Abstract
Dravet syndrome (DS) is a genetic form of severe epilepsy often associated with mutation of the SCN1A gene encoding the voltage gated sodium channel Nav1.1. Typically refractive to conventional therapy, serotonin neurotransmission may be an innovative target for treatment. To further understand the role of serotonin in this disorder, in this study we examined the state of the endogenous serotonin system in an Scn1a+/− mouse model of DS. Examined at an age before seizures appear, we found the hypothermic effect of 5-HT1A receptor agonist administration was attenuated. HPLC analysis of brain monoamine content revealed modestly reduced serotonin levels in tissue samples of the midbrain that included the dorsal raphe nucleus but no changes elsewhere in the brain. The reduced sensitivity to 5-HT1A agonist administration seen at young ages reversed after the age of seizure development when mice showed an exaggerated hypothermic response. Likewise, adult DS mice showed a pronounced hypersensitivity to a 5-HT2A/2C agonist. As adults however monoamine levels were not detectably altered. Thus there are alterations in the endogenous serotonin system that both precede and follow the appearance of seizure in DS mice, most strikingly in the response to agonist administration.
Keywords: seizure, epilepsy, raphe, serotonin, Scn1A, Nav1.1
1. Introduction
Dravet syndrome (DS) is a genetic form of severe-intractable epilepsy that begins in infancy. Seizures are frequently triggered by hyperthermia and can be highly refractive to standard treatment strategies. Additional motor and cognitive impairments develop with age resulting in severe handicap. Intellectual disability is very common with DS, and a patient subpopulation meets the criteria for autism. Individuals with DS are also at high risk for sudden unexpected death in epilepsy (Dravet, 2011; Genton et al., 2011; Li et al., 2011). The incidence of DS is about 1:16,000 and the most common cause is a mutation of a sodium channel gene, SCN1A encoding Nav1.1. (Kalume, 2013; Meisler and Kearney, 2005).
Mice carrying loss of function mutations of the Scn1a gene recapitulate many of the features of the human disease, although the phenotype depends on the genetic background strain (Ogiwara et al., 2007; Yu et al., 2006). C57BL/6 mice are relatively vulnerable to the deleterious effects of an Scn1A mutation whereas 129/SvJ mice are resistant. On a mixed (F1) background of these strains, mice carrying the Scn1a mutation exhibit seizure that develops after postnatal day (P) 19 (Yu et al., 2006). The mutation is partially lethal due to postictal respiratory depression with death occurring before P35, with little further lethality later in life (Kalume, 2013; Kalume et al., 2013). In addition, mice exhibit behavioral modifications including hyperactivity accompanied by mild deficits in tests of cognitive function and sociability (Sawyer et al., 2016). Mouse genetic studies have shown that impairment of Nav1.1 in subpopulations of inhibitory (GABAergic) interneurons is sufficient to recapitulate many of the deficits of the global loss of function (Cheah et al., 2012; Ogiwara et al., 2013).
There have been several positive reports of the utility of the serotonin-selective amphetamine fenfluramine for DS (Boyd et al., 2019; Ceulemans et al., 2012; Schoonjans et al., 2017). As such, fenfluramine is also called a ‘serotonin releaser’ because it causes the release endogenous serotonin into the extracellular space where it may act on its receptors (Rothman and Baumann, 2002). Although there is a long-recognized ability of serotonin neurotransmission to modify seizure activity, it remains poorly understood if there are changes in the endogenous serotonin system in DS that might be relevant to understanding why fenfluramine has efficacy in this disorder such as changes in the sensitivity of serotonin receptors or the amount of endogenous serotonin itself. Therefore in this study we examined several endpoints related to the endogenous serotonin system in the DS mouse before and/or after the age when seizures appear and mice are vulnerable to early death. Specifically we measured the sensitivity of 5-HT1A and 5-HT2A/2C receptors using common bioassays and the brain content of serotonin and it’s metabolite using HPLC.
2. Results
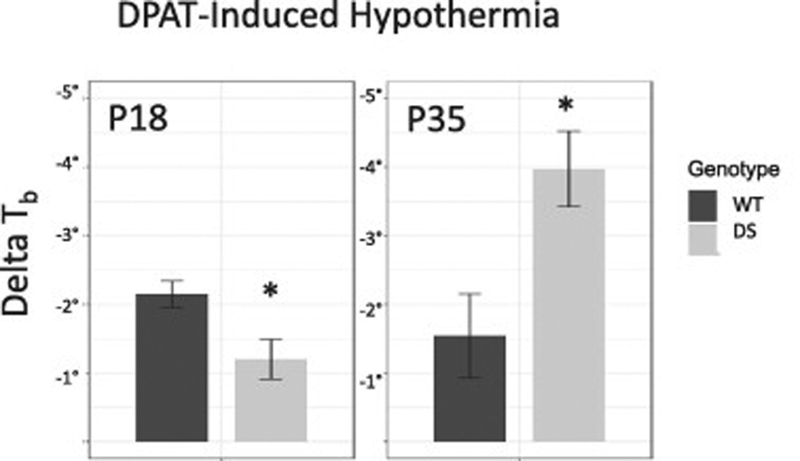
To gauge the function of endogenous 5-HT1A receptors in DS mice we tested the sensitivity of DS and littermate wild-type (WT) mice to the 5-HT1A receptor agonist DPAT by measuring the level of hypothermia produced by this drug, which is used as a bioassay for 5-HT1A receptor function (Richardson-Jones et al., 2010) We found a significant interaction between age and genotype in the sensitivity to DPAT(P = 0.006, Fig. 1). At a young age, DS mice were less sensitive to DPAT then WT mice and had a smaller hypothermic response (P = 0.03). This effect reversed at P35 when DS mice had more profound hypothermic response then their WT littermates (P = 0.03).
Figure 1.
Hypothermia induced by the 5-HT1A receptor agonist DPAT was slightly attenuated in DS mice before the age of seizure onset (P18), while after seizures were established (P35) DS mice were hypersensitive to DPAT.
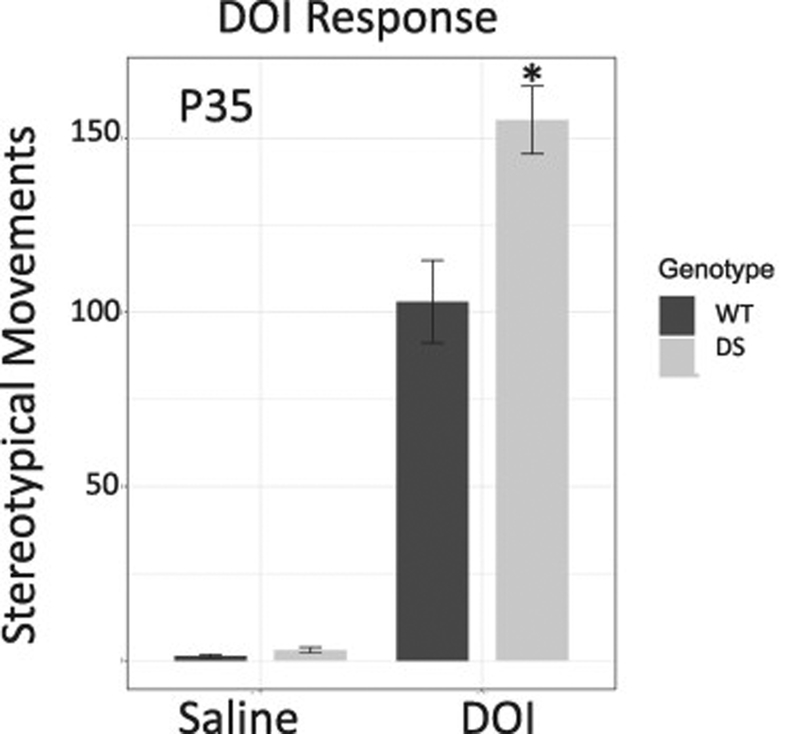
In order to evaluate another class of serotonin receptors we measured the response to the 5-HT2A/2C receptor agonist, DOI. DOI produces stereotypical movements that are easily observed and used as a bioassay for these receptors (Halberstadt and Geyer, 2013), however these responses appear later on in development and therefore we couldn’t detect effects of the drug in either genotype at the younger age, before seizures would appear in DS mice. However, we did test their effects at P35. At this age, DS mice exhibited significantly more headshake/ear scratch responses to DOI then their WT littermates (P = 0.01, Fig. 2).
Figure 2.
Stereotypy produced by the 5-HT2A/2C agonist DOI-induced is not present before P21 however at P35 DS mice exhibited enhanced response to DOI.
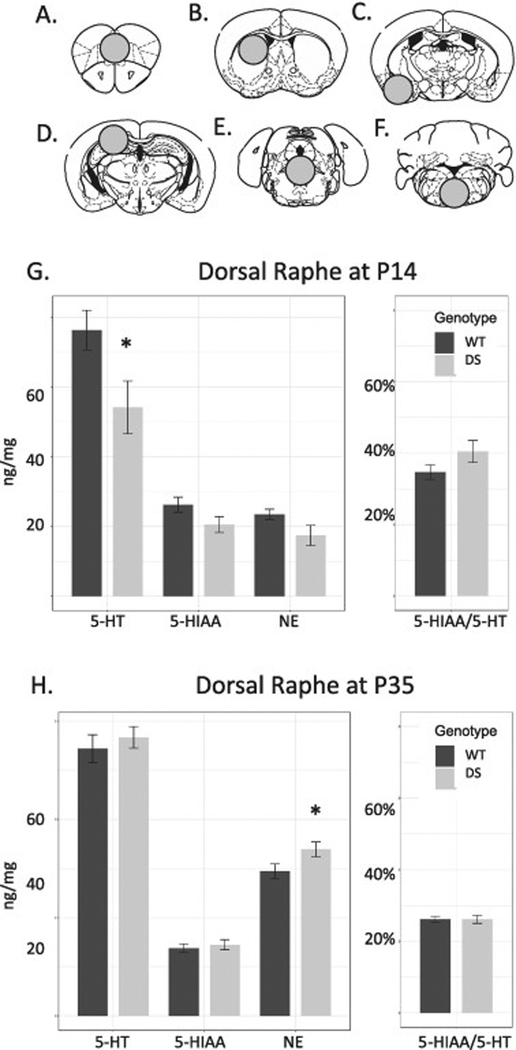
To evaluate how these changes related to levels of endogenous monoamines we sampled different regions representing projection targets and areas where serotonin neuron cell bodies are located (Fig. 3). HPLC analysis revealed a non-significant interaction between genotype and age in the dorsal raphe (DR) region (P = .186), while 5-HIAA and 5-HT groups were of unequal variance using the Levene Test. Testing 5-HIAA and 5-HT at P14 using a Mann-Whitney U-test, DS mice had lower serotonin levels than WT controls in the DR region (P = .046, Fig. 3). In the same region at this age, levels of 5-HIAA and NE trended lower but these changes did not reach statistical significance (P = .089 and .068 respectively). The 5-HIAA to 5-HT ratio, known as serotonin turnover, was nominally elevated in DS mice but this change also did not pass the threshold of significance (P = .12) because of the trend for lower 5-HIAA levels.
Figure 3.
A.-F. Areas sampled for HPLC analysis included: A. Frontal cortex. B. Dorsal striatum. C. Greater amygdala. D. Hippocampus, possibly including some portions of cortex but excluding thalamus. E. The dorsal raphe and surrounding area. F. Ventromedial medulla including raphe magnus. G. At P14, DS mice had reduced serotonin (5-HT, P = .028), with trends for less of the serotonin metabolite, 5-HIAA and norepinephrine (NE, P < .1), while serotonin turnover (5-HIAA/5-HT) was nominally but not statistically elevated compared to WT (P = .12). H. At P35, levels of 5-HT and 5-HIAA normalized between genotypes (P = .5–.6) while NE was significantly increased (P = .05) and serotonin turnover was convincingly normal (P = .989).
At P35, no serotonin metrics were detectably different from controls in the DR sample. The trend for lower NE in mutants in the DR seen at P14 reversed at P35 to an increase, this change at the threshold for significance (P = .050). In the remaining areas sampled there was no significant effect of genotype or interaction effects noted.
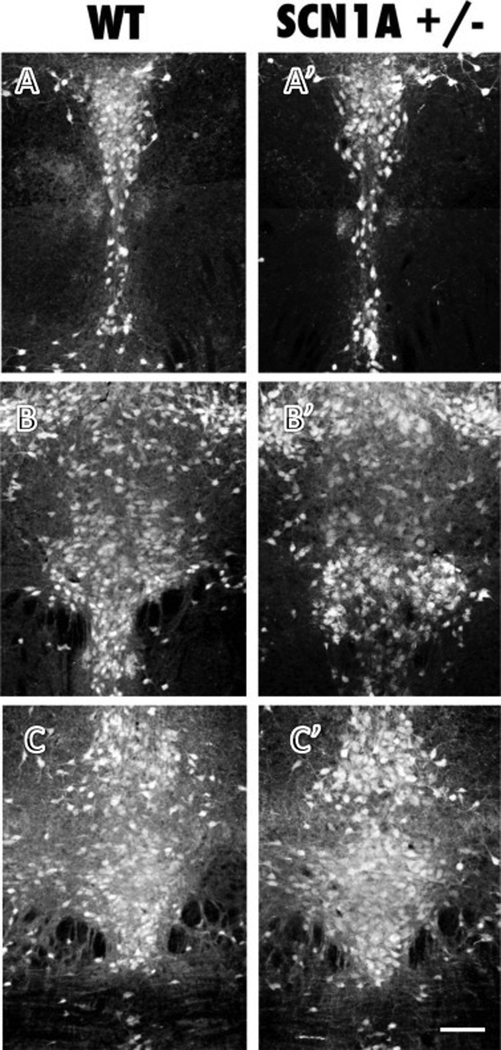
Given the alteration of serotonin in the midbrain, we evaluated the cytoarchitecture of the DR at P14. We found that there were no obvious developmental alterations and the organization of the DR appeared equivalent between genotypes (Fig. 4). Quantitative estimates of number of serotonin neurons in different subregions of the DR showed no detectable effects of genotype (P > .05, data not shown).
Figure 4.
Histological analysis of serotonin immunolabeling in the dorsal raphe at caudal (A, A’), middle (B, B’) and rostral levels (C, C’) showed comparable patterns of neuronal organization in WT and DS (Scn1A+/−) mice. Quantitative analysis of serotonin neuron number did not detect differences between genotypes (P>.05, data not shown). Scale bar = 100 um, all panels same scale.
3. Discussion
In this study, we examined some aspects of the endogenous serotonin system in a mouse model of DS. We found that at young ages there was reduced sensitivity to 5-HT1A receptor effects. In addition, there was reduced serotonin content in the region containing serotonin cell bodies of the median and dorsal raphe nuclei whereas in the medulla and in areas receiving serotonin innervation in the forebrain, serotonin levels were not detectably changed. After seizures developed, levels of serotonin and its metabolite were not detectably different. However, as adults the response of DS mice to serotonergic drugs acting at 5-HT1A and 5-HT2A/2C receptors was heightened.
DS mice at young ages had reduced hypothermia in response to the 5-HT1A agonist, DPAT. This observation is intriguing in connection to some observations related to SIDS, Sudden Infant Death Syndrome. SCN1A variants have been identified in 2 infants who died of SIDS, which is consistent with the suggestion that some SIDS cases may be seizure-related (Brownstein et al., 2018; Buchanan, 2019; Kinney et al., 2016). Separately it has been shown that SIDS infants often have reduced 5-HT1A receptor binding in several medullary regions (Kinney et al., 2009). Likewise it is know that 5-HT1A receptors can modify respiratory function in a complex way, both by acting on serotonin neurons themselves as well as non-serotonergic effector neurons (Corcoran et al., 2014). Taken together the findings suggest that DS mice may be a model useful for understanding the mechanism of at least a subset of SIDS cases.
In the literature changes in response to DPAT and DOI do not appear to simply correlate with each other nor with levels of extracellular serotonin. For example, in a mouse model of Smith-Lemli-Opitz syndrome there are profound increases in serotonin content, increased head-twitch response to DOI but no differences in DPAT-induced hypothermia (Waage-Baudet et al., 2003). Rictor-knockout mice have increased hyperthermic response to DPAT but reduced head-twitches to DOI and no changes in the levels of serotonin or serotonin turnover in any brain region (Saunders et al., 2014). In a third example, a mouse model bearing a serotonin transporter gain-of-function allele relevant to autism shows increased response to DPAT and DOI with no detectable effect on serotonin content (Mosienko et al., 2014). Therefor changes in serotonin levels, 5-HT1A and 5HT2A/2C receptor adaptations may all be triggered by different mechanisms and occur independently of each other.
DS mice exhibit an increased functionality of 5-HT1A, 5-HT2A and/or 5-HT 2C receptors, which may be an adaptive change because activation of these receptors should be protective against seizure. In a rat model of absence-seizure, (Groggy, GRY rats), incidence of seizure was reduced by either DPAT or DOI (Ohno et al., 2010). Mice lacking functional 5-HT2C receptors have social deficits, susceptibility to spontaneous death and audiogenic seizures (Heisler et al., 1998; Sejourne et al., 2015). Activation of 5-HT2A receptors also has antiepileptic effects and loss of these receptors reduces the threshold for seizure (Guiard and Di Giovanni, 2015). 5-HT1A receptor activation increases seizure threshold in a picrotoxin model (Pericic et al., 2005). In addition, loss of serotonin results in higher limbic excitability manifest in lower seizure threshold and higher lethality in response to kainic acid administration (Maia et al., 2017). In a zebrafish model relevant to DS, both 5-HT2A and 5-HT2C agonists improved locomotor behavior and reduce epileptiform activity suggesting activation of these receptors may have positive therapeutic effects (Sourbron et al., 2016).
We found that the sensitivity of DS mice to the 5-HT1A receptor agonist DPAT reverses with age, and an early insensitivity converts to hypersensitivity in adulthood. Likewise the response to the 5-HT2A/2C agonist evolves over the age of seizure appearance. These changes are consistent with other observations noting refinements in serotonin signaling with age. For example even though 5-HT1A receptors are expressed in serotonin neurons early, electrophysiology studies have shown that the 5-HT1A mediated hyperpolarization of serotonin neurons in the dorsal raphe only appears after P21 (Rood et al., 2014). 5-HT2A/2C receptors develop postnatally along the same time course that seizures appear in these mice even though electrophysiological studies show 5-HT2A receptor stimulation of cortical neurons peaks at P10 (Zhang, 2003). Stereotypical behavioral response to DOI only begin to appear after the third week of life in mice and the behavioral response continues to mature over the next few weeks (Darmani et al., 1996; Darmani and Ahmad, 1999). Meanwhile, some phenotypes of the 5-HT2C knockout mouse are not apparent until P90, suggesting continuing evolution of the serotonin system with age (Sejourne et al., 2015). These and possibly other developmental changes in the serotonin system could relate both to the age of onset of seizures as well as the transient age-dependent vulnerability to early death
We found reduced serotonin levels in the DR early in life. This could potentially make DS mice more vulnerable to seizure. In general low extracellular serotonin and thus low receptor occupancy promotes seizures (Bagdy et al., 2007; Statnick et al., 1996; Vermoesen et al., 2012). For example, lower baseline levels of serotonin and other monoamines correlate with more and longer seizures in a kindling paradigm (Shouse et al., 2001). Individuals with reduced serotonin are more susceptible to severe seizure following in a kainate model (Maia et al., 2017). Conversely, increases in extracellular serotonin and enhanced receptor signaling tend to be protective against seizure (Clinckers et al., 2004; Prendiville and Gale, 1993; Smolders et al., 2008; Yan et al., 1994). However there is a limit to the beneficial effects of serotonin as very high levels such as those that are reached in serotonin syndrome can be associated with seizures (Brown et al., 1996).
In mice that survived to P35, levels of serotonin and its metabolite were normal. One explanation of this result is that seizure activity could produce a stimulus, possibly elevated norepinephrine, that normalizes serotonin levels in the model (Meurs et al., 2008). An alternative explanation is that individuals with low serotonin as well as reduced 5-HT1A receptor function could be selectively vulnerable to early death. That is, it may be speculated that due to a survival bias only DS individuals with normal serotonin metabolism survived to P35. Consistent with this idea there is evidence that serotonin facilitates protective responses to life-threatening stressors. For example, mice that lack serotonin have a reduced capacity to recover from repeated bouts of hypoxia (Cummings et al., 2011). Likewise serotonin activation of 5-HT2A receptors is critical for appropriate arousal to high carbon dioxide levels and compromising serotonin neurons reduces seizure threshold and increases seizure-related mortality (Buchanan et al., 2014; Buchanan et al., 2015). In addition, mice that lack functional 5-HT2C receptors exhibit partial lethality and seizures (Sejourne et al., 2015). Optogenetic activation of serotonin neurons reduces respiratory arrest and is anticonvulsant in DBA/1 mouse (Zhang et al., 2018). Taken together these observations are consistent with the hypothesis that lower levels of serotonin early in life could confer vulnerability to early death in this model.
Overall the present set of experiments show that changes in the endogenous serotonin system exists in a mouse model of DS. Before seizures develop DS mice have lower levels of serotonin in the area containing the serotonin neuronal somata in the midbrain/hindbrain border. This effect either resolves or individuals with low serotonin do not survive to P35. Also at an early age they are less sensitive to the 5-HT1A receptor agonist, DPAT. At older ages after the appearance of seizures there are changes in 5-HT1A and 5-HT2A/2C receptor responses indicating ongoing alterations in endogenous serotonin system function in this mouse model. These observations may be relevant to understanding the mechanism of action of fenfluramine in this disorder since fenfluramine releases serotonin into the extracellular space where it may act on its receptors. Specifically the results would support the hypothesis that hypersensitivity of 5-HT1A and 5-HT2A/2C receptors could contribute to the beneficial effects of fenfluramine in DS. Further elucidation of importance and underlying mechanism of these changes may drive innovative strategies for pharmacotherapy and further insight into the developmental changes responsible for age-dependent phenotypes in this mouse.
4. Experimental Procedures
129S-Scn1Atm1Kea/Mmjax (Scn1a−/+) (MMRRC Stock No: 37107-JAX) mice were purchased from Jackson Labs. To generate experimental mice at Boston Children’s Hospital, male 129S- Scn1a−/+ mice were bred with normal female C57BL/6J (B6) (Jackson stock 000664) mice such that maternal behavior would not be impacted by the genetic deficiency. Both male and female F1 progeny [129S X B6] Scn1a −/+ (DS mice) born at Boston Children’s Hospital were used for analysis. Before weaning mice were housed with both parents while after weaning mice were housed with at least 2 but no more than 5 individuals of the same sex per cage, with free access to food and water. Controls consisted of wild-type (WT) littermates. Care and use of animals in these studies was approved by the Institutional Care and Use Committee at Boston Children’s Hospital under protocol approval number 15-03-2904R.
Hypothermia
Separate groups of DS and WT mice at P18 and P35 were monitored for hypothermia in response to a single acute injection of (+/−)-8-hydroxy-2-(dipropylamino)tetralin hydrobromide (DPAT) (0.5 mg/kg). For this a flexible temperature probe (RET-3 rectal probe for mice, Physitemp Instruments, Clifton New Jersey) was carefully inserted rectally and the mouse was given about 5 minutes to adapt to the probe and the chamber. Baseline temperature was recorded and an injection of DPAT was given. Following the injection temperature was continuously followed for 15 minutes, when mice were returned to their home cage.
Head Twitch and Ear Scratch Response
A separate group of DS and WT mice were injected intraperitoneally and monitored for stereotypical movements a P35. For this, (+/−)-2,5-dimethoxy-4-iodoamphetamine hydrochloride (DOI) (Sigma-Aldrich, St Louis, MO, USA) was dissolved in 0.9% normal saline and administered intraperitoneally in a single acute dose of 3.0 mg/kg. Control mice received vehicle injections. The mice were then singly placed into an empty cage where their behavior was video recorded for 10 minutes. The two movements that were counted were head twitch responses (rapid rotational side-to-side head movement) and ear scratch responses (a rapid scratching movement of the ear, head, or neck are by either hind limb). Stereotypical response to DOI is age dependent and not present before P21, therefore only P35-age mice were tested (Darmani et al., 1996; Darmani and Ahmad, 1999).
HPLC Analysis
Mice were sampled for HPLC at P14, an age before seizures develop, and at P35, after the vulnerable period for early death ends. For sampling, mice were anesthetized using isoflurane and whole brains were rapidly extracted and snap-frozen in 2-methyl butane cooled with dry ice. The regions sampled were punched out of serial 300-micron thick slices cut on a cryostat with a 2 mm diameter punch and included areas centered on the frontal cortex, dorsal striatum, amygdala, hippocampus, dorsal raphe nucleus and the ventromedial medulla (Fig. 3). These areas were selected because they represent termination fields with distinct areas of origin within the raphe nuclei, they were implicated as areas where serotonin neurotransmission may be compromised in DS mice and they could be sampled reproducibly from animal to animal (Panzini et al., 2017). HPLC analysis was completed at the Neurochemistry Core Facility at Vanderbilt University. There, frozen brain tissue samples were homogenized by using a tissue dismembrator (Misonix XL-2000; Qsonica) in a solution containing 100 mM TCA, 10 mM NaC2H3O2, 100 μM EDTA, 5 ng/mL isoproterenol (an internal standard), and 7.5% (vol/vol) methanol (pH 3.8). Using a Waters 2707 autosampler 10μL of supernatant from each sample was injected onto a Phenomenex Kintex (2.6 μm, 100 A) C18 HPLC column (100 X4.6 mm). Biogenic amines were eluted with a mobile phase [100 mM TCA, 10mM NaC2H3O2, 100μM EDTA, and 7.5% methanol (pH 3.8)] delivered at 0.6 mL/min by using a Waters 515 HPLC pump. Analytes were detected by using an Antec Decade II (oxidation: 0.4) electrochemical detector operated at 33°C. Empower software was used to manage HPLC instrument control and data acquisition.
Immunohistochemistry
For analysis of the cytoarchitecture of serotonin neurons, mice were anesthetized using pentobarbital and perfused with 4% paraformaldehyde in 0.1 M phosphate buffer. After equilibration in 30% sucrose, the brains were frozen and sectioned. Immunofluorescence processing was performed on floating sections. Serotonin was detected by incubating sections in primary antisera raised in rabbit (ImmunoStar, product ID: 20080, RRID:AB_572263_) diluted 1:10,000 in 0.1 M phosphate buffered saline with 0.3% Triton X-100, 0.1% bovine serum albumin and 0.01% sodium azide at 4° C for 72 hours, followed by incubation in CY3 anti-rabbit (Jackson Labs, diluted 1:500) raised in donkey at room temperature for 90 minutes. Sections were rinsed in 0.05 M phosphate buffer, mounted, dried, and cover-slipped with a glycerol-based mounting medium containing a nuclear stain 4’,6-diamidino-2-phenylindole (DAPI).
For image analysis, tissue was sampled in every third section through the region of interest. Three rostrocaudal divisions of the DR were made: rostral was −4.16 to −4.24mm, middle was −4.36 to −4.84mm, and caudal was −4.96 to −5.20mm relative to Bregma. These three divisions were divided in half into dorsal and ventral subregions. Using NIH Image J Software, images containing DAPI labeling for nuclei and serotonin labeling were split into separate color channels. For each color channel, the background was subtracted and a threshold was applied to define labeled areas. A selection area generated by the area imunolabeled for serotonin was applied to the cognate image of DAPI-defined nuclei. The number of nuclei within the serotonin-selection area were counted using Analyze-Measure. The mean number of cells per section per region was determined for each genotype. Widefield images were obtained using an Olympus IX-81 fluorescence microscope with a 10× objective, a Hamamatsu Orca ER camera and Slidebook software (3i).
Statistics
For the hypothermic effect of DPAT, the delta, or the change in body temperature from baseline to the 15-minute time-point was analyzed using a two factor (age and genotype) ANOVA. For the effect of DOI on stereotypical movement we used a two factor (drug treatment and genotype) ANOVA. In both cases the subsequent relevant comparisons were between two groups therefore a student’s t-test was used as posthoc test. For the HPLC, data points were rejected from the final dataset if they were greater than two standard deviations from the mean value of the associated group. Subsequently a two factor (age and genotype) ANOVA was used for each region. If there was evidence for inequality of variance from the Levene test, a Mann-Whitney U test was conducted. Subsequent relevant comparisons were also two groups such that a student’s t-test was used for posthoc testing. For analysis of serotonin neurons in the DR a one-way ANOVA for genotype was used. If there was no main effect of genotype, no follow up tests were conducted.
Before the age where seizures appear in Dravet syndrome mice they have reduced response to 5-HT1A agonists and less serotonin in the dorsal raphe nucleus
After the age of seizure development Dravet syndrome mice are hypersensitive to both 5-HT1A and 5-HT2A/2C agonists.
Acknowledgements:
Declaration of Interest: KGC was a consultant for Zogenixs, Inc. on the topic of Dravet Syndrome.
Abbreviations:
- DS
Dravet Syndrome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: none
References
- Bagdy G, et al. , 2007. Serotonin and epilepsy. J Neurochem. 100, 857–73. [DOI] [PubMed] [Google Scholar]
- Boyd B, et al. , 2019. A phase I, randomized, open-label, single-dose, 3-period crossover study to evaluate the drug-drug interaction between ZX008 (fenfluramine HCl oral solution) and a regimen of stiripentol, clobazam, and valproate in healthy subjects. Int J Clin Pharmacol Ther. 57, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TM, Skop BP, Mareth TR, 1996. Pathophysiology and management of the serotonin syndrome. Ann Pharmacother. 30, 527–33. [DOI] [PubMed] [Google Scholar]
- Brownstein CA, et al. , 2018. SCN1A variants associated with sudden infant death syndrome. Epilepsia. 59, e56–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, et al. , 2014. Serotonin neurones have anti-convulsant effects and reduce seizure-induced mortality. J Physiol. 592, 4395–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, et al. , 2015. 5-HT2A receptor activation is necessary for CO2-induced arousal. J Neurophysiol. 114, 233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, 2019. Impaired CO2-Induced Arousal in SIDS and SUDEP. Trends Neurosci. 42, 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceulemans B, et al. , 2012. Successful use of fenfluramine as an add-on treatment for Dravet syndrome. Epilepsia. 53, 1131–9. [DOI] [PubMed] [Google Scholar]
- Cheah CS, et al. , 2012. Specific deletion of NaV1.1 sodium channels in inhibitory interneurons causes seizures and premature death in a mouse model of Dravet syndrome. Proc Natl Acad Sci U S A. 109, 14646–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinckers R, et al. , 2004. Anticonvulsant action of hippocampal dopamine and serotonin is independently mediated by D and 5-HT receptors. J Neurochem. 89, 834–43. [DOI] [PubMed] [Google Scholar]
- Corcoran AE, et al. , 2014. Dual effects of 5-HT(1a) receptor activation on breathing in neonatal mice. J Neurosci. 34, 51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KJ, et al. , 2011. Failed heart rate recovery at a critical age in 5-HT-deficient mice exposed to episodic anoxia: implications for SIDS. J Appl Physiol (1985). 111, 825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani NA, Shaddy J, Gerdes CF, 1996. Differential ontogenesis of three DOI-induced behaviors in mice. Physiol Behav. 60, 1495–500. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Ahmad B, 1999. Long-term sequential determination of behavioral ontogeny of 5-HT1A and 5-HT2 receptor functions in the rat. J Pharmacol Exp Ther. 288, 247–53. [PubMed] [Google Scholar]
- Dravet C, 2011. Dravet syndrome history. Dev Med Child Neurol. 53 Suppl 2, 1–6. [DOI] [PubMed] [Google Scholar]
- Genton P, Velizarova R, Dravet C, 2011. Dravet syndrome: the long-term outcome. Epilepsia. 52 Suppl 2, 44–9. [DOI] [PubMed] [Google Scholar]
- Guiard BP, Di Giovanni G, 2015. Central serotonin-2A (5-HT2A) receptor dysfunction in depression and epilepsy: the missing link? Front Pharmacol. 6, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA, 2013. Characterization of the head-twitch response induced by hallucinogens in mice: detection of the behavior based on the dynamics of head movement. Psychopharmacology (Berl). 227, 727–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Tecott LH, 1998. Epilepsy and obesity in serotonin 5-HT2C receptor mutant mice. Ann N Y Acad Sci. 861, 74–8. [DOI] [PubMed] [Google Scholar]
- Kalume F, 2013. Sudden unexpected death in Dravet syndrome: respiratory and other physiological dysfunctions. Respir Physiol Neurobiol. 189, 324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalume F, et al. , 2013. Sudden unexpected death in a mouse model of Dravet syndrome. J Clin Invest. 123, 1798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, et al. , 2009. The brainstem and serotonin in the sudden infant death syndrome. Annu Rev Pathol. 4, 517–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, et al. , 2016. Hippocampal Formation Maldevelopment and Sudden Unexpected Death across the Pediatric Age Spectrum. J Neuropathol Exp Neurol. 75, 981–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BM, et al. , 2011. Autism in Dravet syndrome: prevalence, features, and relationship to the clinical characteristics of epilepsy and mental retardation. Epilepsy Behav. 21, 291–5. [DOI] [PubMed] [Google Scholar]
- Maia GH, et al. , 2017. Serotonin depletion increases seizure susceptibility and worsens neuropathological outcomes in kainate model of epilepsy. Brain Res Bull. 134, 109–120. [DOI] [PubMed] [Google Scholar]
- Meisler MH, Kearney JA, 2005. Sodium channel mutations in epilepsy and other neurological disorders. J Clin Invest. 115, 2010–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurs A, et al. , 2008. Seizure activity and changes in hippocampal extracellular glutamate, GABA, dopamine and serotonin. Epilepsy Res. 78, 50–9. [DOI] [PubMed] [Google Scholar]
- Mosienko V, et al. , 2014. Adaptive changes in serotonin metabolism preserve normal behavior in mice with reduced TPH2 activity. Neuropharmacology. 85, 73–80. [DOI] [PubMed] [Google Scholar]
- Ogiwara I, et al. , 2007. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci. 27, 5903–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiwara I, et al. , 2013. Nav1.1 haploinsufficiency in excitatory neurons ameliorates seizure-associated sudden death in a mouse model of Dravet syndrome. Hum Mol Genet. 22, 4784–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno Y, et al. , 2010. Serotonergic modulation of absence-like seizures in groggy rats: a novel rat model of absence epilepsy. J Pharmacol Sci. 114, 99–105. [DOI] [PubMed] [Google Scholar]
- Panzini CM, et al. , 2017. 16p11.2 Deletion Syndrome Mice Perseverate with Active Coping Response to Acute Stress - Rescue by Blocking 5-HT2A Receptors. J Neurochem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pericic D, et al. , 2005. Stimulation of 5-HT 1A receptors increases the seizure threshold for picrotoxin in mice. Eur J Pharmacol. 527, 105–10. [DOI] [PubMed] [Google Scholar]
- Prendiville S, Gale K, 1993. Anticonvulsant effect of fluoxetine on focally evoked limbic motor seizures in rats. Epilepsia. 34, 381–4. [DOI] [PubMed] [Google Scholar]
- Richardson-Jones JW, et al. , 2010. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 65, 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood BD, et al. , 2014. Dorsal raphe serotonin neurons in mice: immature hyperexcitability transitions to adult state during first three postnatal weeks suggesting sensitive period for environmental perturbation. J Neurosci. 34, 4809–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, 2002. Serotonin releasing agents. Neurochemical, therapeutic and adverse effects. Pharmacol Biochem Behav. 71, 825–36. [DOI] [PubMed] [Google Scholar]
- Saunders C, et al. , 2014. Neuronal ablation of p-Akt at Ser473 leads to altered 5-HT1A/2A receptor function. Neurochem Int. 73, 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer NT, et al. , 2016. Scn1a dysfunction alters behavior but not the effect of stress on seizure response. Genes Brain Behav. 15, 335–47. [DOI] [PubMed] [Google Scholar]
- Schoonjans A, et al. , 2017. Low-dose fenfluramine significantly reduces seizure frequency in Dravet syndrome: a prospective study of a new cohort of patients. Eur J Neurol. 24, 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejourne J, et al. , 2015. Social Behavioral Deficits Coincide with the Onset of Seizure Susceptibility in Mice Lacking Serotonin Receptor 2c. PLoS One. 10, e0136494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouse MN, et al. , 2001. Monoamines and seizures: microdialysis findings in locus ceruleus and amygdala before and during amygdala kindling. Brain Res. 892, 176–92. [DOI] [PubMed] [Google Scholar]
- Smolders I, et al. , 2008. Direct enhancement of hippocampal dopamine or serotonin levels as a pharmacodynamic measure of combined antidepressant-anticonvulsant action. Neuropharmacology. 54, 1017–28. [DOI] [PubMed] [Google Scholar]
- Sourbron J, et al. , 2016. Serotonergic Modulation as Effective Treatment for Dravet Syndrome in a Zebrafish Mutant Model. ACS Chem Neurosci. 7, 588–98. [DOI] [PubMed] [Google Scholar]
- Statnick MA, et al. , 1996. Effect of 5,7-dihydroxytryptamine on audiogenic seizures in genetically epilepsy-prone rats. Life Sci. 59, 1763–71. [DOI] [PubMed] [Google Scholar]
- Vermoesen K, et al. , 2012. The antidepressants citalopram and reboxetine reduce seizure frequency in rats with chronic epilepsy. Epilepsia. 53, 870–8. [DOI] [PubMed] [Google Scholar]
- Waage-Baudet H, et al. , 2003. Abnormal serotonergic development in a mouse model for the Smith-Lemli-Opitz syndrome: implications for autism. Int J Dev Neurosci. 21, 451–9. [DOI] [PubMed] [Google Scholar]
- Yan QS, et al. , 1994. Role of serotonin in the anticonvulsant effect of fluoxetine in genetically epilepsy-prone rats. Naunyn Schmiedebergs Arch Pharmacol. 350, 149–52. [DOI] [PubMed] [Google Scholar]
- Yu FH, et al. , 2006. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci. 9, 1142–9. [DOI] [PubMed] [Google Scholar]
- Zhang H, et al. , 2018. Optogenetic activation of 5-HT neurons in the dorsal raphe suppresses seizure-induced respiratory arrest and produces anticonvulsant effect in the DBA/1 mouse SUDEP model. Neurobiol Dis. 110, 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZW, 2003. Serotonin induces tonic firing in layer V pyramidal neurons of rat prefrontal cortex during postnatal development. J Neurosci. 23, 3373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]