Abstract
L-carnitine (LC) is well known for its antioxidant activity. In this study, we explored the potential mechanistic effects of LC supplementation on aged bovine oocytes in vitro. We showed that in-vitro maturation could enhance the subsequent developmental capacity of aging oocytes, when supplemented with LC. After in vitro fertilization, the blastocyst formation rate in the aged oocytes post-LC treatment significantly increased compared to that in untreated aged oocytes (29.23 ± 2.20% vs. 20.90 ± 3.05%). Furthermore, after LC treatment, the level of intracellular reactive oxygen species in aged oocytes significantly decreased, and glutathione levels significantly increased, compared to those in untreated aged oocytes. Mitochondrial membrane potential, the percentage of early apoptotic oocytes, and caspase-3 activity were significantly reduced in LC-treated aged oocytes compared to those in untreated aged oocytes. Furthermore, during in vitro aging, the mRNA levels of the anti-apoptotic genes, Bcl-xl and survivin in LC-treated aged oocytes were significantly higher than those in untreated aged oocytes. Overall, these results indicate that at least in in vitro conditions, LC can prevent the aging of bovine oocytes and improve the developmental capacity of bovine embryo.
Keywords: Bovine, Embryo development, L-carnitine, Oocyte aging
Mammalian oocytes are arrested in the metaphase of the second meiosis (MII) phase, where they await fertilization. If no fertilization occurs within an appropriate time, the quality of oocytes gradually deteriorates, a process termed as “postovulatory aging” [1]. In humans and livestock, it is well known that postovulatory aging of oocytes may affect the results of assisted reproductive technologies (ARTs), such as artificial insemination [2], in vitro fertilization (IVF) [3, 4], and intracytoplasmic sperm injection [5, 6]. In bovine, both in vivo and in vitro aging of oocytes can result in reduced fertilization and embryonic development [2, 3, 7,8,9,10]. Extensive research on aged bovine oocytes may help in the development of a method to prevent aging in matured bovine oocytes, resulting in improved efficacy of ARTs.
It has been demonstrated that, following ovulation, intracellular reactive oxygen species (ROS) accumulation increases in oocytes with time [11, 12]. Oocytes exhibit an intracellular defense [(via the antioxidant glutathione (GSH)] mechanism against an oxidative attack. However, this defense response decreases with aging after ovulation [13]. Thus, aging oocytes after ovulation undergo oxidative stress due to an increase in ROS level, and a decrease in antioxidant defenses, causing multiple oxidative damages in cell structures, including lipid peroxidation of membranes, enzyme inactivation, protein oxidation, and DNA damage [14, 15]. The imbalance between ROS and their normal scavenger antioxidants leads to oxidative stress, which adversely affects embryonic development through structural and functional alterations. Increased production of ROS in aging oocytes reduces intracellular ATP concentration [16] and glutathione disulfide ratio [17,18,19]. This outcome adversely affects fertilization and subsequent embryonic development, thereby increasing the risk of an early miscarriage and abnormal development of offspring [20, 21].
L-carnitine (LC), the biologically active form of carnitine (3-hydroxy-4-N-trimethyl amino butyrate, C7H15NO3), is a naturally occurring, vitamin-like water-soluble quaternary ammonium compound. It is mainly synthesized from the amino acids lysine and methionine, in the liver. LC is required to transport fatty acids from the cytosol to the mitochondria during the breakdown of lipids (fats), to generate metabolic energy. As an antioxidant, LC neutralizes free radicals, especially superoxide anions, and protects cells from oxidative damage-induced apoptosis [22]. Although the effects of LC on the in vitro development of bovine embryos [23], pig embryos [24], and mouse embryos [25] have been previously reported, there are no reports regarding the effects of LC on aging bovine oocytes.
The best mature culture period for bovine embryo production is 20–22 h. Upon extension of this period, the blastocyst formation rate relatively decreases [3]. Previous studies have considered bovine oocytes at about 30 h after in-vitro maturation (IVM), as aged or slightly aged and used them to investigate age-related changes [8, 26, 27]. Oocytes after 30 h of IVM showed a low blastocyst development rate [8].
In the present study, aging bovine oocytes treated with LC were evaluated for ROS and GSH levels, mitochondrial membrane potential (ΔΨm), early apoptosis levels, and caspase-3 activity indicators in order to identify whether LC treatment improved the performance of oocytes. The aim of this study was to investigate the potential of LC in delaying aging via reducing oxidative stress in bovine oocytes.
Materials and Methods
All the chemicals and reagents used for this study were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated.
IVM and aging of bovine oocytes
Bovine ovaries were collected from a local abattoir and transported to the laboratory within 2 h at 38ºC in phosphate buffered saline (PBS). Bovine cumulus-oocyte complexes (COCs) were aspirated from small antral follicles (2–8 mm in diameter). Oocytes surrounded by intact cumulus layers were washed 5 times in IVM medium composed of TCM199 (Earle’s salts; 11150-59, Gibco, NY, USA), 0.57 mmol/l cysteine, 10% fetal bovine serum (FBS), 10 μg/ml follicle-stimulating hormone, 0.04 mg/ml pyruvate, 1 μg/ml estradiol, 10 ng/ml epidermal growth factor, and 1% penicillin-streptomycin solution. The COCs were randomly divided into the following three groups: fresh, aged, and aged + LC. The fresh group was cultured in IVM medium (200 μl) for 24 h, the aged group was cultured in IVM medium (200 μl) for 30 h, and the aged + LC group was cultured with 2.5 mM LC (L-carnitine hydrochloride; C0283) [28] during the entire 30 h duration of IVM (200 μl) in a humidified atmosphere of 5% CO2 at 38.5°C. The IVM dishes had 4 wells per plate (10034; SPL Lifesciences, Pocheon, Korea) and all the oocytes were covered with mineral oil. A frozen stock solution of LC was used.
IVF and embryo culture
After the fresh, aged, and aged + L-carnitine groups were cultured for 24, 30, and 30 h, respectively, the matured oocytes were washed and cultured in fertilization medium (100 μl) (IVF100; Research Institute for the Functional Peptides, Higashine, Japan), overlaid with mineral oil, and incubated in a humidified atmosphere of 5% CO2 at 38.5°C. Frozen bull semen straws were thawed by immersing them in a water bath at 37.5°C for 30 sec. The sperm was then centrifuged twice with Brackett Oliphant (BO) medium at 25°C at 1000 rpm for 6 min. The BO medium comprised of 6.63 mg/ml NaCl, 0.299 mg/ml KCl, 0.25 mg/ml CaCl2, 0.12 mg/ml NaH2PO4, 0.11 mg/ml MgCl2, 2.1 mg/ml NaHCO3, 2.5 mg/ml glucose, 2.98 mg/ml HEPES, 3.88 mg/ml caffeine, 0.01 mg/ml heparin, 0.13 mg/ml pyruvate, and 6.25 mg/ml bovine serum albumin (BSA; fatty acid-free BSA; A8806). The matured oocytes were co-incubated with spermatozoa in fertilization medium for 6 h in a humidified atmosphere of 5% CO2 at 38.5°C. After fertilization (day 0), the presumptive zygotes of the three groups were washed three times with 0.4% BSA in Charles Rosenkrans medium (CRI) (fatty acid-free BSA; A8806; BSA-CRI), maintained in BSA-CRI medium (10 μl), overlaid with mineral oil, and cultured to the 8-cell stage (72 h). CRI medium comprised 6.7 mg/ml NaCl, 0.23 mg/ml KCl, 2.2 mg/ml NaHCO3, 0.15 mg/ml L-glutamine, 0.05 mg/ml gentamycin, 0.01 ml Non-Essential Amino Acid (MEM), 0.02 ml Amino Acid (BME), 0.04 mg/ml pyruvate, and 0.55 mg/ml L (+)-Lactate. Subsequently, oocytes in the three groups were washed three times using 10% FBS in CRI (Gibco; 04-002-1B), placed in 10% FBS-CRI medium (10 μl), overlaid with mineral oil, and cultured to the blastocyst stage (96 h).
Terminal deoxynucleotidyl transferase-mediated 2′-deoxyuridine 5′-triphosphate (dUTP) nick-end labeling (TUNEL) assay
TUNEL assay was used to measure the intracellular apoptosis rates of blastocysts using the In Situ Cell Death Assay Kit (Cat #11684795910, Roche Diagnostics, Mannheim, Germany). The day-7 blastocysts were fixed in 3.7% paraformaldehyde for 30 min at 25°C and then permeabilized by incubating in 0.5% Triton X-100 at 37.5°C for 30 min. Following this, they were blocked in PBS containing 1% BSA (BSA-PBS) for 1 h. The embryos were then incubated with fluorescein-conjugated dUTP and terminal deoxynucleotidyl transferase enzyme for 1 h at 37.5°C, and subsequently washed three times with 0.1% BSA-PBS. Post end labeling, the embryos were treated with 10 µg/ml Hoechst 33342 for 20 min at 37.5°C, washed three times with 0.1% BSA-PBS, and mounted onto glass slides. Images were captured by fluorescence microscopy (Nikon, Tokyo, Japan) using the blue (for DNA) and green fluorescence filters (for apoptosis), and analyzed by ImageJ software [29]. The apoptosis index was denoted as the percentage of TUNEL-positive nuclei based on the total number of nuclei.
Measurement of ΔΨm, ROS, and GSH levels
To assess ΔΨm, denuded MII-stage oocytes were incubated with 2 μM JC-1 (Invitrogen, Waltham, MA, USA) for 1 h at 37.5°C in the dark. The ΔΨm of oocytes was then calculated as the ratio of red fluorescence intensity (J-aggregates; corresponding to activated mitochondria) to green fluorescence intensity (J-monomers; corresponding to inactive mitochondria) using ImageJ software. The fluorescence intensity of the resulting oocytes was analyzed using a fluorescence microscope (Nikon). ROS levels were measured by a 2′,7′-dichlorofluorescein assay (H2DCFDA; Thermo Fisher Scientific, Waltham, MA, USA). In brief, denuded MII-stage oocytes were cultured in 0.1% BSA-PBS containing 10 μM H2DCFDA for 15 min at 37.5°C in the dark, and then visualized at an excitation of 485 nm and emission of 535 nm. GSH levels were quantified with the CellTracker™ Blue dye (4-chloromethyl-6, 8-difluoro-7-hydroxycoumarin, CMF2HC; Invitrogen). In brief, denuded MII-stage oocytes were incubated in 0.1% BSA-PBS medium containing 10 μM CMF2HC for 15 min at 37.5°C in the dark, and then visualized at an excitation of 371 nm and emission of 464 nm. The fluorescence intensity (1 sec after the shutter opening with 10 msec exposure for H2DCFDA; 3 sec after the shutter opening with 100 msec exposure for CMF2HC) of the resulting oocytes was analyzed by fluorescence microscopy (Nikon) using ImageJ.
Immunofluorescence and Annexin V-FITC assay
Approximately 10 oocytes from each of the three (fresh, aged, and aged + LC) groups were washed in 0.1% BSA-PBS, fixed for 30 min in 3.7% formaldehyde in PBS with 1% Polyvinyl alcohol (PVA), and permeabilized with 0.5% Triton X-100 in 1% BSA-PBS for 30 min at room temperature. The oocytes were then blocked using 1% BSA-PBS. Next, the oocytes were incubated with rabbit anti-caspase-3 antibody (Sigma-Aldrich) at 4°C overnight, followed by incubation with an Alexa Fluor 488-conjugated secondary antibody (1:200; Sigma-Aldrich) for 1–2 h at 25°C. Hoechst 33342 (10 µg/ml in PBS) was used for DNA counterstaining.
An Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection Kit (Vazyme, Nanjing, China) was used to stain the oocytes with Annexin V- FITC to detect the externalization of phosphatidylserine in early apoptotic MII oocytes, according to the manufacturer’s instructions. Briefly, 20–30 MII oocytes were washed three times in 0.1% BSA-PBS and then incubated for 30 min in the dark at room temperature in 100 µl binding buffer containing 5 µl Annexin V-FITC. The oocytes were again washed three times in 0.1% BSA-PBS, following which Hoechst 33342 (10 µg/ml in PBS) was used for DNA counterstaining. Oocytes were then mounted on a glass slide. Annexin-V-positive oocytes were identified using a confocal microscope (Zeiss LSM 710 META; Carl-Zeiss Jena, Germany). Specifically, a green circle observed on the cellular membrane indicated the presence of an Annexin-V-positive oocyte.
Real-time reverse transcriptase-polymerase chain reaction (RT-PCR)
MII oocytes were harvested, and mRNA was extracted from each of the 15 oocyte pools using the DynaBeads mRNA Direct Kit (Cat #61012; Dynal Asa, Oslo, Norway) according to the manufacturer’s instructions. cDNA was synthesized by reverse transcription of mRNA using oligo(dT)12-18 primers and SuperScript III reverse transcriptase (Invitrogen). RT-PCR was performed using KAPA SYBR® FAST kit (KK4601; Kapa Biosystem, Salt River Cape Town, South Africa), wherein each reaction contained 10 μl SYBR Green, 1 μl of each forward and reverse primers, and 2 μl of cDNA template (10 ng/μl) in a final reaction volume of 20 µl. The amplification cycle was programmed as follows: 95°C for 3 min, followed by 40 cycles of 95°C for 3 sec, 60°C for 30 sec, and 72°C for 20 sec. The target genes were B-cell lymphoma-extra-large (Bcl-xl), Bcl-2-associated X (Bax), and survivin. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a reference gene. The primer sequences used to amplify each gene are listed in Table 1. mRNA quantification data were analyzed using the 2−ΔΔCT method.
Table 1. Sequences of primers used for RT-PCR.
| Genes | Primer sequences | Product size (bp) |
|---|---|---|
| Bax | F: AGAAGGATGATCGCAGCTGTG | 198 |
| R: AGTCCAATGTCCAGCCCATG | ||
| Survivin | F: GCCAGATGACGACCCCATAG | 199 |
| R: GGCACAGCGGACTTTCTTTG | ||
| Bcl-xl | F: AGGCAGGCGATGAGTTTGAA | 159 |
| R: AGAAAGAGGGCCAVAATGCGA | ||
| Gapdh | F: ACAGTCAAGGCAGAGAACGG | 235 |
| R: GGTTCACGCCCATCACAAAC |
The annealing temperature for all reactions was 60°C. F: forward primer, R: reverse primer.
Statistical analyses
Statistical analysis was performed by one-way analysis of variance (ANOVA), followed by least significant difference (LSD) test, using SPSS software, version 19.0 (SPSS, Chicago, IL, USA). Figures were generated using the GraphPad Prism software package (version 6.01; GraphPad, La Jolla, CA, USA). Data are expressed as the mean ± standard deviation (SD). P < 0.05 was considered to be statistically significant. The total number (N) of oocytes/embryos used in each group are shown in the data columns and replicates (R) in each experiment are mentioned in the figure legends.
Results
Effect of LC on the development and quality of aged bovine oocytes in vitro
The aim of our study was to determine whether LC supplementation could maintain the quality of aged oocytes, especially for subsequent embryo development after IVF (Fig. 1A). The blastocyst formation rate in the aged + LC group was comparable to that in the fresh group (29.23 ± 2.20% vs. 31.37 ± 2.93%; Fig. 1B), while the rate in the aged group was significantly lower than that in the aged + LC group (20.90 ± 3.05% vs. 29.23 ± 2.20%; Fig. 1B). Moreover, the number of cells per blastocyst in the aged + LC group was higher compared to that in the aged group (97.29 ± 17.70 vs. 72.10 ± 6.44; Fig. 1C), but similar to that in the fresh group (97.29 ± 17.70 vs. 103.3 ± 15.06; Fig. 1C). Furthermore, the apoptotic rate of blastocysts derived from the aged + LC group was lower than that of blastocysts derived from the aged group (1.08 ± 0.12 vs. 1.52 ± 0.14; Fig. 1D).
Fig. 1.
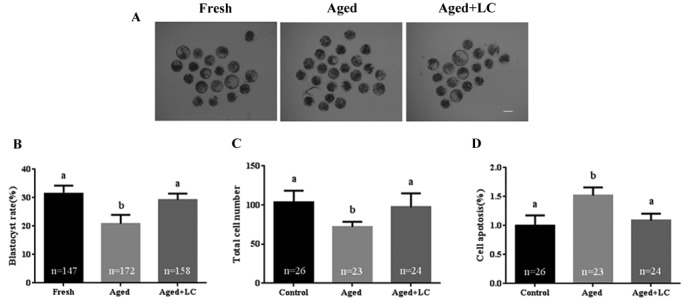
Effect of L-carnitine (LC) on the development and quality of aged bovine oocytes in vitro. (A) Blastocyst formation on day 7. Scale bar: 100 µm. (B) Blastocyst rate. R = 5. (C) Total cell number in each day-7 blastocyst. R = 3. (D) The rate of cell apoptosis in the day-7 blastocysts, R = 3. Statistically significant differences are represented with different letters (P < 0.05).
Effect of LC on ROS and GSH levels in aged bovine oocytes in vitro
Oxidative stress is a potential threat to developmental potential; therefore, we evaluated ROS levels, as shown in Fig. 2A. The ROS levels in aged oocytes were significantly higher than those in LC-treated aged oocytes (1.73 ± 0.31 vs. 1.07 ± 0.29; Fig. 2B), while the levels in LC-treated oocytes were similar to those in fresh oocytes (1.07 ± 0.29 vs. 1 ± 0.21; Fig. 2B). Though GSH levels vary among different cell types, it exerts a powerful antioxidant function in protecting cells against oxidative stress damage. Thus, quantification of intracellular GSH was performed, as shown in Fig. 2C. The GSH levels in aged oocytes were significantly lower than those in LC-treated oocytes (0.67 ± 0.19 vs. 0.81 ± 0.20; Fig. 2D), while the levels in LC-treated aged oocytes were significantly lower than those in fresh oocytes (0.81 ± 0.20 vs. 1 ± 0.13; Fig. 2D).
Fig. 2.
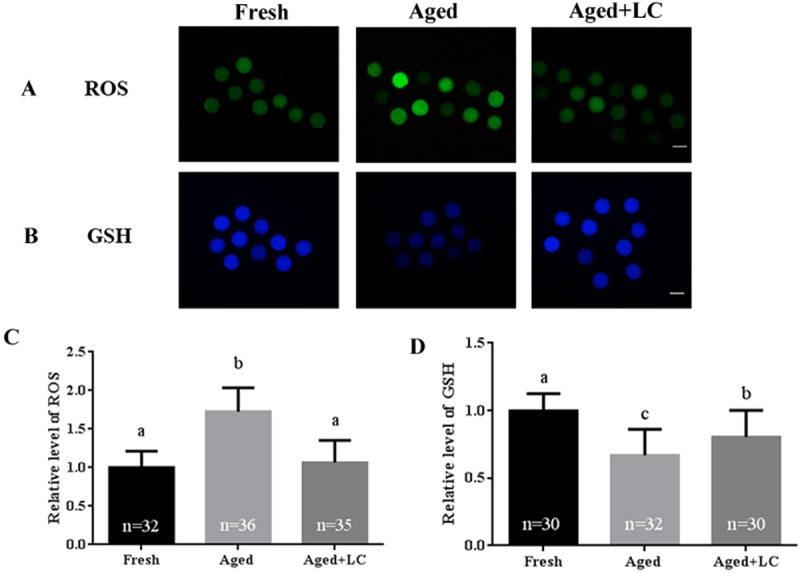
Effect of L-carnitine (LC) on ROS and GSH levels in aged bovine oocytes in vitro. (A) Oocytes were stained with H2DCFDA to detect the intracellular levels of ROS. Scale bar: 100 µm, R = 3. (B) Oocytes were stained with Tracker Blue CMF2HC dye to detect the intracellular levels of GSH. Scale bar: 100 µm, R = 3. (C) and (D) The relative intracellular levels of ROS and GSH in bovine oocytes from the three groups (fresh, aged, and aged + LC). Statistically significant differences are represented with different letters (P < 0.05).
Effect of LC on the ΔΨm of aged bovine oocytes in vitro
In cells, the mitochondria play a crucial role in maintaining normal metabolic functions [30]. Thus, we evaluated mitochondrial function (as indicated by ΔΨm) during in vitro aging of oocytes, with and without LC treatment. Representative images of JC-1 staining are shown in Fig. 3A. The ΔΨm of the aged group was higher than that of the fresh group (3.49 ± 1.30 vs. 1.33 ± 0.41; Fig. 3B), while the ΔΨm of the aged + LC group was lower than that of the aged group (3.49 ± 1.30 vs. 1.69 ± 0.61; Fig. 3B).
Fig. 3.
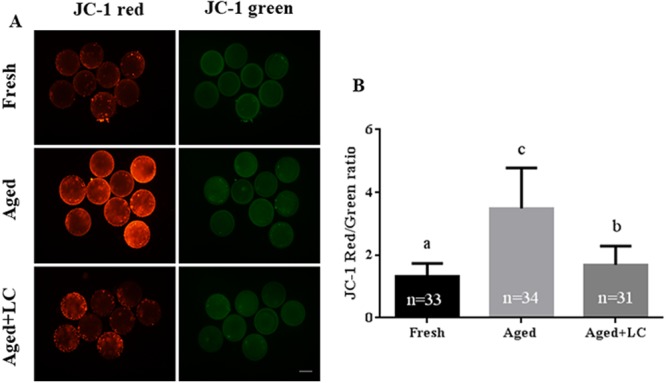
Effect of L-carnitine (LC) on the mitochondrial membrane potential (ΔΨm) of aged bovine oocytes in vitro. (A) Representative fluorescent images of JC-1-stained oocytes after in vitro aging. Scale bar: 200 µm, R = 3. (B) Quantification of JC-1 fluorescence intensity. Statistically significant differences are represented with different letters (P < 0.05).
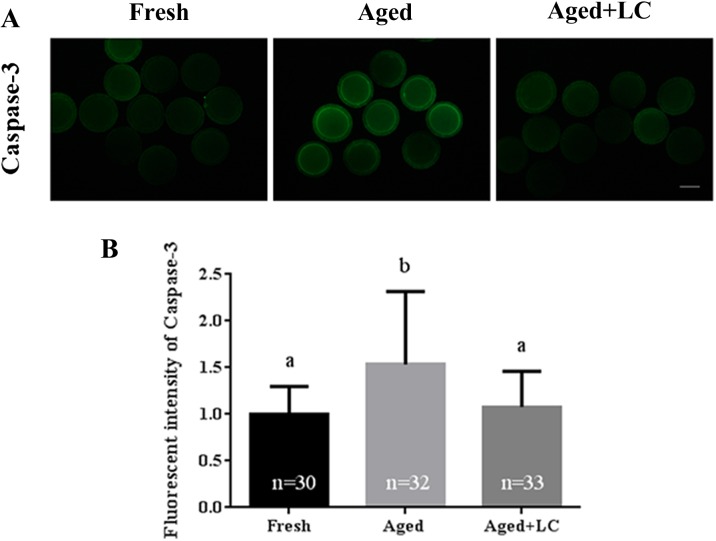
Effect of LC on caspase-3 activity in aged bovine oocytes in vitro
As caspase-3 is an important apoptosis marker, we measured the caspase-3 activity in aging oocytes (Fig. 4A). The caspase-3 activity of LC-treated aged oocytes was significantly lower than that of untreated aged oocytes (1.08 ± 0.38 vs. 1.54 ± 0.78; Fig. 4B). The level of caspase-3 in fresh oocytes was significantly lower than that in aged oocytes (1.00 ± 0.30 vs. 1.54 ± 0.78; Fig. 4B), but similar to that in LC-treated aged oocytes.
Fig. 4.
Effect of L-carnitine (LC) on the caspase-3 activity of aged bovine oocytes in vitro. (A) Representative images showing caspase-3 activity in fresh, aged, and LC-treated aged MII oocytes. Scale bar: 200 μm, R = 3. (B) Quantified fluorescence intensity for caspase-3 in oocytes. Statistically significant differences are represented with different letters (P < 0.05).
Effect of LC on the level of early apoptosis in aged bovine oocytes in vitro
Oocyte aging is accompanied by apoptosis. Therefore, we detected the proportion of aged oocytes undergoing early apoptosis by the Annexin V-FITC assay. In this assay, a green circle indicating the position of the oocyte on the outer cell membrane was defined as Annexin V-positive (Figs. 5A and 5B). The results showed that the percentage of oocytes undergoing early apoptosis in the fresh group was significantly lower than that in the aged group (12.43 ± 3.09% vs. 38.97 ± 10.38%; Fig. 5C), but was similar to that in the LC-treated aged group (12.43 ± 3.09% vs. 18.23 ± 5.09%; Fig. 5C).
Fig. 5.
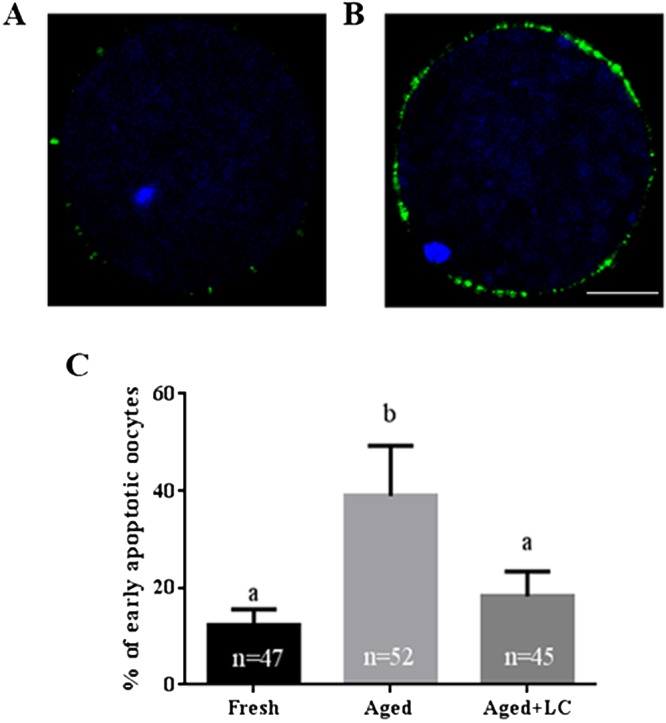
Effect of L-carnitine (LC) on the percentage of early apoptotic aged bovine oocytes in vitro. (A) Negative control. (B) Annexin V-positive. (C) The percentage of Annexin V-positive oocytes in the fresh, aged, and aged + LC groups. Scale bar: 100 μm, R = 3. Statistically significant differences are represented with different letters (P < 0.05).
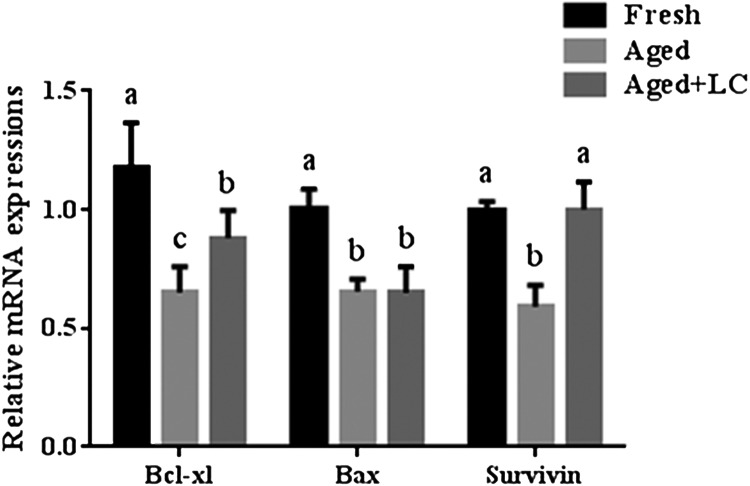
Effect of LC on the mRNA levels of apoptosis-related genes in aged bovine oocytes in vitro
To determine the effect of LC on the mRNA levels of apoptosis-related genes, we measured the transcript levels of Bcl-xl, Bax, and survivin in the oocytes from each group (Fig. 6). The mRNA levels of Bcl-xl and survivin were significantly lower in the aged oocytes than those in the fresh oocytes. However, the mRNA levels of Bcl-xl and survivin in LC-treated aged oocytes were higher than those in aged oocytes. No significant differences in survivin transcript levels were observed between the LC-treated aged oocytes and fresh oocytes. Moreover, the mRNA levels of Bcl-xl were significantly lower in the aged + LC group than those in the fresh group, while the mRNA levels of Bax were significantly lower in the aged + LC and aged groups than those in the fresh group. No significant difference in Bax transcript level was observed between the aged + LC and aged groups.
Fig. 6.
Effect of LC on apoptosis-related gene expression in aged bovine oocytes in vitro. The relative mRNA levels of apoptosis-related genes encoding Bcl-xl, Bax, and survivin, as analyzed by RT-PCR; R = 3. Statistically significant differences are represented with different letters (P < 0.05).
Discussion
Oocyte aging is a complex and irreversible biological process that may lead to several changes in the structure and functional states of mammalian oocytes, including DNA damage, reduced fertilization rates, abnormal mitochondrial structure, oxidative damage, and early oocyte apoptosis [31, 32, 33]. Here, we demonstrated that LC treatment may effectively delay the aging of oocytes and enhance subsequent embryo development.
Oocyte quality is a major determinant of subsequent embryo development. Oocyte aging has been shown to severely reduce the quality of oocytes, significantly affecting embryo development before and after implantation [1, 34]. To demonstrate that LC can improve oocyte quality and delay oocyte aging, we investigated the in vitro developmental capacity of oocytes after IVF. We found that prolonged IVM significantly impaired blastocyst formation. A higher proportion of bovine embryos developed into blastocysts in LC-treated aged oocytes compared to that in untreated aged oocytes after IVF, following IVM treatment for 30 h. Previous studies have shown that LC supplementation can increase blastocyst formation rates [28, 35]. Furthermore, we determined the number of cells in day-7 blastocysts, and found that the number of cells per blastocyst in the aged + LC group was higher than that in the aged group. The results of TUNEL assay showed that the aged + LC group showed a smaller percentage of apoptotic cells in the blastocyst at 30 h than the aged group. Previous studies have shown that increased DNA fragmentation in developing aged oocytes has negative effects on subsequent embryonic development, resulting in developmental arrest and apoptosis [1, 12, 36]. Our results indicate that LC can maintain the capacity of aged oocytes to develop into the blastocyst stage and downregulate apoptosis in the resultant blastocysts.
Oocyte quality is usually affected by oxidative stress or oxidative damage [37, 38]. As the oocyte ages, ROS accumulate [39]. GSH levels are a key factor affecting the quality of oocytes. GSH is known to be present in varying amounts in a diverse range of cells and exerts strong antioxidant effects to protect cells against oxidative stress-induced damage [40]. It has been reported that ROS accumulate in oocytes during aging, and affect their subsequent fertilization capacity [41]. Previous studies have shown the beneficial effects of LC as an antioxidant that reduces ROS levels during oocyte maturation and increases ATP content and GSH levels [24, 42]. In our study, results showed that LC supplementation decreased ROS levels and increased GSH levels in aging oocytes, indicating that LC may improve the quality of aging oocytes via reducing oxidative stress.
Mitochondria play a crucial role in maintaining cellular metabolic functions [43]. As oocytes age after ovulation, the impaired mitochondrial function may seriously affect the quality of oocytes. Therefore, we carried out a mitochondrial membrane potential assay to evaluate the mitochondrial membrane potential of aged oocytes. A previous study reported a lower ΔΨm in bovine oocytes after an extended IVM time of up to 30 h [36]. Another study showed that the ΔΨm and ATP content of oocytes subjected to 40 h of IVM were higher than those in oocytes subjected to 20 h of IVM [44]. After 30 h of IVM, the oocytes presented an intermediate value [44]. In our study, the ΔΨm of the aged group rapidly increased, while that of the aged+LC group markedly decreased. However, we were unable to elucidate a direct relationship between the enhanced ΔΨm and low developmental competence of bovine oocytes. Thus, further research is required to investigate the ΔΨm of embryos (2-cell stage, 4-cell stage, 8-cell stage, and morula stage) derived from oocytes from extended IVM culture.
Previous studies have shown that in vitro aged oocytes are affected by oxidative stress, which can result in apoptosis [31, 45]. Further, previous reports also indicate that LC has antioxidant properties, whereby it reduces oxidative stress by enhancing the activity of several antioxidant enzymes like superoxide dismutase and glutathione peroxidase [42, 46]. Meanwhile, studies have shown that LC upregulates glutathione peroxidase (GPx) and downregulates superoxide dismutase 2 (SOD2) at mRNA level in oocytes and embryos [35]. We presumed that LC may prevent apoptosis due to its resistance to oxidation. Phosphatidylserine on the outer cell membrane is a marker of early apoptosis in mature oocytes. Annexin V has a high binding affinity to phosphatidylserine, therefore we used the Annexin V-FITC Apoptosis Detection Kit to detect early apoptosis in aged oocytes [47]. Our results showed that LC treatment decreased the percentage of early apoptotic cells, which is in accordance with a previous finding that antioxidants reduce the level of early apoptosis in aging oocytes [48]. Caspase-3 is a member of the cysteine-aspartic protease (caspase) family [49]. Caspases are crucial mediators of programmed cell death (apoptosis). Among them, caspase-3 is a frequently activated death protease that catalyzes the specific cleavage of many key cellular proteins [50]. The sequential activation of caspases plays an important role in apoptosis [51]. Previous research has shown that caspase-3 activity is associated with oocyte quality [52]. Bcl-xl, which belongs to the Bcl-2 family, prevents apoptosis by blocking the leakage of cytochrome c through the mitochondrial membrane pores [53]. Survivin is the smallest member of the inhibitor of apoptosis protein family, which regulates cell cycle/apoptosis balance [54]. A previous study showed that oocyte aging eventually leads to cell death via an apoptotic pathway characterized by phosphatidylserine externalization [47], caspase activation [55], accumulation of the apoptotic signaling protein Bax, suppression of Bcl-xl [56, 57], and DNA fragmentation [58]. In our study, the mRNA levels of Bcl-xl and survivin were significantly lower in the aged group than those in the fresh and aged + LC groups. No significant differences in survivin transcript levels were observed between the aged + LC and fresh groups. Moreover, the mRNA levels of Bcl-xl were significantly lower in the aged + LC group than those in fresh group. These results suggest that LC may affect the mRNA levels of apoptosis-related genes.
Finally, our results, as well as previous reports [28, 42] suggest that LC is not only likely to be involved in mitochondrial function and lipid metabolism, but is also involved in regulation of other vital cellular functions, such as apoptosis, which may enhance the developmental capacity of aged oocytes.
In conclusion, our results demonstrate that treating aging oocytes with LC may improve oocyte quality and maintain their developmental capacity. We thus propose LC as a suitable agent to delay oocyte aging in vitro and to prevent the developmental loss of bovine oocytes in ARTs.
Supplementary
Acknowledgments
This study was supported by the project of the Jilin Province Education Department (JJKH20191132KJ) and Yanbian University Research project (No. 2018-09). Additionaly, we would like to thank Professor Xue-Jun Jin for providing assistance with confocal microscopy.
References
- 1.Miao Y-L, Kikuchi K, Sun Q-Y, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update 2009; 15: 573–585. [DOI] [PubMed] [Google Scholar]
- 2.Saacke RG, Dalton JC, Nadir S, Nebel RL, Bame JH. Relationship of seminal traits and insemination time to fertilization rate and embryo quality. Anim Reprod Sci 2000; 60–61: 663–677. [DOI] [PubMed] [Google Scholar]
- 3.Agung B, Otoi T, Wongsrikeao P, Taniguchi M, Shimizu R, Watari H, Nagai T. Effect of maturation culture period of oocytes on the sex ratio of in vitro fertilized bovine embryos. J Reprod Dev 2006; 52: 123–127. [DOI] [PubMed] [Google Scholar]
- 4.Harrison KL, Wilson LM, Breen TM, Pope AK, Cummins JM, Hennessey JF. Fertilization of human oocytes in relation to varying delay before insemination. Fertil Steril 1988; 50: 294–297. [DOI] [PubMed] [Google Scholar]
- 5.Emuta C, Horiuchi T. Effects of timing of activation and aging of bovine oocytes fertilized by intracytoplasmic sperm injection (ICSI) on cleavage and subsequent embryonic development in vitro. J Reprod Dev 2001; 47: 399–405. [Google Scholar]
- 6.Yanagida K, Yazawa H, Katayose H, Suzuki K, Hoshi K, Sato A. Influence of oocyte preincubation time on fertilization after intracytoplasmic sperm injection. Hum Reprod 1998; 13: 2223–2226. [DOI] [PubMed] [Google Scholar]
- 7.Roelofs JB, Graat EA, Mullaart E, Soede NM, Voskamp-Harkema W, Kemp B. Effects of insemination-ovulation interval on fertilization rates and embryo characteristics in dairy cattle. Theriogenology 2006; 66: 2173–2181. [DOI] [PubMed] [Google Scholar]
- 8.Rispoli LA, Lawrence JL, Payton RR, Saxton AM, Schrock GE, Schrick FN, Middlebrooks BW, Dunlap JR, Parrish JJ, Edwards JL. Disparate consequences of heat stress exposure during meiotic maturation: embryo development after chemical activation vs fertilization of bovine oocytes. Reproduction 2011; 142: 831–843. [DOI] [PubMed] [Google Scholar]
- 9.Long CR, Damiani P, Pinto-Correia C, MacLean RA, Duby RT, Robl JM. Morphology and subsequent development in culture of bovine oocytes matured in vitro under various conditions of fertilization. J Reprod Fertil 1994; 102: 361–369. [DOI] [PubMed] [Google Scholar]
- 10.Ward F, Enright B, Rizos D, Boland M, Lonergan P. Optimization of in vitro bovine embryo production: effect of duration of maturation, length of gamete co-incubation, sperm concentration and sire. Theriogenology 2002; 57: 2105–2117. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi T, Takahashi E, Igarashi H, Tezuka N, Kurachi H. Impact of oxidative stress in aged mouse oocytes on calcium oscillations at fertilization. Mol Reprod Dev 2003; 66: 143–152. [DOI] [PubMed] [Google Scholar]
- 12.Lord T, Nixon B, Jones KT, Aitken RJ. Melatonin prevents postovulatory oocyte aging in the mouse and extends the window for optimal fertilization in vitro. Biol Reprod 2013; 88: 67. [DOI] [PubMed] [Google Scholar]
- 13.Lord T, Aitken RJ. Oxidative stress and ageing of the post-ovulatory oocyte. Reproduction 2013; 146: R217–R227. [DOI] [PubMed] [Google Scholar]
- 14.Lim J, Luderer U. Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biol Reprod 2011; 84: 775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeo S, Kimura K, Shirasuna K, Kuwayama T, Iwata H. Age-associated deterioration in follicular fluid induces a decline in bovine oocyte quality. Reprod Fertil Dev 2017; 29: 759–767. [DOI] [PubMed] [Google Scholar]
- 16.Kujjo LL, Perez GI. Ceramide and mitochondrial function in aging oocytes: joggling a new hypothesis and old players. Reproduction 2012; 143: 1–10. [DOI] [PubMed] [Google Scholar]
- 17.Ruder EH, Hartman TJ, Blumberg J, Goldman MB. Oxidative stress and antioxidants: exposure and impact on female fertility. Hum Reprod Update 2008; 14: 345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asano Y. Age-related accumulation of non-heme ferric and ferrous iron in mouse ovarian stroma visualized by sensitive non-heme iron histochemistry. J Histochem Cytochem 2012; 60: 229–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang D-W, Fang Y, Liu Z-X, Wu Y, Wang X-L, Zhao S, Han G-C, Zeng S-M. The disturbances of endoplasmic reticulum calcium homeostasis caused by increased intracellular reactive oxygen species contributes to fragmentation in aged porcine oocytes. Biol Reprod 2013; 89: 124. [DOI] [PubMed] [Google Scholar]
- 20.Lacham-Kaplan O, Trounson A. Reduced developmental competence of immature, in-vitro matured and postovulatory aged mouse oocytes following IVF and ICSI. Reprod Biol Endocrinol 2008; 6: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi T, Igarashi H, Kawagoe J, Amita M, Hara S, Kurachi H. Poor embryo development in mouse oocytes aged in vitro is associated with impaired calcium homeostasis. Biol Reprod 2009; 80: 493–502. [DOI] [PubMed] [Google Scholar]
- 22.Ye J, Li J, Yu Y, Wei Q, Deng W, Yu L. L-carnitine attenuates oxidant injury in HK-2 cells via ROS-mitochondria pathway. Regul Pept 2010; 161: 58–66. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi T, Inaba Y, Somfai T, Kaneda M, Geshi M, Nagai T, Manabe N. Supplementation of culture medium with L-carnitine improves development and cryotolerance of bovine embryos produced in vitro. Reprod Fertil Dev 2013; 25: 589–599. [DOI] [PubMed] [Google Scholar]
- 24.You J, Lee J, Hyun S-H, Lee E. L-carnitine treatment during oocyte maturation improves in vitro development of cloned pig embryos by influencing intracellular glutathione synthesis and embryonic gene expression. Theriogenology 2012; 78: 235–243. [DOI] [PubMed] [Google Scholar]
- 25.Abdelrazik H, Sharma R, Mahfouz R, Agarwal A. L-carnitine decreases DNA damage and improves the in vitro blastocyst development rate in mouse embryos. Fertil Steril 2009; 91: 589–596. [DOI] [PubMed] [Google Scholar]
- 26.Sugimura S, Matoba S, Hashiyada Y, Aikawa Y, Ohtake M, Matsuda H, Kobayashi S, Konishi K, Imai K. Oxidative phosphorylation-linked respiration in individual bovine oocytes. J Reprod Dev 2012; 58: 636–641. [DOI] [PubMed] [Google Scholar]
- 27.Somfai T, Kikuchi K, Kaneda M, Akagi S, Watanabe S, Mizutani E, Haraguchi S, Dang-Nguyen TQ, Inaba Y, Geshi M, Nagai T. Cytoskeletal abnormalities in relation with meiotic competence and ageing in porcine and bovine oocytes during in vitro maturation. Anat Histol Embryol 2011; 40: 335–344. [DOI] [PubMed] [Google Scholar]
- 28.Knitlova D, Hulinska P, Jeseta M, Hanzalova K, Kempisty B, Machatkova M. Supplementation of l-carnitine during in vitro maturation improves embryo development from less competent bovine oocytes. Theriogenology 2017; 102: 16–22. [DOI] [PubMed] [Google Scholar]
- 29.Dorr R, Ozu M, Parisi M. Simple and inexpensive hardware and software method to measure volume changes in Xenopus oocytes expressing aquaporins. J Neurosci Methods 2007; 161: 301–305. [DOI] [PubMed] [Google Scholar]
- 30.Steenbergen R, Nanowski TS, Beigneux A, Kulinski A, Young SG, Vance JE. Disruption of the phosphatidylserine decarboxylase gene in mice causes embryonic lethality and mitochondrial defects. J Biol Chem 2005; 280: 40032–40040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amaral S, Oliveira PJ, Ramalho-Santos J. Diabetes and the impairment of reproductive function: possible role of mitochondria and reactive oxygen species. Curr Diabetes Rev 2008; 4: 46–54. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki H. Cytoskeleton and Regulation of Mitochondrial Translocation in Mammalian Eggs. The Cytoskeleton in Health and Disease Springer; 2015: 169–186. [Google Scholar]
- 33.Au HK, Yeh TS, Kao SH, Tzeng CR, Hsieh RH. Abnormal mitochondrial structure in human unfertilized oocytes and arrested embryos. Ann N Y Acad Sci 2005; 1042: 177–185. [DOI] [PubMed] [Google Scholar]
- 34.Lu F, Jiang J, Li N, Zhang S, Sun H, Luo C, Wei Y, Shi D. Effects of recipient oocyte age and interval from fusion to activation on development of buffalo (Bubalus bubalis) nuclear transfer embryos derived from fetal fibroblasts. Theriogenology 2011; 76: 967–974. [DOI] [PubMed] [Google Scholar]
- 35.Mishra A, Reddy IJ, Gupta PS, Mondal S. L-carnitine mediated reduction in oxidative stress and alteration in transcript level of antioxidant enzymes in sheep embryos produced in vitro. Reprod Domest Anim 2016; 51: 311–321. [DOI] [PubMed] [Google Scholar]
- 36.Liang S, Guo J, Choi J-W, Kim N-H, Cui X-S. Effect and possible mechanisms of melatonin treatment on the quality and developmental potential of aged bovine oocytes. Reprod Fertil Dev 2017; 29: 1821–1831. [DOI] [PubMed] [Google Scholar]
- 37.Wu Y, Zhang N, Li Y-H, Zhao L, Yang M, Jin Y, Xu Y-N, Guo H. Citrinin exposure affects oocyte maturation and embryo development by inducing oxidative stress-mediated apoptosis. Oncotarget 2017; 8: 34525–34533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeelani R, Khan SN, Shaeib F, Kohan-Ghadr H-R, Aldhaheri SR, Najafi T, Thakur M, Morris R, Abu-Soud HM. Cyclophosphamide and acrolein induced oxidative stress leading to deterioration of metaphase II mouse oocyte quality. Free Radic Biol Med 2017; 110: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goud AP, Goud PT, Diamond MP, Gonik B, Abu-Soud HM. Reactive oxygen species and oocyte aging: role of superoxide, hydrogen peroxide, and hypochlorous acid. Free Radic Biol Med 2008; 44: 1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuelke KA, Jeffay SC, Zucker RM, Perreault SD. Glutathione (GSH) concentrations vary with the cell cycle in maturing hamster oocytes, zygotes, and pre-implantation stage embryos. Mol Reprod Dev 2003; 64: 106–112. [DOI] [PubMed] [Google Scholar]
- 41.Jeon H-J, You SY, Kim DH, Jeon HB, Oh JS. Protective effects of ethanol extracts of Artemisia asiatica Nakai ex Pamp. on ageing-induced deterioration in mouse oocyte quality. Zygote 2017; 25: 472–479. [DOI] [PubMed] [Google Scholar]
- 42.Fathi M, El-Shahat KH. L-carnitine enhances oocyte maturation and improves in vitro development of embryos in dromedary camels (Camelus dromedaries). Theriogenology 2017; 104: 18–22. [DOI] [PubMed] [Google Scholar]
- 43.Dai X, Lu Y, Zhang M, Miao Y, Zhou C, Cui Z, Xiong B. Melatonin improves the fertilization ability of post-ovulatory aged mouse oocytes by stabilizing ovastacin and Juno to promote sperm binding and fusion. Hum Reprod 2017; 32: 598–606. [DOI] [PubMed] [Google Scholar]
- 44.Koyama K, Kang S-S, Huang W, Yanagawa Y, Takahashi Y, Nagano M. Aging-related changes in in vitro-matured bovine oocytes: oxidative stress, mitochondrial activity and ATP content after nuclear maturation. J Reprod Dev 2014; 60: 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miao Y, Zhou C, Cui Z, Zhang M, ShiYang X, Lu Y, Xiong B. Postovulatory aging causes the deterioration of porcine oocytes via induction of oxidative stress. FASEB J 2018; 32: 1328–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rizzo AM, Berselli P, Zava S, Montorfano G, Negroni M, Corsetto P, Berra B. Endogenous antioxidants and radical scavengers. Bio-Farms for Nutraceuticals. Springer; 2010: 52–67. [DOI] [PubMed] [Google Scholar]
- 47.Lobascio A-M, Klinger F-G, De Felici M. Isolation of apoptotic mouse fetal oocytes by AnnexinV assay. Int J Dev Biol 2007; 51: 157–160. [DOI] [PubMed] [Google Scholar]
- 48.Wang T, Gao Y-Y, Chen L, Nie Z-W, Cheng W, Liu X, Schatten H, Zhang X, Miao Y-L. Melatonin prevents postovulatory oocyte aging and promotes subsequent embryonic development in the pig. Aging (Albany NY) 2017; 9: 1552–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao X, Jiang H, Liang S, Shen X, Gao Q, Xu YN, Kim N-H. Laminarin enhances the quality of aged pig oocytes by reducing oxidative stress. J Reprod Dev 2018; 64: 489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ 1999; 6: 99–104. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi H, Nakamura S, Asano K, Kinouchi M, Ishida-Yamamoto A, Iizuka H. Fas antigen modulates ultraviolet B-induced apoptosis of SVHK cells: sequential activation of caspases 8, 3, and 1 in the apoptotic process. Exp Cell Res 1999; 249: 291–298. [DOI] [PubMed] [Google Scholar]
- 52.Nicholas B, Alberio R, Fouladi-Nashta AA, Webb R. Relationship between low-molecular-weight insulin-like growth factor-binding proteins, caspase-3 activity, and oocyte quality. Biol Reprod 2005; 72: 796–804. [DOI] [PubMed] [Google Scholar]
- 53.Yang S-E, Hsieh M-T, Tsai T-H, Hsu S-L. Down-modulation of Bcl-XL, release of cytochrome c and sequential activation of caspases during honokiol-induced apoptosis in human squamous lung cancer CH27 cells. Biochem Pharmacol 2002; 63: 1641–1651. [DOI] [PubMed] [Google Scholar]
- 54.Jarrin M, Mansergh FC, Boulton ME, Gunhaga L, Wride MA. Survivin expression is associated with lens epithelial cell proliferation and fiber cell differentiation. Mol Vis 2012; 18: 2758–2769. [PMC free article] [PubMed] [Google Scholar]
- 55.Takai Y, Matikainen T, Jurisicova A, Kim MR, Trbovich AM, Fujita E, Nakagawa T, Lemmers B, Flavell RA, Hakem R, Momoi T, Yuan J, Tilly JL, Perez GI. Caspase-12 compensates for lack of caspase-2 and caspase-3 in female germ cells. Apoptosis 2007; 12: 791–800. [DOI] [PubMed] [Google Scholar]
- 56.Perez GI, Jurisicova A, Matikainen T, Moriyama T, Kim M-R, Takai Y, Pru JK, Kolesnick RN, Tilly JL. A central role for ceramide in the age-related acceleration of apoptosis in the female germline. FASEB J 2005; 19: 860–862. [DOI] [PubMed] [Google Scholar]
- 57.Perez GI, Knudson CM, Leykin L, Korsmeyer SJ, Tilly JL. Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nat Med 1997; 3: 1228–1232. [DOI] [PubMed] [Google Scholar]
- 58.Fujino Y, Ozaki K, Yamamasu S, Ito F, Matsuoka I, Hayashi E, Nakamura H, Ogita S, Sato E, Inoue M. DNA fragmentation of oocytes in aged mice. Hum Reprod 1996; 11: 1480–1483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.