Abstract
In this short review, we will focus on the uniqueness of ciliary extracellular vesicles (EVs). In particular, we will review what has been learned regarding EVs produced by cilia of model organisms. Model systems including Chlamydomonas, C. elegans, and mouse revealed the fundamental biology of cilia and flagella and provide a paradigm to understand the roles of cilia and flagella in human development, health, and disease. Likewise, we propose that general principles learned from model systems regarding ciliary EV biogenesis and functions may provide a framework to explore the roles of ciliary EVs in human development, health, and disease.
Probably the most important question we can ask ourselves is how trillions of cells communicate and cooperate with each other to generate the human body and to fulfill cognitive functions of the brain. After nearly a century of studying the biology of the cell, we have gained extensive knowledge about the making and inner workings of the cell and about intercellular communication. Extracellular vesicles (EVs) are emerging as an evolutionarily conserved method of intercellular communication within an organism or for cellular crosstalk between organisms, species, and kingdoms. This is a most exciting time to review some of the EV communication principles and speculate how these principles may illuminate how cells and tissues crosstalk using EVs to influence organismal biology.
EVs are membrane-bound vesicles that contain signaling proteins, nucleic acids, and lipids and that are shed by all three domains of life, yet we are only beginning to understand the cell biology of EVs (1). Two major types of EVs are exosomes, which are 30–100 nm in diameter and derived from the fusion of multivesicular bodies (MVBs) to the plasma membrane, and microvesicles that bud from the plasma membrane and are larger (50 nm −1,000 nm). In this review and per ISEV nomenclature, we will use the term EV to mean either exosome or microvesicle, and will specifically refer to exosome or microvesicle when the origin of the EV is known.
Prokaryotes, unicellular eukaryotes and lower metazoans all exhibit collective behavior. The human pathogen Pseudomonas aeruginosa packages its quorum sensing signal in membrane vesicles that are environmentally released (2). Microbial liposome-like membrane vesicles may also contain DNA that plays a role in horizontal gene transfer or toxins that transfer virulent factors to cells or to counteract environmental predation (reviewed by (3)). Trypanosoma brucei secretes exosomes that enter recipient trypanosome cells to influence social motility (4). In the green alga Chlamydomonas and the nematode Caenorhabditis elegans, EVs signal between organisms to influence mating and mating-related behaviors, respectively (5, 6). We propose that the principles of the EV social communication between individuals in the microbial community or lower metazoans may resemble those through which cells interact with each other within a tissue, organ or organism. Insights learned from lower organisms may create a framework from which to understand how EVs organize the social biology of the cells within human body in healthy and diseased states. Consistent with a role in orchestrators of intercellular communication, EVs transport lipid-modified morphogens such as Hedgehog (Hh) and Wnt, which are evolutionarily conserved regulators of animal development and body plan (reviewed by (7)).
Several databases have been built to collect published components of EVs. However, a holistic understanding of EV biology is lacking, largely due to technical limitations that include the tiny size of some EVs, the challenge of tracking EV dynamics from its originating cellular source to its target, and the difficulty of measuring EV function in real time and in a physiologically relevant context. It is here where the alga Chlamydomonas reinhardtii and the nematode Caenorhabditis elegans prove invaluable model systems to study EV biogenesis and function in vivo. In these transparent, living organisms, one has the ability to visualize EVs labeled with fluorescent-tagged cargos, to monitor EV shedding, release, and ultimately capture by target cells. Coupled with in vivo assays to measure bioactivity and a powerful molecular genetic toolkit, it is possible to study the fundamental biology of EVs. In this short review, we will focus on the uniqueness of EVs as universal communication devices, especially from what we have learned about EVs shed from cilia and flagella of model systems (reviewed by (8–10)).
Cilia and Ciliary EVs
Cilia project off the surface of most non-dividing cells in the human body. Until the turn of the 21st century, the human cilium was viewed as a vestigial organelle. Studies in Chlamydomonas, C. elegans, and mouse revealed that genes important for cilia development and function had human counterparts that, when mutated, resulted polycystic kidney disease, providing first connection between cilia and human disease (reviewed by (11)). Cilia play important and essential roles in development, signaling, and homeostasis, with defects in ciliary genes causing human ciliopathies that display a spectrum of symptoms including embryonic lethality, polydactyly, polycystic kidney disease, obesity, and hydrocephalus (12).
Cilia act as cell towers to both receive extracellular signals and to send information via ciliary EVs (13). Given the importance of cilia in receiving extracellular signals and EVs in sending signals, ciliary EVs - named for their source or target - are emerging at the forefront of these two fields of study. As cilia evolved before the divergence of the last eukaryotic common ancestor (14–16), we propose that the last common eukaryotic ancestor may have used cilia and ciliary EVs to communicate.
The cilium displays an architecturally conserved microtubule axonemal core and is constructed from over 600 proteins, many of which are evolutionarily conserved (12). The cilium itself occupies a privileged space in the eukaryotic cell. The transition zone physically separates the cilium from the cell and acts as a guard – regulating access of proteins into and out of this restricted ciliary compartment (Figure). In thousands of TEM (transmission electron microscopy) images of well-fixed cilia and flagella in the literature over decades, internal vesicles have not been observed, likely due to the presence of the transition zone blockade. The cilium therefore relies on microtubule-based intraflagellar transport (IFT) driven by anterograde kinesin-2 and retrograde dynein to deliver structural and signaling cargoes.
Figure:
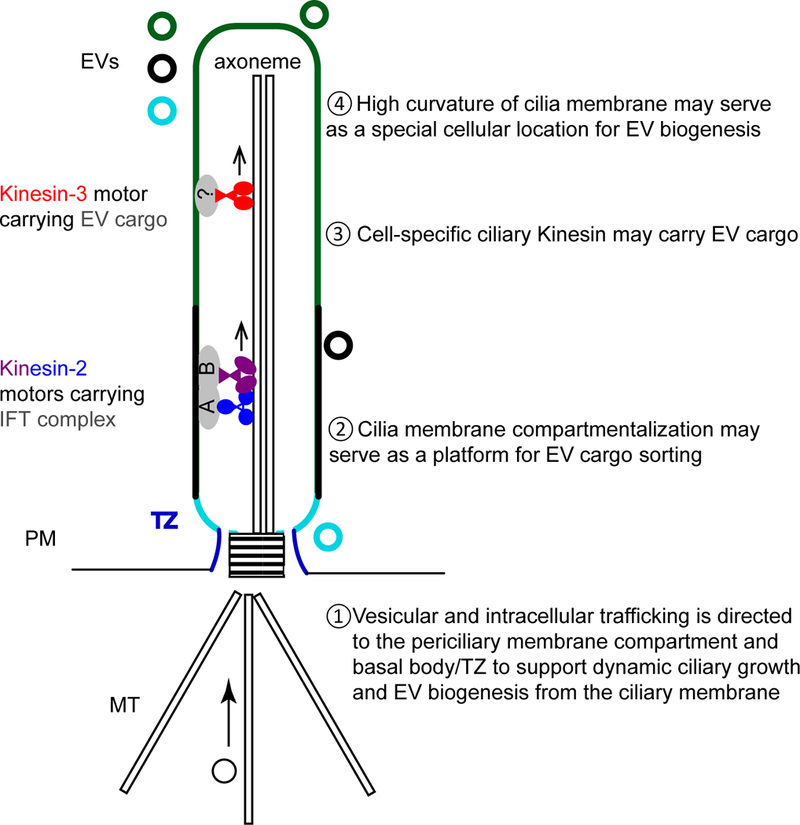
Model of the cilium as a special cellular device and location for EV biogenesis and shedding. Property 1: The cilium is derived from centriole and templated by the basal body/transition zone (TZ). In the periciliary region (cilia base), the basal body/TZ may direct vesicular trafficking necessary for ciliary membrane growth and EV biogenesis. Property 2: The ciliary membrane and axoneme are highly compartmentalized. The ciliary membrane is distinct from the plasma membrane and physically separated from the rest of the cell via TZ gating. Hence, the ciliary membrane may serve as EV cargo sorting platform. Property 3: All cilia possess canonical kinesin-2 motors that drive anterograde IFT transport and dynein motor mediated retrograde transport (not shown). C. elegans EV-producing neurons possess an additional cell-specific ciliary kinesin-3 that may be required for proper EV cargo sorting and/or for EV cargo transport. Property 4: The ciliary tip represents a highly curved membrane surface may also contribute to EV biogenesis. There are three possible sources of ciliary EVs: proximal (ciliary base, light blue), lateral (ciliary length, black), and distal (ciliary tip, dark green). The data-to-date favors that EVs are released from the ciliary base (light blue) and/or ciliary tip (dark green). Those EVs derived from the ciliary base may be exosomes arise from MVB exocytosis or from microvesicle budding near the TZ (light blue membrane and ciliary EV). Microvesicle/ectosome shedding occurs at the ciliary tip (dark green ciliary membrane and ciliary EV). Abbreviations: MT, microtubule; PM, plasma membrane; TZ, transition zone; IFT, intraflagellar transport.
Cilia and flagella of protists, Chlamydomonas, C. elegans, mouse, and cultured human cell lines have the ability to shed EVs (8, 10, 17–20). In Chlamydomonas, flagellar tips shed microvesicles referred to as “ectosomes” in the literature (8, 21). Nomarski microscopy reveals microvesicles/ectosomes budding in real time from ciliary tips of Chlamydomonas (21). Likewise, TEM images show microvesicles/ectosomes budding from ciliary tips in fixed cells (21). In Chlamydomonas, components of the endosomal sorting complex required for transport (ESCRT) are found in isolated ciliary transition zones, ciliary membranes, and ciliary EVs (22, 23), which may mediate membrane budding and the formation of ciliary EVs.
In C. elegans, only a subset of ciliated sensory neurons is capable of producing EVs – those whose ciliary tips protrude through cuticular pores to the outside world (6). Using the worm as an in vivo system, we developed methods to visualize and count EVs that are labeled with fluorescently-tagged cargo. Live imaging of fluorescently-labeled EVs coupled with TEM and electron tomography (EM/ET) of fixed animals provides a basic picture of EV biogenesis in C. elegans. Using this strategy, we showed that ciliary EV biogenesis is regulated by evolutionarily conserved IFT and ciliary resident proteins (6, 24–26). Each EV-releasing sensillum contains the cilium of an EV-releasing neuron and an associated mechanosensory neuron along with glial socket and sheath cells that create a lumen surrounding the cilium (6). By fluorescence microscopy and EM/ET, EVs are observed to accumulate in the lumen, hence we conclude that EVs are shed at the ciliary base into the lumen. Using TEM, we do not see MVBs in the region of the ciliary base and therefore these ciliary EVs are not likely exosomes. Instead, we observe omega-shaped vesicle budding from the ciliary base suggesting that these C. elegans ciliary EVs are microvesicles. By fluorescence microscopy, we observe GFP-tagged EVs being shed from tips of cilia and released into the media. These data suggest two distinct sites of ciliary EV shedding: at the ciliary base and at the ciliary tip. Alternatively, EVs may be shed at the ciliary base, propelled by IFT and an accessory ciliary kinesin-3 motor KLP-6 along the ciliary membrane surface to the cuticular pore, and then released into the environment (proposed in (27)). We are currently using high-speed super-resolution microscopy to distinguish between these possibilities (Wang and Barr, unpublished).
EVs have been observed along the mammalian primary cilium. Scanning electron microscopy of mammalian primary cilia reveals cilia-associated vesicles (28, 29). Ward and colleagues first showed that cilia are associated with exosome-like vesicles and that cilia might absorb EVs rapidly (28). In the kidney, EVs abnormally accumulate along cilia of autosomal recessive PKD human patients and mouse mutants (28), hinting at a connection between EVs and ciliopathies (10). In hTERT-RPE1 cell lines (human telomerase reverse transcriptase immortalized human retinal pigment epithelial) expressing the GPCR Smoothened tagged with pH-sensitive GFP (pH-SMO), ultra-high resolution 3D imaging of whole cells revealed pH-SMO decorating the ciliary membrane and vesicle-like buds along ciliary membrane (refer to Figures 6F and 6H and Supplemental Movie S5 in (30)). The aforementioned study using fixed cells could not resolve whether EVs are budding off or fusing with the cilium. As discussed in the next section, two recent studies convincingly show that EV shedding occurs at the ciliary tip of mammalian cells (18, 19).
Ciliary EV function
Why do cilia shed EVs?
In mammalian cells, ciliary microvesicles/ectosomes regulate ciliary receptor abundance and signaling (18). Under pathological conditions when BBSome-mediated ciliary receptor endocytic retrieval and removal is blocked, Nachury and colleagues found that mammalian cilia shed activated SSTR3 and GPR161 GPCR-containing microvesicles in an actin-dependent process referred to as “ectocytosis”. A pathological form of ectocytosis is caused by mutations in β-arrestin-2, BBSome components, and the BBSome regulator GTPases Ift27 or Arl6, and correlates with decreased ciliary length. Hence, cilia on cultured mammalian cells may use microvesicles/ectosomes to control receptor-mediated signaling and ciliary length.
Ciliary EV shedding influences ciliary assembly/disassembly (19). Ciliary assembly and disassembly is regulated by cell cycle (31, 32). Serum-starved mammalian cells exit the cell cycle and these non-dividing cells grow primary, non-motile cilia. Conversely, ciliary disassembly and proliferation is induced by serum after nutrient deprivation. In several mammalian cell types (NIH/3T3, hTERT RPE-1, and mIMCD-3), Inoue and colleagues showed that the distal most tips of cilia are shed or “decapitated” with growth stimulation or high ciliary PIP2 levels (19). Upon growth stimulation, the phosphoinositide 5-phosphatase INPP5E exits and F-actin enters the cilium, with F-actin accumulation correlating with the site of ciliary decapitation. In this context, ciliary decapitated EV shedding precedes cilia disassembly and drives cell cycle (19).
Ciliary EVs also function in membrane homeostasis and development (20, 33). Vertebrate photoreceptors undergo outer segment disc formation. Photoreceptors discs are constantly shed and renewed throughout life. The mammalian photoreceptor cilium has the ability to mass produce ciliary microvesicles, but this potential is inhibited by the disc-specific protein peripherin in order to elaborate the photoreceptor outer segment (20, 33). In peripherin-2 mutants, the photoreceptor cilium instead produces massive amounts of microvesicles/ectosomes (20). This study by Arshavsky and colleagues suggests that EVs help to maintain membrane homeostasis as ciliary membranes cannot be directly returned to the plasma membrane in mammalian photoreceptors.
Ciliary EVs may act as signaling devices. Rosenbaum and colleagues first demonstrated bioactivity of ciliary EVs - Chlamydomonas flagella shed microvesicles/ectosomes containing a lytic enzyme necessary for hatching from the mother cell wall (21). Building upon these studies, the Snell lab provided the first example of signal-dependent ciliary EV shedding. During mating, Chlamydomas flagellar tips release EVs abundantly and the cleaved product of the mating agglutinin polypeptide receptor SAG1-C65 is shed into the environment (5). We speculate that by releasing EVs during mating, other cells may initiate and synchronize sexual reproduction within the population in order to maximize reproductive success. In principle, a similar EV signaling mechanism may be used to synchronize cell populations during development and normal tissue homeostasis in animals.
C. elegans ciliary EVs function in inter-organismal communication to modulate mating-related locomotory changes in male behaviors (6). Intriguingly, defects in EV cargo sorting correlate with defects in EV bioactivity (6, 25). This EV bioactivity is cargo-dependent: proper sorting of the TRP channel PKD-2 to EVs is required (6). PKD-2 is a conserved TRP polycystin 2 that genetically interacts the polycystin 11-transmembrane spanning adhesion GPCR-like protein LOV-1 (reviewed by (34, 35)). Both PKD-2 and LOV-1 locate to cilia and ciliary EVs. C. elegans pkd-2 and lov-1 genes are homologous to human PKD1 and PKD2, which are mutated in autosomal dominant polycystic kidney disease (ADPKD).
The mammalian PKD1 and PKD2 encoding proteins polycystin 1 and polycystin 2 are found in cilia and in urinary exosome-like vesicles (28, 36–38). The EV functions of the polycystins are not known. One possibility is that the polycystins play a role in maintaining EV structure. Alternatively, the huge extracellular domain of the polycystin 1 family of proteins may cover the EV surface and inhibit non-specific fusion of EV to membranes. In this scenario, upon activation by an unknown ligand or stimulus, polycystin 1 may undergo a conformational change to an open state, similar to that of a tethered adhesion GPCR (39). In an activated state, the polycystins may potentially facilitate EV recognition by the target membrane. Other TRP channels including TRPC4, TRPC6, TRPM4, TRPV2 and TRPV4 are found on EVs, suggesting the TRP channel family plays a broad role on EVs (40). Members of another family of channels, the acid sensing ion channels (ASIC) and degenerin/sodium epithelial channels (DEG/ENaC), are found on EVs and, in C. elegans, on ciliary EVs (24, 41). Future studies are required to determine whether the polycystins or other channels play evolutionarily conserved roles on ciliary EVs.
Generation of left-right symmetry in vertebrates relies on exquisite coordination of multiple cellular pathways (reviewed by (42)). In the mammalian embryonic node, nodal cilia generate a leftward fluid flow that is implicated in breaking symmetry. Over a decade ago, Hirokawa and colleagues showed that nodal vesicular parcels containing Shh are moved from right to left, resulting in the activation of non-canonical Hedgehog signaling (43). The origin and function of these nodal vesicular parcels remains mysterious. It is tempting to speculate that these nodal vesicular parcels are ligand-containing EVs with the potential to synchronize developmental decisions. This synchronizing activity of EVs has been demonstrated in stress spreading among tissues - EVs orchestrate immune cell action during inflammation and immune response in mice and humans (44). Determining EV functions in synchronizing cellular actions within the tissue holds great potential for understanding complex processes such as aging and pathology of diseased state.
The cilium as a specialized device for EV biogenesis
The cilium has several unique features that make it an ideal cellular location for EV biogenesis and launching. In this section, we discuss four properties of the cilium that may enable ciliary EV production (for a comprehensive review of ciliary composition, see (45)).
Both the cilium and the immunological synapse are directed by centrosomes (46) and serve as EV biogenesis sites (10, 47, 48). We propose that the cilium and immunological synapse are special cellular locations for EV biogenesis. The centrosome matures into a basal body that templates the transition zone and cilium. The transition zone serves a hub for microtubule-based transport, from the cell body to the periciliary region, from the periciliary region into the cilium, and then to and from the ciliary tip. The periciliary region has been shown to support the demanding intracellular trafficking required for ciliogenesis (45) – we propose that the periciliary region may be appropriated for ciliary EV biogenesis (Property 1 in Model Figure).
Although cilia are not membrane-enclosed organelles, the ciliary transition zone physically separates the ciliary and plasma membranes and regulates ciliary entry and exit of proteins. Cilia regulate cargo selection by size exclusion and through the transition zone and ciliogenesis machinery (reviewed in (49)). Not only is the cilium distinct from the rest of the cell, within a single cilium, both the membrane and the enclosed axoneme are highly differentiated and compartmentalized (reviewed by (45, 50)). For example, the cilium has a distinct phosphoinositide (PI) composition (51). Ciliary protein trafficking and transition zone functions are regulated by PI kinases and phosphatases (52–56). Perturbing this phosphoinositide balance triggers ciliary tip decapitation (19). Hence, we propose that ciliary compartmentalization might influence both ciliary membrane trafficking and EV cargo sorting (Property 2 in Model Figure).
The ciliogenesis machinery and regulators, including ciliary kinesin-2 and kinesin-3 motors, IFT complexes, tubulin, and tubulin glutamylases and deglutamylases are required for ciliary EV biogenesis and cargo sorting in C. elegans (Property 3 of Figure) (6, 25, 26). In C. elegans EV-releasing cilia, an accessory kinesin-3 called KLP-6 is important for EV cargo sorting – perturbing KLP-6 activity by mutation in itself or in the axonemal building block α-tubulin TBA-6 results in abnormal EV cargo sorting. Hence, we propose that accessory ciliary motors like KLP-6 regulate EV biogenesis and may transport cargo to the site of EV shedding and release.
Flagellar tips of Trypanosoma brucei form membrane nanotubes, which then produce EVs, suggesting that high membrane curvature may support EV biogenesis (Property 4 in Model Figure) (17). Consistent with this idea is that prominin 1 and 2, ciliary tip or microvilli tip protein markers, are also commonly found in EVs (57) (http://exocarta.org/gene_summary?gene_id=8842, http://exocarta.org/gene_summary?gene_id=150696). Moreover, EVs are observed budding from tips of Chlamydomonas flagella (21) and mammalian cilia (18, 19). Chlamydomonas flagellar EVs are enriched for ESCRT components (23), which may act as sensors of membrane curvature and mediate membrane budding and ciliary microvesicle shedding. Therefore, high curvature of the ciliary tip membrane may provide an advantage for the cell to use the cilium as a subcellular location for EV biogenesis.
Very little is known about how ciliary EVs are formed. However, it is clear that the cilium provides several sites for EV launching. The ciliary base may be the source of ciliary microvesicles (as demonstrated in C. elegans) or MVB-derived exosomes (as shown for mammalian polycystin-containing urinary exosome-like vesicles) (6, 28, 58). Microvesicles/ectosomes are directly observed budding from ciliary tips in Chlamydomonas and cultured mammalian cells (18, 19, 21). EVs are occasionally observed along the length of the cilium in Chlamydomonas and cultured mammalian cells (5, 30). The cilium is devoid of MVB and hence ciliary-membrane derived EVs cannot be categorized as exosomes. Hence, ciliary-membrane derived EVs are microvesicles referred to as ectosomes that are formed by budding of the ciliary membrane. Ciliary EVs may also form during disruption of normal ciliary membrane trafficking or during ciliary resorption (18–20). These results indicate that cilia are specialized EV biogenesis sites in normal and pathological conditions.
Unresolved questions and concluding points
One of the most mysterious aspects about ciliary EVs is how they function in the extracellular environment, which raises several fundamental and unresolved questions. How do signaling ciliary EVs survive the extracellular environment? How much of the originating cellular function do ciliary EVs possess? Does ciliary EV stability depend on the purpose of the EV, i. e. the cargo that EVs carry? Do ciliary EVs play a role human ciliopathies, as proposed in (10)? Profiling of ciliary EVs from different cellular origins may identify cargoes that play specific signaling roles and general machineries that enable ciliary EV cargo sorting, biogenesis, and navigation through extracellular environments to destination targets.
Another open question is how cargoes are targeted to ciliary EVs. In wild-type mammalian cells, ciliary membrane proteins lacking BBSome endocytic retrieval signals undergo ectocytosis (18), suggesting that cargoes that are recycled may not be enriched in ciliary microvesicles/ectosomes unless their sorting is impaired. N-myristoylation may target proteins to cilia or EVs (27, 59, 60). In C. elegans EV-releasing neurons, two EV cargoes CIL-7 and tubulin glutamylase TTLL-11B possess N-terminal myristoylation sequences (26, 27). The EV biogenesis regulator and cargo CIL-7 requires its N-terminal myristoylation sequence for its localization and function in PKD-2::GFP-labeled EV release, indicating that N-myristoylation serves as a ciliary EV targeting sequence in vivo.
In the last two decades, the cilium went from being viewed as a vestigial organelle of the eukaryotic cell to a critical signaling nexus. In the last five years, we learned that the cilium also transmits signals via ciliary EVs. Multi-color super resolution microscopy combined with real time imaging and in vivo readouts of ciliary EV bioactivity will be the next wave for understanding ciliary EV biology.
Summary points.
Cilia of protists, Chlamydomonas, C. elegans, mouse, and cultured human cell lines shed EVs.
Ciliary EVs function in disposal, ciliary length regulation, ciliary assembly/disassembly, membrane homeostasis, signal transduction, development, and reproduction.
Cilia are specialized EV biogenesis sites in normal and pathological conditions.
Model systems are powerful tools to study ciliary EV biology.
Acknowledgements
Our research is funded by grants from the NIH (DK59418 and DK116606 to M.M.B.) and the Kansas University Medical Center PKD Center (to J.W.). We thank the anonymous reviewers for valuable critiques. We thank our labmates for ongoing discussions and Joel Rosenbaum for asking the hardest questions. M.M.B. thanks Bruce Springsteen for reminding her that you can’t start a fire without a spark.
References
- 1.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 2018;19(4):213–228. [DOI] [PubMed] [Google Scholar]
- 2.Mashburn LM, Whiteley M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 2005;437(7057):422–5. [DOI] [PubMed] [Google Scholar]
- 3.Hasegawa Y, Futamata H, Tashiro Y. Complexities of cell-to-cell communication through membrane vesicles: implications for selective interaction of membrane vesicles with microbial cells. Front Microbiol 2015;6:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eliaz D, Kannan S, Shaked H, Arvatz G, Tkacz ID, Binder L, et al. Exosome secretion affects social motility in Trypanosoma brucei. PLoS Pathog 2017;13(3):e1006245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao M, Ning J, Hernandez-Lara CI, Belzile O, Wang Q, Dutcher SK, et al. Uni-directional ciliary membrane protein trafficking by a cytoplasmic retrograde IFT motor and ciliary ectosome shedding. Elife 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Silva M, Haas LA, Morsci NS, Nguyen KC, Hall DH, et al. C. elegans Ciliated Sensory Neurons Release Extracellular Vesicles that Function in Animal Communication. Curr Biol 2014;24(5):519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parchure A, Vyas N, Mayor S. Wnt and Hedgehog: Secretion of Lipid-Modified Morphogens. Trends Cell Biol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood CR, Rosenbaum JL. Ciliary ectosomes: transmissions from the cell’s antenna. Trends Cell Biol 2015;25(5):276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beer KB, Wehman AM. Mechanisms and functions of extracellular vesicle release in vivo-What we can learn from flies and worms. Cell Adh Migr 2017;11(2):135–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Barr MM. Ciliary Extracellular Vesicles: Txt Msg Organelles. Cell Mol Neurobiol 2016;36(3):449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satir P CILIA: before and after. Cilia 2017;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reiter JF, Leroux MR. Genes and molecular pathways underpinning ciliopathies. Nat Rev Mol Cell Biol 2017;18(9):533–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avasthi P, Marshall W. Ciliary secretion: switching the cellular antenna to ‘transmit’. Curr Biol 2013;23(11):R471–3. [DOI] [PubMed] [Google Scholar]
- 14.Cavalier-Smith T The evolutionary origin and phylogeny of microtubules, mitotic spindles and eukaryote flagella. Biosystems 1978;10(1–2):93–114. [DOI] [PubMed] [Google Scholar]
- 15.Cavalier-Smith T The evolutionary origin and phylogeny of eukaryote flagella. Symp Soc Exp Biol 1982;35:465–93. [PubMed] [Google Scholar]
- 16.Jékely G, Arendt D. Evolution of intraflagellar transport from coated vesicles and autogenous origin of the eukaryotic cilium. BioEssays : news and reviews in molecular, cellular and developmental biology 2006;28(2):191–198. [DOI] [PubMed] [Google Scholar]
- 17.Szempruch AJ, Sykes SE, Kieft R, Dennison L, Becker AC, Gartrell A, et al. Extracellular Vesicles from Trypanosoma brucei Mediate Virulence Factor Transfer and Cause Host Anemia. Cell 2016;164(1–2):246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nager AR, Goldstein JS, Herranz-Perez V, Portran D, Ye F, Garcia-Verdugo JM, et al. An Actin Network Dispatches Ciliary GPCRs into Extracellular Vesicles to Modulate Signaling. Cell 2017;168(1–2):252–263 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phua SC, Chiba S, Suzuki M, Su E, Roberson EC, Pusapati GV, et al. Dynamic Remodeling of Membrane Composition Drives Cell Cycle through Primary Cilia Excision. Cell 2017;168(1–2):264–279 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salinas RY, Pearring JN, Ding JD, Spencer WJ, Hao Y, Arshavsky VY. Photoreceptor discs form through peripherin-dependent suppression of ciliary ectosome release. J Cell Biol 2017;216(5):1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood CR, Huang K, Diener DR, Rosenbaum JL. The cilium secretes bioactive ectosomes. Curr Biol 2013;23(10):906–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diener DR, Lupetti P, Rosenbaum JL. Proteomic Analysis of Isolated Ciliary Transition Zones Reveals the Presence of ESCRT Proteins. Curr Biol 2015;25(3):379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long H, Zhang F, Xu N, Liu G, Diener DR, Rosenbaum JL, et al. Comparative Analysis of Ciliary Membranes and Ectosomes. Curr Biol 2016;26(24):3327–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Kaletsky R, Silva M, Williams A, Haas LA, Androwski RJ, et al. Cell-Specific Transcriptional Profiling of Ciliated Sensory Neurons Reveals Regulators of Behavior and Extracellular Vesicle Biogenesis. Curr Biol 2015;25(24):3232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva M, Morsci N, Nguyen KC, Rizvi A, Rongo C, Hall DH, et al. Cell-Specific alpha-Tubulin Isotype Regulates Ciliary Microtubule Ultrastructure, Intraflagellar Transport, and Extracellular Vesicle Biology. Curr Biol 2017;27(7):968–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Hagan R, Silva M, Nguyen KCQ, Zhang W, Bellotti S, Ramadan YH, et al. Glutamylation Regulates Transport, Specializes Function, and Sculpts the Structure of Cilia. Curr Biol 2017;27(22):3430–3441 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maguire JE, Silva M, Nguyen KCQ, Hellen E, Kern AD, Hall DH, et al. Myristoylated CIL-7 regulates ciliary extracellular vesicle biogenesis. Molecular Biology of the Cell 2015;26(15):2823–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hogan MC, Manganelli L, Woollard JR, Masyuk AI, Masyuk TV, Tammachote R, et al. Characterization of PKD protein-positive exosome-like vesicles. J Am Soc Nephrol 2009;20(2):278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pampliega O, Orhon I, Patel B, Sridhar S, Diaz-Carretero A, Beau I, et al. Functional interaction between autophagy and ciliogenesis. Nature 2013;502(7470):194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang F, Sirinakis G, Allgeyer ES, Schroeder LK, Duim WC, Kromann EB, et al. Ultra-High Resolution 3D Imaging of Whole Cells. Cell 2016;166(4):1028–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang Y, Meng D, Zhu B, Pan J. Mechanism of ciliary disassembly. Cell Mol Life Sci 2016;73(9):1787–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Izawa I, Goto H, Kasahara K, Inagaki M. Current topics of functional links between primary cilia and cell cycle. Cilia 2015;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molday RS, Goldberg AFX. Peripherin diverts ciliary ectosome release to photoreceptor disc morphogenesis. J Cell Biol 2017;216(5):1227–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langenhan T, Barr MM, Bruchas MR, Ewer J, Griffith LC, Maiellaro I, et al. Model Organisms in G Protein-Coupled Receptor Research. Molecular pharmacology 2015;88(3):596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Hagan R, Wang J, Barr MM. Mating behavior, male sensory cilia, and polycystins in Caenorhabditis elegans. Semin Cell Dev Biol 2014;33:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol 2002;12:R378–R380. [DOI] [PubMed] [Google Scholar]
- 37.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 2002;13(10):2508–16. [DOI] [PubMed] [Google Scholar]
- 38.Hogan MC, Bakeberg JL, Gainullin VG, Irazabal MV, Harmon AJ, Lieske JC, et al. Identification of Biomarkers for PKD1 Using Urinary Exosomes. J Am Soc Nephrol 2015;26(7):1661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liebscher I, Schoneberg T. Tethered Agonism: A Common Activation Mechanism of Adhesion GPCRs. Handb Exp Pharmacol 2016;234:111–125. [DOI] [PubMed] [Google Scholar]
- 40.Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J Mol Biol 2016;428(4):688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salih M, Fenton RA, Zietse R, Hoorn EJ. Urinary extracellular vesicles as markers to assess kidney sodium transport. Curr Opin Nephrol Hypertens 2016;25(2):67–72. [DOI] [PubMed] [Google Scholar]
- 42.Grimes DT, Burdine RD. Left-Right Patterning: Breaking Symmetry to Asymmetric Morphogenesis. Trends Genet 2017;33(9):616–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirokawa N, Tanaka Y, Okada Y, Takeda S. Nodal Flow and the Generation of Left-Right Asymmetry. Cell 2006;125(1):33–45. [DOI] [PubMed] [Google Scholar]
- 44.Wen C, Seeger RC, Fabbri M, Wang L, Wayne AS, Jong AY. Biological roles and potential applications of immune cell-derived extracellular vesicles. J Extracell Vesicles 2017;6(1):1400370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blacque OE, Sanders AA. Compartments within a compartment: what C. elegans can tell us about ciliary subdomain composition, biogenesis, function, and disease. Organogenesis 2014;10(1):126–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de la Roche M, Asano Y, Griffiths GM. Origins of the cytolytic synapse. Nat Rev Immunol 2016;16(7):421–32. [DOI] [PubMed] [Google Scholar]
- 47.Choudhuri K, Llodrá J, Roth EW, Tsai J, Gordo S, Wucherpfennig KW, et al. Polarized release of T-cell-receptor-enriched microve... [Nature. 2014] - PubMed - NCBI. Nature 2014;507(7490):118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finetti F, Onnis A, Baldari CT. Regulation of vesicular traffic at the T cell immune synapse: lessons from the primary cilium. Traffic 2015;16(3):241–9. [DOI] [PubMed] [Google Scholar]
- 49.Goncalves J, Pelletier L. The Ciliary Transition Zone: Finding the Pieces and Assembling the Gate. Mol Cells 2017;40(4):243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mukhopadhyay S, Badgandi HB, Hwang SH, Somatilaka B, Shimada IS, Pal K. Trafficking to the primary cilium membrane. Mol Biol Cell 2017;28(2):233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phua SC, Nihongaki Y, Inoue T. Autonomy declared by primary cilia through compartmentalization of membrane phosphoinositides. Curr Opin Cell Biol 2018;50:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bae Y-K, Kim E, L'hernault SW, Barr MM. The CIL-1 PI 5-phosphatase localizes TRP Polycystins to cilia and activates sperm in C. elegans. Current Biology VL - 2009;19(19):1599–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia-Gonzalo FR, Phua SC, Roberson EC, Garcia G 3rd, Abedin M, Schurmans S, et al. Phosphoinositides Regulate Ciliary Protein Trafficking to Modulate Hedgehog Signaling. Dev Cell 2015;34(4):400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jensen VL, Li C, Bowie RV, Clarke L, Mohan S, Blacque OE, et al. Formation of the transition zone by Mks5/Rpgrip1L establishes a ciliary zone of exclusion (CIZE) that compartmentalises ciliary signalling proteins and controls PIP2 ciliary abundance. EMBO J 2015;34(20):2537–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu Q, Zhang Y, Wei Q, Huang Y, Hu J, Ling K. Phosphatidylinositol phosphate kinase PIPKIgamma and phosphatase INPP5E coordinate initiation of ciliogenesis. Nat Commun 2016;7:10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dyson JM, Conduit SE, Feeney SJ, Hakim S, DiTommaso T, Fulcher AJ, et al. INPP5E regulates phosphoinositide-dependent cilia transition zone function. J Cell Biol 2017;216(1):247–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corbeil D, Marzesco AM, Wilsch-Brauninger M, Huttner WB. The intriguing links between prominin-1 (CD133), cholesterol-based membrane microdomains, remodeling of apical plasma membrane protrusions, extracellular membrane particles, and (neuro)epithelial cell differentiation. FEBS Lett 2010;584(9):1659–64. [DOI] [PubMed] [Google Scholar]
- 58.Chacon-Heszele MF, Choi SY, Zuo X, Baek JI, Ward C, Lipschutz JH. The exocyst and regulatory GTPases in urinary exosomes. Physiol Rep 2014;2(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wright KJ, Baye LM, Olivier-Mason A, Mukhopadhyay S, Sang L, Kwong M, et al. An ARL3-UNC119-RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes Dev 2011;25(22):2347–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen B, Wu N, Yang JM, Gould SJ. Protein targeting to exosomes/microvesicles by plasma membrane anchors. J Biol Chem 2011;286(16):14383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]


