Abstract
This retrospective analysis of patients aims to show the blood levels of preoperative inflammatory markers in patients with glioblastoma and brain metastasis and to provide the diagnostic accuracy of the neutrophil–lymphocyte (NLR), lymphocyte–monocyte (LMR), and platelet–lymphocyte (PLR) ratios between the 2 groups of patients.
The retrospective reviews of the neutrophil, lymphocyte, monocyte, and platelet counts were analyzed in 80 patients with newly diagnosed glioblastoma and 70 patients with brain metastasis. The NLR, LMR, and PLR were calculated in each group. The differences in all the parameters were compared between the 2 groups.
Although the neutrophil, monocyte, and platelet counts were higher and the lymphocyte count was lower in patients with metastasis, the difference was not significant. A significantly higher PLR (P = .004) and a lower LMR (P = .01) were found in patients with brain metastasis. Although both PLR and LMR had diagnostic accuracy in differentiating glioblastoma from brain metastasis, LMR showed the highest diagnostic accuracy. NLR showed no diagnostic accuracy.
Systemic inflammation is more severe in glioblastoma than in brain metastasis, and LMR is more sensitive and/or specific than PLR in differentiating glioblastoma from brain metastasis. Therefore, LMR (less likely PLR) can be used as an index for differentiating between glioblastoma and brain metastasis before surgery.
Keywords: glioma, lymphocyte–monocyte ratio, metastasis, neutrophil–lymphocyte ratio, platelet–lymphocyte ratio
1. Introduction
Glioblastoma or glioblastoma multiforme (GBM) and intracranial metastasis are the 2 malignant neoplasms of the brain, and even advanced treatment modalities, such as surgery plus radiotherapy or chemotherapy, cannot extend patients’ survival much longer. For both tumors, survival takes 12 to 15 months with a high recurrence rate.[1,2] One of the most important diagnostic modalities of these 2 intracranial pathologies is magnetic resonance imaging (MRI). Similar findings such as peripheral contrast enhancement, edema, and central necrosis can be visible in both patient groups. Even though similar images can be seen on the MRI, further investigations and treatments are not completely identical. Distinguishing these 2 patient groups in the preoperative period can be rewarding for patients’ clinical management.
Inflammation is now accepted as one of the hallmarks of cancer,[3] and the role of inflammation in cancer initiation, development, and progression has been well demonstrated in solid cancers, including glioma and intracranial metastasis.[4,5] Lymphocyte infiltration around a tumor is associated with a good prognosis, but the infiltration of tumors by neutrophils may be associated with a poor prognosis. As a tumor progresses, the relative levels of neutrophils, platelets, and monocytes change, resulting in higher and lower levels of neutrophil and lymphocyte blood counts, respectively.[6,7]
Previous studies showed that preoperative inflammatory markers such as neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR), or lymphocyte–monocyte ratio (LMR) could be used as an index of tumor progression in solid cancers including gliomas.[7–10] Significantly higher preoperative neutrophil and monocyte counts and lower lymphocyte counts were found in high-grade glioma, especially GBM, in comparison with nonlesional epilepsy, meningioma, acoustic neuroma, and healthy controls.[7] Higher NLR and PLR or lower LMR is associated with glioma grade and progression and predicts poor prognosis. Moreover, an NLR greater than 4 was found to be a poor prognostic factor prior to the 2nd surgery of GBM.[9]
Although preoperative inflammatory markers have been studied extensively in other solid cancers such as prostate,[8] colorectal,[10] and lung,[11] the number of studies evaluating brain tumors is limited. Moreover, no study has yet evaluated the preoperative inflammatory markers in intracranial metastasis. As NLR, PLR, or LMR can be used as an index of malignancy and to differentiate gliomas from other intracranial tumors,[9] we hypothesize that these preoperative inflammatory markers can be used to differentiate intracranial metastasis from GBM prior to surgery. Therefore, this study aims to show whether a difference exists in the absolute levels of preoperative inflammatory markers between intracranial metastasis and GBM and to determine whether NLR, PLR, or LMR has a diagnostic value in differentiating metastasis from GBM before surgery.
2. Materials and methods
2.1. Study population
This inflammatory markers in patients newly diagnosed with GBM and metastasis was conducted through the collaboration of 2 hospitals. Patients with the following criteria were included in the study: those who had no hematological and/or infectious disease, had no previous surgery for either GBM or metastasis or primary cancer causing metastasis, had no chemo or radiation therapy, and signed the informed consent. All patients underwent resective surgery and were referred to have radiotherapy plus temozolomide for GBM and radiation therapy for metastasis after surgery. Depending on the selection criteria, 150 patients were included in the study. The histopathologic diagnosis of GBM and metastasis was made according to the World Health Organization criteria.[2] Our local ethics committee informed us that no approval was needed for this study because the study did not include data that could reveal the patients’ identity.
2.2. Data collection
Demographic, clinical, radiological, and histopathological data were retrieved from the patients’ medical records. After hospitalization, blood samples were taken for a complete blood count and other tests, including hepatic functions, serology, and electrolyte as a standard preoperative work-up. Neutrophil (103/mm3), lymphocyte (103/mm3), monocyte (103/mm3), and platelet (103/mm3) counts were recorded. The preoperative NLR (quotient of the absolute number of neutrophil count to lymphocyte count), PLR (quotient of the absolute number of platelet to lymphocyte count), and LMR (quotient of the absolute number of lymphocyte to monocyte count) were calculated.
3. Statistical analysis
Statistical analysis was performed using SPSS version 22.0. The results were reported here as the mean ± standard deviation. Comparisons between the groups were made using the independent samples t test, and the chi-square test was used for the categorical variables. The diagnostic value of NLR, PLR, and LMR was evaluated using the receiver operator characteristic (ROC) curve analysis. A probability value (P value) <.05 was considered statistically significant.
4. Results
4.1. Study population
The study population in this study was composed of 150 patients. The first group included 80 patients who underwent resective surgery for GBM. This group had 48 males (60%) and 32 females (40%) with a mean age of 51.65 ± 16.71 years. The second group was composed of 70 patients who underwent resective surgery for intracranial metastasis, mainly in the lung. This group had 49 males (70%) and 21 females (30%) with a mean age of 57.30 ± 10.20 years. Regarding gender, no significant difference was found between the GBM and the metastasis groups (x2 test; P = .08). The patients with metastasis were older than those with GBM, and the difference was significant (t test; P = .01).
4.2. Comparison of preoperative inflammatory markers between the groups
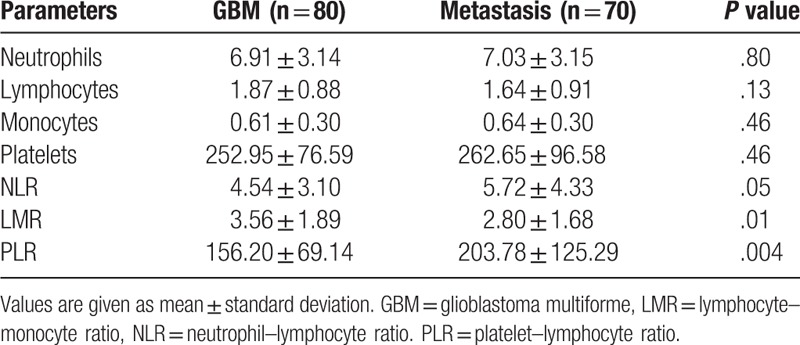
Table 1 summarizes the statistical results in both groups. The neutrophil, monocyte, and platelet counts were higher, and the lymphocyte count was lower in patients with metastasis than in those with GBM. However, none of the parameters showed a significant difference between the 2 groups. As expected, NLR and PLR were higher and LMR was lower in the metastasis group. NLR showed a trend of being significantly higher in the metastasis group (P = .05). The patients with metastasis showed a significantly higher PLR (P = .004) and lower LMR (P = .01) than those with GBM.
Table 1.
Statistical summary of preoperative inflammatory markers in the groups.
4.3. Diagnostic efficacy of the preoperative inflammatory markers between the groups
Figure 1A–C show the results of the ROC analysis for NLR, LMR, and PLR, respectively. According to the ROC analysis, the area under the curve (AUC) was 0.58 (CI 95% 0.48–0.67) with a P value of .08. The cutoff point for NLR was 3.96 (NLR > 3.96 predicts metastasis) with 55.71% sensitivity and 57.5% specificity. The AUC for LMR was 0.64 (P = .03; CI 95% 0.55–0.72). The cutoff point was 2.97 (LMR < 2.97 predicts metastasis) with 64% sensitivity and 58.7% specificity. The AUC for PLR was 0.61 (P = .01; CI 95% 0.52–0.70). The cutoff point was 121.69 (PLR > 120 predicts metastasis) with 78.57% sensitivity and 40% specificity. Depending on the ROC analysis, the best diagnostic value for differentiating GBM from metastasis prior to surgery was LMR followed by PLR. No high diagnostic accuracy was found for NLR.
Figure 1.
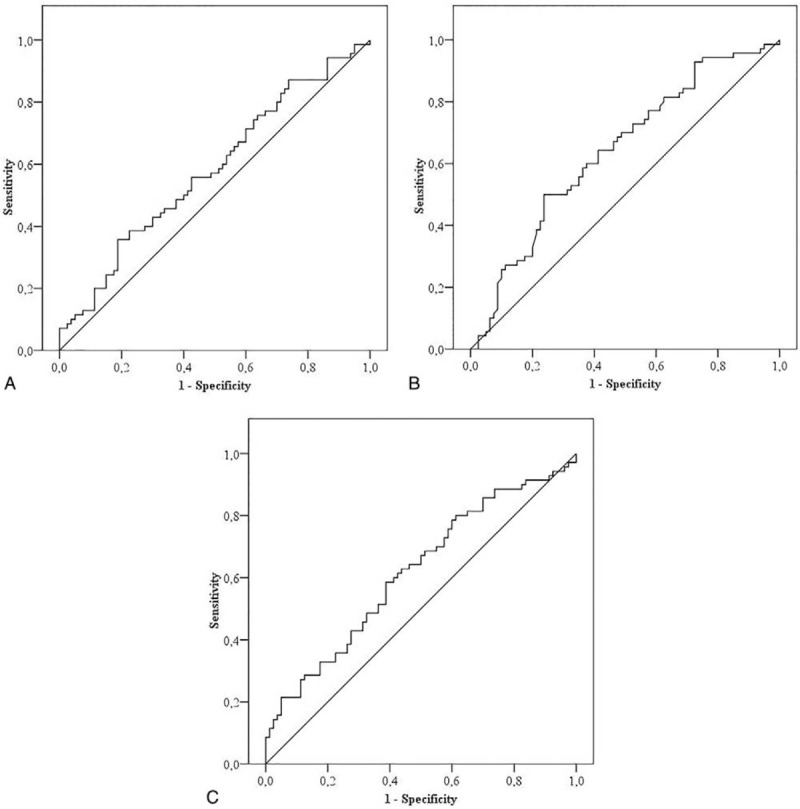
ROC analysis showing diagnostic accuracy for NLR (A; AUC = 0.58, 95% CI 0.48–0.67), LMR (B; AUC = 0.64, 95% CI 0.55–0.72), and PLR (C; AUC = 0.61, 95% CI 0.52–0.70). LMR showed the best diagnostic accuracy in differentiating glioblastoma from brain metastasis. AUC = area under the curve, LMR = lymphocyte–monocyte ratio, NLR = neutrophil–lymphocyte ratio. PLR = platelet–lymphocyte ratio, ROC = receiver operator characteristic.
5. Discussion
For the first time, this study shows that systemic inflammation is more severe in metastasis than in GBM, the most malignant form of brain tumor. Although no significant difference is found in the neutrophil, lymphocyte, monocyte, and platelet counts between the 2 groups, higher neutrophil, monocyte, and platelet counts and lower lymphocyte counts are found in intracranial metastasis. Conversely, the patients with metastasis show significantly higher levels of PLR and lower levels of LMR than those with GBM. NLR shows an increasing trend in metastasis. Our results are consistent with the current literature that systemic inflammation is more severe in most malignant tumors.[7,9–11] Studies in which intracranial metastasis is the focus of discussion are limited. One study showed that a high preoperative PLR value is a predictor of brain metastasis due to lung adenocarcinoma.[12] Another clinical study showed that an elevated preoperative NLR is a predictor of poor prognosis or worse survival after surgery for brain metastasis.[13] Our results with respect to PLR or NLR support the abovementioned studies in that metastasis has higher levels of PLR or NLR. The current study aims to show whether preoperative inflammatory ratios have a diagnostic value in differentiating GBM from metastasis, both of which are contrast-enhancing lesions demonstrated on the MRI. According to our results, we speculate that LMR has the most diagnostic value for differentiating between GBM and metastasis, and an LMR of less than 2.97 may predict metastasis. Furthermore, PLR has a diagnostic value after LMR, and a higher PLR with a cutoff point of 121.69 can predict metastasis. Unexpectedly, NLR may not have the ability to differentiate GBM from metastasis. Patients with metastasis have a primary site, so that they have tumors in more than 1 organ. This can explain our results and why patients with intracranial metastasis show more severe preoperative inflammatory reactions than those with GBM.
Among the preoperative inflammatory markers, NLR has been the most studied one, and a high preoperative NLR has been recognized as a prognostic factor in GBM.[7,9,11,14] Although the diagnostic value of PLR is not as strong as that of NLR, relatively higher levels of PLR have been found in high-grade gliomas.[7] Interestingly, little information is known about LMR. A decreased level of LMR as a poor prognostic index has extensively been demonstrated in several cancers such as renal cell carcinoma[15] and soft tissue sarcoma.[16] However, little is known about LMR in brain tumors. Only one recent study showed that a significantly lower level of LMR was found in high-grade glioma and inversely associated with glioma grade. LMR was concluded to be a useful biomarker for differentiating glioma from other intracranial diseases.[7]
The reason behind the changes in the blood levels of preoperative inflammatory markers is speculative. Cancer cells produce myeloid growth factors that lead to increase neutrophil production.[17] Circulating neutrophils secrete vascular endothelial growth factors, tumor necrosis factors, and some cytokines that can stimulate tumor angiogenesis, which in turn leads to tumor progression.[3] Moreover, an increase in neutrophils inhibits the cytolytic activity of lymphocytes, natural killer cells, and activated T cells.[3,17] Our data suggest that a decreased LMR may be due to the reduction of lymphocyte count and the elevation of monocyte count in metastasis, which has more severe inflammatory reactions than GBM because of the existence of more than 1 tumor in the brain and in other organs.
Finding reliable, easily available, and affordable tests, such as circulating serum or blood biomarker, for differentiating intracranial contrast-enhancing brain lesions from each other such as GBM, metastasis, or abscess can be helpful. Depending only on the MRI cannot differentiate the type of contrast-enhancing lesions. Moreover, neurosurgeons usually have difficulty in deciding whether the lesion is high-grade glioma or metastasis, and thus they refer patients to MR spectroscopy. Even advanced MRI provides a low diagnostic specificity of 50% to 80% for differentiating GBM from low-grade glioma, lymphoma, metastasis, abscess, and subacute infarcts.[18] Evaluating preoperative inflammatory markers such as NLR, LMR, or PLR or their combinations can help to find a new diagnostic tool for differentiating intracranial contrast-enhancing intra-axial tumors.
5.1. Limitations
This study has 3 limitations. First, our patient sample consisted of a relatively small proportion of patients with glioma and metastasis. Second, the retrospective nature of our study could have introduced a selection bias. Third, acute stress in patients with brain tumors could have caused changes in the blood levels of the inflammatory markers.
6. Conclusions
Despite the study's limitations, we provide the first evidence that patients with intracranial metastasis have more severe systemic inflammation than patients with GBM, which is the most malignant form of brain tumors. The LMR and PLR values are significantly different in metastasis. LMR has the most diagnostic accuracy and may serve as a biomarker for differentiating GBM from metastasis. Even PLR can help clinicians to differentiate GBM from intracranial metastasis. We strongly encourage further studies to include more patients with contrast-enhancing different intracranial space-occupying lesions such as high-grade gliomas, intracranial abscess, intracranial lymphomas, and metastasis.
Acknowledgments
The authors thank the patients and their families for their great help and collaboration.
Author contributions
Conceptualization: Taner Tanriverdi.
Data curation: Oguz Baran, Taha Sukru Korkmaz, Ahmet Kayhan.
Formal analysis: Oguz Baran, Taha Sukru Korkmaz.
Investigation: Oguz Baran, Rahsan Kemerdere, Taha Sukru Korkmaz, Taner Tanriverdi.
Methodology: Taha Sukru Korkmaz, Ahmet Kayhan.
Resources: Oguz Baran, Rahsan Kemerdere, Ahmet Kayhan.
Software: Taha Sukru Korkmaz.
Supervision: Oguz Baran, Rahsan Kemerdere, Taner Tanriverdi.
Writing – original draft: Oguz Baran.
Writing – review and editing: Rahsan Kemerdere, Taner Tanriverdi.
Footnotes
Abbreviations: AUC = area under the curve, GBM = glioblastoma multiforme, LMR = lymphocyte–monocyte ratio, MRI = magnetic resonance imaging, NLR = neutrophil–lymphocyte ratio, PLR = platelet–lymphocyte ratio, ROC = receiver operator characteristic.
How to cite this article: Baran O, Kemerdere R, Korkmaz TS, Kayhan A, Tanriverdi T. Can preoperative neutrophil to lymphocyte, lymphocyte to monocyte or platelet to lymphocyte ratios differentiate glioblastoma from brain metastasis?. Medicine. 2019;98:50(e18306).
The authors have no funding and conflicts of interest to disclose.
References
- [1].Ostrom QT, Gittleman H, Stetson L, et al. Epidemiology of gliomas. Cancer Treat Res 2015;163:1–4. [DOI] [PubMed] [Google Scholar]
- [2].Wesseling P, von Deimling A, Aipape KD. Louis DN, Ohgaki H, Wiestler OD. Metastatic tumours of the CNS. WHO Classification of Tumours of the Central Nervous System. Lyon:IARC; 2007. 248–51. [Google Scholar]
- [3].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- [4].Franchino F, Ruda R, Soffietti R. Mechanisms and therapy for cancer metastasis to the brain. Front Oncol 2018;8:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res 2011;71:2411–6. [DOI] [PubMed] [Google Scholar]
- [6].Kawata A, Une Y, Hosokawa M, et al. Tumor-infiltrating lymphocytes and prognosis of hepatocellular carcinoma. J Clin Oncol 1992;22:256–63. [PubMed] [Google Scholar]
- [7].Zheng SH, Huang JL, Chen M, et al. Diagnostic value of preoperative inflammatory markers in patients with glioma: a multicenter cohort study. J Neurosurg 2017;3:1–0. [DOI] [PubMed] [Google Scholar]
- [8].Kawahara T, Fukui S, Sakamaki K, et al. Neutrophil-to-lymphocyte ratio predicts prostatic carcinoma in men undergoing needle biopsy. Oncotarget 2015;31:32169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].McNamara MG, Lwin Z, Jiang H, et al. Factors impacting survival following second surgery in patients with glioblastoma in the temazolamide treatment era, incorporating neutrophil/lymphocyte ratio and time to first progression. J Neurooncol 2014;117:147–52. [DOI] [PubMed] [Google Scholar]
- [10].You J, Zhu GQ, Xie L, et al. Preoperative platelet to lymphocyte ratio is a valuable prognostic biomarker in patients with colorectal cancer. Oncotarget 2016;7:25516–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pinato DJ, Shiner RJ, Seckl MJ, et al. Prognostic performance of inflammation-based prognostic indices in primary operable non-small cell lung cancer. Sci Rep 2014;110:1930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang W, Bian C, Xia D, et al. Combining carcinoembryogenic antigen and platelet to lymphocyte ratio to predict brain metastasis of resected lung adenocarcinoma patients. Biomed Res Int 2017;2017:8076384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mitsuya K, Nakasu Y, Kurakane T, et al. Elevated preoperative neutrophil-to-lymphocyte ratio as a predictor of worse survival after resection in patients with brain metastasis. J Neurosurg 2017;127:433–7. [DOI] [PubMed] [Google Scholar]
- [14].Zadora P, Dabrowski W, Czarko K, et al. Preoperative neutrophil-lymphocyte count ratio helps predict the grade of glial tumor − a pilot study. Neurol Neurochir Pol 2015;49:41–4. [DOI] [PubMed] [Google Scholar]
- [15].Gu L, Li H, Chen L, et al. Prognostic role of lymphocyte to monocyte ratio for patients with cancer: evidence from a systemic review and meta-analysis. Oncotarget 2016;7:31926–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Szkandera J, Gerger A, Liegl-Atzwanger B, et al. The lymphocyte/monocyte ratio predicts poor clinical outcome and improves the predictive accuracy in patients with soft tissue sarcomas. Int J Cancer 2014;135:362–70. [DOI] [PubMed] [Google Scholar]
- [17].Teramukai S, Kitano T, Kishida Y, et al. Pretreatment neutrophil count as an independent prognostic factor in advancd non-small-cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00-03. Eur J Cancer 2009;45:1950–8. [DOI] [PubMed] [Google Scholar]
- [18].Hochberg FH, Atai NA, Gonda D, et al. Glioma diagnostics and biomarkers: an ongoing challenge in the field of medicine and science. Expert Rev Mol Diagn 2014;14:439–52. [DOI] [PMC free article] [PubMed] [Google Scholar]


