Abstract
In this study, we examined the effects of 5-hydroxytryptamine (5-HT) on the motility and hyperactivation of mouse spermatozoa. In addition, we examined whether 5-HT increases the success of in vitro fertilization (IVF) in mice. Interestingly, 5-HT and agonists of the 5-HT2, 5-HT3, 5-HT4, and 5-HT7 receptors significantly increased the percentage of hyperactivated spermatozoa but did not affect the percentage of motile spermatozoa. Moreover, agonists of the 5-HT2, 5-HT3, and 5-HT4 receptors significantly affected the velocities, linearity, straightness, wobbler coefficient, amplitude and/or frequency of spermatozoa. In particular, the improvement of hyperactivation by 5-HT was strongly inhibited by antagonists of the receptors 5-HT4 and 5-HT7 and was completely inhibited by a mixture of the four 5-HT-receptor antagonists. The increase in hyperactivation by the agonists was significantly inhibited by the corresponding 5-HT-receptor antagonist. Moreover, 5-HT significantly increased the percentage of two-cell embryos. The increase in the IVF success rate by 5-HT was significantly inhibited by a 5-HT4-receptor antagonist. These results suggest that 5-HT increased hyperactivation through the 5-HT receptors and increased the success of IVF in mice.
Keywords: 5-Hydroxytryptamine (5-HT), Hyperactivation, In vitro fertilization (IVF), Spermatozoa
Spermatozoa are activated after ejaculation, and activated mammalian spermatozoa are capacitated before fertilization [1, 2]. Activated spermatozoa exhibit small-bend amplitude in flagellar movement and linear swimming patterns [3, 4]. However, capacitation is inhibited by the binding of a decapacitation factor [2] and cannot occur until the decapacitation factor is lost. Capacitated spermatozoa then show hyperactivation of flagellar movement, and acrosome reactions (AR) occur in the head [1, 2]. Hyperactivated spermatozoa have a large amplitude and large asymmetric flagellar beating pattern [3,4,5]; therefore, hyperactivation provides increased motility and propulsive force to facilitate passage of the spermatozoa through the zona pellucida and the cumulus cell layers [3]. Whereas, AR is a form of exocytosis that exposes an enzyme system that promotes binding to the oocyte [1]. Finally, acrosome-reacted spermatozoa are able to fertilize the oocyte.
In order for spermatozoa to be capacitated and hyperactivated in vitro, three essential components, albumin, Ca2+, and, HCO3–, are required in the capacitation medium. Albumin removes cholesterol from the spermatozoal plasma membrane [6], and Ca2+ and HCO3– stimulate soluble adenylate cyclase (sAC) and induce cAMP production [2, 7, 8]. Moreover, Ca2+ and cAMP activate several protein kinases and phosphatases, thereby inducing protein phosphorylation and de-phosphorylation [2, 5, 9, 10]. It has been reported that the spermatozoal capacity for hyperactivation is correlated with the success rate of in vitro fertilization (IVF) [11].
Several oviductal hormones have been observed to affect hyperactivation processes [4], including the induction of hyperactivation by progesterone (P4), melatonin, and 5-hydroxytryptamine (5-HT) [12,13,14]. In contrast, 17β-estradiol suppresses the effects of P4 and melatonin [15,16,17]. Moreover, γ-aminobutyric acid (GABA) induces hyperactivation in humans [18], although it suppresses the effects of P4 and 5-HT in hamsters [19, 20].
The hormone 5-HT is known to regulate numerous functions by activating specific receptors [21]. The receptors for 5-HT consist of seven subtypes (5-HT1, 5-HT2, 5-HT3, 5-HT4, 5-HT5, 5-HT6, and 5-HT7). The receptors 5-HT1 and 5-HT5 suppress transmembrane adenylate cyclase (tmAC); while 5-HT4, 5-HT6, and 5-HT7 stimulate tmAC. The 5-HT2 receptors stimulate phospholipase C (PLC), which regulates the release of Ca2+ from Ca2+-stores. Whereas, the 5-HT3 receptor is a ligand-gated cation channel receptor.
The spermatozoal functions of several mammal species are affected by 5-HT. In hamster spermatozoa [13, 22], 5-HT induces AR and hyperactivation through 5-HT2 and 5-HT4 receptors. In addition, 5-HT dose-dependently induces hyperactivation [13]. Low concentrations (fM to pM) of 5-HT increased hyperactivation through the 5-HT2 receptor, whereas high concentrations (nM) improved hyperactivation through the 5-HT4 receptor. The hormone receptors, 5-HT1, 5-HT2, and 5-HT3, have been found in human and stallion spermatozoa [23, 24]. Furthermore, it has been suggested that 5-HT increases straight-line velocity (VSL), curvilinear velocity (VCL), and average-path velocity (VAP) of human spermatozoa [23]. It is assumed that 5-HT also affects fertilization, as 5-HT and the 5-HT1, 5HT2, and 5HT7 receptors are found in oocytes, cumulus-oocyte complexes (COCs), follicular fluid, oviducts, and embryos of humans and mice [21]. In this study, we examined whether 5-HT affects the motility and hyperactivation of mouse spermatozoa and investigated the effects of 5-HT on mouse IVF.
Materials and Methods
Chemicals
We purchased 5-HT, sumatriptan succinate (Sumatriptan), α-methylserotonin maleate salt (MS), 1-(3-chlorophenyl)biguanide hydrochloride (mCPBG), 5-methoxytryptamine (MT), WAY-208466 dihydrochloride (WAY), LP12 hydrochloride hydrate (LP12), cyproheptadine hydrochloride sesquihydrate (Cypro), dolasetron mesylate hydrate (DS), GR113808 (GR), and SB-258719 (SB25) from Sigma-Aldrich (St. Louis, MO, USA). Pregnant mare serum gonadotropin (PMSG) (Serotropin®) and human chorionic gonadotropin (hCG) (Gonatropin®) were purchased from ASKA Pharmaceutical (Tokyo, Japan). Other reagent-grade chemicals were purchased from FUJIFILM Wako Pure Chemical Corp. (Osaka, Japan).
Animals
ICR mice were bred in the Laboratory Animal Research Center of Dokkyo Medical University. The present study was approved by the Animal Care and Use Committee of the university (experimental permission number: 0107) and performed in accordance with the University’s Guidelines for Animal Experimentation.
Preparation of hyperactivated spermatozoa
Hyperactivated spermatozoa were prepared according to the method described previously [9] with some modifications. Modified Tyrode’s albumin lactate pyruvate (mTALP) medium [25] was used as a capacitation medium. Spermatozoa were collected from the cauda epididymis of male mice (10–20 weeks of age). One drop (approximately 3 μl) of spermatozoa was placed on a culture dish (35 mm diameter), and 3 ml mTALP medium was carefully added to the dish. The spermatozoa were incubated for 5 min at 37°C to allow diffusion into the medium, and the supernatant containing motile spermatozoa were collected and placed in a new dish containing a vehicle or antagonists. After incubation for 5 min, the supernatant was transferred to a new dish containing the vehicle, 5-HT, or agonists. The spermatozoa were incubated for 2 h at 37°C in an atmosphere containing 5% CO2 to induce hyperactivation. Stock solutions of 5-HT (100 μM), sumatriptan (100 μM), MS (100 pM), mCPBG (100 mM), WAY (7.3 μM), LP12 (0.13 μM), and DS (3.8 μM) were dissolved in de-ionized and distilled water. Stock solutions of MT (10 nM) and Cypro (1 mM) were dissolved in ethanol. Stock solutions of GR (1 mM) and SB25 (10 mM) were dissolved in dimethyl sulfoxide (DMSO). In all experiments, the maximum concentration of the vehicle was 0.5%.
Measurement of motile and hyperactivated spermatozoa
The analyses of motile and hyperactivated spermatozoa were performed as follows: motile spermatozoa were recorded on a Blu-ray disc recorder (DIGA DMR-BRW520; Panasonic, Osaka, Japan) using a CCD camera (Model XC-77; Kyoshin, Tokyo, Japan) attached to a microscope (CX41, Olympus, Tokyo, Japan) with phase-contrast illumination and a warm plate (MP-1000; Kitazato, Shizuoka, Japan). The suspension containing motile spermatozoa (50 μl) was transferred to an observation chamber (0.125 mm deep, 16 mm wide, and 18 mm long) made of vinyl tape attached to the glass slide in two parallel strips, and then covered with a cover glass. Observations were performed for 1 min at 37°C. Visual analyses of the movies comprised manual counts of the numbers of total spermatozoa, motile spermatozoa, and hyperactivated spermatozoa. The analyses were performed in a blinded manner for all experiments. Motile spermatozoa that exhibited asymmetric flagellar movement and moved in a circular and/or figure of eight pattern were defined as hyperactivated [1, 4] (see Supplemental Movie
). The percentages of motile and hyperactivated spermatozoa were defined as the number of motile spermatozoa/number of total spermatozoa × 100, and the number of hyperactivated spermatozoa/number of total spermatozoa × 100, respectively. Each experiment was performed four times on four different mice. When the proportion of motile spermatozoa was equal to or below 70% of levels seen before incubation, the experiment was repeated. The data were statistically analyzed by Student’s t-test using Microsoft Excel (Microsoft Japan, Tokyo, Japan) or by one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test using Microsoft Excel with the Statcel2 (OMS Publishing, Saitama, Japan) add-on. Statistical significance was considered for P values of < 0.05.
Motility assay by the Sperm Motility Analysis System (SMAS)
The motility assay was evaluated by using SMAS for animals (Ver. 3.18) with the loaded parameter file mouse_BM10×_640nm_Bright59_150fps-shutter200.ini (Ditect, Tokyo, Japan) [26]. The suspension containing motile spermatozoa (20 μl) was transferred to an observation chamber (0.1 mm deep, 18 mm wide, and 18 mm long) made of mending tape attached to the glass slide in two parallel strips, and then covered with a cover slips. Spermatozoal movement was recorded for 1 sec on the hard disk drive of the SMAS via a high-speed digital camera (HAS-L2; Ditect) attached to a microscope (ECLIPSE E2000; Nikon, Tokyo, Japan) with phase-contrast illumination, a 650 nm band-pass filter, and a warm plate (MP10DM; Kitazato). The SMAS analyzed 150 consecutive images obtained from a single field at 10 × magnification in a negative phase-contrast. VSL (μm/sec), VCL (μm/sec), VAP (μm/sec), linearity (LIN; defined as VSL/VCL), straightness (STR; defined as VSL/VAP), amplitude of lateral head displacement (ALH, μm), and beat-cross frequency (BCF, Hz), were automatically calculated by the SMAS; wobbler coefficient (WOB; defined as VAP/VCL) was calculated manually [27]. The SMAS analysis was repeated five times on five different mice. In each experiment, ≥ 300 spermatozoa were detected. Only motile spermatozoa judged as significant were analyzed. The effects of 5-HT and agonists were statistically analyzed by a Student’s t-test performed using Microsoft Excel or by one-way ANOVA with Tukey’s multiple comparisons test using Microsoft Excel with the add-in software Statcel2. Differences were considered significant for P values of < 0.05.
IVF
IVF was performed in accordance with the method described by Takeo et al. [28] with some modifications. For the superovulation treatment, female mice (8–12 weeks of age) were intraperitoneally (i.p.) administered 10 units PMSG at 1600 h, three days before IVF was conducted, followed by 10 units hCG (i.p.) at 1630 h, one day before IVF was performed. At 0950 h on the day IVF was performed, one drop (approximately 3 μl) of the dense mass of spermatozoa obtained from the cauda epididymis of male mice (10–20 weeks of age) were introduced to 300 μl drops of mTALP medium in the presence and absence of a vehicle, 100 pM 5-HT, and/or antagonists. Spermatozoa at 20 × 106 cell/ml were incubated for 1 h at 37°C in an atmosphere containing 5% CO2. The eggs were collected at 1030 h on the day of IVF (18 h after the hCG injection) from PMSG/hCG-treated female mice. COCs were dissected from the ampullae of both oviducts and incubated in 300 μl drops of the mTALP medium in the presence and absence of a vehicle, 100 pM 5-HT, and/or antagonists. At 1050 h on the day of IVF, 10 μl of the suspension containing the pre-incubated spermatozoa was added to the medium containing the COCs to start the insemination process. The spermatozoa and COCs were incubated together for 0.5 or 5 h at 37°C in an atmosphere containing 5% CO2. After incubation, the eggs or COCs were collected and washed with the mTALP medium to remove spermatozoa and cumulus cells from the medium, and insemination was stopped. The washed eggs or COCs were observed after incubation at 1600 h on the day of IVF using an inverted microscope (Leica DM IRB; Leica Microsystems, Wetzlar, Germany), and the total number of eggs was manually counted. The eggs or COCs were observed again at 1600 h after re-incubation on the day after IVF using an inverted microscope. The two-cell embryos were manually counted. The percentage of two-cell embryos was defined as the number of two-cell embryos/total number of eggs × 100. The analysis was performed in a blinded manner. Experiments were performed four times on four different male and four different female mice. The data were statistically analyzed by Student’s t-test using Microsoft Excel or one-way ANOVA with Tukey’s multiple comparisons test using Microsoft Excel with the add-in software Statcel2, with differences considered significant for P values of < 0.05.
Results
Effects of 5-HT and 5-HT-receptor agonists on percentages of motile and hyperactivated spermatozoa, and the motility assay
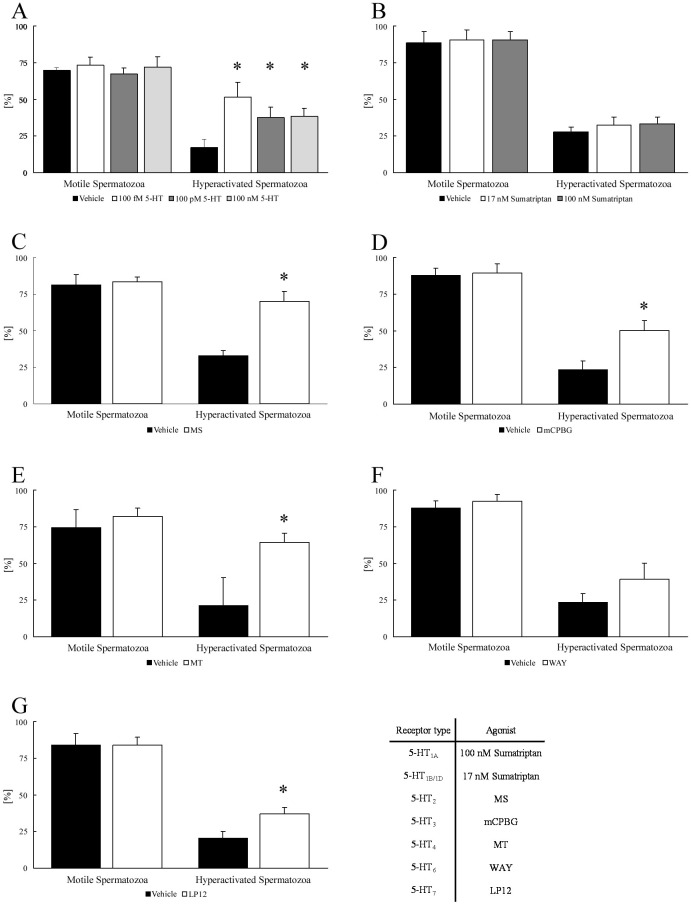
Whether 5-HT affects the percentages of motile and hyperactivated spermatozoa was investigated (Fig. 1A). When spermatozoa were exposed to 100 fM, 100 pM, and 100 nM 5-HT, all concentrations of 5-HT significantly increased the percentage of hyperactivated spermatozoa but did not affect the percentage of motile spermatozoa. Moreover, spermatozoa treated with 5-HT were evaluated using SMAS (Table 1A). All concentrations of 5-HT did not affect VSL, VCL, VAP, LIN, STR, WOB, ALH, and BCF.
Fig. 1.
Effects of 5-hydroxytryptamine (5-HT) and 5-HT-receptor agonists on percentages of motile and hyperactivated spermatozoa. The percentages of motile and hyperactivated spermatozoa are shown after spermatozoa were exposed to 100 fM, 100 pM, or 100 nM 5-HT (A), 17 nM or 100 nM sumatriptan succinate (Sumatriptan) (B), 100 fM α-methylserotonin maleate salt (MS) (C), 100 μM 1-(3-chlorophenyl)biguanide hydrochloride (mCPBG) (D), 10 pM 5-methoxytryptamine (MT) (E), 7.3 nM WAY-208466 dihydrochloride (WAY) (F), and 0.13 nM LP12 hydrochloride hydrate (LP12) (G). Data represent the mean ± SD. (A) (Vehicle) the medium with 0.1% (v/v) pure water as vehicle; (respective concentrations of 5-HT) the medium with indicated concentration of 5-HT and vehicle. (B) (Vehicle) same as above; (respective concentrations of Sumatriptan) the medium with indicated concentrations of Sumatriptan and vehicle. (C) (Vehicle) same as above; (MS) the medium with MS and vehicle. (D) (Vehicle) same as above; (mCPBG) the medium with mCPBG and vehicle. (E) (Vehicle) medium with 0.1% (v/v) ethanol as vehicle; (MT) medium with MT and vehicle. (F) (Vehicle) medium with 0.1% (v/v) pure water as vehicle; (WAY) medium with WAY and vehicle. (G) (Vehicle) same as above; (LP12) medium with LP12 and vehicle. * Significant difference compared with “Vehicle” (P < 0.05).
Table 1. Effects of 5-hydroxytryptamine (5-HT) and 5-HT-receptor agonists on motility assay by Sperm Motility Analysis System (SMAS).
| VSL (µm/sec) | VCL (µm/sec) | VAP (µm/sec) | LIN | ||
|---|---|---|---|---|---|
| A | Vehicle | 84.27 ± 13.67 | 314.31 ± 32.18 | 142.65 ± 21.96 | 0.27 ± 0.04 |
| 100 fM 5-HT | 83.57 ± 10.96 | 306.68 ± 23.74 | 143.82 ± 24.20 | 0.29 ± 0.05 | |
| 100 pM 5-HT | 66.41 ± 13.65 | 295.51 ± 48.02 | 137.68 ± 22.24 | 0.23 ± 0.06 | |
| 100 nM 5-HT | 85.88 ± 16.68 | 296.61 ± 20.17 | 141.89 ± 10.85 | 0.30 ± 0.05 | |
| B | Vehicle | 84.92 ± 9.21 | 322.45 ± 17.50 | 152.97 ± 11.02 | 0.27 ± 0.03 |
| MS | 64.04 ± 4.05 * | 293.27 ± 17.27 * | 142.12 ± 15.50 | 0.22 ± 0.01 * | |
| C | Vehicle | 78.75 ± 4.15 | 313.24 ± 12.88 | 150.75 ± 26.40 | 0.26 ± 0.02 |
| mCPBG | 57.95 ± 10.67 * | 327.22 ± 84.36 | 129.49 ± 38.58 | 0.21 ± 0.03 * | |
| D | Vehicle | 75.67 ± 18.79 | 287.99 ± 29.08 | 137.33 ± 14.02 | 0.27 ± 0.05 |
| MT | 68.86 ± 21.91 | 262.32 ± 28.38 * | 138.90 ± 13.31 | 0.26 ± 0.07 | |
| E | Vehicle | 76.00 ± 14.89 | 317.11 ± 9.74 | 130.51 ± 21.00 | 0.24 ± 0.05 |
| LP12 | 74.26 ± 15.24 | 334.24 ± 32.32 | 137.80 ± 37.73 | 0.23 ± 0.04 | |
| STR | WOB | ALH (µm) | BCF (Hz) | ||
| A | Vehicle | 0.59 ± 0.10 | 0.47 ± 0.10 | 7.41 ± 0.40 | 8.78 ± 1.20 |
| 100 fM 5-HT | 0.61 ± 0.09 | 0.48 ± 0.05 | 7.40 ± 0.91 | 8.29 ± 0.48 | |
| 100 pM 5-HT | 0.51 ± 0.14 | 0.48 ± 0.04 | 7.72 ± 1.31 | 7.85 ± 0.35 | |
| 100 nM 5-HT | 0.62 ± 0.11 | 0.48 ± 0.03 | 7.48 ± 0.29 | 8.36 ± 0.66 | |
| B | Vehicle | 0.57 ± 0.09 | 0.48 ± 0.04 | 8.19 ± 0.74 | 7.99 ± 0.84 |
| MS | 0.48 ± 0.08 * | 0.49 ± 0.06 | 7.41 ± 0.78 * | 8.44 ± 0.48 | |
| C | Vehicle | 0.54 ± 0.05 | 0.49 ± 0.06 | 7.25 ± 1.09 | 9.88 ± 1.19 |
| mCPBG | 0.49 ± 0.07 | 0.42 ± 0.07 * | 7.60 ± 2.20 | 7.53 ± 0.49 * | |
| D | Vehicle | 0.56 ± 0.10 | 0.48 ± 0.04 | 7.70 ± 0.95 | 7.37 ± 0.70 |
| MT | 0.51 ± 0.14 | 0.53 ± 0.02 * | 7.05 ± 0.74 * | 8.34 ± 0.84 | |
| E | Vehicle | 0.57 ± 0.09 | 0.42 ± 0.08 | 7.35 ± 1.35 | 8.45 ± 1.24 |
| LP12 | 0.55 ± 0.05 | 0.42 ± 0.09 | 8.00 ± 2.00 | 7.53 ± 0.90 | |
Straight-line velocity (VSL), curvilinear velocity (VCL), average-path velocity (VAP), linearity (LIN), straightness (STR), wobbler coefficient (WOB), amplitude of lateral head displacement (ALH), and beat-cross frequency (BCF) are shown after spermatozoa were exposed to 100 fM, 100 pM, or 100 nM 5-HT (A), 100 fM α-methylserotonin maleate salt (MS) (B), 100 µM 1-(3-chlorophenyl)biguanide hydrochloride (mCPBG) (C), 10 pM 5-methoxytryptamine (MT) (D), and 0.13 nM LP12 hydrochloride hydrate (LP12) (E). Data represent the mean ± SD. (A) (Vehicle) the medium with 0.1% (v/v) pure water as vehicle; (respective concentrations of 5-HT) the medium with indicated concentration of 5-HT and vehicle. (B) (Vehicle) same as above; (MS) the medium with MS and vehicle. (C) (Vehicle) same as above; (mCPBG) the medium with mCPBG and vehicle. (D) (Vehicle) the medium with 0.1% (v/v) ethanol as vehicle; (MT) medium with MT and vehicle. (E) (Vehicle) the medium with 0.1% (v/v) pure water as vehicle; (LP12) medium with LP12 and vehicle. * Significant difference compared with “Vehicle” in the same condition (P < 0.05). Experiments were repeated five times on five different mice.
In the next step, the effects of 5-HT-receptor agonists on the percentages of motile and hyperactivated spermatozoa were examined (Figs. 1B–G). Sumatriptan (5-HT1-receptor agonist) and WAY (5-HT6-receptor agonist) did not affect the percentages of motile and hyperactivated spermatozoa (Figs. 1B and 1F). However, MS (5-HT2-receptor agonist), mCPBG (5-HT3-receptor agonist), MT (5-HT4-receptor agonist), and LP12 (5-HT7-receptor agonist) significantly increased the percentage of hyperactivated spermatozoa, but they did not affect the percentage of motile spermatozoa (Figs. 1C–E and 1G).
Spermatozoa treated with 5-HT-receptor agonists were evaluated by SMAS. MS significantly decreased VSL, VCL, LIN, STR, and ALH but it did not affect VAP, WOB, and BCF (Table 1B). Treatment by mCPBG significantly decreased VSL, LIN, WOB, and BCF but it did not affect VCL, VAP, STR, and ALH (Table 1C). MT significantly decreased VCL and ALH and significantly increased WOB, whereas it did not affect VSL, VAP, LIN, STR, and BCF (Table 1D). LP12 did not affect VSL, VCL, VAP, LIN, STR, WOB, ALH, and BCF (Table 1E).
Effects of 5-HT-receptor antagonists on the increase of hyperactivation by 5-HT and 5-HT-receptor agonists
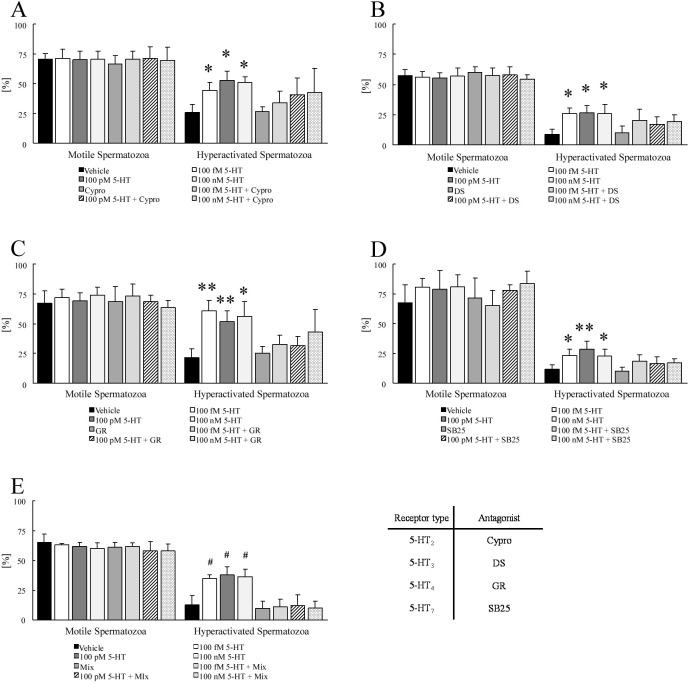
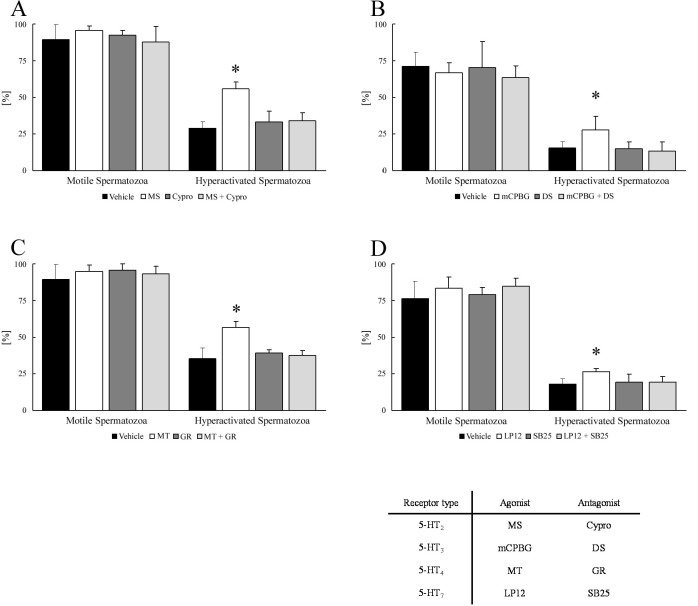
As the 5-HT and four 5-HT-receptor agonists increased hyperactivation (Fig. 1), the effects of the 5-HT-receptor antagonists on these increases were investigated (Fig. 2). Cypro (5-HT2-receptor antagonist) at 1 μM [13, 22] and 3.8 nM DS (5-HT3-receptor antagonist) [29] did not significantly suppress the improvement of hyperactivation by any concentration of 5-HT (Figs. 2A and B). As shown in Fig. 2C, 1 μM GR (5-HT4-receptor antagonist) [13, 22] significantly suppressed the increase of hyperactivation by 100 fM and 100 pM 5-HT, although 1 μM GR did not suppress the increase of hyperactivation by 100 nM 5-HT. Although 10 μM SB25 (5-HT7-receptor antagonist) [30] did not suppress the improvement of hyperactivation by 100 fM and 100 nM 5-HT, it significantly suppressed the increase by 100 pM 5-HT (Fig. 2D). In addition, a mixture of the four 5-HT-receptor antagonists significantly suppressed the increase of hyperactivation by all concentrations of 5-HT (Fig. 2E). As shown in Fig. 3, the increase of hyperactivation by each agonist was significantly suppressed by the corresponding antagonists.
Fig. 2.
Suppressive effects of 5-hydroxytryptamine (5-HT)-receptor antagonists on the increase of hyperactivation by 5-HT. The percentages of motile and hyperactivated spermatozoa are shown after spermatozoa were exposed to 100 fM, 100 pM, or 100 nM 5-HT after exposure to 5-HT-receptor antagonists (A–D) or a mixture of antagonists (E) for 5 min. The mixture contained 1 μM cyproheptadine hydrochloride sesquihydrate (Cypro), 3.8 nM dolasetron mesylate hydrate (DS), 1 μM GR113808 (GR), and 10 μM SB-258719 (SB). The data represent the mean ± SD. (A) (Vehicle) medium with 0.1% (v/v) pure water and 0.1% (v/v) ethanol as vehicle; (respective concentrations of 5-HT) medium with respective concentration of 5-HT and vehicle; (Cypro) the medium with 1 μM Cypro and vehicle; (respective concentrations of 5-HT + Cypro) medium with respective concentration of 5-HT, 1 μM Cypro, and vehicle. (B) (Vehicle) medium with 0.2% (v/v) pure water as vehicle; (respective concentrations of 5-HT) medium with respective concentration of 5-HT and vehicle; (DS) medium with 3.8 nM DS and vehicle; (respective concentrations of 5-HT + DS) medium with respective concentration of 5-HT, 3.8 nM DS, and vehicle. (C) (Vehicle) medium with 0.1% (v/v) pure water and 0.1% (v/v) dimethyl sulfoxide (DMSO) as vehicle; (respective concentrations of 5-HT) medium with respective concentration of 5-HT and vehicle; (GR) medium with 1 μM GR and vehicle; (respective concentrations of 5-HT + GR) medium with respective concentration of 5-HT, 1 μM GR, and vehicle. (D) (Vehicle) same as above; (all concentrations of 5-HT) medium with respective concentration of 5-HT and vehicle; (SB25) medium with 10 μM SB-258719 (SB25) and vehicle; (respective concentrations of 5-HT + SB25) medium with respective concentration of 5-HT, 10 μM SB25, and vehicle. (E) (Vehicle) medium with 0.2% (v/v) pure water, 0.1% (v/v) ethanol, and 0.2% (v/v) DMSO as vehicle; (respective concentrations of 5-HT) medium with the respective concentrations of 5-HT and vehicle; (Mix) medium with the mixture of four antagonists and vehicle; (respective concentrations of 5-HT + Mix) medium with all concentrations of 5-HT, the mixture of four antagonists, and vehicle. * Significant difference compared with “Vehicle” and “antagonist” (P < 0.05). ** Significant difference compared with “Vehicle”, “antagonist”, and “respective concentrations of 5-HT + antagonist” (P < 0.05). # Significant difference compared with “Vehicle”, “Mix”, and “respective concentrations of 5-HT + Mix” (P < 0.05).
Fig. 3.
Inhibition of the effect of 5-hydroxytryptamine (5-HT)-receptor agonists by 5-HT-receptor antagonists. The percentages of motile and hyperactivated spermatozoa are shown when spermatozoa were exposed to 5-HT-receptor agonists after exposure to 5-HT-receptor antagonists for 5 min. The data represent the mean ± SD. (A) (Vehicle) medium with 0.1% (v/v) pure water and 0.1% (v/v) ethanol as vehicle; (MS) medium with 100 fM α-methylserotonin maleate salt (MS) and vehicle; (Cypro) medium with 1 μM cyproheptadine hydrochloride sesquihydrate (Cypro) and vehicle; (MS + Cypro) the medium with 100 fM MS, 1 μM Cypro, and vehicle. (B) (Vehicle) medium with 0.2% (v/v) pure water as vehicle; (mCPBG) medium with 100 μM 1-(3-chlorophenyl)biguanide hydrochloride (mCPBG) and vehicle; (DS) medium with 3.8 nM dolasetron mesylate hydrate (DS) and vehicle; (mCPBG + DS) medium with 100 μM mCPBG, 3.8 nM DS, and vehicle. (C) (Vehicle) medium with 0.1% (v/v) ethanol and 0.1% (v/v) dimethyl sulfoxide (DMSO) as vehicle; (MT) medium with 10 pM 5-methoxytryptamine (MT) and vehicle; (GR) medium with 1 μM GR113808 (GR) and vehicle; (MT + GR) medium with 10 pM MT, 1 μM GR, and vehicle. (D) (Vehicle) medium with 0.1% (v/v) pure water and 0.1% (v/v) DMSO as vehicle; (LP12) medium with 0.13 nM LP12 hydrochloride hydrate (LP12) and vehicle; (SB25) medium with 10 μM SB-258719 (SB25) and vehicle; (LP12 + SB25) medium with 0.13 nM LP12, 10 μM SB25, and vehicle. * Significant difference compared with “Vehicle”, “antagonist”, and “agonist + antagonist” for the same incubation time (P < 0.05).
Effects of 5-HT on IVF in mice
Finally, the effect of 5-HT on the success rate of IVF was examined (Table 2). The addition of 100 pM of 5-HT to the medium did not affect the percentage of two-cell embryos when spermatozoa were co-incubated with COCs for 5 h. However, 100 pM 5-HT significantly increased the percentage of two-cell embryos when spermatozoa were co-incubated with COCs for 0.5 h.
Table 2. Effects of 5-hydroxytryptamine (5-HT) on in vitro fertilization (IVF).
| No. of total eggs | No. of two-cell embryos | Two-cell embryo (%) | ||
|---|---|---|---|---|
| A 5 h insemination | ||||
| Vehicle | 120 | 39 | 34.35 ± 14.11 | |
| 100 pM 5HT | 112 | 33 | 30.17 ± 9.06 | |
| B 0.5 h insemination | ||||
| Vehicle | 112 | 11 | 9.23 ± 4.62 | |
| 100 pM 5HT | 101 | 27 | 26.59 ± 10.37 * | |
The percentages of the two-cell embryos are shown when IVF was performed in the medium in the presence and absence of 100 pM 5-HT. The data represent the mean ± SD. The insemination times of IVF were 5 h (A) and 0.5 h (B). (Vehicle) medium with 0.1% (v/v) pure water as vehicle; (100 pM 5-HT) medium with 100 pM 5-HT and vehicle. * Significant difference compared with “Vehicle” (P < 0.05). Experiments were performed four times on four different male and four different female mice.
We then investigated the 5-HT-receptor antagonists effects on the increase of two-cell embryos by 5-HT (Table 3). Cypro at 1 μM (Table 3A), 3.8 nM DS (Table 3B), 10 μM SB25 (Table 3D), and the mixture of antagonists (Table 3E) did not suppress the percentage increase of two-cell embryos by 100 pM 5-HT. In contrast, 1 μM GR significantly suppressed the effect of 5-HT (Table 3C). Additionally, 1 μM GR significantly decreased the percentage of two-cell embryos.
Table 3. Inhibition of the increase in in vitro fertilization (IVF) by 5-hydroxytryptamine (5-HT)-receptor antagonists.
| No. of total eggs | No. of two-cell embryos | Two-cell embryo (%) | ||
|---|---|---|---|---|
| A | Vehicle | 154 | 25 | 14.96 ± 5.29 |
| 100 pM 5HT | 164 | 57 | 35.96 ± 10.69 * | |
| Cypro | 73 | 19 | 25.25 ± 8.58 | |
| 100 pM 5HT + Cypro | 89 | 20 | 22.09 ± 5.81 | |
| B | Vehicle | 128 | 13 | 6.64 ± 10.59 |
| 100 pM 5HT | 107 | 39 | 34.76 ± 7.79 ** | |
| DS | 67 | 2 | 3.35 ± 3.88 | |
| 100 pM 5HT + DS | 102 | 30 | 25.71 ± 19.39 | |
| C | Vehicle | 138 | 29 | 21.33 ± 3.73 |
| 100 pM 5HT | 130 | 48 | 35.91 ± 6.75 # | |
| GR | 91 | 5 | 4.96 ± 4.53 ## | |
| 100 pM 5HT + GR | 97 | 16 | 18.25 ± 6.04 | |
| D | Vehicle | 103 | 14 | 12.15 ± 8.11 |
| 100 pM 5HT | 129 | 52 | 40.55 ± 9.96 ** | |
| SB25 | 109 | 3 | 3.11 ± 2.98 $ | |
| 100 pM 5HT + SB25 | 110 | 26 | 22.31 ± 12.31 | |
| E | Vehicle | 141 | 15 | 9.43 ± 6.47 |
| 100 pM 5HT | 144 | 51 | 35.18 ± 11.53 ** | |
| Mix | 51 | 4 | 6.25 ± 12.50 | |
| 100 pM 5HT + Mix | 60 | 13 | 17.39 ± 14.58 | |
The percentages of the two-cell embryos are shown when IVF was performed in the medium with 100 pM 5-HT and 5-HT-receptor antagonists (A–D) or a mixture of antagonists (E). The mixture contained 1 µM cyproheptadine hydrochloride sesquihydrate (Cypro), 3.8 nM dolasetron mesylate hydrate (DS), 1 µM GR113808 (GR), and 10 µM SB-258719 (SB25). The data represent the mean ± SD. (A) (Vehicle) medium with medium with 0.1% (v/v) pure water and 0.1% (v/v) ethanol as vehicle; (100 pM 5-HT) medium with 100 pM 5-HT and vehicle; (Cypro) medium with 1 µM Cypro and vehicle; (100 pM 5-HT + Cypro) medium with 100 pM 5-HT, 1 µM Cypro, and vehicle. (B) (Vehicle) medium with medium with 0.2% (v/v) pure water as vehicle; (100 pM 5-HT) medium with 100 pM 5-HT and vehicle; (DS) medium with 3.8 nM DS and vehicle; (100 pM 5-HT + DS) medium with 100 pM 5-HT, 3.8 nM DS, and vehicle. (C) (Vehicle) medium with medium with 0.1% (v/v) pure water and 0.1% (v/v) dimethyl sulfoxide (DMSO) as vehicle; (100 pM 5-HT) medium with 100 pM 5-HT and vehicle; (GR) medium with 1 µM GR113808 (GR) and vehicle; (100 pM 5-HT + GR) medium with 100 pM 5-HT, 1 µM GR, and vehicle. (D) (Vehicle) same as above; (100 pM 5-HT) medium with 100 pM 5-HT and vehicle; (SB25) medium with 10 µM SB-258719 (SB25) and vehicle; (100 pM 5-HT + SB25) medium with 100 pM 5-HT, 10 mM SB25, and vehicle. (E) (Vehicle) medium with medium with 0.2% (v/v) pure water, 0.1% (v/v) ethanol, and 0.2% (v/v) DMSO as vehicle; (100 pM 5-HT) medium with 100 pM 5-HT and vehicle; (Mix) medium with the mixture of antagonists and vehicle; (100 pM 5-HT + Mix) medium with 100 pM 5-HT, the mixture of antagonists, and vehicle. * Significant difference compared with “Vehicle” (P < 0.05). ** Significant difference compared with “Vehicle”, and “antagonist” (P < 0.05). # Significant difference compared with “Vehicle”, “antagonist”, and “100 pM 5-HT + antagonist” (P < 0.05). ## Significant difference compared with “Vehicle”, “100 pM 5-HT”, and “100 pM 5-HT + antagonist” (P < 0.05). $ Significant difference compared with “100 pM 5-HT”, and “100 pM 5-HT + antagonist” (P < 0.05). Experiments were performed four times on four different male and four female mice.
Discussion
In mammalian reproduction, 5-HT (serotonin) regulates several important functions of gametes and organs [13, 21,22,23,24]. Because 5-HT and 5-HT receptors are found in female reproductive organs [21], serotonergic signaling is thought to be involved in the regulation of steroidogenesis, oocyte maturation, and embryonic development. For example, the 5HT2 receptor has been detected in mouse cumulus cells [21]. The concentration of 5-HT in rat oviducts is between 2.06 and 3.34 µg/g fresh tissue [31]; whereas, in humans, the concentration of 5-HT in the preovulatory follicles and the cystically degenerated follicles is 14.3 ± 8.9 µg/100 ml and 12.2 ± 6.2 µg/100 ml, respectively [32]. Moreover, 5-HT induces the release of P4 from granulose cells [33]. In hamsters, 5-HT and MT induce the AR in the spermatozoa, and this induction can be inhibited by Cypro [22], and 5-HT dose-dependently enhances hyperactivation through the 5-HT2 and 5-HT4 receptors [13]. In addition, the 5HT1B, 5HT2A, and 5HT3 receptors have been found in human and stallion spermatozoa [23, 24], and 5-HT increases the VSL, VCL, and VAP of human spermatozoa [23]. It is assumed that the velocities of human spermatozoa are regulated by the 5-HT2 and 5-HT3 receptors because the 5-HT1 receptor suppresses tmAC and decreases cAMP concentration [21]. In mice, 5-HT and LP12 did not affect the VSL, VCL, and VAP of the spermatozoa (Tables 1A and E), but MS decreased VSL and VCL (Table 1B). Moreover, mCPBG and MT decreased VSL and VCL, respectively (Tables 1C and D). In either case, the 5-HT2 receptor appears to be associated with the regulation of spermatozoa velocities in humans and mice. The 5-HT2 receptor stimulates Ca2+ signals related to the PLC [21] and, Ca2+ stimulates the sAC to induce motility and hyperactivation via the production of cAMP [8]. Therefore, it is likely that 5-HT regulates spermatozoal velocities and hyperactivation through the activation of sAC by Ca2+ signals associated with the 5-HT2 receptor. As for the other parameters, 5-HT and LP12 had no effects (Tables 1A and E). As MS decreased VSL and VCL, it decreased LIN and STR, which are calculated as VSL/VCL and VSL/VAP, respectively (Table 1B). As mCPBG decreased VSL, LIN also decreased (Table 1C). In addition, mCPBG decreased WOB, which is calculated as VAP/VCL, although it did not affect VCL or VAP (Table 1C). Therefore, as MT decreased VCL, WOB increased (Table 1D). Both MS and MT decreased ALH, and mCPBG decreased BCF (Tables 1B, C and D).
Previously, we found that 100 fM, 100 pM, and 100 nM 5-HT significantly improved the hyperactivation of hamster spermatozoa through the 5-HT2 and 5-HT4 receptors [13]. Although not all concentrations of 5-HT affected the percentage of motile mouse spermatozoa, they significantly increased the percentage of hyperactivated spermatozoa through the 5-HT2, 5-HT3, 5-HT4, and 5-HT7 receptors (Figs. 1 and 3). In hamster spermatozoa, 100 fM and 100 pM 5-HT enhanced hyperactivation through the 5-HT2 receptor only [13]. In contrast, hyperactivation was not always improved through the receptors in mouse spermatozoa (Fig. 2A). The improvement by 100 nM 5-HT was not affected by Cypro in either hamsters [13] or mice (Fig. 2A). In hamsters, 100 nM 5-HT enhanced hyperactivation through the 5-HT4 receptor only [13]; however, the same experiment had no effect in mice, although 100 fM and 100 pM 5-HT did increase hyperactivation (Fig. 2C). In addition, the 5-HT3 receptor was associated with improved hyperactivation by 5-HT, but 5-HT3 was not the main regulatory receptor for this effect (Figs. 1D and 2B). Moreover, 100 pM 5-HT increased hyperactivation through the 5-HT7 receptor (Fig. 2D). Furthermore, the mixture of Cypro, DS, GR, and SB25 completely inhibited the improvement by 5-HT in all conditions (Fig. 2E). These results suggest that 5-HT simultaneously stimulated these receptors to increase hyperactivation in mice (Fig. 2). In addition, it is likely that the Ca2+ signals associated with PLC and the cAMP signals associated with the tmAC were simultaneously stimulated in the increase of hyperactivation of mouse spermatozoa by 5-HT. However, it was not possible to show how the 5-HT3 receptor regulated the increase of hyperactivation by 5-HT in mice in this study.
In human studies, it has been suggested that the capability for hyperactivation is correlated with the success of IVF [11]. P4 is an inducer of hyperactivation in human spermatozoa [34, 35], but P4-induced hyperactivation did not increase the success of IVF [11]. Several hormones induce hyperactivation in mammals [12,13,14, 18], although, no studies have reported an increase in the success rate of IVF. In the present study, 5-HT increased the success rate of IVF in mice (Tables 2 and 3). Because 5-HT significantly improved hyperactivation of mouse spermatozoa, it is likely that 5-HT increases IVF success through an increase in hyperactivation. In addition, the percentage of two-cell embryos tended to decrease when the increase of hyperactivation by 5-HT was suppressed by 5-HT-receptor antagonists (Fig. 2 and Table 3). In particular, the 5-HT4-receptor antagonists inhibited the increase of hyperactivation by 5-HT, resulting in a decrease in the success of IVF (Fig. 2C and Table 3).
In this study, Cypro was used as a 5-HT2-receptor antagonist (Figs. 2 and 3 and Table 3A). Cypro significantly suppressed the increase of hyperactivation of mouse spermatozoa by MS (Fig. 3A) but tended to suppress the 5-HT-increased hyperactivation and IVF success (Fig. 2A and Table 3A). We found that a mixture of Cypro and other antagonists significantly suppressed the increase of hyperactivation and slightly suppressed the increase in IVF success by 5-HT (Fig. 2E and Table 3E). In hamsters [13, 22], Cypro significantly suppressed the induction of AR and hyperactivation by 5-HT. These results suggest that Cypro may induce infertility in rodents. It is thought that Cypro suppresses the effects of 5-HT on the reproductive functions in humans because the 5-HT2 receptor is expressed in the spermatozoa and oocytes [21, 23]. Cypro has been approved for use in humans and is used as an antihistamine for the treatment of allergic reactions [36].
In conclusion, this study investigated whether 5-HT regulates spermatozoal hyperactivation and IVF in mice. The experimental results indicate that 5-HT significantly increases hyperactivation through the 5-HT2, 5-HT3, 5-HT4, and 5-HT7 receptors. Moreover, the results show that 5-HT substantially increases the success rate of IVF through these receptors. It is expected that 5-HT could be used as a stimulatory agent to increase the success rate of IVF, as the spermatozoal ability to hyperactivate is correlated with the success rate of IVF in humans [11].
Declaration of conflict of interest
The authors declare that there are no conflicts of interest that could be perceived as prejudicing the impartiality of the research reported.
Acknowledgments
The authors would like to thank Mr Masahide Ohyama of the Laboratory Animal Research Center of the Dokkyo Medical University for his excellent technical support for IVF in mice.
This work was partially supported by a Grand-in-Aid for Scientific Research (C) (No. 18K09204 to MF) from the Japan Society for the Promotion of Science (JSPS).
References
- 1.Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill JD (ed.), The Physiology of Reproduction Vol. 2, 2nd ed. New York: Raven Press; 1994: 189–317. [Google Scholar]
- 2.Harayama H. Flagellar hyperactivation of bull and boar spermatozoa. Reprod Med Biol 2018; 17: 442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohri H, Inaba K, Ishijima S, Baba SA. Tubulin-dynein system in flagellar and ciliary movement. Proc Jpn Acad B 2012; 88: 397–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujinoki M, Takei GL, Kon H. Non-genomic regulation and disruption of spermatozoal in vitro hyperactivation by oviductal hormones. J Physiol Sci 2016; 66: 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suarez SS, Ho HC. Hyperactivated motility in sperm. Reprod Domest Anim 2003; 38: 119–124. [DOI] [PubMed] [Google Scholar]
- 6.Langlais J, Roberts KD. A molecular membrane model of sperm capacitation and the acrosome reaction of mammalian spermatozoa. Gamete Res 1985; 13: 183–224. [Google Scholar]
- 7.Xie F, Garcia MA, Carlson AE, Schuh SM, Babcock DF, Jaiswal BS, Gossen JA, Esposito G, van Duin M, Conti M. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev Biol 2006; 296: 353–362. [DOI] [PubMed] [Google Scholar]
- 8.Wertheimer E, Krapf D, de la Vega-Beltran JL, Sánchez-Cárdenas C, Navarrete F, Haddad D, Escoffier J, Salicioni AM, Levin LR, Buck J, Mager J, Darszon A, Visconti PE. Compartmentalization of distinct cAMP signaling pathways in mammalian sperm. J Biol Chem 2013; 288: 35307–35320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujinoki M, Suzuki T, Takayama T, Shibahara H, Ohtake H. Profiling of proteins phosphorylated or dephosphorylated during hyperactivation via activation on hamster spermatozoa. Reprod Med Biol 2006; 5: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki T, Fujinoki M, Shibahara H, Suzuki M. Regulation of hyperactivation by PPP2 in hamster spermatozoa. Reproduction 2010; 139: 847–856. [DOI] [PubMed] [Google Scholar]
- 11.Alasmari W, Barratt CLR, Publicover SJ, Whalley KM, Foster E, Kay V, Martins da Silva S, Oxenham SK. The clinical significance of calcium-signalling pathways mediating human sperm hyperactivation. Hum Reprod 2013; 28: 866–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujinoki M. Melatonin-enhanced hyperactivation of hamster sperm. Reproduction 2008; 136: 533–541. [DOI] [PubMed] [Google Scholar]
- 13.Fujinoki M. Serotonin-enhanced hyperactivation of hamster sperm. Reproduction 2011; 142: 255–266. [DOI] [PubMed] [Google Scholar]
- 14.Noguchi T, Fujinoki M, Kitazawa M, Inaba N. Regulation of hyperactivation of hamster spermatozoa by progesterone. Reprod Med Biol 2008; 7: 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujinoki M. Suppression of progesterone-enhanced hyperactivation in hamster spermatozoa by estrogen. Reproduction 2010; 140: 453–464. [DOI] [PubMed] [Google Scholar]
- 16.Fujinoki M. Regulation and disruption of hamster sperm hyperactivation by progesterone, 17β-estradiol and diethylstilbestrol. Reprod Med Biol 2014; 13: 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujinoki M, Takei GL. Estrogen suppresses melatonin-enhanced hyperactivation of hamster spermatozoa. J Reprod Dev 2015; 61: 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calogero AE, Hall J, Fishel S, Green S, Hunter A, D’Agata R. Effects of γ-aminobutyric acid on human sperm motility and hyperactivation. Mol Hum Reprod 1996; 2: 733–738. [DOI] [PubMed] [Google Scholar]
- 19.Kon H, Takei GL, Fujinoki M, Shinoda M. Suppression of progesterone-enhanced hyperactivation in hamster spermatozoa by γ-aminobutyric acid. J Reprod Dev 2014; 60: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujinoki M, Takei GL. γ-Aminobutyric acid suppresses enhancement of hamster sperm hyperactivation by 5-hydroxytryptamine. J Reprod Dev 2017; 63: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubé F, Amireault P. Local serotonergic signaling in mammalian follicles, oocytes and early embryos. Life Sci 2007; 81: 1627–1637. [DOI] [PubMed] [Google Scholar]
- 22.Meizel S, Turner KO. Serotonin or its agonist 5-methoxytryptamine can stimulate hamster sperm acrosome reactions in a more direct manner than catecholamines. J Exp Zool 1983; 226: 171–174. [DOI] [PubMed] [Google Scholar]
- 23.Jiménez-Trejo F, Tapia-Rodríguez M, Cerbón M, Kuhn DM, Manjarrez-Gutiérrez G, Mendoza-Rodríguez CA, Picazo O. Evidence of 5-HT components in human sperm: implications for protein tyrosine phosphorylation and the physiology of motility. Reproduction 2012; 144: 677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiménez-Trejo F, Coronado-Mares I, Boeta M, González-Santoyo I, Vigueras-Villaseñor R, Arriaga-Canon C, Herrera LA, Tapia-Rodríguez M. Identification of serotoninergic system components in stallion sperm. Histol Histopathol 2018; 33: 951–958. [DOI] [PubMed] [Google Scholar]
- 25.Maleszewski M, Kline D, Yanagimachi R. Activation of hamster zona-free oocytes by homologous and heterologous spermatozoa. J Reprod Fertil 1995; 105: 99–107. [DOI] [PubMed] [Google Scholar]
- 26.Umezu K, Hiradate Y, Oikawa T, Ishiguro H, Numabe T, Hara K, Tanemura K. Exogenous neurotensin modulates sperm function in Japanese Black cattle. J Reprod Dev 2016; 62: 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mortimer ST. A critical review of the physiological importance and analysis of sperm movement in mammals. Hum Reprod Update 1997; 3: 403–439. [DOI] [PubMed] [Google Scholar]
- 28.Takeo T, Hoshii T, Kondo Y, Toyodome H, Arima H, Yamamura K, Irie T, Nakagata N. Methyl-beta-cyclodextrin improves fertilizing ability of C57BL/6 mouse sperm after freezing and thawing by facilitating cholesterol efflux from the cells. Biol Reprod 2008; 78: 546–551. [DOI] [PubMed] [Google Scholar]
- 29.Boeijinga PH, Galvan M, Baron BM, Dudley MW, Siegel BW, Slone AL. Characterization of the novel 5-HT3 antagonists MDL 73147EF (dolasetron mesilate) and MDL 74156 in NG108-15 neuroblastoma × glioma cells. Eur J Pharmacol 1992; 219: 9–13. [DOI] [PubMed] [Google Scholar]
- 30.Harsing LG, Jr, Prauda I, Barkoczy J, Matyus P, Juranyi Z. A 5-HT7 heteroreceptor-mediated inhibition of [3H]serotonin release in raphe nuclei slices of the rat: evidence for a serotonergic-glutamatergic interaction. Neurochem Res 2004; 29: 1487–1497. [DOI] [PubMed] [Google Scholar]
- 31.Juorio AV, Chedrese PJ, Li XM. The influence of ovarian hormones on the rat oviductal and uterine concentration of noradrenaline and 5-hydroxytryptamine. Neurochem Res 1989; 14: 821–827. [DOI] [PubMed] [Google Scholar]
- 32.Bòdis J, Bognàr Z, Hartmann G, Török A, Csaba IF. Measurement of noradrenaline, dopamine and serotonin contents in follicular fluid of human graafian follicles after superovulation treatment. Gynecol Obstet Invest 1992; 33: 165–167. [DOI] [PubMed] [Google Scholar]
- 33.Bódis J, Török A, Tinneberg HR, Hanf V, Papenfuss F, Schwarz H. Serotonin induces progesterone release from human granulosa cells in a superfused granulosa cell system. Arch Gynecol Obstet 1993; 253: 59–64. [DOI] [PubMed] [Google Scholar]
- 34.Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 2011; 471: 387–391. [DOI] [PubMed] [Google Scholar]
- 35.Strünker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 2011; 471: 382–386. [DOI] [PubMed] [Google Scholar]
- 36.The American Society of Health-System Pharmacists (2019, October 1). Cyproheptadine Retrieved from https://medlineplus.gov/druginfo/meds/a682541.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.