Abstract
Objective
Polygraphy (PG) is an attractive alternative for diagnosing obstructive sleep apnea (OSA) in patients with high pre-test probability. However, several patients may not present typical symptoms. In this scenario, it is unclear the performance of PG for diagnosing OSA in non-referred populations to sleep laboratories.
Methods
Data from participants of the ELSA-Brasil cohort were used for this analysis. We performed an overnight home PG (Embletta GoldTM) synchronized with a wrist actigraphy (Actiwatch model 2TM). The validation strategy comprised three scorings from each participant: 1) Original scoring (PG): Routine scoring using data from the exclamation button mark to define “analysis start” and “analysis stop”; 2) Scoring using actigraphy data (PG+actigraphy): total sleep time defined by the actigraphy data; 3) Scoring using diaries (PG+diary): “analysis start” and “analysis stop” based on the diaries. Bland-Altman plots were generated to assess the agreements (Kappa) between each scoring strategy.
Results
A total of 300 participants were included in the final analysis (45% males, mean age: 48±8 years). The frequency of OSA using the PG score was 27.3%. Despite small differences in the OSA severity index, we obtained a high concordance of AHI comparing the PG vs. PG+actigraphy (Kappa: 0.95) as well as PG+diary vs. PG+actigraphy (Kappa: 0.96). No significant changes in the OSA classification (mild, moderate and severe) were observed in the 3 protocols.
Conclusion
Using a pragmatic approach to address OSA at home, our results suggest that PG is a useful tool for OSA diagnosis even in subjects not referred to sleep studies.
Keywords: Sleep Apnea, Diagnosis, Polysomnography, Wrist
INTRODUCTION
Obstructive Sleep Apnea (OSA) is a clinical condition characterized by repetitive upper-airway obstructions during sleep promoting sleep fragmentation, intermittent hypoxia and intrathoracic pressure reduction1. OSA is common in the general population2-4, reaching one third of adults in a recent population study using contemporaneous definitions of apnea and hypopnea4. Consistent evidence from the literature pointed that OSA is associated with increased risk for metabolic and cardiovascular consequences5-8.
In clinical practice, however, OSA is still underdiagnosed9. The reasons for this unrecognition are multiple and include: 1) lack of medical background in sleep medicine; 2) lack of typical symptoms in a significant proportion of patients; 3) the gold standard method for diagnosing OSA, namely polysomnography, has limited access, long waitlists10 and significant costs11. In this scenario, portable sleep monitors, also called polygraphy (PG) have gained growing interest to be used as an attractive alternative to surpass the main above limitations. PGs have been extensively validated against polysomnography in patients with high clinical probability of OSA12-15. However, whether PGs can be used for diagnosing OSA in non-referred populations is unclear. From an epidemiologic perspective, it is important to understand if cohorts evaluating the consequences of OSA may have benefits in using pragmatic and feasible forms of OSA diagnosis in the home environment16. In addition, there is a growing interest for wide spreading sleep evaluation using simplified tools17.
In the present study, we selected non-referred participants derived from the ELSA-Brasil cohort to compare the OSA diagnosis agreement using PGs and wrist actigraphy simultaneously. This approach is approved by the AASM as a validated method for estimating the total sleep time when polysomnography is not available18. We hypothesized that home PG scoring or PG scoring using diary data had good agreement in diagnosing OSA (and the related severity classifications) compared to the PG coupled with actigraphy (the reference group) in a non-referred population.
METHODS
The local ethical committed approved the study and all participants provided an informed consent.
This is an ancillary to the ELSA-Brasil study, which cohort profile and routines were previously reported19-21. Briefly, all active or retired employees (aged 35-74 years) of the six institutions (Federal Universities of Bahia, Espírito Santo, Minas Gerais and Rio Grande do Sul; University of São Paulo, and Oswaldo Cruz Foundation) were eligible for the study. Exclusion criteria were current or recent (<4 months) pregnancy, intention to quit working at the institution in the near future, severe cognitive or communication impairment, and, if retired, residence outside of a study center’s corresponding metropolitan area. As previously described16, the sleep approach in ELSA-Brasil was conducted in the participants of the Sao Paulo site, and the only pre-defined exclusion criterion is the refuse to perform sleep studies.
Overnight Home Sleep Study
Sleep studies were perfomed using the Embletta Gold (Natus Medical Inc., Ontario, Canada) a standardized level-3 portable diagnostic device including the following outputs: nasal airflow (nasal pressure transducer), thoraco-abdominal movements (inductive respiratory bands), arterial oxygen saturation (pulse oximetry), snoring episodes (derived from the integrated pressure transducer), and body position16. This device has an exclamation point button to report events. The participants were actively instructed to push the button when they turn out the lights to sleep and wake-up to help physicians to report total recording time. The Embletta system has been validated in patients with high suspicious of OSA12. All studies are being manually scored by an expert in sleep medicine. The respiratory events are being scored according to the American Academy of Sleep Medicine (AASM) 2012 criteria22 as follows: An apnea was defined as a ≥90% decrease in airflow from the baseline value for ≥10 seconds. Hypopnea was defined as ≥30% drop of airflow lasting at least 10 seconds with a ≥3% O2 drop. Apneas were further classified as obstructive or central based on the presence or absence, respectively, of respiratory-related chest-wall movement. The sum of apnea and hypopneas per hour determined the apnea-hypopnea index (AHI). We excluded participants with predominantly (>50%) central apnea events. Considering that growing evidence suggesting that mild OSA is not associated with increased cardiovascular risk23, we decided for classifying OSA using a more conservative AHI cut-off of ≥15 events/hour. However, we performed a sub-analysis using the standard classification of mild (AHI 5-14.9), moderate (AHI 15-29.9) and severe OSA (AHI ≥30 events/h).
Wrist actigraphy
Participants were instructed to wear the actigraphy (Actiwatch model 2,TM Philips Respironics) in the same night of the sleep monitor recording. As previously described16, participants were asked to press the event marker button on the actigraph each night when they began trying to fall asleep and again when they got out of bed each morning. A validated algorithm was used to calculate sleep duration (calculated as the total time of the epochs classified as sleep between sleep start and sleep end), fragmentation index (the number of interruptions of sleep by physical movement calculated as 100 × the number of groups of consecutive mobile 30-s epochs by the total number of immobile epochs) and sleep efficiency (100% × sleep duration/the time between bedtime and rise-time).
Diary
We instructed all participants for filling diaries reporting routine events, including subjective sleep time.
Validation strategy
The time of portable sleep study and wrist actigraphy were synchronized in the respective softwares. The validation strategy comprised three scorings from each participant: 1) Original scoring (PG): Routine scoring using data from the exclamation button mark to define “analysis start” and “analysis stop”. If the participant did not press the button, we defined “analysis start” when all traces were working properly and the body position sensor suggested the participant is lying in the bed; for the “analysis stop”, we defined the first period of the standing up position and lack of traces suggesting that the device was removed as recommended in the instructions; 2) Scoring using diaries (PG+diary): In this case, “analysis start” and “analysis stop” were based on the related events reported in the diaries; 3) Scoring using actigraphy data (PG+actigraphy): the “analysis start” and “analysis stop” were defined by the timing suggested by the actigraphy as sleep start and stop, respectively. A same trained researcher performed the original (PG) scorings of all participants while another researcher performed two new reports using diary data (PG+diary) or actigraphy data (PG+actigraphy) to recalculate the AHI and the related hypoxemia variables. There two new reports used the same respiratory events but the second researcher did not check the sleep report provided by the original score.
Statistical analysis
Continuous variables with normal distribution were presented as mean±SD. For skewed variables, medians and interquartile ranges were reported and Kruskal-Wallis tests were performed. Categorical variables were presented as frequencies and analyzed using chi-square tests. Bland-Altman plots and Pearson tests were generated to assess agreement (Kappa) and correlations, respectively, of PG alone and PG+diary as compared to the PG+actigraphy results. In addition, we compared the proportion of participants with no, mild, moderate and severe OSA using these three strategies.
Finally, we performed a comparison of participants that did press or not the button mark to define “analysis start” and “analysis stop” (not only for mimicking the real practice but also to extrapolate our findings to PGs that do not have available button mark to define “analysis start” and “analysis stop”). A p value of <0.05 was considered statistically significant (2-sided).
RESULTS
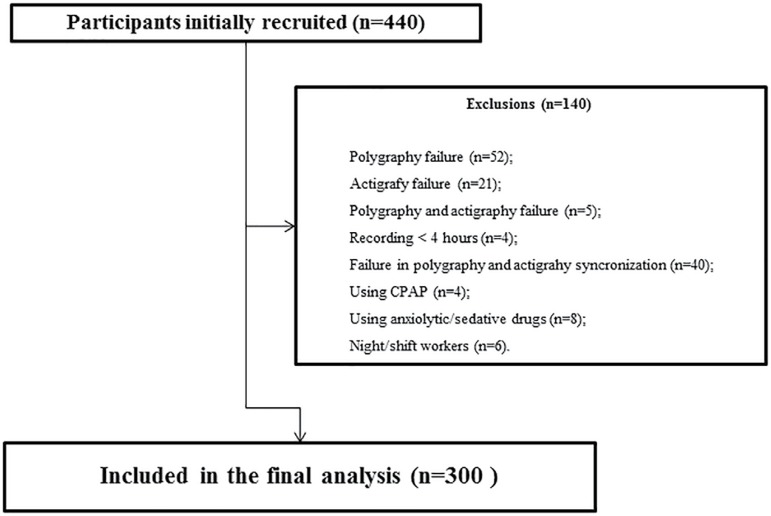
For this study, we initially recruited consecutive 440 participants from the ELSA-Brasil who performed the portable sleep monitor and the wrist actigraphy in a synchronized way. After exclusions, a total sample of 300 participants were included in the final analysis (please see Figure 1 for details).
Figure 1.
Flow chart.
Characteristics of participants were reported on Table 1. Overall, our sample comprised middle-age and overweight participants. Almost half of them were men and two thirds of them were white. Twenty-seven percent of them had OSA. Compared to no OSA, participants with OSA were older, had higher frequency of men, presented higher levels of adiposity parameters as well as higher frequencies of hypertension, diabetes and current smoking. The wrist actigraphy data showed that OSA participants had higher sleep latency, higher time in minutes of wake after sleep onset (WASO), higher frequency of number of awakenings (AWAKE), and lower sleep efficiency than no OSA group.
Table 1.
Characteristics of the participants.
| Characteristics | Total N=300 |
No OSA N= 218 (72.7%) |
OSA N= 82 (27.3%) |
p value |
|---|---|---|---|---|
| Demographic and anthropometric data | ||||
| Male sex, n (%) | 135 (45) | 82 (37.6) | 53 (64.6) | <0.001 |
| Age, years, mean (SD) | 48 (8.3) | 47 (7.8) | 50 (8.9) | 0.001 |
| White (self-reported race), n (%) | 206 (68.7) | 154 (71) | 52 (63.4) | 0.511 |
| Body-mass index, kg/m2, mean (SD) | 26.8 (4.6) | 26 (4.5) | 28.9 (4.5) | <0.001 |
| Classification of BMI, n (%) | ||||
| Normal | 62 (20.7) | 96 (44.1) | 13 (15.9) | |
| Overweight | 129 (43) | 85 (39) | 44 (53.7) | <0.001 |
| Obesity | 109 (36.3) | 37 (17) | 25 (30.5) | |
| Neck circumference, cm, mean (SD) | 35.9 (3.7) | 35.1 (3.4) | 37.9 (3.6) | <0.001 |
| Waist circumference, cm, mean (SD) | 88.8 (11.9) | 85.8 (11) | 96.6 (10.8) | <0.001 |
| Hypertension, n (%) | 65 (21.7) | 37 (17) | 28 (34.1) | 0.001 |
| Diabetes, n (%) | 48 (16) | 24 (11) | 24 (29.3) | <0.001 |
| Current Smoking, n (%) | 35 (11.7) | 22 (10.1) | 13 (16) | 0.159 |
| Depression, n (%) | 16 (5.3) | 14 (6.5) | 2 (2.5) | 0.175 |
| Excessive daytime sleepiness, n (%) | 105 (35) | 83 (38.1) | 22 (26.8) | 0.069 |
| Sleep data (Wrist actigraphy) | ||||
| Sleep time, min, mean (SD) | 396.5 (57.7) | 396.9 (58.1) | 395.5 (55.3) | 0.839 |
| Sleep time <6 hours, n (%) | 74 (24.7) | 55 (25.2) | 19 (23.2) | 0.712 |
| Sleep latency, min, mean (SD) | 21 (14.6) | 19.7 (14) | 24.4 (15.7) | 0.013 |
| Sleep efficiency, min, mean (SD) | 82.8 (6.4) | 83.6 (6.2) | 80.7 (6.5) | <0.001 |
| WASO, min, mean (SD) | 44 (18.9) | 41.1 (17.2) | 51.8 (21.1) | <0.001 |
| AWAKE, n, mean (SD) | 32.7 (11.2) | 31.3 (10.2) | 36.3 (12.9) | <0.001 |
OSA: Obstructive Sleep Apnea
BMI: Body-mass index
WASO: Wake after sleep onset
AWAKE: Number of awakenings
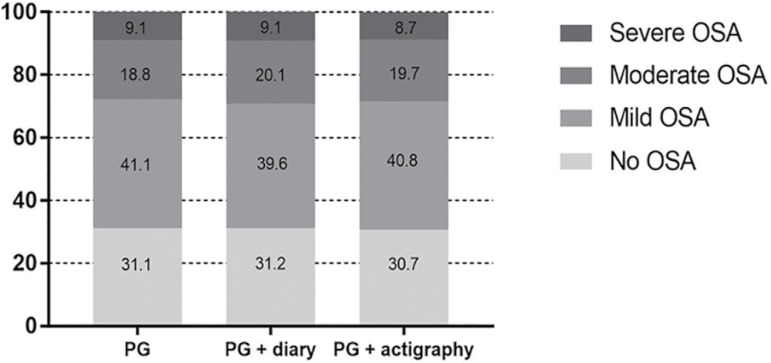
Table 2 describes the comparisons of sleep data using the PG, PG+diary and PG+actigraphy scores. The total time for analysis was lower in the PG+actigraphy as compared to the remaining groups. There was a trend for lower AHI in the PG+actigraphy group but all of them were in the mild OSA range. Significant differences were observed in the hypoxemic parameters, but all absolute values were very similar, suggesting no clinical relevance. Supporting this concept, the percentages of no OSA, mild, moderate and severe OSA were similar in the three scores analysis (Figure 2).
Table 2.
Comparisons of sleep data using the original scoring, combined with actigraphy data and using the diary.
| Sleep study characteristics | PG | PG + diary | PG + actigraphy | p value* |
|---|---|---|---|---|
| Record duration, median (IQR) | 436(383-483) | 430(386-480) | 413(359-459) * | <0.001 |
| AHI, events/hours, median (IQR) | 8.9 (4.1-16.2) | 8.9 (4.1-16.7) | 8.8 (4.1 -16.5) | 0.058 |
| Baseline SpO2%, median (IQR) | 94.4 (93.2-95.5) | 94.4 (93.2-95.5) * | 94.4 (93.2-95.5) | <0.001 |
| Lowest SpO2%, median (IQR) | 87 (83-90) | 87 (82-90) | 87 (83-90) | 0.004 |
| Time SpO2 <90%, median (IQR) | 0.2 (0-2.1) & | 0.2 (0-2.3) | 0.2 (0-2.2) # | <0.001 |
| Supine AHI (events/hours), median (IQR) | 14.6 (4.9-29.5) | 14 (5-29.8) | 14.3 (4.5-29.8) * | <0.001 |
| Supine events, median (IQR) | 28 (10-61) | 28 (10-60) | 26 (8-55) | <0.001 |
IQR: Interquartile range. Some variables presented identical median and IQR but have significant variabilities among the groups.
p<0.05 vs. remains groups
p<0.05 vs. PG
p<0.05 vs. PS + actigraphy
Figure 2.
Frequency of normal, mild, moderate and severe obstructive sleep apnea (OSA) using the PG, PG + diary and PG + actigraphy analysis.
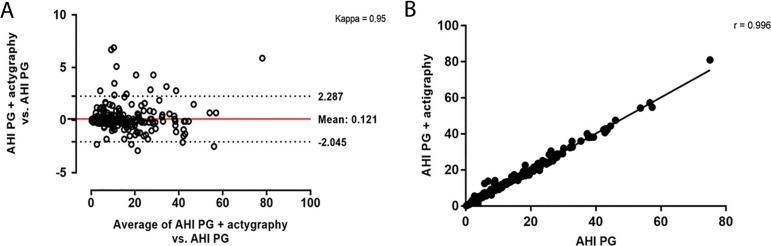
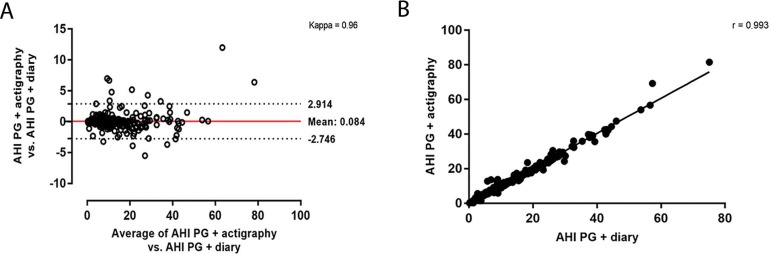
Figures 3 and 4 reported the agreement and correlation of AHI in each method. Using PG+actigraphy as the reference group, a high agreement (Figure 3A) and correlation (Figure 3B) were observed for the comparisons of AHI derived from PG vs. PG+actigraphy as well as in the comparisons of AHI derived from PG+diary vs. PG+actigraphy (Figures 4A and 4B).
Figure 3.
Bland-Altman (A) and correlation (B) between the apnea-hypopnea index (AHI) using the PG versus PG + actigraphy data.
Figure 4.
Bland-Altman (A) and correlation (B) between the apnea-hypopnea index (AHI) using the PG + diary versus PG + actigraphy data.
Finally, we also compared the AHI in patients that did (n=241, 80%) and did not (n=59, 20%) press the Embletta GoldTM button to sign the sleep onset and sleep end. We found no differences in the performance and OSA severity classification among those who used versus not used this particular Embletta GoldTM function, suggesting that our result may be applied for all type 3 monitors (please see supplemental file).
DISCUSSION
This is one of the largest studies devoted to validating portable sleep monitors. The novelty here is the validation of this simplified method for diagnosing OSA in a non-referred population using a pragmatic approach (home PG coupled with actigraphy as a reference group). We found an excellent AHI agreement and correlation after performing three different scoring strategies. Despite significant differences in some sleep parameters related to the OSA severity, these findings seems to be not clinically relevant as suggested by the lack of differences in the proportion of each OSA severity classification. Taken together, our results pointed the home PG as a feasible strategy to diagnose OSA even in those participants not referred to sleep studies.
In patients with high clinical suspicious for OSA, several portable sleep monitors have been extensively validated against polysomnography12-14. The correlation values varied from 0.890 to 0.997. This variation may be partially explained by differences in the protocols, including simultaneous versus no simultaneous polysomnography recording. It is important to mention that validation studies in the sleep laboratory as well as excessive monitoring using two methods (including two nasal cannulas) in the same night may compromise the original sleep quality and quantity. For our knowledge, a single study used only one nasal cannula with a connector for sending the signal for both sleep monitor and polysomnography14. In our study, we decided for using a simple strategy trying to resemble the normal sleep pattern using a wrist actigraphy coupled to the sleep monitor. The high agreement and correlation between the three strategies may contribute to expand the indications for using sleep monitors even in a non-referred population. The potential utility of our findings relies on the fact that several patients may not present typical symptoms of OSA. In addition, the most used questionnaires have only modest accuracy in screening OSA24,25.
Therefore, pragmatic and feasible forms of OSA diagnosis ideally in the home environment may increase the awareness and treatment of this sleep disordered breathing. In the same direction, we have observed a tremendous development of technology for monitoring vital parameters using smartphones, including sleep26,27. The development and validation of apps for diagnosing OSA may be useful for OSA diagnosis in patients with no complex and comorbid conditions. Further studies in this research area are warranted.
Two interesting strategies of our study include: 1) the evaluation of having an available diary helping to define sleep start and ending in a subjectively way; 2) the evaluation of having an actigraphy coupled with the PG. In clinical practice, it is not unusual to come across with patients that did not fill the diary. In our study, the rate of participants that did not have the diary report was low (partially explained by the fact that a significant proportion of them filled the diary at the time they dropped off the sleep monitor).
However, the performance of PG was similar in participants who filled or not the diary. Therefore, our results underscore that the utility of the sleep monitor remains even in the lack of the diary data. The second interesting finding was the high agreement of the PG+actigraphy score compared to PG+diary. Our results also pointed to a no specific need of an actigraphy (already included in some devices) to validate the sleep monitor data. These findings may have clinical implications for simplifying the sources of several trademarks available for OSA diagnosis.
The present study has strengths and limitations to be discussed. The used a large sample of consecutive participants performing sleep monitoring at home, since the sleep environment during polysomnography may not mimic real life. We carefully performed a detailed analysis of different scenarios (PG alone and combined with actigraphy and diary) as well as analysis of OSA severity classification in a blinded way. The following limitations deserve comments: 1) we did not use home polysomnography as the gold standard comparator. However, as previously mentioned, the actigraphy is an acceptable alternative for detecting sleep duration18; 2) due to our stringent protocol, we excluded almost 10% of patients that we were not confident about the time synchronization; 3) these results should be extrapolated with cautions for patients with significant comorbidities such as heart failure or chronic obstructive pulmonary disease.
In conclusion, home PG is useful for diagnosing OSA even in a non-referred population. Considering contemporary trends for using portable devices and wearables technologies for surpass the aforementioned obstacles of a formal polysomnography, this study may have implications not only for the ELSA-Brasil cohort but also for future portable and wearable devices validations.
Abbreviations list
- AASM:
American Academy of Sleep Medicine
- AHI:
Apnea-hypopnea index
- AWAKE:
number of awakenings
- BMI:
Body mass index
- ELSA:
Longitudinal Study of Adult Health
- IQR:
Interquartile range
- OSA:
Obstructive Sleep Apnea
- PG:
Polygraphy
- WASO:
wake after sleep onset
REFERENCES
- 1.Jordan AS, McSharry DG, Malhorta A. Adult obstructive sleep apnea. Lancet. 2014;383(9918):736–747. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11(5):441–446. doi: 10.1016/j.sleep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Eng J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 4.Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, et al. Prevalence of sleep-disordered breathing in the general population: the HipnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62(7):569–576. doi: 10.1016/j.jacc.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 7.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163(1):19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 8.Redline S, Sotres-Alvarez D, Loredo J, Hall M, Patel SR, Ramos A, et al. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med. 2014;189(3):335–344. doi: 10.1164/rccm.201309-1735OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costa LE, Uchôa CH, Harmon RR, Bortolotto LA, Lorenzi-Filho G, Drager LF. Potential underdiagnosis of obstructive sleep apnoea in the cardiology outpatient setting. Heart. 2015;101(16):1288–1292. doi: 10.1136/heartjnl-2014-307276. [DOI] [PubMed] [Google Scholar]
- 10.Hirshkowitz M. Polysomnography Challenges. Sleep Med Clin. 2016;11(4):403–411. doi: 10.1016/j.jsmc.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Flemons WW, Douglas NJ, Kuna ST, Rodenstein DO, Wheatley J. Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med. 2004;169(6):668–672. doi: 10.1164/rccm.200308-1124PP. [DOI] [PubMed] [Google Scholar]
- 12.Ng SS, Chan TO, To KW, Ngai J, Tung A, Ko FW, et al. Validation of Embletta portable diagnostic system for identifying patients with suspected obstructive sleep apnoea syndrome (OSAS) Respirology. 2010;15(2):336–342. doi: 10.1111/j.1440-1843.2009.01697.x. [DOI] [PubMed] [Google Scholar]
- 13.Tiihonen P, Hukkanen T, Tuomilehto H, Mervaala E, Töyräs J. Evaluation of a novel ambulatory device for screening of sleep apnea. Telemed J E Health. 2009;15(3):283–289. doi: 10.1089/tmj.2008.0118. [DOI] [PubMed] [Google Scholar]
- 14.Santos-Silva R, Sartori DE, Truksinas V, Truksinas E, Alonso FF, Tufik S, et al. Validation of a portable monitoring system for the diagnosis of obstructive sleep apnea syndrome. Sleep. 2009;32(5):629–636. doi: 10.1093/sleep/32.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polese JF, Santos-Silva R, Kobayashi RF, Pinto IN, Tufik S, Bittencourt LR. Portable monitoring devices in the diagnosis of obstructive sleep apnea: current status, advantages, and limitations. J Bras Pneumol. 2010;36(4):498–505. doi: 10.1590/s1806-37132010000400017. [DOI] [PubMed] [Google Scholar]
- 16.Drager LF, Santos RB, Silva WA, Parise BK, Giatti S, Aielo AN, et al. OSA, Short Sleep Duration and Their Interactions With Sleepiness and Cardiometabolic Risk Factors in Adults: The ELSA-Brasil study. Chest. 2019;155(6):1190–1198. doi: 10.1016/j.chest.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Drager LF. New Challenges for Sleep Apnea Research: Simple Diagnostic Tools, Biomarkers, New Treatments and Precision Medicine. Sleep Sci. 2017;10(1):55–56. doi: 10.5935/1984-0063.20170009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berry RB, Brooks R, Gamaldo C, Harding SM, Lloyd RM, Quan SF, et al. AASM Manual for the Scoring of Sleep and Associated Events-rules, terminology and technical specifications. Darien: American Academy of Sleep Medicine; 2017. [Google Scholar]
- 19.Schmidt MI, Duncan BB, Mill JG, Lotufo PA, Chor D, Barreto SM, et al. Cohort Profile: Longitudinal Study of Adult Health (ELSA-Brasil) Int J Epidemiol. 2015;44(1):68–75. doi: 10.1093/ije/dyu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bensenor IM, Griep RH, Pinto KA, Faria CP, Felisbino-Mendes M, Caetano EI, et al. Routines of organization of clinical tests and interviews in the ELSA-Brasil investigation center. Rev Saude Pública. 2013;47(Suppl 2):37–47. doi: 10.1590/s0034-8910.2013047003780. [Article in Portuguese] [DOI] [PubMed] [Google Scholar]
- 21.Aquino EM, Barreto SM, Bensenor IM, Carvalho MS, Chor D, Duncan BB, et al. Brazilian Longitudinal Study of Adult Health (ELSA-Brasil): objectives and design. Am J Epidemiol. 2012;175(4):315–324. doi: 10.1093/aje/kwr294. [DOI] [PubMed] [Google Scholar]
- 22.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. American Academy of Sleep Medicine Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chowdhuri S, Quan SF, Almeida F, Ayappa I, Batool-Anwar S, Budhiraja R, et al. ATS Ad Hoc Committee on Mild Obstructive Sleep Apnea An Official American Thoracic Society Research Statement: Impact of Mild Obstructive Sleep Apnea in Adults. Am J Respir Crit Care Med. 2016;193(9):e37–e54. doi: 10.1164/rccm.201602-0361ST. [DOI] [PubMed] [Google Scholar]
- 24.Giampá SQC, Pedrosa RP, Gonzaga CC, Bertolami A, Amodeo C, Furlan SF, et al. Performance of NoSAS score versus Berlin questionnaire for screening obstructive sleep apnoea in patients with resistant hypertension. J Hum Hypertens. 2018;32:518–523. doi: 10.1038/s41371-018-0072-z. [DOI] [PubMed] [Google Scholar]
- 25.Marti-Soler H, Hirotsu C, Marques-Vidal P, Vollenweider P, Waeber G, Preisig M, et al. The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med. 2016;4(9):742–748. doi: 10.1016/S2213-2600(16)30075-3. [DOI] [PubMed] [Google Scholar]
- 26.Stippig A, Hübers U, Emerich M. Apps in sleep medicine. Sleep Breath. 2015;19(1):411–417. doi: 10.1007/s11325-014-1009-6. [DOI] [PubMed] [Google Scholar]
- 27.Fino E, Mazzetti M. Monitoring healthy and disturbed sleep through smartphone applications: a review of experimental evidence. Sleep Breath. 2019;23(1):13–24. doi: 10.1007/s11325-018-1661-3. [DOI] [PubMed] [Google Scholar]