Abstract
Objective:
We sought to determine safety and efficacy of the AKT inhibitor, GSK2141795, combined with the MEK inhibitor, trametinib, in endometrial cancer.
Methods:
Patients with measurable recurrent endometrial cancer were eligible. One to two prior cytotoxic regimens were allowed; prior use of a MEK or PI3K pathway inhibitor was excluded. Initial trial design consisted of a KRAS mutation stratified randomized phase II with a safety lead-in evaluating the combination. For the safety lead in, the previously recommended phase 2 dose (RP2D; trametinib 1.5mg, GSK2141795 50mg) was chosen for Dose Level 1 (DL1).
Results:
Of 26 enrolled patients, 14 were treated on DL1 and 12 were treated on DL-1 (trametinib 1.5mg, GSK2141795 25mg). Most common histologies were endometrioid (58%) and serous (27%). Four of 25 (16%) patients were KRAS mutant.
Dose limiting toxicities (DLTs) were assessed during cycle 1. DL1 had 8 DLTs (hypertension (n=2), mucositis (2), rash (2), dehydration, stroke/ acute kidney injury). DL1 was deemed non-tolerable so DL-1 was explored. DL-1 had no DLTs. Sixty-five percent of patients had ≥ grade 3 toxicity.
There were no responses in DL1 (0%, 90%CI 0 ~ 15%) and 1 response in DL-1 (8.3%, 90%CI 0.4 ~ 33.9%). Proportion PFS at 6 months for DL1 is 14%, and 25% for DL-1.
Conclusion:
The combination of trametinib and GSK2141795 had high levels of toxicity in endometrial cancer at the previously RP2D but was tolerable at a reduced dose. Due to insufficient preliminary efficacy at a tolerable dose, the Phase II study was not initiated.
Keywords: Endometrial Cancer, MEK inhibition, AKT inhibition, PI3K, KRAS mutation, NRG Oncology
INTRODUCTION
With increasing incidence over the last 5 years, endometrial cancer remains the most common gynecologic malignancy1. Patients with advanced and recurrent disease have a poor prognosis and limited options for effective treatment2. Currently, only hormonal therapy and pembrolizumab for MSI-high tumors are FDA-approved for this disease and the National Comprehensive Cancer Network (NCCN) strongly encourages participation of these patients in clinical trials3. There are other compendium-listed chemotherapeutic agents available, including doxorubicin, pegylated liposomal doxorubicin, cisplatin, topotecan, and docetaxel. However, response rates to these agents are modest and response duration quite short2,3. Thus, the exploration of novel agents and targeted therapy has been of great interest.
The phosphatidylinositol 3-kinase (PI3K)/AKT pathway plays a strong role in tumorigenesis through activation of growth and survival characteristics as well as avoidance of apoptosis4. Mutational activation of this pathway has also been implicated in chemotherapy resistance, especially to paclitaxel and carboplatin in multiple model systems5. Importantly, the PI3K/AKT pathway is characterized by extensive feedback loops and crosstalk with the rat sarcoma virus (RAS)/RAF, AMPK, and hormone signaling pathways6,7. It has become increasingly apparent that the PI3K pathway is important in the pathogenesis of endometrial cancer, evidenced by the finding that up to 80% of endometrial cancers will have an aberration in this pathway. These aberrations include activating mutations in PIK3CA and inactivating mutations in PIK3R1 or PTEN, as well as loss of PTEN protein expression, with these frequently occurring concurrently8–13.
The RAS/RAF pathway is equally important for tumorigenesis across a number of solid tumors, in which it has been shown to contribute to increased proliferation, metastasis, and avoidance of apoptosis. This pathway is activated through mutations in key pathway nodes such as KRAS, NRAS, and BRAF as well as through receptor tyrosine kinase activation14. Further KRAS can directly activate the PI3K pathway thus providing an additional mechanism for PI3K pathway activation6,7. Interestingly, activation of this pathway has been implicated in resistance to multiple targeted therapies, including PI3K-directed agents. Indeed, activating mutations in KRAS (which are identified in 10-30% of endometrial cancers9,10,15) have been shown to be dominant predictors of resistance to targeted therapies (e.g. erlotinib, cetuximab, PI3K inhibitors) in lung and colorectal cancers16–18.
The frequent mutational activation in endometrial cancer justifies exploration of the PI3K/AKT and RAS/RAF pathways as therapeutic targets in this recalcitrant disease. Thus far, clinical trials in recurrent endometrial cancer have predominantly focused on PI3K-directed agents as a single agent or in combination with chemotherapy. Results have been modest, with the highest efficacy seen in the chemonaive patient population19–25. Similarly, a single agent study targeting the RAS/RAF pathway with MEK inhibition had limited success in endometrial cancer26.
To date, aberrations in the PI3K/AKT pathway have not correlated directly with activity of agents directed at pathway nodes in endometrial cancer, potentially due to a dominant role for this pathway in the majority of endometrial cancers. However, two studies have implicated the potential for KRAS mutation to select for nonresponse to these agents27–29. Factors such feedback loops, crosstalk and toxicity resulting in inadequate target inhibition have been proposed as reasons for the limited efficacy of single agent pathway inhibition. Thus, the combination of PI3K and RAS/RAF pathway inhibitors may be necessary for optimal growth inhibition of endometrial cancers and other solid tumors where PI3K or RAS pathway aberrations are common18,30. The combination of AKT and MEK inhibition has been deemed safe in phase I trials, although high levels of toxicity have been observed depending on the specific agent combination31–34. Further, whether doses that are safe in patients adequately inhibit the pathways remains to be determined. The objective of the safety lead in portion of this study was to determine tolerability and estimate early efficacy of the combination of MEK and AKT inhibitors in women with recurrent or persistent endometrial cancer.
METHODS
Eligibility.
Patients were eligible for this study if they had recurrent or persistent endometrial cancer, measurable disease, and a GOG/ECOG performance status of 0 or 1. All histologies were eligible except carcinosarcoma, sarcoma, mucinous, or squamous cell cancers. Adequate organ function was required including ANC ≥ 1500/mm3, platelets ≥100,000/mm3, Hg ≥ 9 g/dL, INR and PTT ≤ 1.5 x institutional upper limit of normal (ULN) or in range INR for patients on warfarin, creatinine ≤ 1.5 x ULN or creatinine clearance ≥ 50ml/min (calculated or 24 hour), total bilirubin ≤ 1.5 x ULN, AST/ALT/Alkaline phosphatase ≤ 2.5 x ULN, albumin ≥ 2.5g/dL, and normal TSH. Hemodynamic parameters included systolic blood pressure < 140 mmHg, diastolic blood pressure < 90 mmHg, and left ventricular function ejection fraction ≥ lower limit of normal (LLN) by echocardiogram or MUGA. Patients were not permitted to have symptomatic or untreated leptomenigeal or brain metastasis, spinal cord compression, or history of interstitial lung disease/pneumonitis. Patients with uncontrolled intercurrrent illness, including active infection, symptomatic cardiac dysfunction, and Hepatitis B/C infection were excluded. One to two prior cytotoxics were allowed, but prior treatment with a MEK inhibitor or a PI3K/AKT pathway-directed therapy was not permitted. In addition, patients were required to have archival tissue available for KRAS mutation testing. Patients with Type I or poorly controlled Type II diabetes mellitus were not eligible. Patients with Type II diabetes mellitus were allowed if their hemoglobin A1C was ≤ 8. All patients with a screening fasting glucose ≥160 were excluded. Given the impact of MEK inhibition, a history of current evidence or risk of retinal vein occlusion was an exclusion criterion. As both agents were orally administered, patients had to be able to swallow and retain oral medications.
Treatment.
The trial was designed as a randomized, phase II, open label trial to compare the MEK inhibitor, trametinib, alone or combination with the AKT inhibitor, GSK2141795. Given prior experience with adverse events among this patient population when treated with PI3K pathway-directed agents, the trial included a planned 12-15 patient safety lead in to insure the combination was tolerable before opening the larger randomized phase II trial. The study was reviewed and approved by the Cancer Therapy Evaluation Program (CTEP) in the National Cancer Institute (NCI).
Prior to starting the study, patients submitted archived formalin fixed paraffin-embedded (FFPE) primary, metastatic or recurrent tumor tissue for performance of KRAS testing. All molecular testing was performed in a CLIA-compliant manner by Baylor College of Medicine. Patients were to be stratified based on genomic profile into two distinct subgroups defined as KRAS mutant or KRAS wild type.
For the first cohort on the safety lead in, patients received trametinib 1.5 mg (three, 0.5 mg tablets) daily and GSK2141795 50 mg (two, 25 mg) daily on a continuous schedule over a 28 day cycle. During the safety lead-in, dose limiting toxicities (DLTs) of the study agents were assessed over cycle 1 for the combination of trametinib and GSK2141795. Response assessment was performed every 8 weeks according to RECIST 1.135.
Medications were taken on an empty stomach 2 hours before or 2 hours after a meal. Patients were instructed to not take a missed dose or double the next dose if they vomited or missed a dose. They were encouraged to continue with the assigned dosing schedule. Patient pill calendars were provided to keep track of the date and time of dosing. Patients received prophylaxis for rash during the first 6 weeks of study treatment with twice daily application of topical steroids and antibiotics to body areas including face, chest and upper back.
A review of safety was planned after the first 12 evaluable patients on the combination regimen were treated and completed one 28 day cycle or had a DLT prior to completing the first cycle. All patients that received any treatment were considered evaluable for DLT, unless they withdrew from the trial for reasons other than toxicity before completing cycle 1.
DLTs, for the purposes of the safety evaluation, were defined as either study treatment related hematologic or non-hematologic toxicity occurring during cycle 1 of therapy based on the NCI Common Terminology Criteria (CTCAE) Version 4. Criteria defining DLT included dose delays of greater than 14 days due to failure to recover counts, febrile neutropenia, grade 4 neutropenia lasting >7 days, grade 4 thrombocytopenia or clinically significant bleeding with grade 3 thrombocytopenia, treatment delay of greater than 14 days, any drug related death, or grade 3 or grade 4 non-hematologic toxicity (excluding anorexia, constipation, fatigue, hypersensitivity/allergic reactions, grade 4 nausea and vomiting ≤ 48 hours with maximum medical management, grade 3 electrolyte imbalance, including hypocalcemia, hypokalemia, hypomagnesemia, hyponatremia, and hypophosphatemia as a result of diarrhea, Grade 3 dehydration as a result of nausea and vomiting, grade 3 rash that does not decrease the activities of daily living or recovers to grade 1 within one week, and grade 3 hypertension that can be controlled within one week). Grade 4 nausea and vomiting for >48 hours despite maximum medical management was considered a DLT. Grade 4 electrolyte imbalance that could be reduced within 48 hours to grade 2 or less was not be considered a DLT. Subsequent treatment was not re-started until resolution of prior toxicity to grade 1.
Frequency and severity of all adverse events were assessed during all cycles of therapy. Toxicity was managed by prescribed delays and dose modifications. Subsequent cycles of therapy did not start until the toxicity was ≤ grade 1, ANC ≥ 1500/mm3 and platelets ≥ 100,000/mm3. Therapy was delayed for a maximum of three weeks.
As part of a separate IRB-approved protocol at the lead site, patients gave consent for prospective tissue collection. Characterization of change in expression levels of key cancer-related total and phosphorylated proteins was planned using reverse phase protein array (RPPA) in post-treatment biopsies (day 3 and day 28) compared to baseline as pre-treatment control in frozen tumor specimens.
RPPA.
RPPA was performed in the University of Texas MD Anderson Cancer Center (MDACC) Cancer Center Support Grant Core facility. As described previously13, the phosphorylation status of proteins or phosphorylated forms of proteins were evaluated in RPPA analyses using antibodies preselected for signaling pathways well known to be involved in tumor development, encompassing both the PI3K and RAS pathways. Briefly, cellular proteins from frozen tissue were denatured by 1% SDS (with Beta-mercaptoethanol) and diluted in five 2-fold serial dilutions in dilution lysis buffer, and then serial diluted lysates were arrayed on nitrocellulose-coated slides (Grace Bio Lab) by Aushon 2470 Arrayer (Aushon BioSystems). Total 5808 array spots were arranged on each slide including 48 negative control spots and 5760 spots corresponding to serial diluted: 1) “Standard Lysates”; 2) positive controls prepared from mixed cell lysates or dilution buffer, respectively. The signal intensities on the RPPA arrays were quantitated using Array-Pro (Media Cybernetics, MD), and were processed using the R package SuperCurve available at (version-1.01, available at http://bioinformatics.mdanderson.org/OOMPA) to estimate the relative protein expression levels.
Statistics.
Approximately 14 to 15 patients were targeted for recruitment at the first dose-level to assure at least 12 DLT-evaluable patients were enrolled. The following criteria were utilized to determine the safety of the combination regimen. If 3 or fewer patients out of 12 experienced DLTs in cycle 1 (including treatment delays for cycle 2 of greater than 2 weeks due to toxicities), then the regimen was deemed safe for administration in the phase II study. If 4 or more patients out of 12 experience DLTs, then the regimen was declared too toxic. This study had a 78% chance of declaring a toxic regimen as being too toxic (probability of DLT equal to 40%). The study had a 79.5% chance of declaring a safe regimen as being safe (probability of DLT equal to 20%). The first dose-level was deemed too toxic, so the study was amended to evaluate another dose-level with a 2-stage safety assessment design enrolling 6 evaluable patients in each stage. If 0 patients experienced a DLT in cycle 1, then the study could conclude that the regimen was safe and proceed to the randomized phase II. If 3 or more patients experienced DLTs, then the study would have terminated early and concluded that the regimen was too toxic for further study. If the number of DLTs was either 1 or 2, then the study would proceed to a second stage to accrue 6 additional patients. If the total number of DLTs was 3 or less in 12, then the regimen could be considered safe for further investigation in the randomized phase II study. The operating characteristics were about the same.
Once safety was assured, a randomized trial was designed to assess Regimen II (the combination regimen) against Regimen I (Trametinib only). The trial was not conducted because of data outside this study. However, on March 13, 2014, the original continuous dosing combination arm was deemed too toxic for further exploration in the endometrial cancer population. Thus, an additional safety lead was planned to assess the safety and tolerability of an alternative dosing regimen. The regimen chosen was trametinib 1 mg daily and GSK2141795 25mg daily. The trametinib was not reduced due to concern that the dose would fall out of the therapeutic window.
Progression free survival (PFS) and overall survival (OS) were calculated for the cohort. Endpoints for PFS included progression or death. Endpoints for overall survival was death from any cause. Patients without an endpoint were censored at the date last seen. Tumor response was assessed using RECIST v1.1.
In the RPPA assay, a total of 287 good-quality antibodies were used. The log2 expression data obtained from the SuperCurve fitting were normalized by median-centering protein expression across the samples and then across the antibodies. After normalization, the proteins were ranked for each sample based on their expression levels in the sample and then the ranks were summed for each protein across the samples. The proteins’ rank sums were sorted and used to supervise the protein order to generate rank-sum ordered heatmap that used the normalized RPPA data. The color of the rank-sum ordered heatmap ranging from green to red represents the ranges of the protein expression levels from low to high. The analysis was done using R version 3.3.2 (https://www.r-project.org).
RESULTS
Subjects.
The study opened to accrual on 9/30/2013. From 1/29/2014 to 1/9/2015, 26 patients were enrolled over the two dosing cohorts, 14 patients on Dose Level 1 and 12 patients on Dose Level −1. Patient characteristics are summarized in Table 1. Median age among both cohorts was 62, the majority of patients were White, had endometrioid or serous histology, and a performance status of 0. In addition, prior radiation dose was equivalent by cohort. More patients in DL-1 had only 1 prior chemo while more patients in DL1 had 2 prior regimens. Four of 26 patients (15.4%) had a KRAS mutation in their tumor.
Table 1.
Demographic and clinical characteristics of enrolled patients
| Factor | n=26 |
|---|---|
| Age in years (median, range) | 62 (29 – 80) |
| Race (n, %) | |
| White | 23 (88.5) |
| Black | 2 (7.7) |
| Hispanic | 1 (3.8) |
| Histology (n, %) | |
| Endometrioid | 15 (57.8) |
| Serous | 7 (27.0) |
| Clear cell | 1 (3.8) |
| Mixed | 1 (3.8) |
| Adenocarcinoma, unspecified | 1 (3.8) |
| Complex atypical hyperplasia | 1 (3.8) |
| Performance Status | |
| 0 | 20 (76.9) |
| 1 | 6 (23.1) |
| Prior Regimens | |
| 1 | 12 (46.2) |
| 2 | 14 (53.8) |
| Prior Radiation | |
| 0 | 13 (50.0) |
| 1 | 13 (50.0) |
| KRAS mutation status | |
| mutant | 4 (15.4) |
| wildtype | 22 (84.6) |
Adverse Events.
Within 35 days of the first patient entering, 14 patients were enrolled in the safety lead in to evaluate the continuous dosing regimen. Among evaluable patients, dose-limiting toxicities occurred in 8 patients including: G3 fatigue/G3 hyponatremia (n=1), grade 3 hypertension > 7 days (n=2), grade 3 mucositis (n=2), grade 3 dehydration/G3 acute kidney injury/G3 stroke (n=1), and grade 3 rash lasting > 7 days (n=2). Five of the patients had complete resolution of toxicity and were restarted on DL-1. Three patients came off study because of significant residual toxicity. Given the toxicity at DL1, we proceeded with an immediate dose level reduction to DL-1 for all patients on trial. In addition, we added the DL-1 for evaluation in a new safety lead in cohort prior to proceeding with the randomized phase II portion. All patients completed DL-1 without a DLT.
Table 2 provides all treatment-related, maximum toxicities experienced by patients on the trial. Aside from anemia, there were minimal hematologic adverse events across both dosing cohorts. As noted above, gastrointestinal toxicities such as nausea, vomiting, and diarrhea were the most common adverse events with this combination. The majority of patients experienced fatigue and rash. There were no treatment-related deaths. Patients received a median of 2 cycles of treatment regardless of the starting dose level.
Table 2.
Treatment-related Adverse Events by Dose Level
Adverse events were graded with CTCAE version 4
| Trametinib+GSK (High) (n=14) | Trametinib+GSK (Low) (n=12) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. and (%) of Patients by Grade | No. and (%) of Patients by Grade | |||||||||
| System Organ Class | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 |
| Overall Highest Grade | 1 (7.1) |
4 (28.6) |
9 (64.3) |
0 (0.0) |
0 (0.0) |
2 (16.7) |
6 (50.0) |
4 (33.3) |
0 (0.0) |
0 (0.0) |
| Blood and Lymphatic System Disorders | 3 (21.4) |
2 (14.3) |
2 (14.3) |
0 (0.0) |
0 (0.0) |
3 (25.0) |
3 (25.0) |
1 (8.3) |
0 (0.0) |
0 (0.0) |
| Cardiac Disorders | 1 (7.1) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Eye Disorders | 2 (14.3) |
1 (7.1) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (8.3) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Gastrointestinal Disorders | 4 (28.6) |
5 (35.7) |
3 (21.4) |
0 (0.0) |
0 (0.0) |
6 (50.0) |
4 (33.3) |
2 (16.7) |
0 (0.0) |
0 (0.0) |
| General Disorders and Administration Site Conditions | 5 (35.7) |
4 (28.6) |
1 (7.1) |
0 (0.0) |
0 (0.0) |
3 (25.0) |
4 (33.3) |
2 (16.7) |
0 (0.0) |
0 (0.0) |
| Immune System Disorders | 0 (0.0) |
0 (0.0) |
1 (7.1) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Infections and Infestations | 0 (0.0) |
1 (7.1) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Injury, Poisoning and Procedural Complications | 1 (7.1) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Investigations | 7 (50.0) |
3 (21.4) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
4 (33.3) |
4 (33.3) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Metabolism and Nutrition Disorders | 0 (0.0) |
6 (42.9) |
3 (21.4) |
0 (0.0) |
0 (0.0) |
4 (33.3) |
5 (41.7) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Musculoskeletal and Connective Tissue Disorders | 2 (14.3) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (8.3) |
1 (8.3) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Nervous System Disorders | 2 (14.3) |
0 (0.0) |
1 (7.1) |
0 (0.0) |
0 (0.0) |
4 (33.3) |
1 (8.3) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Psychiatric Disorders | 0 (0.0) |
1 (7.1) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Renal and Urinary Disorders | 2 (14.3) |
0 (0.0) |
1 (7.1) |
0 (0.0) |
0 (0.0) |
2 (16.7) |
1 (8.3) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Respiratory, Thoracic and Mediastinal Disorders | 4 (28.6) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
5 (41.7) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Skin and Subcutaneous Tissue Disorders | 7 (50.0) |
1 (7.1) |
3 (21.4) |
0 (0.0) |
0 (0.0) |
4 (33.3) |
2 (16.7) |
1 (8.3) |
0 (0.0) |
0 (0.0) |
| Vascular Disorders | 2 (14.3) |
3 (21.4) |
3 (21.4) |
0 (0.0) |
0 (0.0) |
2 (16.7) |
3 (25.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
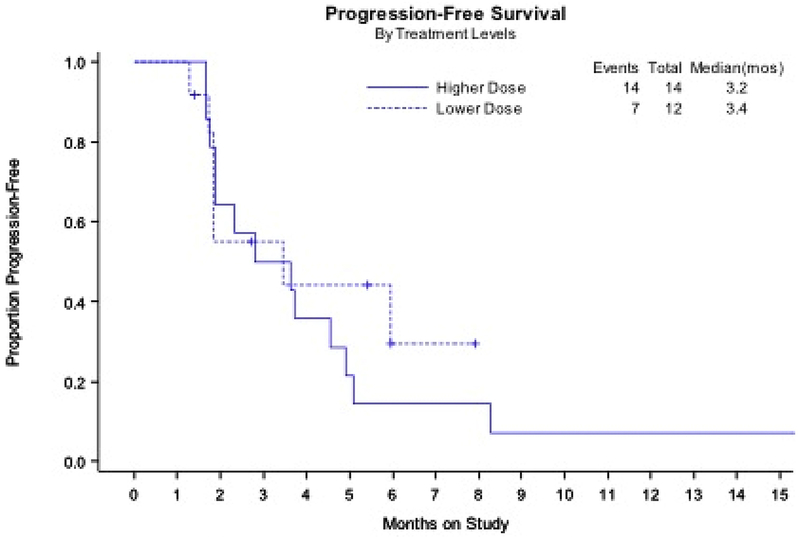
This was a phase 1/2 study, however, the phase II portion was not completed because the study was terminated early due to the lack of efficacy in the safety lead-in. There was only one partial response in 26 evaluable patients. However, 11 patients out of 26 achieved stable disease of limited duration. At a median follow up of 24.6 months (range 2.3 to 27.6 months), 14% of DL 1 and 25% of DL-1 were alive and progression free at the 6 month time point (Figure 1). Median progression free survival was 3.1 months across the entire cohort. Median PFS was 3.22 months (90% CI 1.87-4.57) in DL 1 and 2.97 months (90%CI 1.84 – 5.95) in DL-1. Median overall survival was 11.5 months across the entire cohort, achieving an OS of 12.91 months (90% CI 3.65 – 15.38) in DL1 and 10.32 months (90% CI 5.95-16.43) in DL-1.
Figure 1. Progression-free survival by dose level.
Note: enrollment to two dose levels was not randomized.
There was only one patient who underwent serial biopsies as part of the companion translational study. This patient had a PR on DL-1. Figure 2 demonstrates the RPPA analyses, representing protein expression changes between baseline, day 3 and day 28 on study. In terms of the effects on individual proteins, there was a marked and persistent increase in pAKT. This is to be expected from the inhibition of AKT and the inactivation of the feedback pathways noted above. We have previously demonstrated that inhibition of the PI3K pathway as well as the MAPK pathway increases Bim levels, an observation that is recapitulated here36,37. Strikingly, the cells undergo a shift to more epithelial characteristics as indicated by a marked increase in E cadherin and in Epithelial Membrane Antigen (EMA, Figure 3).
Figure 2. Rank-sum ordered heatmap analysis of RPPA data from frozen tissue samples from a patient with treatment of trametinib in combination with GSK2141795.
The heatmap by RPPA analysis demonstrates differences in protein expression between day 3 and day 28 on combination of trametinib with GSK2141794 compared to baseline.
Figure 3. Pathway analysis of RPPA data from frozen tissue samples from a patient with treatments of trametinib in combination with GSK2141795.
Data from baseline are grouped based on key pathways and targets in endometrial cancer.
DISCUSSION
In this study, the combination of the MEK inhibitor, trametinib, and the AKT inhibitor, GSK2141795, had high levels of toxicity at the previously defined recommended phase II dose. A reduced dose was better tolerated, however, there was insufficient activity to warrant moving to the planned randomized phase II in recurrent endometrial cancer.
This is not the first study to experience and report significant toxicity with the combination of a PI3K directed agent and an MEK inhibitor. In fact, thus far, this combination has been challenging to administer successfully in patients due to toxicity, despite encouraging preclinical results demonstrating efficacy. In studies of PI3K/MEK and AKT/MEK, high levels of toxicity, including significant rash, stomatitis, diarrhea, and fatigue, have been reported. This has limited identification of a recommended phase II dose in a number of therapy combinations31,33,34. Among those that did achieve a RP2D, further studies have not moved forward. Certainly, these combinations remain of great interest, however, it is unclear which combination, dose and schedule of agents may be able to move forward for further study in advanced solid tumors.
Evaluation of single agent PI3K pathway inhibition has had only modest clinical effect19–25. Aside from toxicity considerations, several factors limit the antitumor activity of single agent inhibition. The PI3K pathway can be visualized as a pyramid, with PI3K at the apex and multiple signaling branches downstream from PI3K that PI3K utilizes to mediate its effects. Thus, effects of the inhibition of a single node on the pathway may be bypassed by other signaling branches downstream from PI3K7,16,18. Additionally, feedback loops that likely play a normal role in the maintenance of PI3K pathway homeostasis are now known to be induced by most mTOR (TORC1) inhibitors. These feedback loops paradoxically result in upstream activation of AKT and therefore potentially blunt the antitumor efficacy of mTOR inhibition38. It was hypothesized that agents higher in the pathway, such as AKT or PI3K, would yield increased activity. Unfortunately, activity has remained modest. Targeting multiple related pathways still holds the potential to achieve great tumor response, although toxicity must be mitigated.
KRAS mutation has been implicated in resistance to therapy, including chemotherapy as well as agents targeting EGFR and PI3K16,17. Several studies in endometrial cancer revealed the presence of a KRAS mutation, either alone or in combination with increased phosphorylated s6 expression, to serve as a predictor of nonresponse to mTORC1 inhibition27,28. Our ability to assess the presence of KRAS mutations as a predictive factor for response was limited by the overall number of responders on trial. However, none of the patients with KRAS mutation had response or clinical benefit from this therapy. Although single agent MEK inhibition in endometrial cancer has not had clinically meaningful effects26, the combination of MEK inhibition with other agents is still of interest. Although the combination of AKT and MEK inhibition in this trial was limited by toxicity, further study of cross-pathway combinations can be considered.
The RPPA data demonstrate the feasibility of obtaining and analyzing serial biopsies in an early phase trial. Importantly, the data presented herein demonstrate the possible role of RPPA to confirm target engagement as well as provide hypothesis-generating data to guide design of future combination studies. In terms of therapeutic opportunities exposed by the RPPA data, the increase in apoptotic index as well as the increase in Bim suggest that the addition of a BCL2 family inhibitor may increase the depth and duration of response. Indeed, we have previously demonstrated that inhibition of the PI3K pathway increases the apoptotic index and sensitizes cells to BCL2 family inhibitors 36,39 The marked alteration in the DNA damage response pathway suggests potential activity of PARP inhibitors or DNA damage checkpoint inhibitors such as those targeting ATM, ATR or Wee1. Importantly, previously we have demonstrated marked synergy between PARP and MEK and PARP and PI3K pathway inhibitors in model systems and in patients, respectively37,40. The shift to an epithelial characteristic could indicate sensitivity to conventional chemotherapy agents based on the increased sensitivity of cells that have undergone MET compared to those that have undergone EMT to therapeutic challenge. As a whole, these data provide an opportunity to explore novel rational combinations in future clinical trials.
In summary, the combination of the MEK inhibitor, trametinib, and the AKT inhibitor, GSK2141795, was ultimately found to be tolerable at reduced dose levels from the initial recommended phase II dose. The reduced dose had minimal activity, which precluded further evaluation in the planned randomized phase II trial.
Research Highlights.
The combination of trametinib (MEK inhibitor) and GSK2141795 (AKT inhibitor) led to high levels of adverse events.
Clinical activity of the combination of trametinib and GSK2141795 was low in patients with recurrent endometrial cancer.
Use of serial biopsies in early phase trials is feasible and may yield information regarding therapeutic opportunities.
ACKNOWLEDGEMENTS
This work was supported by National Cancer Institute grants to NIH SPORE in Uterine Cancer (NIH 2P50 CA098258-06 (SW, RC, GBM); MD Anderson Cancer Center Support Grant (NIH P30CA016672); NCI Grant to NRG Oncology (U10CA180822), NRG Operations Grant (U10 CA180868) and Andrew Sabin Family Fellowship. Dr. Aghajanian is supported in part by the MSK Cancer Center Support Grant P30 CA008748. Dr. Schilder is supported in part by the Thomas Jefferson University Cancer Center Support Grant, P30 CA056036. Dr. Coleman is supported in part by the Ann Rife Cox Chair in Gynecology.
The following institutions participated in the primary treatment studies: University of Oklahoma Health Sciences Center, Case Western Reserve University, Women and Infants Hospital, Memorial Sloan Kettering Cancer Center, University of Virginia, MD Anderson Cancer Center, Thomas Jefferson University Hospital, Fox Chase Cancer Center and Johns Hopkins University/Sidney Kimmel Cancer Center.
Funding: NIH SPORE in Uterine Cancer (NIH 2P50 CA098258-06) (SW, RC), NCI Grant to NRG Oncology/GOG (U10CA180822), NRG Operations Grant (U10CA180868) and Andrew Sabin Family Fellowship (SW). NCI Cancer Center Support Grants to MD Anderson Cancer Center, Memorial Sloan Kettering Cancer Center, and Thomas Jefferson University (P30CA016672, P30 CA008748, P30 CA056036). Dr. Coleman is supported in part by the Ann Rife Cox Chair in Gynecology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This original research was presented in part at the 47th Annual Meeting of the Society of Gynecologic Oncology, San Diego, CA, March 18 – 22, 2016.
Conflict of Interest
Dr. Shannon Westin reports that she has received research support from ArQule, AstraZeneca, Clovis, Tesaro, Bayer, Roche/Genentech, Cotinga Pharmaceuticals and Novartis. In addition, Dr. Westin has served as Consultant for AstraZeneca, Clovis, Tesaro, Bayer, Novartis, Roche/Genentech, Pfizer, Takeda and Merck.
Drs. Sill, Ju, Waggoner, Birrer, Modesitt, Mathews, Schilder and Lee have no conflict of interest to declare.
Dr. Robert Coleman reports research funding from AstraZeneca, AbbVie, Clovis, Roche/Genentech, Janssen and Merck. Dr. Coleman has served on the Scientific Steering Committee for AbbVie, AstraZeneca, Clovis, Immunogen, Tesaro, Array, Janssen, Genmab and Gamamab.
Dr. Kathleen Moore reports that she served on advisory boards for AstraZeneca, Advaxis, Clovis, Immunogen, Jannsen, Genentech/Roche, Aravive, VBL Therapeutics, OncoMed, Tesaro and Merck (all honoraria paid to Dr. Moore). Dr. Moore reports serving on steering committees for Genentech Roche, Tesaro, Immunogen and Aravive (unpaid).
Dr. Lainie Martin reports that she has served on advisory boards for ImmunoGen and Tesaro.
Dr. Gordon Mills reports that he has served on Scientific Advisory boards or as consultant for AstraZeneca, Catena Pharmaceuticals, Critical Outcome Technologies, ImmunoMET, Ionis, Signalchem Lifesciences, Symphogen and Takeda/Millennium Pharmaceuticals. Dr. Mills reports that he has Tarveda, Stock options/financial Catena Pharmaceuticals, ImmunoMet, SignalChem, Spindle Top Ventures, Tarveda. He also has licensed technology HRD assay to Myriad Genetics, DSP patent with Nanostring. Additionally, Dr. Mills has sponsored research performed for Adelson Medical Research Foundation, AstraZeneca, Breast Cancer Research Foundation, Immunomet, Ionis, Komen Research Foundation, Nanostring, Ovarian Cancer Research Foundation, Pfizer, Prospect Creek Foundation and Takeda/Millennium Pharmaceuticals.
Dr. Paula Fracasso reports that she has no conflicts to disclose. Dr. Fracasso worked on this study while employed by the University of Virginia and, since May of 2014, has been an employee of Bristol-Myers Squibb and has stock with the company.
Dr. Carol Aghajanian reports personal fees from Tesaro, personal fees from Immunogen, personal fees from Clovis, personal fees from Mateon Therapeutics, personal fees from Cerulean Pharmaceutical, outside of the submitted work.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017; 67(1): 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Burke WM, Orr J, Leitao M, et al. Endometrial cancer: a review and current management strategies: part II. Gynecol Oncol 2014; 134(2): 393–402. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Uterine Neoplasms. 2017. (accessed April 25 2017).
- 4.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov 2005; 4(12): 988–1004. [DOI] [PubMed] [Google Scholar]
- 5.West KA, Castillo SS, Dennis PA. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Updat 2002; 5(6): 234–48. [DOI] [PubMed] [Google Scholar]
- 6.Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci 2011; 36(6): 320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene 2008; 27(41): 5527–41. [DOI] [PubMed] [Google Scholar]
- 8.Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013; 497(7447): 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oda K, Stokoe D, Taketani Y, McCormick F. High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer Res 2005; 65(23): 10669–73. [DOI] [PubMed] [Google Scholar]
- 10.Hayes MP, Wang H, Espinal-Witter R, et al. PIK3CA and PTEN mutations in uterine endometrioid carcinoma and complex atypical hyperplasia. Clin Cancer Res 2006; 12(20 Pt 1): 5932–5. [DOI] [PubMed] [Google Scholar]
- 11.Shoji K, Oda K, Nakagawa S, et al. The oncogenic mutation in the pleckstrin homology domain of AKT1 in endometrial carcinomas. Br J Cancer 2009; 101(1): 145–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung LW, Hennessy BT, Li J, et al. High Frequency of PIK3R1 and PIK3R2 Mutations in Endometrial Cancer Elucidates a Novel Mechanism for Regulation of PTEN Protein Stability. Cancer Discov 2011; 1(2): 170–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang H, Cheung LW, Li J, et al. Whole-exome sequencing combined with functional genomics reveals novel candidate driver cancer genes in endometrial cancer. Genome research 2012; 22(11): 2120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoshino R, Chatani Y, Yamori T, et al. Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signaling pathway in human tumors. Oncogene 1999; 18(3): 813–22. [DOI] [PubMed] [Google Scholar]
- 15.Koul A, Willen R, Bendahl PO, Nilbert M, Borg A. Distinct sets of gene alterations in endometrial carcinoma implicate alternate modes of tumorigenesis. Cancer 2002; 94(9): 2369–79. [DOI] [PubMed] [Google Scholar]
- 16.Massarelli E, Varella-Garcia M, Tang X, et al. KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Clin Cancer Res 2007; 13(10): 2890–6. [DOI] [PubMed] [Google Scholar]
- 17.Jimeno A, Messersmith WA, Hirsch FR, Franklin WA, Eckhardt SG. KRAS mutations and sensitivity to epidermal growth factor receptor inhibitors in colorectal cancer: practical application of patient selection. J Clin Oncol 2009; 27(7): 1130–6. [DOI] [PubMed] [Google Scholar]
- 18.Engelman JA, Chen L, Tan X, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med 2008; 14(12): 1351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oza AM, Elit L, Tsao MS, et al. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. J Clin Oncol 2011; 29(24): 3278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oza AM, Pignata S, Poveda A, et al. Randomized Phase II Trial of Ridaforolimus in Advanced Endometrial Carcinoma. J Clin Oncol 2015; 33(31): 3576–82. [DOI] [PubMed] [Google Scholar]
- 21.Tsoref D, Welch S, Lau S, et al. Phase II study of oral ridaforolimus in women with recurrent or metastatic endometrial cancer. Gynecol Oncol 2014; 135(2): 184–9. [DOI] [PubMed] [Google Scholar]
- 22.Fleming GF, Filiaci VL, Marzullo B, et al. Temsirolimus with or without megestrol acetate and tamoxifen for endometrial cancer: a gynecologic oncology group study. Gynecol Oncol 2014; 132(3): 585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slomovitz BM, Lu KH, Johnston T, et al. A phase 2 study of the oral mammalian target of rapamycin inhibitor, everolimus, in patients with recurrent endometrial carcinoma. Cancer 2010; 116(23): 5415–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ray-Coquard I, Favier L, Weber B, et al. Everolimus as second- or third-line treatment of advanced endometrial cancer: ENDORAD, a phase II trial of GINECO. British journal of cancer 2013; 108(9): 1771–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colombo N, McMeekin DS, Schwartz PE, et al. Ridaforolimus as a single agent in advanced endometrial cancer: results of a single-arm, phase 2 trial. British journal of cancer 2013; 108(5): 1021–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coleman RL, Sill MW, Thaker PH, et al. A phase II evaluation of selumetinib (AZD6244, ARRY-142886), a selective MEK-1/2 inhibitor in the treatment of recurrent or persistent endometrial cancer: an NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol 2015; 138(1): 30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackay HJ, Eisenhauer EA, Kamel-Reid S, et al. Molecular determinants of outcome with mammalian target of rapamycin inhibition in endometrial cancer. Cancer 2013. [DOI] [PubMed] [Google Scholar]
- 28.Meyer LA, Slomovitz BM, Djordjevic B, et al. The search continues: looking for predictive biomarkers for response to mammalian target of rapamycin inhibition in endometrial cancer. Int J Gynecol Cancer 2014; 24(4): 713–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tredan O, Treilleux I, Wang Q, et al. Predicting everolimus treatment efficacy in patients with advanced endometrial carcinoma: a GINECO group study. Target Oncol 2013; 8(4): 243–51. [DOI] [PubMed] [Google Scholar]
- 30.Di Nicolantonio F, Arena S, Tabernero J, et al. Deregulation of the PI3K and KRAS signaling pathways in human cancer cells determines their response to everolimus. J Clin Invest 2010; 120(8): 2858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juric D, Soria JC, Sharma S, et al. A phase 1b dose-escalation study of BYL719 plus binimetinib (MEK162) in patients with selected advanced solid tumors. J Clin Oncol 2014; 32: 5s (suppl; abstr 9051). [Google Scholar]
- 32.Kurzrock R, Patnaik A, Rosenstein L, et al. Phase I dose-escalation of the oral MEK1/2 inhibitor GSK1120212 (GSK212) dosed in combination with the oral AKT inhibitor GSK2141795 (GSK795). J Clin Oncol 2011; 29 (suppl: abstr 3085). [Google Scholar]
- 33.Bedard PL, Tabernero J, Janku F, et al. A phase Ib dose-escalation study of the oral pan-PI3K inhibitor buparlisib (BKM120) in combination with the oral MEK1/2 inhibitor trametinib (GSK1120212) in patients with selected advanced solid tumors. Clin Cancer Res 2015; 21(4): 730–8. [DOI] [PubMed] [Google Scholar]
- 34.Tolcher AW, Patnaik A, Papadopoulos KP, et al. Phase I study of the MEK inhibitor trametinib in combination with the AKT inhibitor afuresertib in patients with solid tumors and multiple myeloma. Cancer Chemother Pharmacol 2015; 75(1): 183–9. [DOI] [PubMed] [Google Scholar]
- 35.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45(2): 228–47. [DOI] [PubMed] [Google Scholar]
- 36.Muranen T, Selfors LM, Worster DT, et al. Inhibition of PI3K/mTOR leads to adaptive resistance in matrix-attached cancer cells. Cancer Cell 2012; 21(2): 227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun C, Fang Y, Yin J, et al. Rational combination therapy with PARP and MEK inhibitors capitalizes on therapeutic liabilities in RAS mutant cancers. Sci Transl Med 2017; 9(392). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 2006; 66(3): 1500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zervantonakis IK, Iavarone C, Chen HY, et al. Systems analysis of apoptotic priming in ovarian cancer identifies vulnerabilities and predictors of drug response. Nature communications 2017; 8(1): 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matulonis UA, Wulf GM, Barry WT, et al. Phase I dose escalation study of the PI3kinase pathway inhibitor BKM120 and the oral poly (ADP ribose) polymerase (PARP) inhibitor olaparib for the treatment of high-grade serous ovarian and breast cancer. Ann Oncol 2017; 28(3): 512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]