Abstract
Gamma-glutamyl transpeptidase-to-platelet ratio (GPR) and fibrosis-4 (FIB-4) index have been reported to be useful predictors in predicting hepatocellular carcinoma (HCC) development in chronic hepatitis B (CHB) patients. However, their predictive performances on HCC development have not been validated in elderly patients. Thus, the aim of this study was to evaluate the predictive values of the GPR and FIB-4 index on HCC in elderly CHB patients with in China.
Between January 2007 and December 2016, 1011 CHB patients older than 60 years were enrolled in the study, and their data were retrospectively analyzed. Receiver-operating characteristic (ROC) curve analysis was used to determine the optimal cutoff points of GPR and the FIB-4 index. Cumulative HCC incidence rates were calculated by the Kaplan–Meier method and compared by the log-rank test. Univariate and multivariate analyses were performed to detect risk factors for HCC development. The prediction performances of GPR and FIB-4 index were compared based on time-dependent ROC analyses.
After a median follow-up of 6.8 (interquartile range 3.9–8.4) years, 39 (3.9%) patients developed HCC. The ROC analysis of GPR and the FIB-4 index at the 5-year time point revealed that the optimal cutoff point was 0.23 for GPR and 4.15 for the FIB-4 index. When stratified by low and high GPR values and FIB-4 indices, the patients’ subgroups showed significantly different cumulative incidences of HCC. The multivariate analysis revealed that high GPR (hazard ratio [HR] 4.224; 95% confidence interval [CI] 1.891–9.434, P < .001) was an independent risk factor for HCC development, whereas a high FIB-4 index was not (HR 0.470; 95% CI 0.212–1.043; P = .063). In the time-dependent ROC analysis, GPR showed higher area under curve (AUC) values than the FIB-4 index did at all time points and reached statistical significance at the 5-, 7-, and 10-year time points (GPR vs FIB-4 index, AUC 0.725 vs 0.549 at 5 years, P = .005; GPR vs FIB-4 index, AUC 0.733 vs 0.578 at 7 years, P = .001; GPR vs FIB-4 index, AUC 0.837 vs 0.475 at 10 years, P < .001).
In conclusion, our study suggests GPR is superior to the FIB-4 index in predicting HCC development in elderly CHB patients in China.
Keywords: chronic hepatitis B, Fib-4, gamma-glutamyl transpeptidase-to-platelet ratio, hepatocellular carcinoma, noninvasive index
1. Introduction
Hepatocellular carcinoma (HCC), the 5th most common malignancy in males and the 7th most common malignancy in females, accounts for the second major cause of cancer-related mortality with a rising incidence worldwide.[1,2] Chronic hepatitis B (CHB) is a well-known primary cause of HCC, especially in sub-Saharan Africa and east Asia, and accounts for >50% of newly diagnosed HCC cases.[3] A recent systematic review estimated that >240 million people around the world are carriers of the hepatitis B surface antigen (HBsAg).[4] Therefore, methods to recognize CHB patients with a high incidence of HCC are urgently needed.
Although liver biopsy is regarded as the most reliable method for hepatic fibrosis assessment, potential complications of liver biopsy have spawned many noninvasive indices. The fibrosis-4 (FIB-4) index, first introduced by Vallet-Pichard et al[5] to evaluate the degree of hepatic fibrosis in patients with chronic hepatitis C (CHC), is calculated by a simple formula based on age, alanine aminotransferase (ALT) levels, aspartate aminotransferase (AST) levels, and platelet (PLT) counts. Subsequent studies have proven that the FIB-4 index is able to identify liver fibrosis not only in patients with CHC but also in patients with various hepatic diseases, such as non-alcohol fatty liver disease and CHB, with acceptable sensitivity and accuracy.[6,7] The gamma-glutamyl transpeptidase (GGT)-to-platelet ratio (GPR), another noninvasive laboratory marker, was first introduced by Lemoine et al[8] to predict hepatic fibrosis in patients with CHB in West Africa and showed good accuracy. A later study from China confirmed the reliability of GPR in predicting hepatic fibrosis in CHB patients in China.[9] Because liver cirrhosis is a well-known risk factor for HBV-related HCC,[10,11] a study from Korea investigated the longitudinal value of the FIB-4 index and reported that it may be a good indicator to predict hepatocarcinogenesis in CHB patients.[12] Recently, another report from Korea suggested that GPR can serve as a good noninvasive predicator of hepatocarcinogenesis in CHB patients in Korea.[13]
However, the predictive performance of GPR and the FIB-4 index on HCC development has not been validated in elderly patients with CHB. Aging has been widely accepted as a significant risk factor for various malignancies, including HCC.[14,15] In addition, aging is a well-known factor associated with chronic changes in liver sinusoidal function and various transforms of each hepatic cell type.[16] Moreover, although the prevalence of hepatitis B virus (HBV) infection has declined in adolescents and young adults due to the implementation of universal infant vaccination policy since 1992, the HCC incidence in the elderly may not taper off in the near future.[4,14,15,17] Thus, an effective tool to predict HBV-related HCC incidence risk in elderly CHB patients is warranted.
The aim of current research is to evaluate the predictive value of GPR and the FIB-4 index on HBV-related HCC incidence risk in elderly CHB patients with in China.
2. Methods
2.1. Patients
Between January 2007 and December 2016, civil servants older than 60 years who had access to free routine physical examinations covered by the government who were diagnosed with CHB at the Physical Examination Center, West China Hospital of Sichuan University, were recruited. CHB was defined as the persistent presence of serum HBsAg for >6 months. Patients lacking clinical data were excluded. Patients who met the following exclusion criteria were also excluded: coinfection with hepatitis C virus or human immunodeficiency virus; alcohol intake ≥80 g/day[18]; history of HCC or diagnosed with HCC at enrollment; and HBV DNA copy >103 cells/mL or having an antiviral therapy history or developed decompensated liver cirrhosis. Patients with HBV DNA copy >103 cells/mL or with a history of antiviral therapy history or with decompensated liver cirrhosis were transferred to the infectious disease department for further management. The Ethics Committee of West China Hospital, Sichuan University approved this study, written informed consent was waived because this was a retrospective study, and all participants were anonymous.
2.2. Clinical data and assessments
Detailed demographic data and medical history were collected at enrollment. The initial assessment included blood biochemical examination, routine blood examination, serum HBV DNA level, alpha fetoprotein (AFP) level, and abdominal ultrasound findings. Biochemical examination was performed by electrochemiluminescence detection of an immunoassay using a Roche Cobas modular p800 automatic biochemical analyzer, whereas AFP level was detected by an electrochemiluminescence immunoassay using a Roche E170 modular analytics immunoassay analyzer. Liver cirrhosis was clinically diagnosed if patients met the following criteria: PLT count <100 × 109/L and manifestation of liver regenerative nodules, congestive splenomegaly or ascites on abdominal ultrasonography; evidence of portal hypertension.
2.3. GPR and FIB-4 index calculation
Noninvasive indices were calculated based on the formulas in previous reports[5,8] as follows: FIB-4 = (age [years] × AST [IU/L])/([PLT count {109 cells/L} ×ALT {IU/L}]1/2) and GPR = GGT (IU/L)/PLT count (109 cells/L) × 100. All these markers were measured at enrollment.
2.4. HCC surveillance and follow-up
After enrollment, all patients were regularly followed up every ≥6 months frequently if clinically needed. Abdominal ultrasonography and blood tests, including liver function tests and AFP levels, were performed at each follow-up. For any subject with a positive finding for AFP level (≥100 ng/mL) or abdominal ultrasound, enhanced contrast ultrasound or computed tomography (CT)/magnetic resonance imaging was conducted immediately. The HCC diagnosis was based on pathology or noninvasive criteria according to the Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition).[19] The follow-up duration was defined as the interval between enrollment and diagnosis of HCC or the interval between enrollment and last follow-up for patients without HCC development.
2.5. Statistical analysis
Data are expressed as the median (interquartile range [IQR]) or number (%) as appropriate. Receiver-operating characteristic (ROC) curve analysis of GPR and the FIB-4 index to predict HCC development at the 5-year time point (approximately two-thirds of patients achieved this duration of follow-up) was carried out. The cutoff point with the maximized sum of specificity and sensitivity was defined as the optimal cutoff point. Cumulative HCC incidence rates were calculated by the Kaplan–Meier method and compared by the log-rank test. Potential risk factors for HCC development include but not limited to increasing age, male sex, underlying diseases, advanced liver fibrosis or cirrhosis, and HBeAg positivity. To determine which factors were independent predictors of subsequent HCC development, variables with a P < .05 in the univariate analyses were entered into the multivariate Cox proportional hazard regression analysis, with hazard ratios (HRs) and 95% confidence intervals (CIs) calculated. The time-dependent ROC curve analysis was carried out to evaluate the predictive performance.[20] Area under curve (AUC) values were calculated. The AUC values were compared using the method of DeLong et al.[21] SPSS statistical software (SPSS Standard version 22.0; Chicago, IL) and R 3.6.1 software (Institute of Statistics and Mathematics, Vienna, Austria) were used to perform all statistical analyses. A significant difference was considered if the P value from a 2-tailed test was <.05.
3. Result
3.1. Baseline characteristics
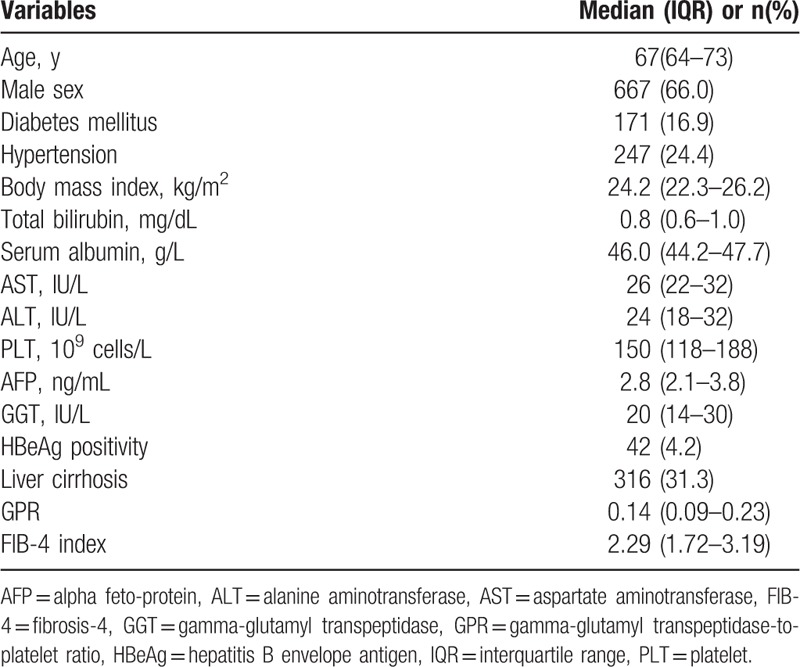
Patient selection inflows are shown in Figure 1. Overall, a total of 1011 patients, including 667 men and 344 women, were included in this study for statistical analysis. The baseline characteristics of all elderly CHB patients who were included are listed in Table 1. The median age of the entire study population was 67 (IQR, 64–73) years. At enrollment, 171 patients (16.9%) had diabetes mellitus (DM), 247 patients (24.4%) had hypertension, and 316 patients (31.3%) were clinically diagnosed with liver cirrhosis based on the principles mentioned above. All patients exhibited preserved Child-Pugh class A liver function at enrollment. The median GPR and FIB-4 index values were 0.14 (IQR, 0.09–0.23) and 2.29 (IQR, 1.72–3.19), respectively.
Figure 1.
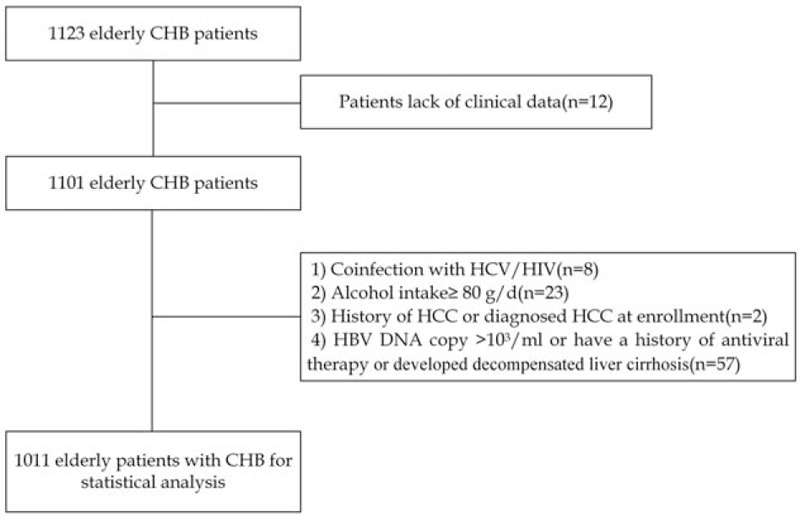
Patient selection flow chart. CHB = chronic hepatitis B; HCC = hepatocellular carcinoma, HCV = hepatitis C virus.
Table 1.
Baseline characteristics at enrollment (n = 1011).
3.2. ROC analyses for selecting the optimal cutoff values for GPR and the FIB-4 index
HCC incidence at the 5-year time point (approximately two-thirds of patients achieved this duration of follow-up) was used for ROC analyses, and the cutoff point with the maximized sum of specificity and sensitivity was defined as the optimal cutoff point. The optimal cutoff point for GPR was 0.23 (AUC value = 0.725; sensitivity, 74.1%; and specificity, 76.8%), and the optimal cutoff point for the FIB-4 index was 4.15 (AUC value = 0.549; sensitivity, 29.6%; and specificity, 87.0%). Thus, we divided patients with GPR >0.23 into the high GPR group (n = 243) and patients with GPR <0.23 into the low-GPR group (n = 768). Similarly, patients with an FIB-4 index >4.15 were divided into the high FIB-4 index group (n = 125), and patients with an FIB-4 index <4.15 were divided into the low FIB-4 index group (n = 886).
3.3. Cumulative HCC incidence rate
After a median follow-up of 6.8 (IQR 3.9–8.4) years, 39 (3.9%) patients developed HCC. In the whole cohort, the 1-, 3-, 5-, and 7-year cumulative incidences of HCC were 0.4%, 1.9%, 3.0%, and 4.2%, respectively (Fig. 2A). Cumulative incidence rates of HCC in the different subgroups based on GPR and FIB-4 index cutoff values were analyzed. Significantly different cumulative incidences of HCC were identified among the patients who were categorized by low and high GPR and FIB-4 index values. Regarding GPR, the 1-, 3-, 5-, and 7-year cumulative incidences of HCC were 1.2%, 6.1%, 8.8%, and 12.4%, respectively, in the high GPR group and 0.1%, 0.5%, 1.2%, and 1.6%, respectively, in the low GPR group (P < .001) (Fig. 2B). For the FIB-4 index subgroups, the 1-, 3-, 5-, and 7-year cumulative incidences of HCC were 1.6%, 5.8%, 6.9%, and 10.2%, respectively, in the high FIB-4 group and 0.2%, 1.3%, 2.4%, and 3.3%, respectively, in the low FIB-4 group (P = .001) (Fig. 2C).
Figure 2.
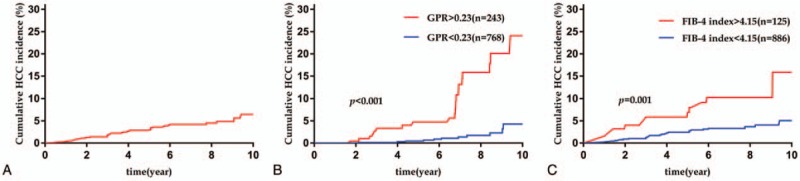
Cumulative hepatocellular carcinoma incidence rate. (A) Cumulative HCC incidence rate for all patients, (B) cumulative HCC incidence rate for different GPR groups, (C) cumulative HCC incidence rate for different FIB-4 index groups. FIB-4 = fibrosis-4, GPR = gamma-glutamyl transpeptidase-to-platelet ratio, HCC = hepatocellular carcinoma.
3.4. Univariate and multivariate analyses to detect the risk factors for HCC development
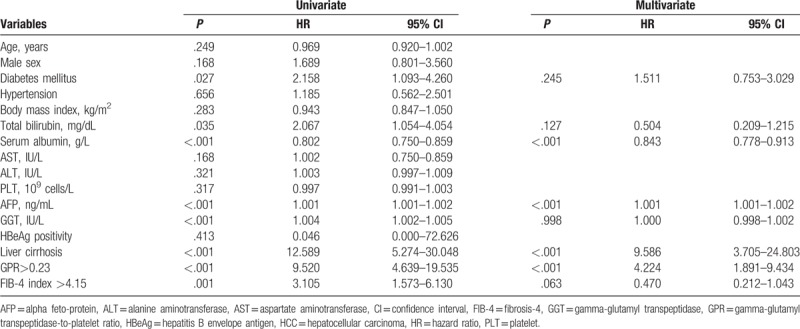
The results of the univariate and multivariate analyses for HCC risk factors are listed in Table 2. The following factors were found to be related to HCC incidence in the univariate analyses: DM, total bilirubin level, serum albumin level, AFP level, GGT level, and clinically diagnosed liver cirrhosis; HCC development was also related to GPR >0.23 and an FIB-4 index >4.15. Further multivariate analysis was based on the 8 factors with P < .05 in the univariate analyses. Serum albumin level (HR 0.843; 95% CI 0.778–0.913; P < .001), AFP level (HR 1.001; 95% CI 1.001–1.002; P < .001), clinically diagnosed liver cirrhosis (HR 9.586; 95% CI 3.705–24.803; P < .001), and GPR >0.23 (HR 4.224; 95% CI 1.891–9.434, P < .001) were found to be independent risk factors correlated with HCC development.
Table 2.
Univariate and multivariate analyses to detect risk factors for HCC development.
3.5. Comparison of the predictive performance between GPR and the FIB-4 index on HCC development
During the follow-up of up to 10 years, the numbers of HCC cases were 4 (in 1007) at 1 year, 18 (in 842) at 3 years, 27 (in 676) at 5 years, and 35 (in 442) at 7 years. Time-dependent ROC analysis for GPR and the FIB-4 index to predict HCC development were analyzed and are shown in Figure 3. The AUC values of GPR and the FIB-4 index to predict HCC development in all patients at different time points are listed in Table 3 and shown in Figure 4A. GPR showed higher AUC values than the FIB-4 index did at all time points and reached statistical significance at the 5-, 7-, and 10-year time points (GPR vs FIB-4 index, AUC 0.725 vs 0.549 at 5 years, P = .005; GPR vs FIB-4 index, AUC 0.733 vs 0.578 at 7 years, P = .001; GPR vs FIB-4 index, AUC 0.837 vs 0.475 at 10 years, P < .001).
Figure 3.
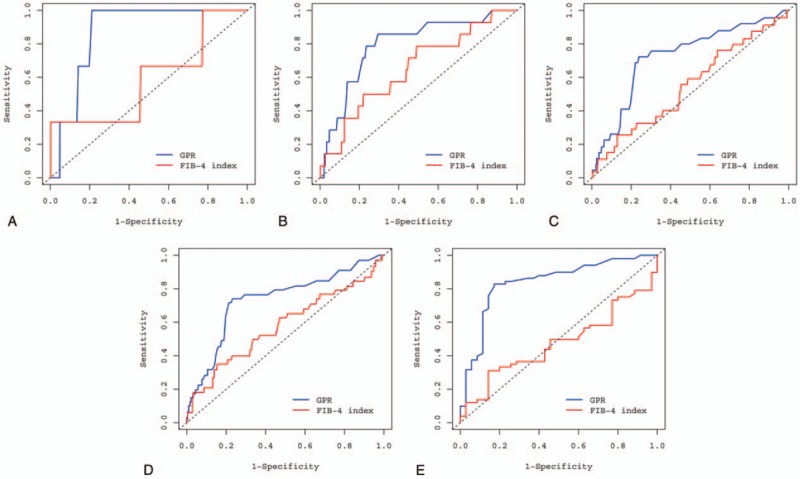
Time-dependent ROC curves for GPR and the FIB-4 index to predict hepatocellular carcinoma development. (A) Receiver-operating characteristic (ROC) curve for GPR and the FIB-4 index at the 1-year time point, (B) ROC curve for GPR and the FIB-4 index at the 3-year time point, (C) ROC curve for GPR and the FIB-4 index at the 5-year time point, (D) ROC curve for GPR and the FIB-4 index at 7-year time point, (E) ROC curve for GPR and the FIB-4 index at 10-year time point. FIB-4 = fibrosis-4, GPR = gamma-glutamyl transpeptidase-to-platelet ratio.
Table 3.
AUC values of GPR and the FIB-4 index to predict HCC development in all patients at different time points.
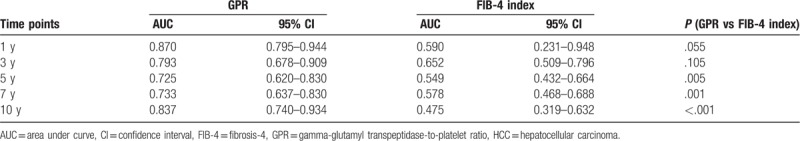
Figure 4.
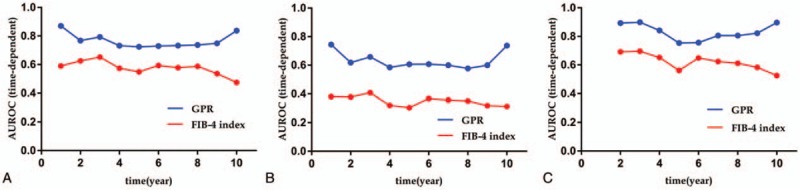
Area under the curve (AUC) values of GPR and the FIB-4 index to predict HCC development. (A) AUC values of GPR and the FIB-4 index to predict hepatocellular carcinoma (HCC) development for all patients (n = 1011), (B) AUC values of GPR and the FIB-4 index to predict HCC development for patients with cirrhosis (n = 316), (C) AUC values of GPR and the FIB-4 index to predict HCC development for patients without cirrhosis (n = 695). AUROC = area under receiver-operating characteristic curve, FIB-4 = fibrosis-4, GPR = gamma-glutamyl transpeptidase-to-platelet ratio.
3.6. Comparison of the predictive accuracy of HCC development in patients with and without liver cirrhosis between GPR and the FIB-4 index
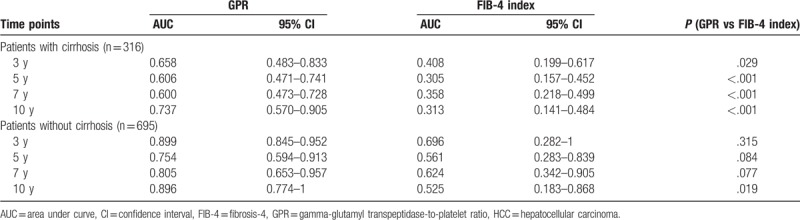
Given the highest HR among all risk factors and the well-known relationship with HCC carcinogenesis, liver cirrhosis was further considered in the subgroup analysis. To confirm the predictive performance of GPR and the FIB-4 index in patients with/without liver cirrhosis, time-dependent ROC analyses of GPR and the FIB-4 index were carried out. The results of time-dependent ROC analyses of GPR and the FIB-4 index in patients with liver cirrhosis (n = 316) are listed in Table 4 and shown in Figure 4B. The AUC values of GPR to predict HCC development were higher than those of the FIB-4 index at every time point. Moreover, the results of the time-dependent ROC analyses of GPR and the FIB-4 index in patients without liver cirrhosis (n = 695) are shown in Table 4 and Figure 4C. The AUC values of GPR to predict HCC development were higher than those of the FIB-4 index at all time points.
Table 4.
AUC values of GPR and the FIB-4 index to predict HCC development in patients with/without cirrhosis.
4. Discussion
Although previous reports demonstrated that noninvasive markers could be used to assess the risk of HCC development in patients with CHB,[12,13,22–25] the predicted values of GPR and the FIB-4 index have not been validated in elderly patients with CHB. The aim of the current study was to evaluate the predictive values of GPR and the FIB-4 index on HBV-related HCC incidence risk in elderly patients with CHB in China. To the best of our knowledge, this is the first study focusing on the predicted values of GPR and the FIB-4 index on HCC incidence in elderly patients with CHB. To guarantee a homogeneous population, the current study only included civil servants who had access to free routine physical examinations covered by the government. Focusing on the civil servant population was expected to reduce confounding effects caused by different social statuses, lifestyles, and education levels, which may possibly affect the risk of malignancy.[26] In addition, the follow-up period (median 6.8 years, IQR 3.9–8.4 years) was relatively longer in our study than in previous studies.[13,22,25]
Patients with HBV DNA copy >103 cells/mL or having a history of antiviral therapy, deemed as a population with a high risk of HCC incidence, were excluded from the final cohort. As expected, the 5-year cumulative HCC incidence rates were lower (3.0% for all patients with CHB, 7.8% for patients with hepatic cirrhosis, and 0.8% for patients without hepatic cirrhosis) than those in a previous study.[27] However, the 5-year cumulative HCC incidence in the current study was still lower than that in another study focused on CHB patients with low-level viremia from Korea, which reported that the cumulative HCC incidence at 5 years was 13.9% for patients with hepatic cirrhosis and 2.0% for patients without hepatic cirrhosis.[25] The differences in baseline characteristics, ethic characteristics, and HBV-DNA genotypes may account for the morbidity difference.[11,28,29]
Park et al[13] studied 1109 CHB patients and reported that GPR can serve as a good noninvasive predicator for the risk of HCC development in CHB patients. Consistent with their study, the results from our study suggest that GPR can be a good predictor for HCC development in elderly CHB patients in China. Given that high GPR can predict advanced liver fibrosis,[8,9,30,31] this result is not surprising because liver cirrhosis is one of the most significant factors related to HCC development in CHB patients.[10,11] Optimal cutoff points of GPR and the FIB-4 index were determined based on ROC analyses to predict HCC development at the 5-year time point. Patients who harbored low and high GPR values and FIB-4 indices showed significantly different cumulative incidences of HCC. Furthermore, the multivariate analysis revealed that high GPR was an independent risk factor for HCC development, whereas a high FIB-4 index was not (Table 2). Moreover, our study compared the prediction performance between GPR and the FIB-4 index based on time-dependent ROC analyses. The results showed that the prediction performance of GPR was superior to that of the FIB-4 index for all elderly CHB patients included in our study at every study time point, and GPR maintained its superiority in the subgroup analyses according to patients with and without cirrhosis (Fig. 4).
It is worth noting that the median FIB-4 index of the current study population was 2.19 (IQR 1.73–3.19), which is higher than the value of 1.62 (IQR 1.02–2.72) in Park et al's.[13] In addition, the optimal cutoff point of the FIB-4 index was 4.15 in our study, which was higher than that in a previous similar study (3.66) reported by Nishikawa et al.[22] One possible explanation may be that the cirrhosis rate (31.6%) was higher in our study than in previous studies (18.6% in Park et al's [13] study and 26.9% in Nishikawa et al's [22] study). More importantly, focusing on elderly CHB patients may have resulted in a higher FIB-4 index in our study because the FIB-4 index is calculated based on age, ALT level, AST level, and PLT count. Furthermore, the FIB-4 index, which has been reported a useful predictor associated with HCC development in patients with CHB in many previous studies,[12,22,24,32] showed a poor predictive performance in elderly CHB patients in our study. One reasonable explanation may be that our study included only elderly CHB patients, and the narrowed age differential in the current study made FIB-4 less heterogeneous among our study population and affected the predictive value of FIB-4 on HCC development. This issue of the FIB-4 index was also proposed by Kurosaki and Izumi,[33] who found that the FIB-4 index displays satisfactory diagnostic value in identifying advanced fibrosis among young Japanese CHC patients; however, the diagnostic value is limited in elderly Japanese CHC patients. In general, our findings indicate that the FIB-4 index may display a different predictive value in the elderly population and may need to be modified to adapt to different age groups. Recently, Wang et al[34] proposed a new noninvasive index called the modified FIB-4 index (mFIB-4), and mFIB-4 showed better predictive performance than that of FIB-4 in predicting the risk of HCC in their retrospective study of 1325 CHB patients in Taiwan. However, the median follow-up of their study was only 4.1 years, and they included only CHB patients who received entecavir therapy; thus, the generalizability of their results needs further research.
The presence of liver cirrhosis and AFP levels proved to be associated with HCC development in many studies.[11,13,22] In the multivariate analysis, our study confirmed that they were independent risk factors correlated with HCC development in elderly CHB patients. However, older age, a widely accepted risk factor for HCC development in previous studies, was not a significant predictor of HCC development in our study. Indeed, in the elderly CHB patient subgroup, which has more subtle differences in age, biological age rather than chronological age may play a larger role in carcinogenesis.[35] In addition, DM has been reported to be a risk factor associated with HCC development in CHB patients.[18,32,34,36] However, DM was not a significant factor in the multivariate analysis in our study. Considering the retrospective nature of our study, the duration of DM in our study subjects was difficult to assess accurately. Thus, the cumulative effect of hyperglycemia on HCC development was difficult to evaluate, and the potential relationship between DM and HCC development may be underestimated. Further well-designed studies are needed to illuminate the relationship between DM and HCC.
There are several limitations in the current study. First, this is a retrospective study, and our study population consisted of elderly civil servants who had access to free routine physical examinations. There might be selection bias involved, and a well-designed prospective study might still be required. Second, we focused on only elderly CHB patients with HBV DNA copy <103 cells/mL who had no history of antiviral therapy. This patient population may restrict the generalizability of our results. Whether our results can be applied to CHB patients with high-level viremia or those receiving antiviral therapy needs further research. Third, histological evaluation and liver stiffness measurement data were scarce in our study, and liver cirrhosis is mainly clinically diagnosed by ultrasonography, which is operator-dependent. Because ultrasonography measurement errors were independent and nondifferential, ultrasonography may underestimate the correlation between liver cirrhosis and HCC incidence. Despite the aforementioned limitations, the current study has significant clinical value. To the best of our knowledge, this is the first study focusing on the longitudinal values of GPR and the FIB-4 index on HCC incidence in mainland China, and the first study evaluated the predictive values of GPR and the FIB-4 index on HCC development in elderly CHB patients.
In conclusion, we suggest that GPR is superior than the FIB-4 index in predicting HCC development in elderly CHB patients in China. Thus, elderly CHB patients harboring high GPR values should be more carefully monitored for the development of HCC. Further well-designed prospective studies should be performed to confirm our results.
Author contributions
Conceptualization: Yi-Fei Tan, Jia-Yin Yang.
Data curation: Xi Xu, Jin-Li Zheng, Bo-Han Zhang, Huai-Rong Tang.
Methodology: Yun-Feng Zhu, Yi-Fei Tan.
Writing – original draft: Yun-Feng Zhu, Yi-Fei Tan.
Writing – review & editing: Huai-Rong Tang, Jia-Yin Yang.
Footnotes
Abbreviations: AFP = alpha fetoprotein, ALT = alanine aminotransferase, AST = aspartate aminotransferase, AUC = area under curve, CHB = chronic hepatitis B, CHC = chronic hepatitis C, CI = confidence interval, CT = computed tomography, DM = diabetes mellitus, FIB-4 = fibrosis-4, GGT = gamma-glutamyl transpeptidase, GPR = gamma-glutamyl transpeptidase-to-platelet ratio, HBsAg = hepatitis B surface antigen, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HR = hazard ratio, PLT = platelet, ROC = receiver-operating characteristic.
How to cite this article: Zhu YF, Tan YF, Xu X, Zheng JL, Zhang BH, Tang HR, Yang JY. Gamma-glutamyl transpeptidase-to-platelet ratio and the fibrosis-4 index in predicting hepatitis B virus-related hepatocellular carcinoma development in elderly chronic hepatitis B patients in China: a single-center retrospective study. Medicine. 2019;98:50(e18319).
This study was supported by the 1.3.5 project for discipline of excellence, West China Hospital, Sichuan University, No. ZY20170308 and Sichuan Science and Technology Project, No. 2018SZ0261.
The authors report no conflicts of interest.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin 2012;62:394–9. [DOI] [PubMed] [Google Scholar]
- [3].El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118–27. [DOI] [PubMed] [Google Scholar]
- [4].Schweitzer A, Horn J, Mikolajczyk RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015;386:1546–55. [DOI] [PubMed] [Google Scholar]
- [5].Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 2007;46:32–6. [DOI] [PubMed] [Google Scholar]
- [6].McPherson S, Stewart SF, Henderson E, et al. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010;59:1265–9. [DOI] [PubMed] [Google Scholar]
- [7].Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology 2015;61:292–302. [DOI] [PubMed] [Google Scholar]
- [8].Lemoine M, Shimakawa Y, Nayagam S, et al. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut 2016;65:1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang W, Sun M, Chen G, et al. Reassessment of gamma-glutamyl transpeptidase to platelet ratio (GPR): a large-sample, dynamic study based on liver biopsy in a Chinese population with chronic hepatitis B virus (HBV) infection. Gut 2018;67:989–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004;127:S35–50. [DOI] [PubMed] [Google Scholar]
- [11].Yuen M-F, Tanaka Y, Fong DY-T, et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol 2009;50:80–8. [DOI] [PubMed] [Google Scholar]
- [12].Suh B, Park S, Shin DW, et al. High liver fibrosis index FIB-4 is highly predictive of hepatocellular carcinoma in chronic hepatitis B carriers. Hepatology 2015;61:1261–8. [DOI] [PubMed] [Google Scholar]
- [13].Park YE, Kim BK, Park JY, et al. Gamma-glutamyl transpeptidase-to-platelet ratio is an independent predictor of hepatitis B virus-related liver cancer. J Gastroenterol Hepatol 2017;32:1221–9. [DOI] [PubMed] [Google Scholar]
- [14].Hung GY, Horng JL, Yen HJ, et al. Changing incidence patterns of hepatocellular carcinoma among age groups in Taiwan. J Hepatol 2015;63:1390–6. [DOI] [PubMed] [Google Scholar]
- [15].Mittal S, Kramer JR, Omino R, et al. Role of age and race in the risk of hepatocellular carcinoma in veterans with hepatitis B virus infection. Clin Gastroenterol Hepatol 2018;16:252–9. [DOI] [PubMed] [Google Scholar]
- [16].Maeso-Diaz R, Ortega-Ribera M, Fernandez-Iglesias A, et al. Effects of aging on liver microcirculatory function and sinusoidal phenotype. Aging Cell 2018;17:e12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liang X, Bi S, Yang W, et al. Epidemiological serosurvey of hepatitis B in China—declining HBV prevalence due to hepatitis B vaccination. Vaccine 2009;27:6550–7. [DOI] [PubMed] [Google Scholar]
- [18].Hassan MM, Hwang LY, Hatten CJ, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology 2002;36:1206–13. [DOI] [PubMed] [Google Scholar]
- [19].Zhou J, Sun HC, Wang Z, et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 edition). Liver Cancer 2018;7:235–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 2000;56:337–44. [DOI] [PubMed] [Google Scholar]
- [21].DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. [PubMed] [Google Scholar]
- [22].Nishikawa H, Nishijima N, Enomoto H, et al. Comparison of FIB-4 index and aspartate aminotransferase to platelet ratio index on carcinogenesis in chronic hepatitis B treated with entecavir. J Cancer 2017;8:152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tada T, Kumada T, Toyoda H, et al. Impact of FIB-4 index on hepatocellular carcinoma incidence during nucleos(t)ide analogue therapy in patients with chronic hepatitis B: an analysis using time-dependent receiver operating characteristic. J Gastroenterol Hepatol 2017;32:451–8. [DOI] [PubMed] [Google Scholar]
- [24].Kim JH, Kim JW, Seo JW, et al. Noninvasive tests for fibrosis predict 5-year mortality and hepatocellular carcinoma in patients with chronic hepatitis B. J Clin Gastroenterol 2016;50:882–8. [DOI] [PubMed] [Google Scholar]
- [25].Paik N, Sinn DH, Lee JH, et al. Non-invasive tests for liver disease severity and the hepatocellular carcinoma risk in chronic hepatitis B patients with low-level viremia. Liver Int 2018;38:68–75. [DOI] [PubMed] [Google Scholar]
- [26].Saran U, Humar B, Kolly P, et al. Hepatocellular carcinoma and lifestyles. J Hepatol 2016;64:203–14. [DOI] [PubMed] [Google Scholar]
- [27].Jung KS, Kim SU, Song K, et al. Validation of hepatitis B virus-related hepatocellular carcinoma prediction models in the era of antiviral therapy. Hepatology 2015;62:1757–66. [DOI] [PubMed] [Google Scholar]
- [28].Sunbul M. Hepatitis B virus genotypes: global distribution and clinical importance. World J Gastroenterol 2014;20:5427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang HW, Yin JH, Li YT, et al. Risk factors for acute hepatitis B and its progression to chronic hepatitis in Shanghai China. Gut 2008;57:1713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dong M, Wu J, Yu X, et al. Validation and comparison of seventeen noninvasive models for evaluating liver fibrosis in Chinese hepatitis B patients. Liver Int 2018;38:1562–70. [DOI] [PubMed] [Google Scholar]
- [31].Lian MJ, Zhang JQ, Chen SD, et al. Diagnostic accuracy of gamma-glutamyl transpeptidase-to-platelet ratio for predicting hepatitis B-related fibrosis: a meta-analysis. Eur J Gastroenterol Hepatol 2019;31:599–606. [DOI] [PubMed] [Google Scholar]
- [32].Chiang HH, Lee CM, Hu TH, et al. A combination of the on-treatment FIB-4 and alpha-foetoprotein predicts clinical outcomes in cirrhotic patients receiving entecavir. Liver Int 2018;38:1997–2005. [DOI] [PubMed] [Google Scholar]
- [33].Kurosaki M, Izumi N. External validation of FIB-4: diagnostic accuracy is limited in elderly populations. Hepatology 2008;47:352–3. [DOI] [PubMed] [Google Scholar]
- [34].Wang HW, Lai HC, Hu TH, et al. Stratification of hepatocellular carcinoma risk through modified FIB-4 index in chronic hepatitis B patients on entecavir therapy. J Gastroenterol Hepatol 2019;34:442–9. [DOI] [PubMed] [Google Scholar]
- [35].Rozhok AI, DeGregori J. The evolution of lifespan and age-dependent cancer risk. Trends Cancer 2016;2:552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tan Y, Wei S, Zhang W, et al. Type 2 diabetes mellitus increases the risk of hepatocellular carcinoma in subjects with chronic hepatitis B virus infection: a meta-analysis and systematic review. Cancer Manag Res 2019;11:705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]


