Supplemental Digital Content is available in the text
Keywords: Crohn disease, differentiation, intestinal tuberculosis, primary intestinal lymphoma, proteome
Abstract
The differential diagnosis of Crohn disease (CD) from intestinal tuberculosis (ITB) and primary intestinal lymphoma (PIL) is challenging in patients who exhibit atypical clinical characteristics. The aim of the present study was to explore the serum proteome profiles of CD, PIL and ITB and to identify their differentiations.
Treatment-naïve patients with CD (n = 10), PIL (n = 10) and ITB (n = 10) were enrolled in the present study. Differentially expressed proteins (DEPs) in patient serum samples were compared between groups using tandem mass tag labeled proteomic technology. A principal component analysis (PCA) plot and volcano maps were also visualized. Functional pathway analysis was performed using Reactome. The Area under the Curve (AUC) was calculated for each DEP.
A total of 818 proteins were identified through proteomic quantification. Among them, 108 DEPs were identified to be differentiated between CD and ITB, 105 proteins between CD and PIL and 55 proteins between ITB and PIL. The proteome from the three groups was distinguishable in the PCA plot. The results revealed that 19, 12, and 10 proteins (AUC ≥ 0.95) were differentially expressed between CD and PIL, CD and ITB, and PIL and ITB, respectively. Among these DEPs, tumor necrosis factor ligand superfamily member 13 was higher in CD than in ITB and PIL. Peroxiredoxin-5, T-complex protein 1 subunit Gamma, CutA, and Fibulin-5 were increased in CD and PIL when compared with ITB. The levels of fibrinogen chains were also significantly higher in patients with PIL compared with CD.
The current study demonstrated that serum proteome was distinguishable among patients with CD, PIL, and ITB. The identified proteins may assist in the clinical differentiation among them.
1. Introduction
Crohn disease (CD) is a chronic inflammatory disease that can affect almost any area of the gastrointestinal tract, particularly the terminal ileum and ileocecal area. The diagnosis of CD is based on an evaluation of clinical, radiography, endoscopic and histological characteristics, according to the criteria of the World Health Organization.[1,2] However, none of these characteristics are unique to CD. Therefore, it is difficult to differentiate CD from other intestinal diseases, particularly intestinal tuberculosis (ITB) and primary intestinal lymphoma (PIL). Furthermore, the treatment of CD is different from that of ITB and PIL. Immunomodulatory drugs, such as glucocorticoid and azathioprine, are commonly prescribed to treat moderate to severe CD. As ITB is caused by infection of the intestine by Mycobacterium tuberculosis, these drugs are harmful in ITB treatment and may result in an acceleration of bacterial activity. PIL has the potential to become malignant;[3] therefore, a misdiagnosis or inappropriate management of the disease may prove to be lethal. Consequently, the accurate diagnosis and differential diagnosis of CD, ITB, and PIL is clinically important.
Currently, the differential diagnosis of CD, ITB, and PIL is based on clinical examinations such as computed tomographic (CT), colonoscopy and histological examination. Numerous studies have attempted to establish a clinical model for the differentiation of CD from ITB.[4–7] A previous study developed a differentiating diagnostic model by combining clinical and laboratory parameters, endoscopic parameters and CT enterography parameters,[8] with a receiver operating characteristic curve of 0.989 for the differentiation of CD from PIL. However, these diagnostic methods are either not cost-effective or time consuming, and may be invasive. It is therefore essential to develop more convenient and non-invasive methods to overcome the limitations of current diagnostic methods.
Proteomics has emerged as a promising tool for the identification of biomarkers and has provided valuable insights into disease pathophysiology. Proteomics has been used to investigate inflammatory bowel disease (IBD)[9–12] since last decade. Recently, a comprehensive review summarized the clinical advantages of using proteomics when differentiating CD from ulcerative colitis, predicting the behavior and response to biological treatment and monitoring patient response to treatment.[13] We previously demonstrated that unique proteomic signatures are present in IBD patients, and four serum protein peaks were shown to distinguish CD from ITB patients with a specificity and sensitivity of 76.2% and 80.0%, respectively.[14] However, due to the technical limitations, the identities of these protein signatures were not revealed. To the best of our knowledge, proteomics research has not yet been used to differentiate between CD and PIL. The present study aimed to investigate serum proteome profiles and identify novel serum markers to differentiate CD from ITB and PIL.
2. Materials and methods
2.1. Patients
Treatment-naïve patients with CD (n = 10), PIL (n = 10) and ITB (n = 10) were recruited among inpatients attending The Department of Gastroenterology, the First Affiliated Hospital of Zhejiang University, School of Medicine, between January 2016 and February 2019. The study protocol was approved by The Ethics Committee of The First Affiliated Hospital of Zhejiang University (reference number 2019312). Informed consent was obtained from all patients. CD is diagnosed via assessment of a combination of clinical, radiographic, endoscopic and histological findings, according to the World Health Organization criteria.[1,2] For the diagnosis of ITB, at least one of the following criteria should be fulfilled:[15,16]
-
(i)
Presence of caseating granuloma (s) on histological examination;
-
(ii)
positive acid-fast bacilli (AFB) smear or positive AFB culture;
-
(iii)
remission of symptoms and endoscopic manifestations after anti-tuberculosis treatment;
-
(iv)
presence of proven tuberculosis in other organs.
The diagnosis of PIL was based on the 1961 Dawson proposed standards[17] as follows:
-
(i)
No enlargement of the peripheral or mediastinal lymph nodes;
-
(ii)
normal white blood cell count;
-
(iii)
gastrointestinal lesions with involvement of local lymph nodes only;
-
(iv)
involvement of the liver or spleen.
2.2. Serum protein processing
Venous blood was collected under fasting conditions and centrifuged. For the proteomic study, serum samples were processed using a ProteoMiner column (Bio-Rad Laboratories, Inc.) to remove high-abundance proteins. The ProteoMiner column was loaded with 200 μL serum sample, followed by vortexing for 2 hours. The flow-through fraction was discarded and the column was washed three times using 200 μL PBS. Enriched low-abundance proteins were collected in 40 μL of 4% sodium dodecyl sulfate (SDS) with 25 mM dithiothreitol and incubated for 30 minutes prior to trypsin digestion using the filter-aided sample preparation method. A total of 200 μg protein was quantified using a bicinchoninic acid protein assay and transferred to a 10-kDa spin filter (EMD Millipore).
2.3. Peptide labeling and fractionation
Tryptic peptides (100 μg) from each sample were labeled by 11plex tandem mass tag (TMT) reagent (Thermo) according to manufacturer's instruction. The TMT multiplexed peptides were fractionated with an Acquity Peptide BEH C18 column (1.7 μm, 130 Å, 2.1 mm × 150 mm, Waters) on a 1260 High Performance Liquid Chromatography (HPLC) System (Agilent) at a flow rate of 0.2 mL/min. Mobile phase A contains 0.1% NH4OH and B contains 0.1% NH4OH in acetonitrile (ACN). The 60 minutes LC gradient was set as follows: 5% B within 2 minutes; 5%–18% B in 35 minutes; 18%–32% B in 15 minutes; 32%–95% B in 3 minutes; maintained at 95% B for 5 minutes. For each TMT experiment, eluent was collected per minute and combined into 15 fractions via a concatenated fashion. All peptide fractions were dried by Speed Vac.
2.4. Mass spectrometric acquisition
For each fraction, around 300 ng of peptides suspended in 0.1% FA in 2% ACN were enriched on an Acclaim PepMap 100 Column (75 μm × 2 cm). Peptide separation was performed by an Acclaim PepMap RSLC Column (75 μm × 25 cm) on an Ultimate 3000 nanoUPLC system (Thermo) operated at 400 nL/min. The mobile phase A and B contained 2% and 98% ACN, respectively, and both mobile phases were supplemented with 0.1% FA. The gradient started with 3% of B for 4 minutes and increased to 5% B in 2 minutes, then reached 18% B in 64 minutes and 32% B in 20 minutes. The gradient finally reached 80% B in 10 minutes and was then held for 10 minutes before it returns to 3% B in 2 minutes and kept at the re-equilibration condition for 8 minutes. The total analysis time per injection was 120 minutes.
The nanoLC was coupled to an Orbitrap Q-Exactive HFX mass spectrometer (Thermo). The nanospray source was operated at 2.3 kV. The MS was operated in data-dependent analysis (DDA) mode scheduling a full MS survey scan from 350 to 1500 Th at the 60,000 FWHM resolution (at m/z 200 Th) with automatic gain control (AGC) set to 3e6, followed by 20 MS2 scans of precursors selected for fragmentation by higher-energy collision dissociation with normalized collision energy set to 32%. Isolation window was set to 1.0 Th. Dynamic exclusion was set to 40 seconds. All MS2 spectra were acquired at 45,000 FWHM resolution with AGC of 5e4.
2.5. Proteomic data analysis
The acquired.RAW files were searched against the human UniProtKB database (88,473 sequences, version 09–2015) using MaxQuant (version 1.6.1.0). The database search was performed using the MS2 report ion mode with 11plexTMT option selected. Trypsin with up to 2 missed cleavages was set. Oxidation(M) and carbamidomethyl(C) were set as variable and fixed modification, respectively. Mass tolerance 7 ppm was set for main database search. Reversed sequences were used for false discovery rate (FDR) control, and protein level 1% FDR was set to filter the result. Additionally, 67 common contaminants including immunoglobulins, hemoglobin, keratins were excluded from analyses.
For quantitative analysis, the TMT report ion intensities of each protein were normalized against the median intensity value of all proteins within each sample, and further normalized against the reference ion intensity of 131C label in all three runs to correct run-to-run variations. The results of the quantification and fold change between groups were log2 transformed. For reliable protein quantitation, only proteins with TMT data across all three experiments were included, and proteins with 0 TMT values were discarded from all groups. A heat map was constructed using Perseus software (version 1.6). The protein quantitation data in log2 scale was transformed into Z-score by rows, both sample and protein distances were calculated using Spearman correlation and then clustered using the K-mean algorithm.
2.6. Statistical analysis
A Student's t test was used to identify differentially expressed proteins (DEPs). P < .05 was considered statistically significant. Principal component analysis (PCA) and volcano maps were visualized by SIMCA (Umetrics). Reactome pathway analysis was performed to identify functional pathways between groups. The area under the curve (AUC) was calculated to examine the classification accuracy of each DEP for comparison between any 2 disease groups, as proposed by Peter et al[11] The AUC was calculated using Python 3.0 (Sklearn metrics.roc_auc_score). Proteins with AUC ≥ 0.95 between any 2 disease groups were identified.
3. Results
3.1. Proteomics modulation related to CD, PIL and ITB
A total of 30 patients were enrolled in the present study, with 10 patients in each group. Age, sex and location of intestinal lesions were shown in Supplementary Table 1. There was no significant difference as to age and sex among patients in the 3 groups. However, the jejunum was involved in 2 patients with PIL but not in patients with CD or ITB.
According to the results from MaxQuant using an Andromeda search engine (v.1.5.2.8) at the level of 1% FDR, a total of 1013, 1107, and 995 serum proteins were identified from three independent TMT experiments, respectively (Fig. 1A). Among them, 818 proteins were overlapped with non-zero TMT proteomic quantitative data across all samples. Therefore, these were used in the downstream analyses. The results demonstrated that the overall proteome expression trends may be used to distinguish all 3 groups as presented in the PCA plot (Fig. 1B). Clustering analysis suggested that CD could be separated from the other 2 groups, while separation between the PIL and ITB groups was relatively incomplete (Fig. 1C).
Figure 1.
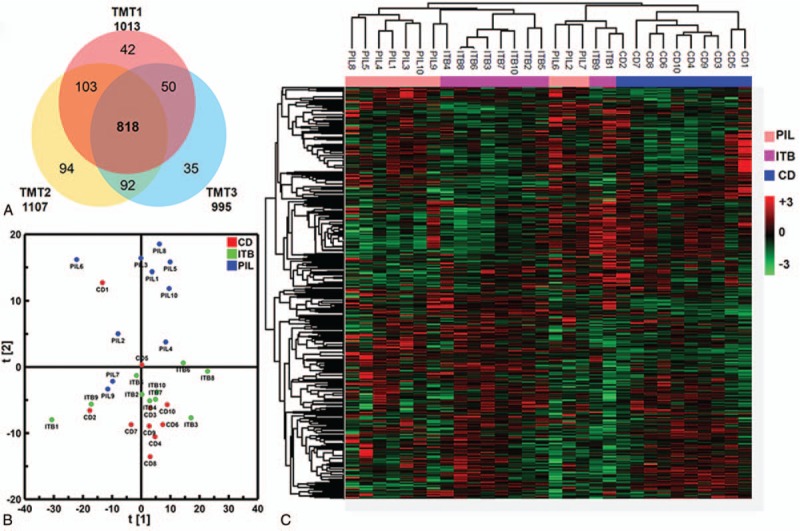
Summary of proteomics analysis of CD, ITB, PIL using TMT quantitation method. (A) Venn diagram illustration of proteins identified across 3 TMT experiments, from which 818 commonly identified proteins were used for downstream analyses. (B) Overall differences of serum proteome between CD, ITB, and PIL were summarized by PCA plot. (C) Heatmap representation of abundance profiles of all 818 proteins in all samples. Color shade correlates with relative protein abundances across each row (red/green for up-/down-regulation).
3.2. Identification of serum DEPs related to CD, PIL, and ITB
As shown in Figure 2A, there were 108 serum DEPs between CD and ITB, 105 DEPs between CD and PIL, and 55 DEPs between ITB and PIL. Among the DEPs in ITB and PIL compared with CD, 41 proteins were overlapping. The volcano map revealed the distribution of DEPs between CD and PIL (Fig. 2B), CD and ITB (Fig. 2C) and ITB and PIL (Fig. 2D).
Figure 2.
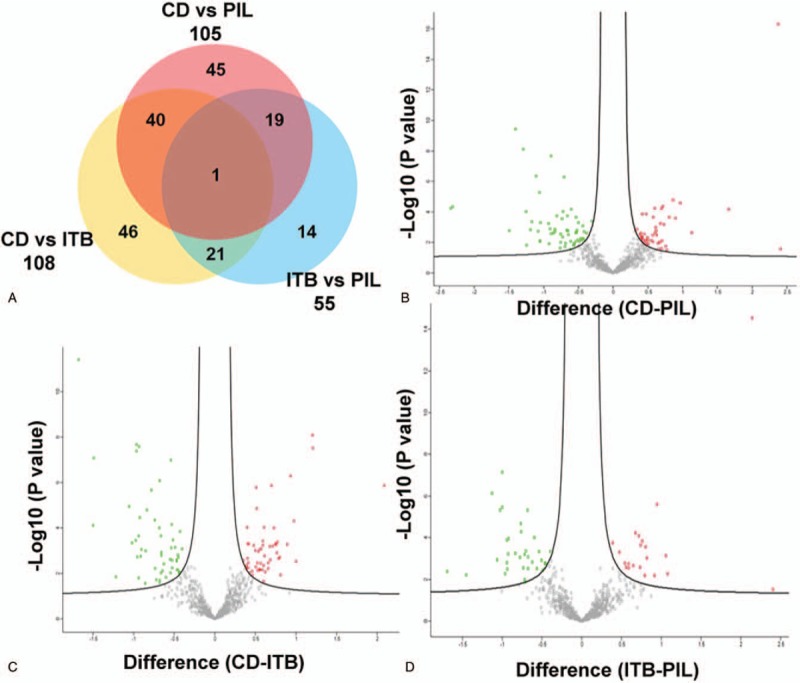
Differentially expressed proteins between groups. (A) Venn diagram illustration of DEPs selected from 3 pair-wise comparisons. Volcano plots of DEPs selection from each pair-wise comparison: DEPs (highlighted by red color) from CD vs PIL (B), CD vs ITB (C), and ITB vs PIL (D). Note that majority of proteins were not significantly changed (Gray).
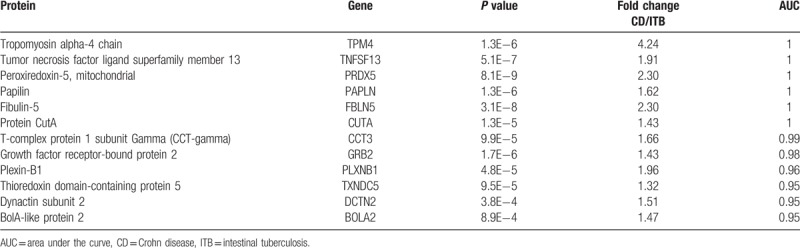
Using AUC ≥ 0.95 as a criterion in the identification of potential markers, 19, 12, and 10 proteins were indicated to differentiate CD/PIL, CD/ITB and ITB/PIL, respectively (Table 1). A summary of these proteins is presented in Tables 2–4, respectively. Among these DEPs, tumor necrosis factor ligand superfamily member 13 (TNFSF13), which induced apoptosis through its interaction with a variety of other TNF receptor family proteins, was higher in CD compared with PIL (CD/PIL 1.74, data not shown in Table 2 because the AUC is below 0.95) and ITB (Table 3). Peroxiredoxin-5 (PRDX5), T-complex protein 1 subunit gamma (CCT3), CutA, and fibulin-5 were increased in CD and PIL compared with ITB. Fibrinogen chains levels (fibrinogen gamma chain, fibrinogen beta chain and fibrinogen alpha chain) were significantly higher in patients with PIL compared with those with CD.
Table 1.
Results of differentially expressed proteins between groups.
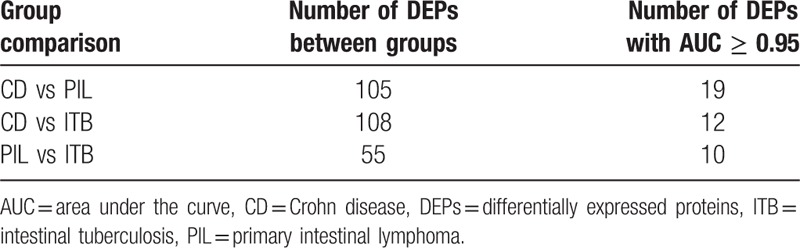
Table 2.
Lists of proteins with AUC ≥ 0.95 that differentiate Crohn disease from primary intestinal lymphoma.
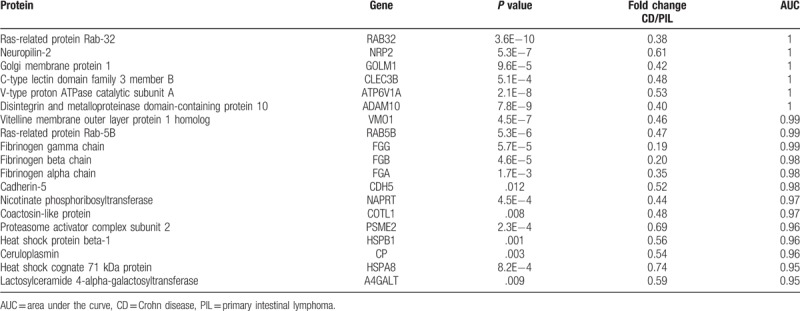
Table 4.
Lists of proteins with AUC ≥ 0.95 that differentiate intestinal tuberculosis from primary intestinal lymphoma.
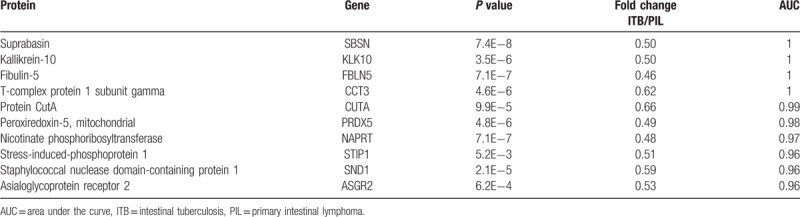
Table 3.
Lists of proteins with AUC ≥ 0.95 that differentiate Crohn disease, from intestinal tuberculosis.
3.3. Functional clustering analysis of pathways between groups
In the functional analysis of DEPs between CD and ITB, the antigen cross-presentation pathway, interferon alpha/beta pathway, adaptive immune system, and ER-phagosome pathways were mainly involved (Fig. S1). In the comparison between patients with CD and PIL, the regulation of mRNA stability by proteins that bind AU-rich elements, AUF1 (hnRNP D0) bound and destabilized mRNA and the NOTCH4 signaling pathway was mainly involved (Fig. S2). Between patients with ITB and PIL, degradation of GLI1 by the proteasome pathway and neutrophil degranulation pathway were mainly involved (Fig. S3).
4. Discussion
The differential diagnosis of CD, PIL, and ITB is a challenge in patients who exhibit atypical clinical characteristics. Due to the varying treatment options, the misdiagnosis of these diseases may lead to serious outcomes for affected patients. Clinically, the diagnosis of these diseases relies on blood biochemistry, colonoscopy, histology and imaging to reach a definitive diagnosis.[2,18] Histological findings are the gold standard for the diagnosis of PIL and ITB. However, the positive histology rate is low for ITB.[15] For the histological diagnosis of PIL, qualified specimens are difficult to obtain under endoscopy due to the small size and superficial location of PILs. The preoperative diagnosis of PIL also remains a challenge.[19] Proteomics is well-established for identifying biomarkers on a large scale.[20,21] Using tissue samples, Lokesh et al performed iTRAQ labeling technology to locate biomarkers for differentiation between CD and ITB. This study screened out six candidate proteins (trefoil factor 3, fatty acid synthase, myosin 14, myosin 11, human thioredoxin 1, IgG Fc-binding protein, transgelin, and tropomyosin) from 63 DEPs.[22] However, this study failed to confirm the expression of these candidate proteins in an independent validation cohort.
The results of the present study indicated that serum proteomic analysis can be used to differentiate CD from PIL and ITB. To the best of our knowledge, this is the first study to investigate serum protein biomarkers in the context of the differentiation among patients with CD, PIL, and ITB. The present study identified many serum proteins that are differentially expressed among the 3 groups. The difference between the proteomic profiles of ITB and PIL is smaller compared with that of CD and ITB or CD and PIL. CD is characterized by the infiltration of immune cells and excessive activation of the immune system along the gastrointestinal tract,[23] whereas the pathogenesis of ITB and PIL is associated with impaired immune function,[24–26] which may partly explain the smaller differences in proteomic profiles between ITB and PIL.
The results also indicated that TNFSF13 was higher in CD compared with PIL and ITB. TNFSF13, also referred to as a proliferation-inducing ligand (APRIL), belongs to the tumor necrosis factor ligand family and modulates B- and T-cell immunity.[27] The serum levels of TNFSF13 were significantly higher in patients with systemic lupus erythematosus when compared with the healthy controls.[28] Significant positive correlations were identified between the serum levels of TNFSF13, IL-17 and IFN-γ.[28,29] Weldon et al[30] demonstrated that surface TNFSF13 on myeloid cells was elevated and was associated with disease activity in patients with rheumatoid arthritis. In TNFSF13-/- mice, the incidence of collagen-induced arthritis was reduced in parallel with lower levels of antigen-specific IgG2a autoantibody and IL-17,[31] suggesting that TNFSF13 may regulate Th17 polarization or Th17-related cytokine production. Whether TNFSF13 correlated with IL-17 in patients with CD remains to be determined. The results of the present study also revealed higher levels of PRDX5, CCT3, CutA and fibulin-5 in CD and PIL compared with ITB, which may be used to exclude ITB. However, these results require verification in future studies.
Furthermore, levels of fibrinogen gamma chain, fibrinogen beta chain, and fibrinogen alpha chain were revealed to be significantly higher in patients with PIL than in patients with CD. Fibrinogen includes alpha, beta, and gamma chains,[32] and the expression of these chains may reflect the level of serum fibrinogen. By using multivariate analysis, a previous study[33] demonstrated that high plasma fibrinogen is an independent marker of a poor 5-year overall survival in patients with DLBL, indicating that fibrinogen may participate in tumor cell growth or invasion. Fibrinogen has also been reported to be increased in patients with CD and UC and was correlated with disease activity.[34] However, Hiroshi et al observed no difference between CD patients with a high Crohn Disease Activity Index (CDAI) and low CDAI (P = .2637). Therefore, the role of fibrinogen in the differentiation of CD and PIL requires further validation and research.
Several limitations should be addressed in this study. The sample size of each group was limited, and the expression of DEPs was not confirmed in an independent patient cohort. Therefore, further research should focus on validating these identified proteins in a larger sample of patients. In the present study, a total of 6 histological types of lymphoma were present in the group of patients with PIL. However, the most common pathogenic subtype of PIL is DLBL.[35] In this case, the identified proteins in the present study may not able to distinguish these subtypes of PIL. Finally, proteomic technologies exhibit low reproducibility. For example, Lysyl oxidase-like 2 has been found to be poorly expressed in CD when compared with ITB in a previous study,[14] but this could not be reproduced in the present study due to the application of varying proteomic methods.
5. Conclusions
The present study revealed that serum proteomic analysis was able to differentiate CD from PIL and ITB. The proteins identified in the present study may be helpful for the differential diagnosis of CD, PIL, and ITB. These findings may prompt further biomarker validation and mechanistic studies on disease pathogenesis.
Author contributions
Conceptualization: Guodong Shan, Guoqiang Xu.
Data curation: Xinhe Lou, Fenming Zhang, Sha Li, Haojie Du, Jinghua Yu.
Formal analysis: Longgui Ning.
Funding acquisition: Guoqiang Xu.
Investigation: Longgui Ning, Zeyu Sun.
Methodology: Haojie Du, Hongtan Chen.
Software: Zeyu Sun.
Writing – original draft: Longgui Ning.
Writing – review & editing: Guodong Shan, Guoqiang Xu.
Guoqiang Xu orcid: 0000-0003-1337-9120.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: ACN = acetonitrile, AFB = acid-fast bacilli, AGC = automatic gain control, APRIL = proliferation-inducing ligand, AUC = Area Under the Curve, CCT3 = T-complex protein 1 subunit gamma, CD = Crohn disease, CDAI = Crohn Disease Activity Index, CT = computed tomographic, DDA = data-dependent analysis, DEPs = differentially expressed proteins, DLBL = diffuse large B-cell lymphoma, FDR = false discovery rate, HPLC = High Performance Liquid Chromatography, IBD = inflammatory bowel disease, ITB = intestinal tuberculosis, MALDI-MS = matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, PCA = principal component analysis, PIL = primary intestinal lymphoma, PRDX5 = Peroxiredoxin-5, SDS = sodium dodecyl sulfate, TMT = tandem mass tag, TNFSF13 = tumor necrosis factor ligand superfamily member 13.
How to cite this article: Ning L, Shan G, Sun Z, Lou X, Zhang F, Li S, Du H, Yu J, Chen H, Xu G. Serum proteome profiles to differentiate Crohn disease from intestinal tuberculosis and primary intestinal lymphoma: A pilot study. Medicine. 2019;98:50(e18304).
This work was supported by Medical Science Research Foundation of Health Bureau of Zhejiang Province (WKJ-ZJ-1516). The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Stange EF, Travis SP, Vermeire S, et al. European evidence based consensus on the diagnosis and management of Crohn's disease: definitions and diagnosis. Gut 2006;55: Suppl 1: i1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bernstein CN, Fried M, Krabshuis JH, et al. World Gastroenterology Organization Practice Guidelines for the diagnosis and management of IBD in 2010. Inflamm Bowel Dis 2010;16:112–24. [DOI] [PubMed] [Google Scholar]
- [3].Arora N, Manipadam MT, Pulimood A, et al. Gastrointestinal lymphomas: pattern of distribution and histological subtypes: 10 years experience in a tertiary centre in South India. Indian J Pathol Microbiol 2011;54:712–9. [DOI] [PubMed] [Google Scholar]
- [4].Huang X, Liao WD, Yu C, et al. Differences in clinical features of Crohn's disease and intestinal tuberculosis. World J Gastroenterol 2015;21:3650–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ko JK, Lee HL, Kim JO, et al. Visceral fat as a useful parameter in the differential diagnosis of Crohn's disease and intestinal tuberculosis. Intest Res 2014;12:42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhao XS, Wang ZT, Wu ZY, et al. Differentiation of Crohn's disease from intestinal tuberculosis by clinical and CT enterographic models. Inflamm Bowel Dis 2014;20:916–25. [DOI] [PubMed] [Google Scholar]
- [7].Limsrivilai J, Shreiner AB, Pongpaibul A, et al. Meta-analytic bayesian model for differentiating intestinal tuberculosis from Crohn's Disease. Am J Gastroenterol 2017;112:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liu X, Zhou W, Zhang X, et al. Dimethyl fumarate ameliorates dextran sulfate sodium-induced murine experimental colitis by activating Nrf2 and suppressing NLRP3 inflammasome activation. Biochem Pharmacol 2016;112:37–49. [DOI] [PubMed] [Google Scholar]
- [9].Chan PP, Wasinger VC, Leong RW. Current application of proteomics in biomarker discovery for inflammatory bowel disease. World J Gastrointest Pathophysiol 2016;7:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Burakoff R, Pabby V, Onyewadume L, et al. Blood-based biomarkers used to predict disease activity in Crohn's disease and ulcerative colitis. Inflamm Bowel Dis 2015;21:1132–40. [DOI] [PubMed] [Google Scholar]
- [11].Townsend P, Zhang Q, Shapiro J, et al. Serum proteome profiles in stricturing crohn's disease: a pilot study. Inflamm Bowel Dis 2015;21:1935–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Starr AE, Deeke SA, Ning Z, et al. Proteomic analysis of ascending colon biopsies from a paediatric inflammatory bowel disease inception cohort identifies protein biomarkers that differentiate Crohn's disease from UC. Gut 2016;66:1573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gisbert JP, Chaparro M. Clinical usefulness of proteomics in inflammatory bowel disease: A comprehensive review. J Crohn's Colit 2018;13:374–84. [DOI] [PubMed] [Google Scholar]
- [14].Zhang F, Xu C, Ning L, et al. Exploration of serum proteomic profiling and diagnostic model that differentiate Crohn's disease and intestinal tuberculosis. PloS One 2016;11:e0167109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Patel N, Amarapurkar D, Agal S, et al. Gastrointestinal luminal tuberculosis: establishing the diagnosis. J Gastroenterol Hepatol 2004;19:1240–6. [DOI] [PubMed] [Google Scholar]
- [16].Makharia GK, Srivastava S, Das P, et al. Clinical, endoscopic, and histological differentiations between Crohn's disease and intestinal tuberculosis. Am J Gastroenterol 2010;105:642–51. [DOI] [PubMed] [Google Scholar]
- [17].Dawson IM, Cornes JS, Morson BC. Primary malignant lymphoid tumours of the intestinal tract. Report of 37 cases with a study of factors influencing prognosis. Br J Surg 1961;49:80–9. [DOI] [PubMed] [Google Scholar]
- [18].Dickson BC, Serra S, Chetty R. Primary gastrointestinal tract lymphoma: diagnosis and management of common neoplasms. Expert Rev Anticancer Ther 2006;6:1609–28. [DOI] [PubMed] [Google Scholar]
- [19].Chen JH, Ho CL, Chen YC, et al. Clinicopathological analysis and prognostic factors of 11 patients with primary non-Hodgkin lymphoma of the small intestine in a single institute. Oncol Lett 2014;8:876–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vaiopoulou A, Gazouli M, Theodoropoulos G, et al. Current advantages in the application of proteomics in inflammatory bowel disease. Dig Dis Sci 2012;57:2755–64. [DOI] [PubMed] [Google Scholar]
- [21].Tyers M, Mann M. From genomics to proteomics. Nature 2003;422:193–7. [DOI] [PubMed] [Google Scholar]
- [22].Rukmangadachar LA, Makharia GK, Mishra A, et al. Proteome analysis of the macroscopically affected colonic mucosa of Crohn's disease and intestinal tuberculosis. Sci Rep 2016;6:23162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007;448:427–34. [DOI] [PubMed] [Google Scholar]
- [24].Wiegeshaus E, Balasubramanian V, Smith DW. Immunity to tuberculosis from the perspective of pathogenesis. Infect Immun 1989;57:3671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cerhan JR, Ansell SM, Fredericksen ZS, et al. Genetic variation in 1253 immune and inflammation genes and risk of non-Hodgkin lymphoma. Blood 2007;110:4455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hu W, Bassig BA, Xu J, et al. Polymorphisms in pattern-recognition genes in the innate immunity system and risk of non-Hodgkin lymphoma. Environ Mol Mutagen 2013;54:72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Stein JV, Lopez-Fraga M, Elustondo FA, et al. APRIL modulates B and T cell immunity. J Clin Invest 2002;109:1587–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Boghdadi G, Elewa EA. Increased serum APRIL differentially correlates with distinct cytokine profiles and disease activity in systemic lupus erythematosus patients. Rheumatol Int 2014;34:1217–23. [DOI] [PubMed] [Google Scholar]
- [29].Eilertsen GO, Nossent JC. APRIL levels strongly correlate with IL-17 in systemic lupus erythematosus. Lupus 2014;23:1383–91. [DOI] [PubMed] [Google Scholar]
- [30].Weldon AJ, Moldovan I, Cabling MG, et al. Surface april is elevated on myeloid cells and is associated with disease activity in patients with rheumatoid arthritis. J Rheumatol 2015;42:749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Xiao Y, Motomura S, Podack ER. APRIL (TNFSF13) regulates collagen-induced arthritis, IL-17 production and Th2 response. Eur J Immunol 2008;38:3450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mosesson MW. Fibrinogen and fibrin structure and functions. JTH 2005;3:1894–904. [DOI] [PubMed] [Google Scholar]
- [33].Troppan KT, Melchardt T, Wenzl K, et al. The clinical significance of fibrinogen plasma levels in patients with diffuse large B cell lymphoma. J Clin Pathol 2016;69:326–30. [DOI] [PubMed] [Google Scholar]
- [34].Dolapcioglu C, Soylu A, Kendir T, et al. Coagulation parameters in inflammatory bowel disease. Int J Clin Exp Med 2014;7:1442–8. [PMC free article] [PubMed] [Google Scholar]
- [35].Lightner AL, Shannon E, Gibbons MM, et al. Primary gastrointestinal non-hodgkin's lymphoma of the small and large intestines: a systematic review. J Gastrointest Surg 2016;20:827–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


