Abstract
Alzheimer disease (AD) is the most common neurodegenerative brain disease that causes cognitive impairment in the elderly. Behavioral and psychological symptoms of dementia (BPSD), also known as neuropsychiatric symptoms, represent a heterogeneous group of non-cognitive symptoms and behaviors for AD patients. Sleep disorder is one closely-related psychiatric symptom of AD. In this cross-section study, we aimed to investigate the characteristics of sleep status and BPSD among AD patients in Eastern China and to assess the relationship among sleep disorder, BPSD, and cognition.
A total of 176 participants were enrolled in the study, including 84 AD patients and 92 healthy individuals as controls. Mini-mental state examination (MMSE), cooperative study-activities of daily living (ADCS-ADL) and clinical dementia rating (CDR) were used to measure cognition, the competence in basic and instrumental activities of daily living, and severity of dementia, respectively. BPSD were evaluated by neuropsychiatric inventory (NPI). Pittsburgh sleep quality index (PSQI) and Epworth sleepiness scale were designed to assess the sleep status and daytime naps. Spearman correlation analyses were performed to determine the relations between PSQI, MMSE, ADCS-ADL, and NPI scores and CDR.
Sleep disorders occurred in 55.9% of AD patients versus only 15.2% of controls. 89.2% of AD patients had BPSD while only 22.9% of controls did, with apathy (64.2%) the most common among AD patients. Among AD patients, PSQI was negatively correlated with both MMSE (r = −0.600, P < .01) and ADCS-ADL (r = −0.725, P < .01), and was positively correlated with total NPI score (r = 0.608, P < .01). PSQI was closely associated with depression (r = 0.653, P < .01) and apathy (r = 0.604, P < .01).
This study showed that AD patients have a higher prevalence of sleep disorders and BPSD than healthy elderly adults. Sleep disorders affect cognition of AD patients and increase apathy and depression. These results can help investigate new therapeutic targets in AD treatments.
Keywords: Alzheimer disease, behavioral and psychological symptoms of dementia, cognitive function, sleep disorders
1. Introduction
Alzheimer disease (AD) is the most common neurodegenerative brain disease and causes cognitive impairment and dementia. AD mainly affects structures of the cerebral cortex and hippocampus, leading to memory impairment and visual space dysfunction. Behavioral and psychological symptoms in dementia (BPSD), a term that is defined as signs and symptoms of disturbed perception, thought content, mood, or behavior of patients with dementia,[1] represents a heterogeneous group of noncognitive symptoms and behaviors in patients with dementia. For a long time, the cognitive symptoms of AD, such as amnesia syndrome BPSD, and noncognitive neuropsychiatric symptoms, were often neglected. The incidence of neuropsychiatric symptoms in AD ranges from 70% to 100%.[2–7] Sleep disorders are closely-related psychiatric symptoms of AD. Sleep disturbance can accelerate cognitive deterioration and the progression of other neuropsychiatric symptoms in AD patients. Sleep disorders also lower the quality of life for patients and increase stress among caregivers.[8–11]
Although current treatments cannot halt or reverse AD progression, effective treatment can delay its progression and improve the life quality in patients.[12,13] Improving sleep disorders and preventing other BPSD are important in treating AD patients. Hence, it is very important to identify sleep disorders in these patients and provide early clinical intervention to slow the progression of cognition deterioration and to improve neuropsychiatric symptoms.[12–14]
In this study, we examined the occurrence and severity of sleep disorders and BPSD in AD patients and healthy elderly adults, to investigate the characteristics of sleep disorders and the correlation between cognition, sleep disorders and other neuropsychiatric symptoms in AD patients.
2. Methods
2.1. Setting and participants
The present study was conducted at the Memory Clinic of Department of Neurology, Qilu Hospital of Shandong University in Eastern China, from May 2015 to September 2015. The 176 enrolled participants comprised 84 patients with newly-diagnosed AD who had not been medicated and 92 age- and sex-matched healthy elderly adults as the control group. The diagnoses of AD were carried out according to criteria of the National Institute of Neurological and Communicative Disorders [14] by 2 experienced psychiatrists with at least 2-year experience at the Memory Clinic. Each subject had a structured clinical interview, routine laboratory tests, physical and neurological examinations, and brain magnetic resonance imaging. We applied the following exclusion criteria: history of significant head trauma, history of neurological disorders, major psychiatric disorders, use of psychoactive medication, and history of abuse of alcohol or other substances. We also excluded patients with leukoaraiosis diagnosed with brain magnetic resonance imaging to avoid the possible influence of other diseases, such as cerebrovascular diseases and toxic and metabolic abnormalities.
This study was approved by the Ethical Committee at Qilu Hospital. Written fully-informed consents were obtained from all participants and their caregivers.
2.2. Behavioral assessment
All participants took the behavioral assessment. The instruments used to evaluate BPSD and sleep quality were applied during AD diagnosis.
2.2.1. Mini-mental state examination (MMSE)
The MMSE is a brief 30-item questionnaire that is used to estimate the severity of cognitive impairment.[15] In this study, we used the Chinese version of MMSE, developed by Raina et al (2009), which has good reliability and validity.[16]
2.2.2. Clinical dementia rating (CDR)
The CDR scale is a staging instrument with good reliability and validity, which is used to evaluate and quantify the severity of dementia symptom.[17]
2.2.3. AD cooperative study-activities of daily living (ADCS-ADL)
The ADCS-ADL is a list of questionnaire with 23 Likert-type questions for functional assessment, in which respondents must express their degrees of agreement or disagreement with each item on the questionnaire with respect to level of independence (independent, partially independent, and totally dependent). The questionnaire was used to separate the performance of patients during the previous month into 3 categories: basic (BADL), instrumental (IADL), and advanced ADL.
The IADL subscale is designed to measure higher-level functional activities such as food preparation and shopping with scores ranging from 0 to 56. The BADL subscale measures more rudimentary activities such as dressing and bathing with scores ranging from 0 to 22.
2.2.4. Neuropsychiatric inventory (NPI)
The NPI is an informant-based rating system used to measure various BPSD in AD, such as delusions, hallucinations, agitation/aggression, dysphoria/depression, anxiety, euphoria/elation, apathy/indifference, disinhibition, irritability/lability, aberrant motor behaviors, night-time behavioral disturbances, and appetite/eating disturbances. The informant rates the frequency and severity of each of these dimensions and the final score is calculated by multiplying the frequency and severity scores. Each domain in the NPI includes a screening question. If the answer for this question is not positive, the interviewer moves to the next domain. If the response to the screening question is positive, specific behavioral symptoms within that domain are assessed. If any of these domain symptoms presents, the symptom is rated on both a 4-point scale (for frequency) and a separate 3-point scale (for severity). Scores for each dimension range from 0 to 12 with the maximum total score for the 12-item version of NPI at 144.[18]
2.2.5. Pittsburgh sleep quality index (PSQI)
The detailed profiles of nighttime sleep problems of all AD patients were analyzed using PSQI. This index is well-validated and widely used. It is based on a self-assessment questionnaire that evaluates problems with sleep occurring during the previous 1 month.[19] The PSQI consists of 19 items and yields a 7-component score for subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. Total PSQI scores range from 0 to 21, with a higher score representing worse sleep status. A patient with a total PSQI score of ≥5 is considered a poor sleeper.[19]
2.2.6. Epworth sleepiness scale (ESS)
All patients completed the ESS[20] to evaluate the level of general daytime sleepiness during the previous few weeks. Higher ESS scores (range: 0–24) indicate better sleep propensity. Normal values are between 2 and 10, and scores >14 indicate a high level of daytime sleepiness.[20,21]
2.3. Statistical analysis
All data were analyzed using SPSS version 17.0 (SPSS Inc, Chicago, IL). Differences between the baseline characteristics of AD patients and controls were assessed using the unpaired, 2-tailed Student t test. Spearman correlation coefficients and significance levels were used to evaluate correlations among cognitive function, BPSD, and sleep problems. The level of statistical significance was set at P < .05.
3. Results
3.1. Demographic factors
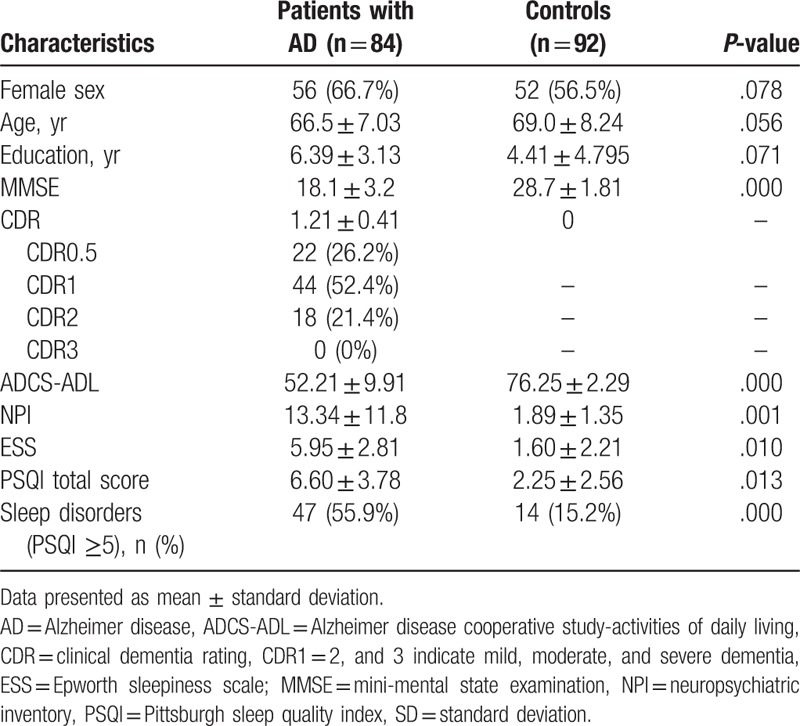
No significant difference was observed between AD and control groups in terms of age, gender, and education background (Table 1). Significant mean differences presented in both PSQI scores and ESS scores (P < .05). Poor sleepers constituted 56.0% of the AD group but only 15.2% of the control group. Sleep disturbance in patients with AD was more severe than that among participants in the control group (PSQI total score: 6.60 ± 3.78 vs 2.25 ± 2.56).
Table 1.
Participant demographics and clinical characteristics.
3.2. Disturbed sleep patterns in AD
In this study, we found that AD patients experienced sleep disorders that comprised all 7 components of the PSQI. As shown in Table 2, sleep disorders were involved in all 7 components of PSQI. Total PSQI score and PSQI components scores in AD patients were higher than those in the control group. Figure 1 showed that disturbed sleep patterns with highest incidences were nighttime sleep disturbances (64.2%), habitual sleep efficiency (58.3%), subjective sleep quality (51.1%), and daytime dysfunction (51.1%). The pattern with the lowest incidence was the use of sleeping medication (10.7%). Impairment in sleep patterns in the control group was similar to that in the experiment group, but the rate of occurrence and degree of impairment were lower in the control group. Significant differences both frequency and severity of sleep problems were found between the 2 groups, as assessed in PSQI components.
Table 2.
Comparison of sleep parameters between patients with AD and controls, according to total PSQI score and PSQI component score.
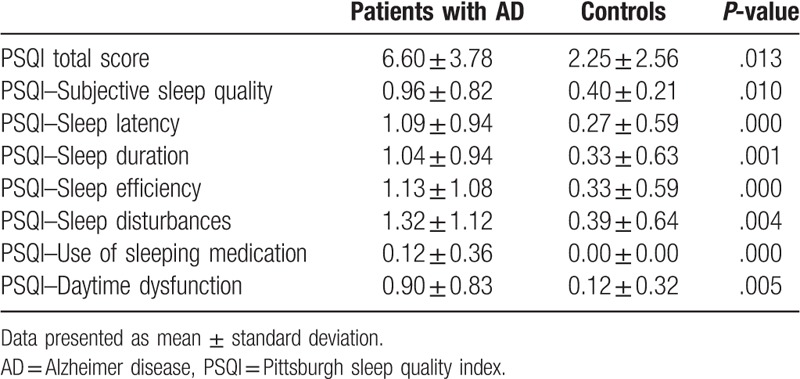
Figure 1.
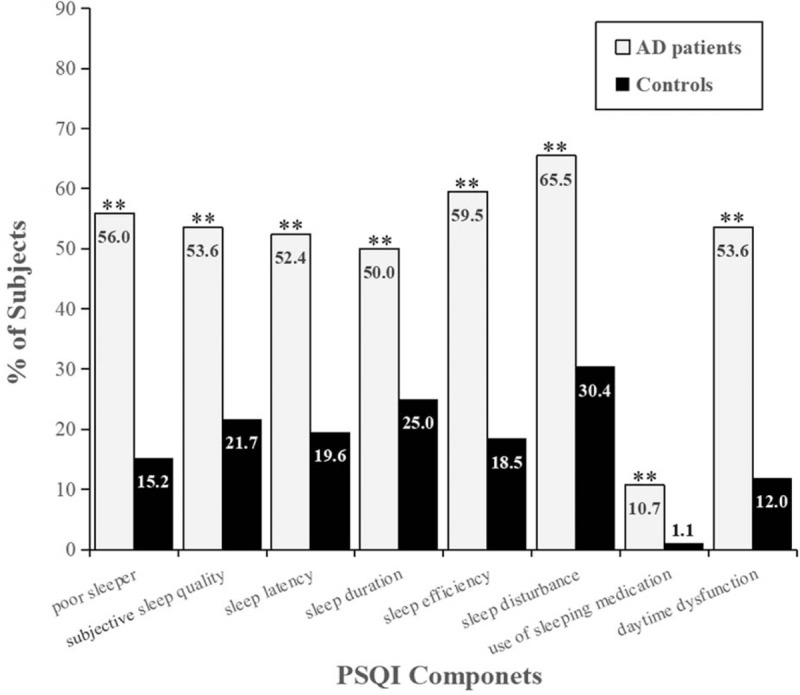
Frequency distribution of PSQI components in AD patients and controls. Percentages were shown for “poor sleeper” (PSQI total score ≤5) and each component of the PSQI. The prevalence of sleep disorders and each PSQI component in AD patients were significantly higher than those in controls. ∗P < .05, ∗∗P < .01. AD = Alzheimer disease, PSQI = Pittsburgh sleep quality index.
3.3. Distribution pattern and severity of BPSD in AD
Significant increases were found in total NPI score and 11 domain scores except appetite change in AD patients (Table 3). As shown in Figure 2, the incidence of BPSD in AD patients was 89.2% and neuropsychiatric symptoms covered all 12 domains of the NPI. The highest rate of BPSD among AD patients in this study was apathy (64.2%), followed by sleep disturbances (48.8%) and depression (46.4%). In addition to appetite change, the frequency and severity of neuropsychiatric symptoms in AD patients were significantly different from those in the control group.
Table 3.
Comparison of neuropsychiatric symptoms between patients with AD and controls, according to total NPI score and each domain score.
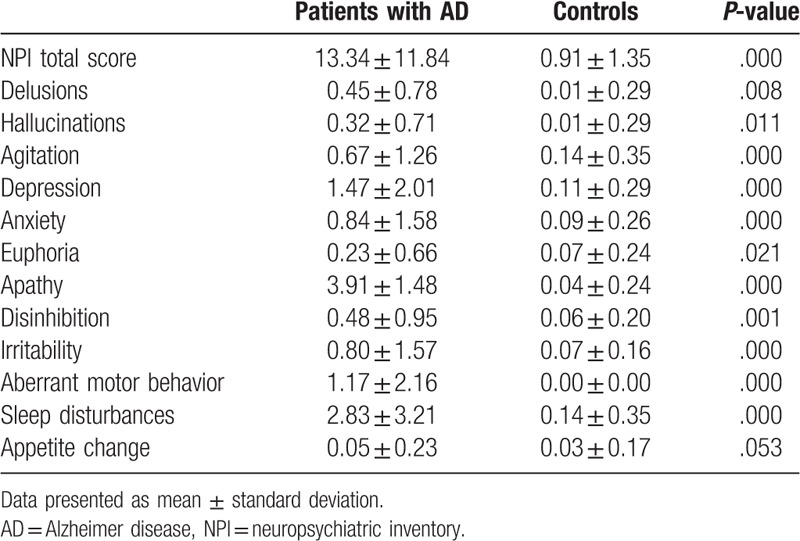
Figure 2.
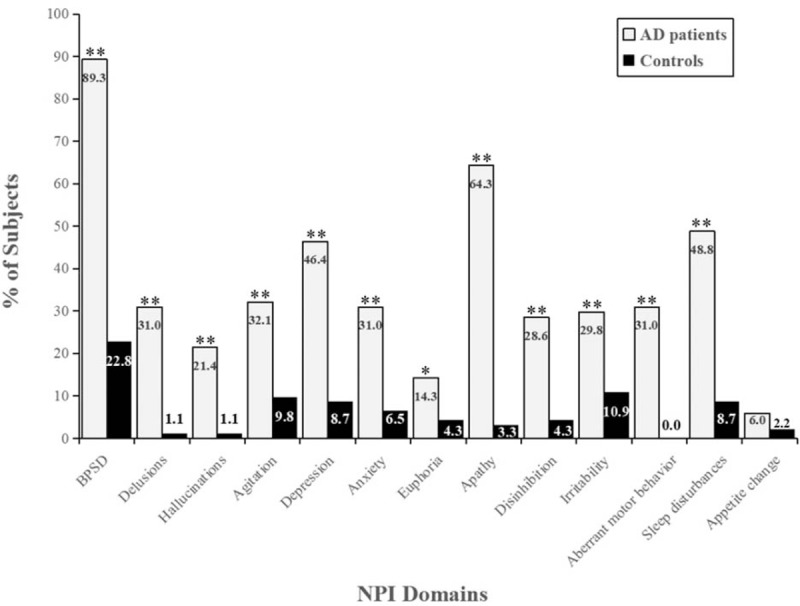
Frequency distribution of BPSD and NPI domains in AD patients and controls. Percentages were shown for BPSD (abnormal NPI) and each domain of the NPI. In addition to appetite change, the prevalence of BPSD and other NPI domains in AD patients were significantly higher than those in controls. ∗P < .05, ∗∗P < .01. AD = Alzheimer disease, BPSD = behavioral and psychological symptoms of dementia, NPI = neuropsychiatric Inventory.
3.4. Correlation analysis between sleep disorders, cognitive function, and daily living ability in AD patients
PSQI scores of both AD and control groups were further analyzed using Spearman correlation together with scores on MMSE, ADCS-ADL, NPI, and CDR. We found that total PSQI scores of AD patients had a negative correlation with MMSE (r = −0.600, P < .01) and ADCS-ADL scores (r = −0.725, P < .01) and a positive correlation with total NPI scores (r = 0.608, P < .01) (Table 4).
Table 4.
Comparison of PSQI score with assessments of cognition, severity of dementia, activities of daily living, and neuropsychiatric symptoms between patients with AD and controls.

3.5. Correlation between sleep disturbances and BPSD in AD patients
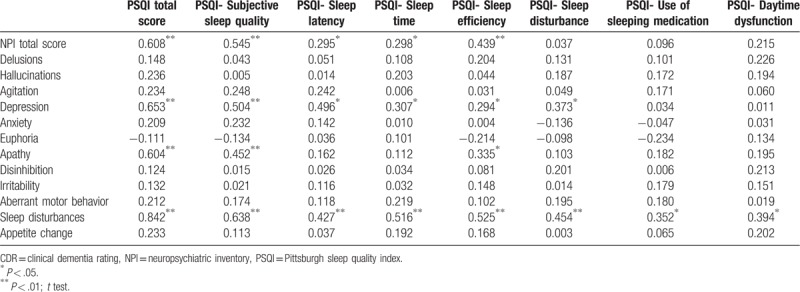
In this study, AD patients experienced prolonged sleep latency and period and decreased subjective sleep quality (r = 0.545, P < .01) and sleep efficiency (r = 0.439, P < .01), which was positively associated with the presence of psychotic symptoms. The decline in subjective sleep quality was positively associated with depression (r = 0.504, P < .01) and apathy (r = 0.452, P < .01) in AD patients. The prolonged sleep latency was positively correlated with depression (r = 0.496, P < .01). PSQI was closely related to total NPI score (r = 0.608, P < .01), especially related to depression (r = 0.653, P < .01) and apathy (r = 0.604, P < .01). Those results showed that there is a relationship between sleep disorders and BPSD in AD patients, especially the association of sleep disorders with apathy and depression (Table 5).
Table 5.
Correlation of PSQI components and NPI domains in patients with AD.
4. Discussion
In the present study, the prevalence of sleep disorders in AD patients was significantly higher and the degree of sleep disorders was greater than those in controls, which is similar to the findings of other previous studies on sleep problems and cognition.[22–31] We also found that there was a negative correlation between PSQI score and MMSE score in AD patients, indicating that the severity of the sleep disorders is associated with the significance of cognitive decline in AD. Andrew et al monitored sleep status in 737 elderly people with normal cognition for 10 consecutive days using actigraphy, with another 6-year follow-up. They found that sleep fragmentation was associated with an increased risk of AD. An individual with high sleep fragmentation had a 1.5-fold greater risk of developing AD as compared with those who had low sleep fragmentation.[32] These data imply that sleep disruption contributes to cognitive decline in AD, and sleep plays a crucial role in memory consolidation.[27]
In this study, 89.2% of AD patients had BPSD, and the most common symptom was apathy (n = 52, 64.2%). In comparison, only 21 participants (22.8%) in the control group developed BPSD.[8] We also found that sleep disorders in AD patients were closely related to apathy and depression. Hye et al reported similar finding after evaluating 63 AD patients. Our results further confirming that BPSD is more common in AD patients with sleep disorders. Interestingly, in Hye's study, 48.1% of participants in the control group with mild cognitive impairment also experienced sleep disorders and had increased BPSD.[23] In the present study, we also found that the degree and frequency of BPSD were greater in AD patients who had sleep disorders than in those who did not.
Collectively, this study suggested that there is a close relationship between sleep, cognition, and BPSD, though the exact mechanism remains unclear and needs further investigation.
Few studies have been done on the relationship between sleep disorders and BPSD in AD patients. De Oliveira et al found that behavioral symptoms have strong associations with sleep satisfaction, which is highly correlated with the sleep length of AD patients. Apathy and Anxiety are associated with the length of sleep in AD patients.[33] Using factorial analysis, Aalten and colleagues clustered neuropsychiatric symptoms in AD patients and distinguished 4 neuropsychiatric subsyndromes among the 12 NPI domains: hyperactivity, psychosis, affective symptoms, and apathy.[34] In that analysis, sleep disturbance was included in the apathy cluster. Further evidence for the association between sleep and apathy comes from the physiology of these systems, both of which are organized by the circadian clock and may have diminished amplitude in individuals with AD. In an actigraphy study, Emmanuel also found that individuals with AD and apathy had poorer nocturnal sleep quality than those who did not. It might be explained that because mood is also under circadian control,[35] apathy may be caused by the same circadian dysfunction. In fact, neurodegeneration of the suprachiasmatic nucleus, the site of the central circadian pacemaker, has been identified in individuals with AD.[36] Apathetic patients with AD who have sleep disorders have markedly disturbed circadian rhythms. Because apathy does not occur in all individuals with AD, diminished circadian oscillation would have to interact with other modulatory systems, such as ventral tegmental dopamine.[37]
Previous studies had reported that sleep disorders had detrimental effects on blood pressure (BP) profile, not only on absolute BP levels but also on short- and long-term BP variability.[38] In this regards, there is accruing evidence suggesting that high BP variability is associated with dementia status[39] and plays a detrimental effect on neurocognitive functioning and cognitive decline in AD patients.[40] It would be, hence, interesting to explore whether BP variability may mediate the relationship between sleep status, BPSD, and cognition in AD patients. This is a promising area in future research and development of new therapeutic strategies.
There are several limitations of our study. First, the evaluation of sleep disruption in the present study was based on PSQI, which is a subjective instrument and is vulnerable to memory processes, misperception, and overt or covert tendency to exaggerate number and gravity of symptoms. The use of objective tools, such as polysomnography or a combination of PSQI and other objective evaluation methods, such as daily diary, to assess sleep status, would be more persuasive. Second, in this study, we paid attention to the sleep quality of AD patients and did not assess correlation of some special sleep disturbances such as sleep apnea, the disturbance in rapid eye movement sleep with cognition in AD patients. Meanwhile, whether some concomitant diseases such as hypertension and cardiovascular-valvular disorders have influence on sleep disorders and BPSD of AD patients should also be investigated. They are crucial for us to understand AD comprehensively. Multi-disciplinary and multi-angle researches on AD are needed in the future. Third, the use of the MMSE as the instrument to estimate the severity of cognitive impairment, especially mild cognitive impairment, is subject to the influence of education level, language, and age. It should be combined with other evaluation tools for cognition. Fourth, the use of correlation instead of regression analysis to interpret data cannot indicate causality between variables and the interpretation of our results is limited by the modest size of our study population. Further studies should include larger sample size and multi-center observations, and regression analysis should be carried to identify the relationship between variables.
In conclusion, the findings of this study suggested a high prevalence of sleep disorders and BPSD in AD patients. Sleep disorders have a strong impact on cognition and daily living ability and can increase or aggravate apathy and depression in this population. We also revealed that there is a close relationship between sleep, cognition, and BPSD in AD patients. This information may be helpful to investigate therapeutic targets in clinical settings.
Acknowledgments
The authors are very grateful to all patients who participated in this project.
Author contributions
Conceptualization: Peiyan Shan.
Data curation: Guoyu Zhou.
Formal analysis: Shuangwu Liu.
Investigation: Shuangwu Liu, Xiaolin Yu, Xinjin Zhao, Lin Ma.
Methodology: Guoyu Zhou.
Project administration: Peiyan Shan.
Software: Shuangwu Liu.
Supervision: Peiyan Shan.
Writing – original draft: Guoyu Zhou.
Peiyan Shan orcid: 0000-0001-9874-2018.
Footnotes
Abbreviations: AD = Alzheimer disease, ADCS-ADL = AD cooperative study-activities of daily living and stroke and the Alzheimer Disease and related disorders association, BADL = basic activities of daily living, BPSD = behavioral and psychological symptoms of dementia, CDR = clinical dementia rating, ESS = Epworth sleepiness scale, IADL = instrumental activities of daily living, MMSE = mini-mental state examination, NPI = neuropsychiatric inventory, PSQI = Pittsburgh sleep quality index.
How to cite this article: Zhou G, Liu S, Yu X, Zhao X, Ma L, Shan P. High prevalence of sleep disorders and behavioral and psychological symptoms of dementia in late-onset Alzheimer disease: a study in Eastern China. Medicine. 2019;98:50(e18405).
The authors have no conflicts of interest to disclose.
References
- [1].Finkel SI, Costa e Silva J, Cohen G, et al. Behavioral and psychological signs and symptoms of dementia: a consensus statement on current knowledge and implications for research and treatment. Int Psychogeriatr 1996;8: Suppl 3: 497–500. [DOI] [PubMed] [Google Scholar]
- [2].Mirakhur A, Craig D, Hart DJ, et al. Behavioural and psychological syndromes in Alzheimer's disease. Int J Geriatr psychiatry 2004;19:1035–9. [DOI] [PubMed] [Google Scholar]
- [3].Guarnieri B, Sorbi S. Sleep and cognitive decline: a strong bidirectional relationship. it is time for specific recommendations on routine assessment and the management of sleep disorders in patients with mild cognitive impairment and dementia. Eur Neurol 2015;74:43–8. [DOI] [PubMed] [Google Scholar]
- [4].Benedict C, Byberg L, Cedernaes J, et al. Self-reported sleep disturbance is associated with Alzheimer's disease risk in men. Alzheimer's Dement 2015;11:1090–7. [DOI] [PubMed] [Google Scholar]
- [5].Ju YE, McLeland JS, Toedebusch CD, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol 2013;70:587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Spira AP, Chen-Edinboro LP, Wu MN, et al. Impact of sleep on the risk of cognitive decline and dementia. Curr Opin Psychiatry 2014;27:478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hahn EA, Wang HX, Andel R, et al. A change in sleep pattern may predict Alzheimer disease. Am J Geriatr Psychiatry 2014;22:1262–71. [DOI] [PubMed] [Google Scholar]
- [8].Garcia-Alberca JM, Lara JP, Cruz B, et al. Sleep disturbances in Alzheimer's disease are associated with neuropsychiatric symptoms and antidementia treatment. J Nerv Ment Dis 2013;201:251–7. [DOI] [PubMed] [Google Scholar]
- [9].Scoralick FM, Camargos EF, Freitas MP, et al. Outpatient treatment of sleep disorders in Alzheimer patients. Einstein 2015;13:430–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lee D, Heo SH, Yoon SS, et al. Sleep disturbances and predictive factors in caregivers of patients with mild cognitive impairment and dementia. J Clin Neurol 2014;10:304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology–a bidirectional relationship. Nat Rev Neurol 2014;10:115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chan KY, Wang W, Wu JJ, et al. Epidemiology of Alzheimer's disease and other forms of dementia in China, 1990-2010: a systematic review and analysis. Lancet 2013;381:2016–23. [DOI] [PubMed] [Google Scholar]
- [13].Urrestarazu E, Iriarte J. Clinical management of sleep disturbances in Alzheimer's disease: current and emerging strategies. Nat Sci Sleep 2016;8:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 1984;34:939–44. [DOI] [PubMed] [Google Scholar]
- [15].Folstein MF, Folstein SE, McHugh PR. Mini-mental state": a practical method for grading cognitive states of patients for the clinician. J Psychiatr Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- [16].Raina SK, Pandita KK, Razdan S. Incidence of dementia in a Kashmiri migrant population. Ann Indian Acad Neurol 2009;12:154–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology 1993;43:2412–4. [DOI] [PubMed] [Google Scholar]
- [18].Cummings JL. The neuropsychiatric inventory: assessing psychopathology in dementia patients. Neurology 1997;48:S10–6. [DOI] [PubMed] [Google Scholar]
- [19].Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- [20].Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991;14:540–5. [DOI] [PubMed] [Google Scholar]
- [21].Johns MW. Sleepiness in different situations measured by the Epworth Sleepiness Scale. Sleep 1994;17:703–10. [DOI] [PubMed] [Google Scholar]
- [22].Bliwise DL. Sleep disorders in Alzheimer's disease and other dementias. Clin Cornerstone 2004;6:S16–28. [DOI] [PubMed] [Google Scholar]
- [23].Shin HY, Han HJ, Shin DJ, et al. Sleep problems associated with behavioral and psychological symptoms as well as cognitive functions in Alzheimer's disease. J Clin Neurol 2014;10:203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dos Santos Silva M, Bazzana CM, de Souza AL, et al. Relationship between perceived sleep and polysomnography in older adult patients. Sleep Sci 2015;8:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lim MM, Gerstner JR, Holtzman DM. The sleep-wake cycle and Alzheimer's disease: what do we know? Neurodegener Dis manag 2014;4:351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Khachiyants N, Trinkle D, Son SJ, et al. Sundown syndrome in persons with dementia: an update. Psychiatry Investig 2011;8:275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mander BA, Marks SM, Vogel JW, et al. β-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci 2015;18:1051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Geda YE, Schneider LS, Gitlin LN, et al. Neuropsychiatric symptoms in Alzheimer's disease: past progress and anticipation of the future. Alzheimer's Dement 2013;9:602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chi S, Yu JT, Tan MS, et al. Depression in Alzheimer's disease: epidemiology, mechanisms, and management. J Alzheimer's Dis 2014;42:739–55. [DOI] [PubMed] [Google Scholar]
- [30].Khundakar AA, Thomas AJ. Neuropathology of depression in Alzheimer's disease: current knowledge and the potential for new treatments. J Alzheimer's Dis 2015;44:27–41. [DOI] [PubMed] [Google Scholar]
- [31].Niitsu T, Okamoto H, Iyo M. Behavioural and psychological symptoms of dementia in an Alzheimer's disease case successfully treated with natural medicine: association with gonadotropins. Psychogeriatrics 2013;13:124–7. [DOI] [PubMed] [Google Scholar]
- [32].Lim AS, Kowgier M, Yu L, et al. Sleep fragmentation and the risk of incident Alzheimer's disease and cognitive decline in older persons. Sleep 2013;36:1027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].De Oliveira FF, Bertolucci PH, Chen ES, et al. Assessment of sleep satisfaction in patients with dementia due to Alzheimer's disease. J Clin Neurosci 2014;21:2112–7. [DOI] [PubMed] [Google Scholar]
- [34].Aalten P, Verhey FR, Boziki M, et al. Neuropsychiatric syndromes in dementia. Results from the European Alzheimer disease consortium: part I. Dement Geriatr Cogn Disord 2007;24:457–63. [DOI] [PubMed] [Google Scholar]
- [35].Boivin DB, Czeisler CA, Dijk DJ, et al. Complex interaction of the sleep-wake cycle and the circadian phase modulates mood in healthy subjects. Arch Gen Psychiatry 1997;54:145–52. [DOI] [PubMed] [Google Scholar]
- [36].Wu YH, Fischer DF, Kalsbeek A, et al. Pineal clock gene oscillation is disturbed in Alzheimer's disease, due to functional disconnection from the “master clock”. FASEB J 2006;20:1874–6. [DOI] [PubMed] [Google Scholar]
- [37].Mulin E, Zeitzer JM, Friedman L, et al. Relationship between apathy and sleep disturbance in mild and moderate Alzheimer's disease: an actigraphic study. J Alzheimers Dis 2011;25:85–91. [DOI] [PubMed] [Google Scholar]
- [38].Lattanzi S, Brigo F, Silvestrini M. Sleep and blood pressure. J Clin Hypertens (Greenwich) 2018;20:1721–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lattanzi S, Viticchi G, Falsetti L, et al. Visit-to-visit blood pressure variability in Alzheimer disease. Alzheimer Dis Assoc Disord 2014;28:347–51. [DOI] [PubMed] [Google Scholar]
- [40].Lattanzi S, Brigo F, Vernieri F, et al. Visit-to-visit variability in blood pressure and Alzheimer's disease. J Clin Hypertens (Greenwich) 2018;20:918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]


