C>T, p.Arg201Cys mutation was detected in 13% of NGS reads in plasma, 29% sequencing reads in pancreatic juice, 8% sequencing reads in IPMN associated cancer and was not detected in non-tumoral pancreas (threshold set at 2%). Reads were visualized by uploading bam files into Integrative Genomics Viewer the variant T allele is shown in red.
3. Discussion
Intraductal papillary mucinous neoplasm (IPMN) can be seen as a “recent” disease and has only been clearly individualized in the mid 80's. Since then, its diagnosis, description, comprehension and management have been significantly improved.[10–13]
The adenoma-carcinoma sequence leading to the development of pancreatic adenocarcinoma is currently under investigation but include as major events telomere shortening, KRAS activating mutation, loss and/or mutation of SMAD4 and p53.[14] Since recent whole-exome analysis, GNAS mutations also appear to have a key role in IPMN pathogenesis.[15] With KRAS, it is therefore, one of the 2 most prevalent mutations in these tumors. It may occur alone or in association with KRAS activating mutations and could define a specific progression pathways in IPMN-associated carcinoma.[15–17]GNAS-activating mutations are reported in both IPMN and MCAS, and IPMN is a MCAS associated lesion.[18,19] This emphasizes the important role of GNAS in pancreatic tumorigenesis.[5]GNAS- driven pancreatic tumorigenesis is associated with IPMN intestinal phenotype[8] and colloid pancreatic adenocarcinoma,[16] and a less aggressive disease, with better long-term outcome.[20] IPMN occur most of the time as a sporadic disease. Some[21–23] previously reported familial forms of IPMN in few kindred, suggesting predisposing genetic alteration. So far they were not found, and neither BRCA2, p16 nor CDKN2A were constitutionally mutated or lost.[21] If familial forms of pancreatic adenocarcinoma are now well known,[24,25] familial forms of pancreatic adenocarcinoma have not been formally described.
First, this observation underlines the need for a specific screening for high-risk patients identified by their known genetic predisposition, and MCAS should be considered as a pancreatic cancer predisposition syndrome. Second, it is now possible to determine preoperatively GNAS status from plasma circulating DNA, duodenum juice DNA collected after secreting stimulation test, or DNA from extracted paraffin-embedded tissue from EUS-FNA and to identify at least intestinal IPMN phenotype. If up to now this information has a limited value,[26] it is likely that in a near future it will help to tailor pancreatic cyst and IPMN management.[27]
Overall, this observation provides additional evidence of MCAS as a new genetic predisposition to IPMN associated pancreatic cancer, and consequently the need for a specific screening in this population.
Author contributions
Conceptualization: Sebastien Gaujoux, Eric Pasmant, Caroline Silve, Frédéric Prat, Karen Leroy.
Data curation: Sebastien Gaujoux, Romain Coriat, Frédéric Prat, Karen Leroy.
Formal analysis: Sebastien Gaujoux, Eric Pasmant, Caroline Silve, Nadia Mehsen-Cetre, Alexandre Rouquette, Bertrand Dousset, Frédéric Prat, Karen Leroy.
Funding acquisition: Sebastien Gaujoux, Romain Coriat, Bertrand Dousset, Frédéric Prat, Karen Leroy.
Investigation: Sebastien Gaujoux, Caroline Silve, Nadia Mehsen-Cetre, Bertrand Dousset, Frédéric Prat, Karen Leroy.
Methodology: Sebastien Gaujoux, Eric Pasmant, Caroline Silve, Romain Coriat, Alexandre Rouquette, Frédéric Prat, Karen Leroy.
Project administration: Sebastien Gaujoux, Eric Pasmant, Romain Coriat, Alexandre Rouquette, Frédéric Prat, Karen Leroy.
Resources: Sebastien Gaujoux, Caroline Silve, Nadia Mehsen-Cetre, Bertrand Dousset, Frédéric Prat, Karen Leroy.
Software: Sebastien Gaujoux, Eric Pasmant, Alexandre Rouquette, Frédéric Prat, Karen Leroy.
Supervision: Sebastien Gaujoux, Caroline Silve, Romain Coriat, Alexandre Rouquette, Frédéric Prat, Karen Leroy.
Validation: Sebastien Gaujoux, Nadia Mehsen-Cetre, Romain Coriat, Bertrand Dousset, Frédéric Prat, Karen Leroy.
Visualization: Sebastien Gaujoux, Eric Pasmant, Alexandre Rouquette, Frédéric Prat, Karen Leroy.
Writing – original draft: Sebastien Gaujoux, Frédéric Prat, Karen Leroy.
Writing – review & editing: Sebastien Gaujoux, Eric Pasmant, Caroline Silve, Nadia Mehsen-Cetre, Romain Coriat, Alexandre Rouquette, Bertrand Dousset, Frédéric Prat, Karen Leroy.
Sebastien Gaujoux orcid: 0000-0002-1072-7639.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: DNA = deoxyribose nucleic acid, EUS-FNA = endoscopic ultrasound guided fine-needle aspiration, GNAS = G-protein alpha stimulatory sub-unit (Gsα subunit) gene, IPMN = intraductal papillary and mucinous neoplasms of the pancreas, MCAS = McCune-Albright syndrome, NGS = next-generation sequencing.
How to cite this article: Gaujoux S, Pasmant E, Silve C, Mehsen-Cetre N, Coriat R, Rouquette A, Douset B, Prat F, Leroy K. McCune Albright syndrome is a genetic predisposition to intraductal papillary and mucinous neoplasms of the pancreas associated pancreatic cancer in relation with GNAS somatic mutation – a case report. Medicine. 2019;98:50(e18102).
FP and KL are co-authors.
The authors have no funding and conflicts of interests to disclose.
Informed written consent was obtained from the patient for publication of this case report and accompanying images.
Supplemental Digital Content is available for this article.
References
- [1].Weinstein LS, Shenker A, Gejman PV, et al. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med 1991;325:1688–95. [DOI] [PubMed] [Google Scholar]
- [2].Nault JC, Fabre M, Couchy G, et al. GNAS-activating mutations define a rare subgroup of inflammatory liver tumors characterized by STAT3 activation. J Hepatol 2012;56:184–91. [DOI] [PubMed] [Google Scholar]
- [3].Gaujoux S, Salenave S, Ronot M, et al. Hepatobiliary and pancreatic neoplasms in patients with McCune-Albright syndrome. J Clin Endocrinol Metab 2014;99:E97–101. [DOI] [PubMed] [Google Scholar]
- [4].Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med 2011;3:92ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wu J, Jiao Y, Dal Molin M, et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A 2011;108:21188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kanda M, Knight S, Topazian M, et al. Mutant GNAS detected in duodenal collections of secretin-stimulated pancreatic juice indicates the presence or emergence of pancreatic cysts. Gut 2013;62:1024–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Furukawa T, Kuboki Y, Tanji E, et al. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep 2011;1:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dal Molin M, Matthaei H, Wu J, et al. Clinicopathological correlates of activating GNAS mutations in intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg Oncol 2013;20:3802–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Robinson JT, Thorvaldsdottir H, Winckler W, et al. Integrative genomics viewer. Nat Biotechnol 2011;29:24–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Falconi M, Crippa S, Chari S, et al. Quality assessment of the guidelines on cystic neoplasms of the pancreas. Pancreatology 2015;15:463–9. [DOI] [PubMed] [Google Scholar]
- [11].Tanaka M, Fernandez-Del Castillo C, Kamisawa T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017;17:738–53. [DOI] [PubMed] [Google Scholar]
- [12].Pancreas ESGoCTot. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018;67:789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Elta GH, Enestvedt BK, Sauer BG, et al. ACG clinical guideline: diagnosis and management of pancreatic cysts. Am J Gastroenterol 2018;113:464–79. [DOI] [PubMed] [Google Scholar]
- [14].Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet 2011;378:607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Patra KC, Bardeesy N, Mizukami Y. Diversity of precursor lesions for pancreatic cancer: the genetics and biology of intraductal papillary mucinous neoplasm. Clin Transl Gastroenterol 2017;8:e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tan MC, Basturk O, Brannon AR, et al. GNAS and KRAS mutations define separate progression pathways in intraductal papillary mucinous neoplasm-associated carcinoma. J Am Coll Surg 2015;220:845–54. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ban S, Naitoh Y, Mino-Kenudson M, et al. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: its histopathologic difference between 2 major types. Am J Surg Pathol 2006;30:1561–9. [DOI] [PubMed] [Google Scholar]
- [18].Wood LD, Noe M, Hackeng W, et al. Patients with McCune-Albright syndrome have a broad spectrum of abnormalities in the gastrointestinal tract and pancreas. Virchows Arch 2017;470:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gaujoux S, Salenave S, Ronot M, et al. Hepatobiliary and pancreatic neoplasms in patients with Mccune-Albright syndrome. J Clin Endocrinol Metab 2014;99:E97–101. [DOI] [PubMed] [Google Scholar]
- [20].Yopp AC, Katabi N, Janakos M, et al. Invasive carcinoma arising in intraductal papillary mucinous neoplasms of the pancreas: a matched control study with conventional pancreatic ductal adenocarcinoma. Ann Surg 2011;253:968–74. [DOI] [PubMed] [Google Scholar]
- [21].Rebours V, Couvelard A, Peyroux JL, et al. Familial intraductal papillary mucinous neoplasms of the pancreas. Dig Liver Dis 2011;44:442–6. [DOI] [PubMed] [Google Scholar]
- [22].Denost Q, Chafai N, Arrive L, et al. Hereditary intraductal papillary mucinous neoplasm of the pancreas. Clin Res Hepatol Gastroenterol 2012;36:e23–5. [DOI] [PubMed] [Google Scholar]
- [23].LaFemina J, Roberts PA, Hung YP, et al. Identification of a novel kindred with familial pancreatitis and pancreatic cancer. Pancreatology 2009;9:273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Petersen GM. Familial pancreatic cancer. Semin Oncol 2016;43:548–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhen DB, Rabe KG, Gallinger S, et al. BRCA1, BRCA2, PALB2, and CDKN2A mutations in familial pancreatic cancer: a PACGENE study. Genet Med 2015;17:569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rong Y, Wang D, Xu C, et al. Prognostic value of histological subtype in intraductal papillary mucinous neoplasm of the pancreas: a retrospective analysis of outcome from one single center. Medicine (Baltimore) 2017;96:e6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Springer S, Wang Y, Dal Molin M, et al. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology 2015;149:1501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


