Table of Contents
| List of Abbreviations ............................................................................................................ | 451 |
| Preface ................................................................................................................................... | 453 |
| Grades of Recommendations and Levels of Evidence ....................................................... | 454 |
| Recommendations ................................................................................................................. | 455 |
| 1. Therapeutic agents ..................................................................................................... | 458 |
| 2. Therapeutic time window .......................................................................................... | 460 |
| 3. Eligibility for treatment .............................................................................................. | 462 |
| 4. Eligibility for treatment in patients receiving anticoagulant therapy ..................... | 466 |
| 5. Required elements for medical institutions .............................................................. | 469 |
| 6. Flow of events from symptom onset to hospital arrival ......................................... | 470 |
| 7. Medical history, medical examination, and laboratory testing ............................... | 470 |
| 8. Diagnostic imaging of the brain and cervical arteries ............................................. | 473 |
| 9. Determination of patient eligibility and informed consent ..................................... | 478 |
| 10. Endovascular therapy ................................................................................................. | 480 |
| 11. Management after initiation of IV thrombolysis ...................................................... | 481 |
| Disclosure Statements .......................................................................................................... | 483 |
| References ............................................................................................................................. | 485 |
List of Abbreviations
1. Common noun
| Abbreviation | Formal name |
|---|---|
| ADC | apparent diffusion coefficient |
| aPTT | activated partial thromboplastin time |
| CI | confidence interval |
| CT | computed tomography |
| CTA | computed tomographic angiography |
| DOAC | direct oral anticoagulant |
| DWI | diffusion-weighted imaging |
| EIC | early ischemic change |
| FLAIR | fluid-attenuated inversion recovery |
| GOR | grade of recommendation |
| IV | intravenous |
| LOE | levels of evidence |
| MRA | magnetic resonance angiography |
| MRI | magnetic resonance imaging |
| PH | parenchymal hematoma |
| PT-INR | prothrombin time international normalized ratio |
| PWI | perfusion-weighted imaging |
| rt-PA | recombinant tissue-type plasminogen activator |
| SCU | stroke care unit |
| sICH | symptomatic intracranial hemorrhage |
| SPECT | single photon emission computed tomography |
| TC-CFI | transcranial color-flow imaging |
| TCD | transcranial Doppler |
2. Trials, organizations, etc.
| Abbreviation | Formal name |
|---|---|
| ASIST-Japan | Acute Stroke Imaging Standardization Group-Japan |
| ASPECTS | Alberta Stroke Program Early CT Score |
| ATLANTIS | Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke |
| DEFUSE | Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution |
| ECASS | European Cooperative Acute Stroke Study |
| ENCHANTED | Enhanced Control of Hypertension and Thrombolysis Stroke Study |
| EPITHET | Echoplanar Imaging Thrombolysis Evaluation Trial |
| ESCAPE | Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion with Emphasis on Minimizing CT to Recanalization Times |
| EXTEND(-IA) | EXtending the time for Thrombolysis in Emergency NeurologicalDeficits(-Intra-Arterial) |
| HERMES | Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials |
| IST-3 | Third International Stroke Trial |
| J-ACT | Japan Alteplase Clinical Trial |
| J-MARS | Japan Post-Marketing Alteplase Registration Study |
| MELT-Japan | Middle Cerebral Artery Embolism Local Fibrinolytic Intervention Trial-Japan |
| MR CLEAN | Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in The Netherlands |
| mRS | modified Rankin Scale |
| NIHSS | National Institutes of Health Stroke Scale |
| NINDS | National Institute of Neurological Disorders and Stroke |
| OHS | Oxford Handicap Score |
| PROACT II | Prolyse in Acute Cerebral Thromboembolism II |
| REVASCAT | Randomized Trial of Revascularization with Solitaire FR Device versus Best Medical Therapy in the Treatment of Acute Stroke Due to Anterior Circulation Large Vessel Occlusion Presenting within Eight Hours of Symptom Onset |
| SAMURAI | Stroke Acute Management with Urgent Risk-factor Assessment and Improvement |
| SITS-MOST | Safe Implementation of Thrombolysis in Stroke-Monitoring Study |
| SWIFT PRIME | Solitaire FR With the Intention For Thrombectomy as PRIMary Endovascular Treatment for Acute Ischemic Stroke |
| THAWS | THrombolysis for Acute Wake-up and Unclear-onset Strokes with Alteplase at0.6 mg/kg Trial |
| WAKE-UP | Efficacy and Safety of MRI-based Thrombolysis in Wake-up Stroke |
Preface
In 2005, intravenous (IV) thrombolysis using alteplase, a recombinant tissue-type plasminogen activator (rt-PA), for patients with acute ischemic stroke was approved in Japan on the basis of the results of the Japan Alteplase Clinical Trial (J-ACT)1) with a specific low-dose regimen. Over the 10 years after approval, intravenous thrombolysis has become common as standard therapy for acute ischemic stroke. During this period, the Japan Stroke Society published the Guidelines for Intravenous Application of rt-PA (Alteplase) in 2005, independently of the Japanese Guidelines for the Management of Stroke in an effort to promote safe, widespread use of intravenous thrombolysis,2) and revised the present guidelines repeatedly according to subsequent accumulated evidence and changing therapeutic attitudes.3,4) The Japan Stroke Society also organized training sessions for proper use using the treatment guidelines as the textbook all over Japan every year and conducted a widespread awareness campaign. This effort was unique in Japan but was highly assessed outside Japan. Since 2018, e-learning has made it easier to participate in these training sessions. Additionally, the Japan Stroke Society published and revised the Guidelines for Mechanical Thrombectomy5) repeatedly for a short time and promoted hyperacute reperfusion therapy in Japan based on these two guidelines.
The following points were included for the revision of the third edition. First, we absolutely considered the time after symptom onset as conventional treatment indications, but this time, we added the options to estimate the approximate onset time and determine the indications using the findings from magnetic resonance imaging (MRI). Second, situations about anticoagulant therapy have been changing recently according to development of new pharmaceuticals. With regard to the eligibility for intravenous thrombolysis in patients on anticoagulation, the contents of the Consensus Guides on Stroke Thrombolysis and Thrombectomy for Anticoagulated Patients, November 20176) published by the Japan Stroke Society in 2017 were included and substantially modified. The contents were also updated in the Section of Acute Endovascular Therapy in coordination with the Guidelines for Mechanical Thrombectomy, the Third Edition.5) In addition, all parts of the present guidelines were reviewed and revised for more realistic treatment guidelines based on new scientific rationales and practical information accumulated in Japan and overseas over the past several years.
For all neurologists, neurosurgeons, physicians, or co-medical staffs involved in emergency medicine, as well as medical students and non-experts who are interested in this therapy, these guidelines for intravenous thrombolysis should be read. All members of the drafting committee carefully developed the guidelines with particular attention not to be technical and esoteric. As it has been mentioned before, it is noted that this therapy is highly effective but increased risks of symptomatic intracerebral hemorrhage due to inappropriate decisions on the indications or inadvertent patient management, and the physicians should provide treatment with care.
The progress of hyperacute reperfusion therapy, including intravenous thrombolysis and acute endovascular therapy, is remarkable, and new information to be reviewed with the guidelines will be available immediately after the release of the present guidelines. In December 2018, the Stroke and Cardiovascular Disease Control Act was enforced, and in 2019, criteria for stroke centers will be newly established to effectively provide hyperacute reperfusion therapy. We will continuously revise the guidelines in accordance with changing circumstances.
March 2019
Kazunori Toyoda, Chair
Grades of Recommendations and Levels of Evidence
| Grade of recommendation (GOR) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade A | Strongly recommended | ||||||||||||||||||||
| Grade B | Recommended | ||||||||||||||||||||
| Grade C1 | May be reasonable but there is insufficient evidence | ||||||||||||||||||||
| Grade C2 | Not recommended because of a lack of scientific evidence | ||||||||||||||||||||
| Grade D | Not recommended because of potentially harmful | ||||||||||||||||||||
| Levels of evidence (LOE) | |
|---|---|
| High | High-quality and consistent evidence from more than one randomized controlled trial or overwhelming evidence from observational studies. Further studies may not change the assessment. |
| Middle | Significantly problematic (inconsistent results, flawed methodology, indirect, or inaccurate) evidence from more than one randomized controlled trial or very strong evidence from observational studies. If further studies are performed, assessment is likely to change. |
| Low | Evidence from observational studies, unstructured clinical experience, or more than one seriously defective randomized controlled trial. All estimate effects are uncertain. |
The classification of grading of treatment recommendations adhered to those presented in the 2015 Guidelines for the Management of Stroke [Supplement 2017].7)
As the level of evidence of recommendations, those used in the 2009 Guidelines for the Management of Stroke were adopted until the second edition, but the level of evidence was established for each reported study in the 2015 Guidelines for the Management of Stroke [Supplement 2017]. Establishment of the level of evidence for each reported study was also reviewed in the third edition, the same policy as in the past was applied to establish the level of evidence for each recommendation, and the criteria used by the American College of Chest Physicians8) and UpToDate (https://www.uptodate.com/ja/home/grading-tutorial#) were adopted.
Recommendations
- Therapeutic agents
- 1. Alteplase should be used for IV thrombolysis [grade of recommendation (GOR) A; level of evidence (LOE) high].
- 2. In Japan, IV alteplase 0.6 mg/kg should be used (GOR A; LOE middle).
- 3. IV rt-PA other than alteplase is not recommended because of insufficient evidence in Japan (GOR C2; LOE high).
- Therapeutic time window
- 4. IV thrombolysis should be provided to patients with ischemic stroke who can be treated within 4.5 h of symptom onset (GOR A; LOE high).
- 5. IV thrombolysis should be initiated as early as possible (at least within 1 h) after the patient’s arrival at the hospital (GOR A; LOE high). Even within 4.5 h of symptom onset, earlier initiation of treatment can be expected to lead to better outcomes.
- 6. If the onset time is unknown, the last time the patient was known to be without signs and symptoms of the current stroke should be considered as the onset time (GOR A; LOE low). However, this shall not apply to the following recommendation.
- 7. If the onset time is unknown, IV thrombolysis may be reasonable in patients whose ischemic change of diffusion-weighted brain MRI is not evident on fluid-attenuated inversion recovery (FLAIR) imaging, because the signs or symptoms of the current stroke highly likely occurred within 4.5 h before MRI imaging (GOR C1; LOE middle).
- Eligibility for treatment
- 8. Patients with any clinical category of ischemic stroke should be considered eligible for IV thrombolysis (GOR A; LOE high).
- 9. Patients after >4.5 h of symptom onset or recognition (GOR D; LOE high), those with a previous history of non-traumatic intracranial hemorrhage, those who are strongly suspected of having thoracic aortic dissection, or those with evidence of extensive early ischemic change on computed tomography (CT) or MRI (GOR all D; LOE low) should be considered ineligible for IV thrombolysis. The use of IV thrombolysis is not recommended if patients meet any of the ineligibility criteria.
- 10. Conditions requiring careful administration refer to those where IV thrombolysis may be considered but where adverse drug reactions are likely to occur, and favorable outcomes may not be always achieved. IV thrombolysis may be initiated in such conditions only if the physician in charge believes that the potential benefits outweigh the possible risks and the patient or their legal representatives provide informed consent (GOR C1; LOE middle).
- 11. The use of IV thrombolysis increases the risk of symptomatic intracranial hemorrhage or death in patients with any deviation from the eligibility criteria (LOE middle).
- Eligibility for treatment in patients receiving anticoagulant therapy
- 12. Eligibility for IV thrombolysis should be carefully considered in patients receiving antithrombotic therapy, especially anticoagulation (GOR A; LOE high).
- 13. IV thrombolysis should not be recommended to patients who are taking anticoagulants and meet any of the ineligibility criteria based on anticoagulant marker value and time after the last dose (GOR D; LOE high).
- 14. IV thrombolysis may be considered after the use of idarucizumab for patients who are taking dabigatran and considered ineligible for IV thrombolysis based on anticoagulant marker value and time after the last dose (GOR C1; LOE low).
- Required elements for medical institutions
- 15. IV thrombolysis should be provided by medical institutions that have the following resources and processes:
- Brain CT or MRI, general blood tests, blood coagulation tests, and electrocardiography available.
- A stroke physician starts evaluation after the patient’s arrival as soon as possible.
- A system in place that makes neurosurgical procedures readily available by neurosurgeons if needed (GOR A; LOE high).
- 16. The telemedicine for acute stroke care (telestroke) can safely provide IV thrombolysis even in the absence of acute stroke physicians in the clinical setting (GOR C1; LOE middle).
- Flow of events from symptom onset to hospital arrival
- 17. To ensure the proper performance of IV thrombolysis, efforts should be made to raise public enlightenment and improve prehospital life support by ambulance staff, facilitating the rapid arrival of patients at the hospital (GOR B; LOE middle).
- 18. When medical personnel inside the hospital receive the initial report on the patient, they should make an effort to proceed with preparations within the hospital so that a prompt response can be made after the patient’s arrival (GOR A; LOE middle).
- Medical history, medical examination, and laboratory testing
- 19. At the initial presentation, efforts should be made to consider differential diagnoses other than stroke to the extent possible (GOR A; LOE low).
- 20. An objective assessment of neurological severity using the National Institutes of Health Stroke Scale (NIHSS) should be performed (GOR A; LOE low).
- 21. In laboratory testing, patients should be assessed for bleeding tendencies or risk factors of symptomatic intracranial hemorrhage (GOR A; LOE low).
- Diagnostic imaging of the brain and cervical arteries
- 22. Intracranial hemorrhage should be ruled out using a non-contrast CT or MRI scan, and the degree of early ischemic change be assessed immediately before IV thrombolysis (GOR A; LOE high).
- 23. IV thrombolysis is not recommended to patients with large early ischemic changes for the consequence of high-risk symptomatic intracranial hemorrhage (GOR D; LOE high).
- 24. Although cerebrovascular assessment is not mandatory prior to IV thrombolysis, it is strongly recommended to assess large vessel occlusion using CTA or MRA immediately after initiation of IV thrombolysis if mechanical thrombectomy should be considered for such patients (GOR A; LOE high).
- 25. Diagnostic imaging should be limited to the minimum necessary to avoid the delay of IV thrombolysis (GOR A; LOE low).
- Determination of patient eligibility and informed consent
- 26. If patients are eligible, it is desirable that the patients or their legal representatives are informed of the potential benefits and risks of IV thrombolysis and provide informed consent to the extent possible (GOR C1; LOE low).
- 27. If patients require careful administration, it is necessary that the patients or their legal representatives provide informed consent (GOR B; LOE low).
- Endovascular therapy
- 28. Patients should receive mechanical thrombectomy regardless of IV thrombolysis if they meet all the following criteria:
- (1) the modified Rankin Scale (mRS) scores before symptom onset of 0–1;
- (2) occlusion of the internal carotid artery or the segment M1 of the middle cerebral artery (MCA);
- (3) Alberta Stroke Program Early CT Score (ASPECTS) ≥6 on brain CT or diffusion-weighted MRI scans;
- (4) the NIHSS score ≥6;
- (5) ≥18 years of age; and (6) treatment can be initiated within 6 h of symptom onset (GOR A; LOE high).
- 29. Patients eligible for IV thrombolysis should receive thrombolysis before initiation of mechanical thrombectomy (GOR A; LOE high).
- 30. The initiation of mechanical thrombectomy should not be delayed due to confirmation of efficacy for IV thrombolysis (GOR D; LOE high).
- 31. Local fibrinolytic therapy with urokinase within 6 h of symptom onset can improve outcomes of patients with the MCA occlusion (GOR B; LOE high).
- Management after initiation of IV thrombolysis
- 32. The dose of alteplase should be 0.6 mg/kg with 10% of the dose given as an initial bolus, and the remainder infused over 1 h (GOR A; LOE middle).
- 33. It is recommended that patients be managed in a stroke care unit or an equivalent ward for at least 24 h after initiation of treatment (GOR B; LOE high).
- 34. During the first 24 h of treatment, control of blood pressure and restriction of antithrombotic therapies are important. If symptoms worsen, a prompt diagnosis should be made, and neurosurgical procedures, such as hematoma evacuation by craniotomy, should be performed as soon as possible as needed (GOR B; LOE low).
1. Therapeutic agents
(Recommendations)
1. Alteplase should be used for IV thrombolysis (GOR A; LOE high).
2. In Japan, IV alteplase 0.6 mg/kg should be used (GOR A; LOE middle).
3. IV rt-PA other than alteplase is not recommended because of insufficient evidence in Japan (GOR C2; LOE high).
Evidence from Overseas Clinical Trials with Alteplase
In 1996, IV thrombolysis with alteplase was first approved in the United States for patients with acute ischemic stroke. In an evidence-based randomized controlled trial conducted by the National Institute of Neurological Disorders and Stroke (NINDS), a significantly higher percentage of subjects after 3 h of symptom onset receiving alteplase (0.9 mg/kg) had a good outcome of complete independence (corresponding to the mRS score of 0–1) at 3 months compared with subjects receiving a placebo (39% vs. 26%), while symptomatic intracranial hemorrhage (sICH) within 36 h of stroke onset also occurred with a significantly higher incidence in the alteplase group (6.4% vs. 0.6%).9) No differences in efficacy were found among the clinical categories of ischemic stroke. The Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke (ATLANTIS) trial10) investigated the efficacy of therapy provided within 3–5 h (in some subjects, within 6 h) of symptom onset according to the methods of this study and found no evidence of improvement in outcomes. In the European Cooperative Acute Stroke Study (ECASS),11) an analysis of patients who complied with the methods of this study among patients within 6 h of symptom onset found that the therapeutic effects of alteplase (1.1 mg/kg) were evident. The ECASS II,12) a repeated trial that used a lower dose of 0.9 mg/kg and stricter entry criteria, concluded that there was no significant difference in the primary endpoint (mRS score of 0–1) between the alteplase and placebo groups, and a significantly greater proportion of patients in the alteplase group had the mRS score of 0–2 (Table 1).13–18)
Table 1.
Major randomized controlled trials using intravenous alteplase cited in the present guidelines
| Trial design | N | Time to initiation (h) | Dose (mg/kg) | mRS score 0–1 at 3 months | Incidence of sICH* in the alteplase group (%) | ||
|---|---|---|---|---|---|---|---|
| rt-PA (%) | Placebo (%) | ||||||
| NINDS (1995)9) | Phase III, placebo controlled | 624 | ≤3 | 0.9 | 39 | 26 | 6.4 |
| ECASS (1995)11) | Phase III, placebo controlled | 620 | ≤6 | 1.1 | 35.7 | 29.3 | 19.8 |
| ECASS-II (1998)12) | Phase III, placebo controlled | 800 | ≤6 | 0.9 | 40.3 | 36.6 | 8.8 |
| ATLANTIS (1999)10) | Phase III, placebo controlled | 579 | 3–5† | 0.9 | 41.7 | 40.5 | 7.2 |
| J-ACT (2006)1) | Phase III, active drug alone | 103 | ≤3 | 0.6 | 36.9 | – | 5.8 |
| DEFUSE (2006)13) | Phase II, active drug alone | 74 | 3–6 | 0.9 | 42‡ | – | 9.5 |
| ECASS-III (2008)14) | Phase III, placebo controlled | 821 | 3–4.5 | 0.9 | 52.4 | 45.2 | 2.4 |
| EPITHET (2008)15) | Phase II, placebo controlled | 100 | 3–6 | 0.9 | 35 | 24 | 7.7 |
| IST-3 (2012)16) | Phase III, non-rt-PA controlled | 3035 | ≤6 | 0.9 | 24§ | 21§ | 7 |
| ENCHANTED (2016)17) | Phase III, active drug alone, dose comparison | 3206 | ≤4.5 | 0.9:0.6 | 48.9 (0.9 mg/kg) | 46.8 (0.6 mg/kg) | 2.1:1.0 |
| WAKE-UP (2018)18) | Phase III, placebo controlled | 503 | ≤4.5 of symptom recognition | 0.9 | 53.3 | 41.8 | 2.8 |
Symptomatic intracranial hemorrhage (sICH) was defined as follows: (1) any worsening [National Institutes of Health Stroke Scale (NIHSS) score ≥ 1] in the NINDS and IST-3; (2) parenchymal hematoma (PH 1 and 2), including an asymptomatic one, in the ESUS; (3) determined by the attending physician in the ATLANTIS; (4) an NIHSS score of ≥2 in the DEFUSE; and (5) an NIHSS score of ≥4 in the other studies as a general rule,
Within 6 h of symptom onset in some subjects,
Includes subjects with an improvement in the NIHSS score of ≥8 at 3 months,
Measured by the Oxford Handicap Score instead of modified Rankin Scale (mRS).
Based on these trial results, especially with reference to the trial method of the NINDS rt-PA Stroke Study, the use of IV alteplase 0.9 mg/kg is strongly recommended overseas.19,20) However, it should be noted that sICH has been observed in 5–20% of subjects receiving alteplase in clinical trials, representing an approximately three to 10-fold increase compared with those receiving a placebo.
Evidence from Japanese Clinical Trials with Alteplase
In the Japan Alteplase Clinical Trial (J-ACT),1) a total of 103 subjects who could be treated within 3 h of symptom onset were exposed to IV alteplase at 0.6 mg/kg (Table 2). The dose of 0.6 mg/kg of alteplase is close to the dose of 20 MU duteplase calculated for a patient weighing 60 kg as described below. The inclusion and exclusion criteria were similar to those of the NINDS rt-PA Stroke Study.9) A total of 37% of subjects had the mRS score of 0–1 at 3 months, and 5.8% of subjects had sICHs within 36 h, which were similar to the results observed in the active drug group in the NINDS rt-PA Stroke Study and the results from the aforementioned references and meta-analyses. In Japan, the use of alteplase 0.6 mg/kg was approved in 2005 on the basis of the J-ACT results. The efficacy and safety of this dose administered within 3 hours of symptom onset have been reconfirmed in a post-marketing clinical study (J-ACT II),21) surveillance [Japan Post-Marketing Alteplase Registration Study (J-MARS)],22) and a smaller multicenter observational study [Stroke Acute Management with Urgent Risk-factor Assessment and Improvement (SAMURAI) rt-PA Registry],23) all of which were conducted after approval in Japan. In the J-ACT II, among 58 subjects with the MCA occlusion as documented by magnetic resonance angiography (MRA) at baseline, 52% and 69% of subjects receiving alteplase 0.6 mg/kg had evidence of recanalization at 6 and 24 h after symptom onset, respectively. The J-MARS enrolled a total of 7492 of 8313 (90%) patients who were estimated to receive IV thrombolysis during the 2 years after approval in Japan and found that 33.1% of subjects had the mRS score of 0–1 at 3 months and 3.5% had sICHs within 36 h. The SAMURAI rt-PA Registry, involving 600 patients, yielded similar results as those in the J-MARS.
Table 2.
Major clinical trials and observational studies in Japan using intravenous alteplase
| Summary | N | Time to initiation (h) | Dose (mg/kg) | mRS score 0–1 at 3 months (%) | Incidence of sICH* (%) | |
|---|---|---|---|---|---|---|
| J-ACT (2006)1) | Phase III trial | 103 | ≤3 | 0.6 | 36.9 | 5.8 |
| J-ACT II (2010)21) | Trial on patients with the MCA occlusion | 58 | ≤3 | 0.6 | 46.6 | 0 |
| J-MARS (2010)22) | Post-marketing surveillance for 2 years | 7492 | ≤3 | 0.6 | 33.1 | 3.5 |
| SAMURAI (2009)23) | Multicenter registry in 10 hospitals | 600 | ≤3 | 0.6 | 33.2 | 1.3 |
| Kimura et al. (2016)24) | Single-center registry | 256 | ≤3 in most subjects | 0.6 | 34.8 | 9.4 |
| ≤4.5 in some subjects | ||||||
| YAMATO (2017)25) | Trial on patients with combined use of edaravone | 165 | ≤4.5 | 0.6 | 55 (mRS 0–2) | 3.6 |
Symptomatic intracranial hemorrhage was defined as parenchymal hematoma (pH 2), including an asymptomatic one in the study conducted by Kimura et al., and as an NIHSS score of ≥4 in the other studies as a general rule.
Optimal Dose of Alteplase
There are still limited data regarding the optimal dose of alteplase either in Japan or overseas. The comparative study with 0.6 mg/kg of the national approved dose and 0.9 mg/kg of the international approved dose was not performed in Japan. The Enhanced Control of Hypertension and Thrombolysis Stroke Study (ENCHANTED),19) a two-dose comparative trial conducted outside Japan, enrolled a majority of subjects from Asian counties, including China, and found that 53.2% of subjects had the mRS score of 2–6 at 90 days, which was similar to the 0.9 mg/kg group (51.1%), and the incidence of sICH, based on criteria in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST),26) was significantly low in the 0.6 mg/kg group (1.0% vs. 2.1%).
Evidence from Clinical Trials with Thrombolytic Agents Other than Alteplase
The indication of rt-PA products other than alteplase for stroke has not been approved in Japan. Before IV alteplase was approved, clinical studies of IV urokinase or streptokinase had been conducted outside Japan but found no evidence of efficacy.27) The placebo-controlled comparative trial using duteplase, a double-chain rt-PA, was conducted in Japan for treatment of patients with embolic stroke within 6 h of symptom onset.28,29) However, duteplase failed to translate into clinical use because of the subsequent discontinuation of development. Monteplase has been developed in Japan as a long half-life t-PA,30) and thrombolysis for acute myocardial infarction and acute pulmonary embolism is covered by insurance. However, the agent cannot be used for stroke thrombolysis because of discontinuation of development for ischemic stroke.
The outcome of tenecteplase that was developed after alteplase was not superior to that of alteplase in patients with comparatively mild ischemic stroke within 4.5 h of symptom onset or within 4.5 h of symptom recognition on wakening with similar safety.31) Additionally, IV tenecteplase before thrombectomy had a significant better recanalization rate immediately after administration and better improvement in functional outcomes compared with alteplase in patients who could receive thrombolysis within 4.5 h of symptom onset, those who had occlusion of the internal carotid artery, MCA, or basilar artery on CT, and those who could receive mechanical thrombectomy within 6 h of symptom onset.32) In contrast, 90 μg/kg of IV desmoteplase did not improve the functional outcome in patients with stroke within 3–9 h of symptom onset significantly at 3 months compared with a placebo, also it was safe and increased artery recanalization, and development of desmoteplase was accordingly discontinued.33)
2. Therapeutic time window
(Recommendations)
4. IV thrombolysis should be provided to patients with ischemic stroke who can be treated within 4.5 h of symptom onset (GOR A; LOE high).
5. IV thrombolysis should be initiated as early as possible (at least within 1 h) after the patient’s arrival at the hospital (GOR A; LOE high). Even within 4.5 h of symptom onset, earlier initiation of treatment can be expected to lead to better outcomes.
6. If the onset time is unknown, the last time the patient was known to be without signs and symptoms of the current stroke should be considered as the onset time (GOR A; LOE low). However, this shall not apply to the following recommendation.
7. If the onset time is unknown, IV thrombolysis may be reasonable in patients whose ischemic change of diffusion-weighted brain MRI is not evident on FLAIR imaging, because the signs or symptoms of the current stroke highly likely occurred within 4.5 h before MRI imaging (GOR C1; LOE middle).
Evidence on Therapeutic Time Window
When IV thrombolysis was first approved overseas, initiation within 3 h after onset of ischemic stroke was strongly recommended, according to the patient entry criteria of the NINDS rt-PA Stroke Study.9) ECASS III,16) a European interventional trial that enrolled patients who could be treated within 3–4.5 h of symptom onset, found that significantly more patients receiving alteplase had the mRS score of 0–1 at 3 months (52.4% vs. 45.2% in the placebo group), the prevalence of sICH was significantly greater in the alteplase group than in the control group but numerically low (2.4%), and there was no difference in mortality between the groups. The Third International Stroke Trial (IST-3),18) which enrolled 3035 patients who could be treated within 6 h of symptom onset, showed that there were no significant differences in the proportion of patients who died (27% in both groups) or who were independent [the Oxford Handicap Score (OHS) of 0–2; 37% vs. 35%] at 6 months with significantly more patients in the alteplase group having a good outcome (OHS of 0–1) (24% vs. 21%), although sICH (7% vs. 1%) and death (11% vs. 7%) within 7 days occurred in significantly more patients in the alteplase group than in the placebo group. A pooled analysis of the NINDS rt-PA Stroke Study, ECASS,11) ECASS II,12) ATLANTIS,10) ECASS III, Echoplanar Imaging Thrombolysis Evaluation Trial (EPITHET)17) and IST-3 revealed that the proportion of patients with the mRS score of 0–1 at 3–6 months after initiation of IV thrombolysis within 4.5 h was significantly greater in thrombolysed patients than the control patients, and no significant difference in the proportion was observed after initiation of therapy beyond 4.5 h.34)
In Japan, the Japan Stroke Society submitted a proposal to extend the time window for IV thrombolysis within 3–4.5 h of symptom onset in the form of changing comments sought by the Japanese Ministry of Health, Labour and Welfare in 2009. Based on a review by the Pharmaceutical Affairs and Food Sanitation Council in 2012, the use of IV thrombolysis within 4.5 h of symptom onset was covered by insurance.
Recommendation for Early Initiation of IV Thrombolysis
Even within 4.5 h of symptom onset, earlier initiation of IV thrombolysis can be expected to lead to better outcomes. The above-mentioned meta-analysis also indicated that the therapeutic effects were reduced with the time after symptom onset.34) In contrast, an increase in incidence of sICH using thrombolysis was similar to treatment within 3 h, within 3–4.5 h, and beyond 4.5 h of symptom onset.35) The TARGET: Stroke Phase II Campaign in the United States established as the primary goal to complete general initial assessments within 10 min of the patient’s arrival, to initiate imaging assessment within 25 min of arrival, to interpret imaging scans within 45 min of arrival, and to determine therapeutic indication and initiate thrombolysis within 60 min of arrival in ≥75% of patients.36) The Campaign also established, as the secondary goal, to initiate CT/MRI within 20 min of patient’s arrival, to interpret CT/MRI within 35 min of arrival, and to initiate thrombolysis within 45 min of arrival in ≥50% of patients. The American Heart Association/American Stroke Association 2018 Guidelines for the early management of patients with acute ischemic stroke established the proportion within 60 min from patient’s arrival to the initiation of IV alteplase of ≥50% as the primary goal, and proportion within 45 min of ≥50% as the secondary goal.13) Recent several studies showed that thrombolysis can be initiated approximately 20–30 min after the emergent arrival.37)
Definition of the Time of Symptom Onset
The time of symptom onset, which is used to calculate the therapeutic time window, is not the first-found abnormal time, but the time reported by the patient or witnesses of symptom onset, or if such information is not available, the last time the patient was known to be symptom-free (last known well). If a patient has symptoms at the time of awakening, the time of symptom onset is the time the patient was known to be symptom-free before or during sleep. If a patient was found lying down, the time of symptom onset is the last time the patient was known by family members or other witnesses to be symptom-free. If stepwise worsening occurs, the time of symptom onset is the time the patient had the first onset of symptoms. If a patient has a preceding transient ischemic attack, the time of symptom onset is the time the patient had the second onset of symptoms after the initial complete resolution of symptoms.
Estimated Time of Symptom Onset Using Brain Diagnostic Imaging
If ischemic changes on diffusion-weighted imaging (DWI) were not evident on FLAIR MRI (i.e., DWI-FLAIR mismatch) in acute stroke, the symptom highly likely occurred within 4.5 h before MRI imaging.38,39) In the Efficacy and Safety of MRI-based Thrombolysis in Wake-up Stroke (WAKE-UP) trial20) conducted in Europe, when treatment was initiated within 1 h of MRI imaging and within 4.5 h of symptom recognition in patients who had stroke with an unknown time of onset or stroke on awakening and had a positive DWI-FLAIR mismatch, the alteplase group had higher rate of the mRS of 0–1 compared with the placebo group (53.3% vs. 41.8%). In contrast, the incidence of intracranial hemorrhage of parenchymal hematomas type 2 increased (2.4% vs. 0.8%), and sICH and death (4.1% vs. 1.2%) after 3 months of symptom onset were more likely to have occurred in the alteplase group. The FLAIR imaging was standardized in the trial, and the details of imaging conditions are given in Section 8 “Diagnostic imaging of the brain and cervical arteries” (see Table 8 in section 8). At the time of this writing, no recommended guidelines have been published in Europe where this trial was conducted. In Japan, the THrombolysis for Acute Wake-up and Unclear-onset Strokes with Alteplase at 0.6 mg/kg Trial (THAWS) using 0.6 mg/kg of alteplase was completed according to the protocol, including standardized FLAIR imaging conditions and criteria in accordance with WAKE-UP trial, and the final analyses are under way.40)
Table 8.
FLAIR imaging conditions in the WAKE-UP trial
|
3. Eligibility for treatment
(Recommendations)
8. Patients with any clinical category of ischemic stroke should be considered eligible for IV thrombolysis (GOR A; LOE high).
9. Patients after >4.5 h of symptom onset or recognition (GOR D; LOE high), those with a previous history of non-traumatic intracranial hemorrhage, those who are strongly suspected of having thoracic aortic dissection, or those with evidence of extensive early ischemic change on CT or MRI (GOR all D; LOE low) should be considered ineligible for IV thrombolysis. The use of IV thrombolysis is not recommended if patients meet any of the ineligibility criteria.
10. Conditions requiring careful administration refer to those where IV thrombolysis may be considered but where adverse drug reactions are likely to occur, and favorable outcomes may not be always achieved. IV thrombolysis may be initiated in such conditions only if the physician in charge believes that the potential benefits outweigh the possible risks and the patient or their legal representatives provide informed consent (GOR C1; LOE middle).
11. The use of IV thrombolysis increases the risk of symptomatic intracranial hemorrhage or death in patients with any deviation from the eligibility criteria (LOE middle).
Importance of Eligibility Criteria
Patients with any clinical category of ischemic stroke (including atherothrombotic infarction, lacunar infarction, cardioembolism, stroke of other determined or undetermined cause, and transient ischemic attack with symptom resolution after thrombolysis) should be considered eligible for IV thrombolysis. This recommendation is based on evidence of certain effects observed in clinical trials of IV alteplase, which included patients with all clinical categories of ischemic stroke.
Table 3 shows the conditions that should be considered ineligible (contraindications) or those that require the careful administration of alteplase. IV thrombolysis is not recommended for patients with any of these contraindications. Conditions requiring careful administration is as follows: (1) treatment may be considered, (2) adverse drug reactions are likely to occur, and (3) favorable outcomes may not always be achieved. Treatment may be started in patients with such conditions only after the patients or their legal representatives are informed of the importance and risks of thrombolysis and provide informed consent and when the physician in charge based on personal experience believes that the potential benefits outweigh the possible risks.
Table 3.
Checklist for intravenous thrombolysis
| Contraindications | Yes | No |
|---|---|---|
| Time from symptom onset or recognition to initiation of IV thrombolysis | ||
| Beyond 4.5 h from symptom onset (time determined) or recognition | □ | □ |
| No DWI-FLAIR mismatch within 4.5 h from symptom recognition, or not yet assessed | □ | □ |
| Medical history | ||
| Non-traumatic intracranial hemorrhage | □ | □ |
| Stroke within 1 month (excluding in case where symptom disappeared for a short period) | □ | □ |
| Significant head/spinal injury or surgery within 3 months | □ | □ |
| Gastrointestinal or urinary tract bleeding within 21 days | □ | □ |
| Major surgery or significant trauma other than head injury within 14 days | □ | □ |
| Drug hypersensitivity to alteplase | □ | □ |
| Clinical findings | ||
| Suspected subarachnoid hemorrhage | □ | □ |
| Concurrent acute aortic dissection | □ | □ |
| Concurrent hemorrhage (e.g., intracranial, gastrointestinal, urinary tract, or retroperitoneal, or hemoptysis) | □ | □ |
| Systolic blood pressure ≥185 mmHg despite emergent antihypertensive therapy | □ | □ |
| Diastolic blood pressure ≥110 mmHg despite emergent antihypertensive therapy | □ | □ |
| Severe hepatic disorder | □ | □ |
| Acute pancreatitis | □ | □ |
| Infective endocarditis (confirmed prior to stroke onset) | □ | □ |
| Blood test (glucose levels and platelet counts should be measured before initiation of thrombolysis) | ||
| Abnormal blood glucose level (<50 or >400 mg/dL even after correction of glucose) | □ | □ |
| Platelet ≤100,000/mm3 (Patients with a history of hepatic cirrhosis and hematologic disease) | □ | □ |
| *Thrombolysis can be initiated before confirmation of the results of blood tests in patients without history of hepatic cirrhosis or hematologic disease. Thrombolysis should be discontinued immediately, if the platelet count is confirmed to be ≤100,000/mm3. | ||
| Blood test (for patients on anticoagulant therapy or those with abnormal coagulation) | ||
| PT-INR > 1.7 | □ | □ |
| Prolonged aPTT (>1.5 times the baseline value [>≈40 s as a guide]) | □ | □ |
| Within 4 h after the last dose of direct oral anticoagulants | □ | □ |
| *The above-mentioned findings are not considered ineligible if IV thrombolysis is considered after the use of idarucizumab in patients treated with dabigatran. | ||
| CT/MR evidence | ||
| Extensive early ischemic change | □ | □ |
| Mass effect (midline shift) | □ | □ |
| Careful administration (eligibility should be determined with care) | Yes | No |
| Age ≥ 81 years | □ | □ |
| Beyond 4.5 h from the last known well and within 4.5 h from symptom recognition, with evidence of DWI-FLAIR mismatch. | □ | □ |
| Medical history | ||
| Biopsy/trauma within 10 days | □ | □ |
| Delivery/abortion or premature labor within 10 days | □ | □ |
| Stroke beyond 1 month of onset (especially cases with concurrent diabetes) | □ | □ |
| Allergy to proteins | □ | □ |
| Neurologic deficit | ||
| NIHSS score ≥26 | □ | □ |
| Minor symptoms | □ | □ |
| Rapidly improving symptoms | □ | □ |
| Convulsion (patients should be ineligible if they are suspected of developing epilepsy based on their medical history) | □ | □ |
| Clinical findings | ||
| Cerebral aneurysm/intracranial neoplasm/arteriovenous malformation/Moyamoya disease | □ | □ |
| Thoracic aortic aneurysm | □ | □ |
| Gastrointestinal ulcer/diverticulitis, colitis | □ | □ |
| Active tuberculosis | □ | □ |
| Diabetic hemorrhagic retinopathy/hemorrhagic ophthalmic conditions | □ | □ |
| On thrombolytic or antithrombotic therapy (especially anticoagulant therapy) | □ | □ |
| Menstruation | □ | □ |
| Severe renal disorder | □ | □ |
| Poorly controlled diabetes | □ | □ |
Note that treatment should not be provided for patients who meet any of the contraindications.
Deviations from the eligibility criteria are critical issues. There was also a significant positive correlation between deviation rate and mortality.41)
While the first edition of guidelines on IV thrombolysis was developed with an emphasis on safety in light of limited therapeutic experience in Japan,2) the second edition is a substantial revision of the first edition mainly in terms of eligibility criteria on the basis of accumulated evidence for thrombolysis and clinical experience accumulated by many Japanese medical institutions. The third edition added management of patients who had an unknown time of onset of stroke, those with concurrent acute aortic dissection, and those awaiting the results of blood tests. When it is necessary to determine patient eligibility, read carefully with the text provided below and use the checklist. The details of eligibility for IV thrombolysis in patients on antithrombotic therapy are given in Section 4 “Eligibility for Treatment of Patients Receiving Anticoagulant Therapy.”
Contraindications
The use of IV thrombolysis is not recommended for patients beyond 4.5 h of symptom onset. There are many other conditions related to medical history, clinical findings, hematologic findings, and imaging findings that should be considered in determining eligibility. Among these criteria, increased blood pressure is the condition that can be resolved with rapid treatment intervention. However, the use of thrombolysis is not recommended if blood pressure of <185/110 mm Hg cannot be maintained by IV administration of antihypertensive agents. Increased blood pressure and hyperglycemia immediately before thrombolysis are risk factors for intracranial hemorrhage and, therefore, necessitate careful determination of patient eligibility even if blood pressure or blood glucose levels meet the eligibility criteria.42–44) Items revised from the second edition and reasons for the revisions are outlined below.
Stroke with unknown time of onset
Intravenous thrombolysis is conventionally not recommended in patients who had stroke with an unknown time of onset and beyond 4.5 h of recognition. In contrast, if FLAIR imaging showed no evident ischemic changes (DWI-FLAIR mismatch) within 4.5 h of symptom recognition in patients who had stroke with an unknown time of onset, the symptom highly likely occurred within 4.5 h before MRI imaging as described in Chapter 2 “Therapeutic Time Window.”38,39) The results of the WAKE-UP trial showed that outcomes improvement could be expected by IV thrombolysis for patients with such conditions.20) In contrast, increasing trends in death or intracranial hemorrhage due to thrombolysis were observed in the WAKE-UP trial, and recommended guidelines have not yet been published in Europe, where the trial was conducted. In the present guidelines, DWI-FLAIR mismatch-positive stroke with an unknown time of onset was added in the list of Careful Administration eligibility. IV thrombolysis should not be provided for patients with stroke beyond 4.5 h from the last known well without an appropriate assessment with MRI.
Concurrent acute aortic dissection
Within 1.5 years after the approval of IV alteplase in Japan, there was a report of 10 stroke patients receiving IV alteplase with unrecognized concurrent thoracic aortic dissection who suddenly deteriorated after thrombolysis, leading to death.45) If patients are strongly suspected of having acute aortic dissection based on their medical history (i.e., preceding chest or back pain), physical findings [e.g., decreased blood pressure, reduced peripheral arterial pulse, asymmetry of branchial systolic blood pressure (>20 mmHg), or aortic regurgitation murmur], or laboratory findings (i.e., plain chest radiography showing evidence of an enlarged superior mediastinum), the possibility of aortic dissection should be ruled out by confirmation of the occlusion or initial flap at the common carotid artery using ultrasonography and by chest CT scans with contrast enhancement to obtain definitive diagnosis of acute thoracic aortic dissection before initiation of IV thrombolysis.46,47) Elevated d-dimer level helps us diagnose as dissection.48) Patients with acute aortic dissection identified based on the clinical assessment are ineligible for IV thrombolysis. Typical symptoms, such as chest pain or back pain, are often unnoticed because of disturbances of consciousness due to acute stroke.
Blood test findings
Blood glucose level and platelet count should be confirmed before IV thrombolysis. When neurological symptoms persist after correction of abnormal blood glucose level, patients can be diagnosed as having stroke, and they are considered eligible for thrombolysis. However, neurological symptoms may prolong after correction of blood glucose level in some patients with hypoglycemia. In such case, caution should be exercised in determining thrombolysis. Thrombolysis is allowed and can be initiated before confirmation of platelet count in patients with no medical history of hepatic cirrhosis and hematologic disease. Thrombolysis should be discontinued, if platelet count is confirmed to be 100,000/mm3 or less after initiation of thrombolysis.
Infective endocarditis (identified before stroke onset)
When IV thrombolysis is performed in patients with stroke associated with infective endocarditis, the outcomes are poor.49–51) However, it is usually difficult to newly diagnose infective endocarditis in restricted time of emergency condition, and IV thrombosis should not be provided to patients who suffered stroke during treatment of infective endocarditis. Patients who were diagnosed as infective endocarditis before stroke onset have been changed from careful administration in the second edition to a contraindication in the present recommendation.
Careful Administration
As items revised from the second edition, myocardial infarction within the last 3 months is deleted from the careful administrations section. The risk of developing cardiac rupture in patients with stroke receiving IV thrombolysis is increased if patients are elderly, having anteroseptal infarction, or are female, but the incidence is <1%.52) Patients with myocardial infarction who received IV thrombolysis for stroke and then developed cardiac tamponade or cardiac rupture had developed myocardial infarction within 7 weeks.53) In addition, if patients with stroke who could receive IV thrombolysis were accompanied with acute myocardial infarction, percutaneous transluminal coronary angioplasty was considered acceptable immediately after thrombolysis.54)
The guidelines for management of acute ischemic stroke in the United States recommended careful investigation of eligibility for IV thrombolysis, if MRI susceptibility-weighted imaging (T2*-weighted images, SWI, SWAN, PRESTO, Multi-Shot RSSG) revealed 11 or more of asymptomatic cerebral microbleeds in the medical history.13) The results of meta-analysis including nine trials showed that the incidence of sICH increased after IV thrombolysis in patients with more than 11 of cerebral microbleeds before treatment [odds ratio (OR) 18.17, 95% confidence interval (CI) 2.39–138.22)].55) Symptomatic intracranial hemorrhage is an important factor requiring careful administration, but this condition was excluded from the checklist because it is difficult to acquire this information at the first medical examination.
4. Eligibility for treatment to patients on anticoagulant therapy
(Recommendations)
12. Eligibility for IV thrombolysis should be carefully considered in patients receiving antithrombotic therapy, especially anticoagulation (GOR A; LOE high).
13. IV thrombolysis should not be recommended to patients who are taking anticoagulants and meet any of the ineligibility criteria based on anticoagulant marker value and time after the last dose (GOR D; LOE high).
14. IV thrombolysis may be considered after the use of idarucizumab for patients who are taking dabigatran and considered ineligible for IV thrombolysis based on anticoagulant marker value and time after the last dose (GOR C1; LOE low).
Key Points of The “Consensus Guides on Stroke Thrombolysis and Thrombectomy for Anticoagulated Patients, November 2017”
Caution should be exercised in determining patient eligibility for IV thrombolysis because the incidence of bleeding complications, including intracranial hemorrhage, is higher in patients receiving antiplatelet therapy or anticoagulation compared with untreated patients. In particular, direct oral anticoagulants (DOACs) obtained commercial approval in Japan and overseas before and after preparing the second edition of these guidelines (2012), and the second edition should be prepared in terms of eligibility for IV thrombolysis during medication with DOAC without sufficient evidence. The Working Group from the Committee for Improvement of Medical Improvement and Social Insurance of the Japan Stroke Society published the Consensus Guides on Stroke Thrombolysis and Thrombectomy for Anticoagulated Patients in 2017 based on further new findings.6) Table 4 shows the key points of recommendations. The changes in recommendations from the second edition of present guideline will be briefly explained. See also the above consensus guides and review of the commentary.56)
Table 4.
Recommendations on intravenous thrombolysis in patients on anticoagulation
|
General Eligibility for IV Thrombolysis in Patients Treated with DOAC
The recommendations since the first edition is continued in patients receiving warfarin or heparin, and such patients should be considered ineligible for IV thrombolysis if they have an international normalized ratio (INR) of prothrombin time (PT) of >1.7 or activated partial thromboplastin time (aPTT) of >1.5 times the baseline value (>approximately 40 s only as a guide, although absolute values vary among reagents). In the second edition, patients treated with DOACs should be ineligible for IV thrombolysis if they have an INR of >1.7 or an aPTT of >1.5 times the baseline value as a tentative cutoff. Subsequently, the results of nationwide questionnaire survey showed that none of the 71 patients treated with DOACs who underwent IV thrombolysis developed sICH with an increase in the NIHSS score of ≥4 occurred.57) The same criteria for coagulation markers applied for patients treated with DOACs in the third edition. Because INR or aPTT does not reflect the intensity of DOACs in a sensitive manner and are not always appropriate indicators to predict bleeding tendency after IV thrombolysis in patients receiving DOACs, there is room to change the criteria, as well as development and dissemination of other coagulation makers. It is appropriate to determine patient eligibility for IV thrombolysis according to the criteria accepted scientifically and ethically within each institution where markers reflecting more exactly the intensity of DOAC such as (diluted) thrombin time, ecarin-clotting time, and anti-factor Xa activity can be measured.
Blood concentration of DOACs reaches the maximum level at 1–4 h, and prothrombin time, international normalized ratio (PT-INR) or aPTT is often within the normal ranges immediately after dosing. The second edition specified that special care should be taken in determining patient eligibility for IV thrombolysis in patients on regular DOAC until approximately 12 h (corresponding to approximately half-life), but treatment within a given time period is not set as a criterion of contraindication. The above-mentioned nationwide questionnaire revealed that asymptomatic intracranial hemorrhage occurred in eight of 13 patients when IV thrombolysis or endovascular therapy was performed within 4 h after the last dosing of DOAC, suggesting that the incidence was higher than treatment beyond 4 h performed in four of 39 patients, and the potential risks were posed to provide IV thrombolysis early after the last dosing.57) A single-center study also demonstrated that intracranial hemorrhage occurred in three of 11 patients treated with IV thrombolysis within 6 h after the last dosing of DOACs and in one of 11 patients receiving treatment beyond 6 h.56) The third edition recommended that patients should be ineligible for IV thrombolysis if it is confirmed that thrombolysis is performed within 4 h of the last dose of DOACs, regardless of coagulation marker levels.
Eligibility for IV Thrombolysis in Patients Treated with Dabigatran
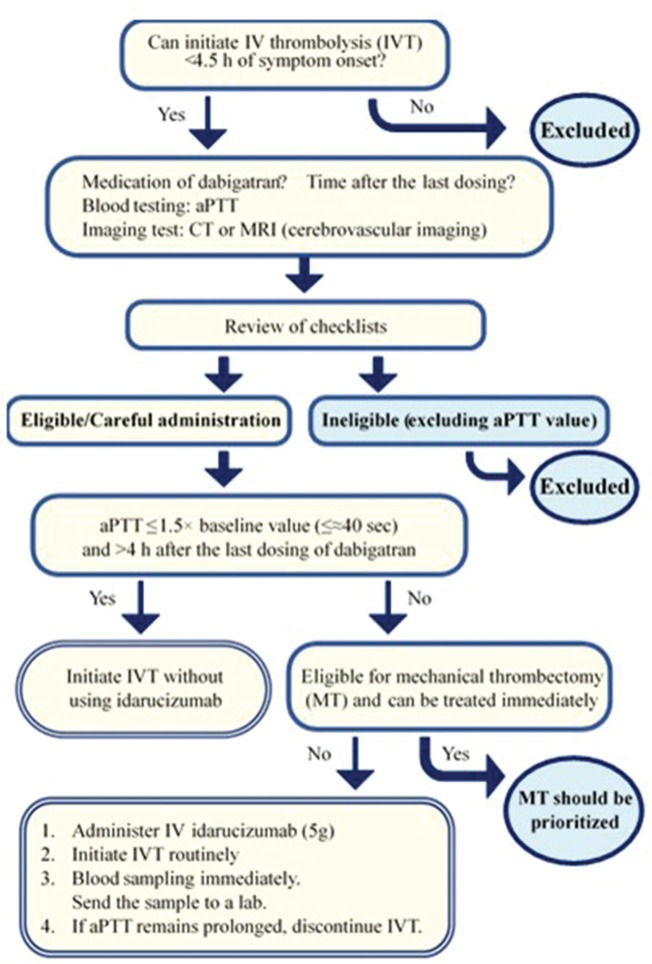
Idarucizumab, a specific neutralizer of dabigatran, is thought to selectively inactivate dabigatran without activating the entire coagulation system. Therefore, if patients treated with dabigatran develop stroke, IV thrombolysis can be safely provided in theory when dabigatran is inactivated by idarucizumab. Of 48 reported patients treated with dabigatran who received IV thrombolysis followed by idarucizumab until February 2018, 30 were considered to show improvement in the mRS score of 0–2, and 3 were dead.56) In Europe, where was reported in 35 of 48 patients, a growing body of clinical guides based on the expert opinion were published to recommend IV thrombolysis followed by idarucizumab.58,59) The Consensus Guides on Stroke Thrombolysis and Thrombectomy for Anticoagulated Patients indicated detailed recommendations on acute reperfusion therapy in patients treated with dabigatran taking account of preceding guides from Europe and eligibility criteria on coagulation markers in Japan (Fig. 1). The outlines are as follows:
IV thrombolysis can be considered without pretreatment with idarucizumab if aPTT is ≤1.5 times the baseline value (≤approximately 40 s only as a guide) and the time of the last dose is >4 h. Additional mechanical thrombectomy can be considered if indicated.
Direct mechanical thrombectomy can be considered without pretreatment with idarucizumab or alteplase if eligible, when aPTT is >1.5 times the baseline value or the time of the last dose is or may be ≤4 h.
Treatment with 5 g idarucizumab, followed immediately by IV thrombolysis, can be considered if mechanical thrombectomy cannot be quickly performed for patients whose aPTT is >1.5 times the baseline value or the time of the last dose is or may be ≤4 h. A blood sample should be collected and sent to the laboratory immediately after initiating thrombolysis. If aPTT is not normalized, alteplase should be discontinued immediately.
Fig. 1.
Guidelines for Practice of IV Thrombolysis for Stroke that Developed in Patients Treated with Dabigatran. Excerpt from Special Working Group in the Committee on Medical Improvement and Social Insurance, Japan Stroke Society.6)
Before and after publishing the Consensus Guides on Stroke Thrombolysis and Thrombectomy for Anticoagulated Patients, IV thrombolysis after idarucizumab was reportedly performed in Japan.60,61) In the third edition, IV thrombolysis is considered after administration of idarucizumab according to the above-mentioned outlines. These recommendations may be changed depending upon the progress of further studies.
5. Required elements for medical institutions
(Recommendations)
- 15. IV thrombolysis should be provided by medical institutions that have the following resources and processes:
- Brain CT or MRI, general blood tests, blood coagulation tests, and electrocardiography available.
- A stroke physician starts evaluation after the patient’s arrival as soon as possible.
- A system in place that makes neurosurgical procedures readily available by neurosurgeons if needed (GOR A; LOE high).
16. The telemedicine for acute stroke care (telestroke) can safely provide IV thrombolysis even in the absence of acute stroke physicians in the clinical setting (GOR C1; LOE middle).
Intravenous thrombolysis has to be initiated within 4.5 h of symptom onset. Therefore, it is desirable that medical institutions accepting stroke patients establish a hotline to collaborate with ambulance staff and provide emergency patient visits. A system in place has to be established where thrombolysis can be initiated by acute stroke physicians who are specialized in the present guidelines as soon as possible after the patient’s arrival at the hospital, and CT and MRI scans or electrocardiography that are necessary for diagnosis of stroke and general blood test and blood coagulation tests required to determine eligibility for IV thrombolysis have to be available. It is desirable that the stroke physicians have taken a training course for e-learning for the proper use of alteplase approved by the Japan Stroke Society, and non-participants try to take training course early. In addition, if neurosurgical procedures are needed, a system in place that makes these procedures immediately available may be necessary.62) In medical institutions that cannot meet these conditions 24 h a day, an acceptable time period should be stated. In a community in the absence of acute stroke physicians who are specialized in the present guidelines or in a medical institution where there is a time period with an absence of full-time stroke physicians, remote stroke treatment (telestroke) may enable IV thrombolysis safely even in the absence of the stroke physicians in the clinical setting and is admissible as a system for remote locations.63–66) In addition, for neurosurgical procedures, if a system is established so that a prompt response can be made within at least 2 h by neurosurgeons, a response could be also made by the transportation of patients to other hospitals or on-call neurosurgeons. A prompt response made within 2 h is only defined as a consensus-based value, and it is desirable to have a system where a response can be made as soon as possible.62)
It is desirable that management after IV thrombolysis is performed in stroke care units (SCU) and their equivalent intensive care units.67) It is known that thrombolysis for patients who did not meet eligibility criteria significantly increases the risk of sICH, and it is desirable to provide the patient database to monitor the treatment and outcomes and to continue the Plan, Do, Study, Act (PDSA) cycles of a program to improve the quality of medicine in hospitals where thrombolysis is performed.62,68) If the hospitals cannot provide enough management for thrombolysed patients, interhospital transfer after thrombolysis (drip and ship) should previously be established in collaboration with neighboring hospitals.
6. Flow of events from symptom onset to hospital arrival
(Recommendations)
17. To ensure the proper performance of IV thrombolysis, efforts should be made to raise public enlightenment and improve prehospital life support by ambulance staff, facilitating the rapid arrival of patients at the hospital (GOR B; LOE middle).
18. When medical personnel inside the hospital receive the initial report on the patient, they should make an effort to proceed with preparations within the hospital so that a prompt response can be made after the patient’s arrival (GOR A; LOE middle).
Educational enlightenment should be raised to indicate that stroke is an emergency for not only medical professionals but also for the public. Stroke includes ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage, but it is difficult to make differential diagnoses of these stroke subtypes in the prehospital setting. The community stroke transportation program should be prepared in accordance with IV thrombolysis or mechanical thrombectomy for stroke with stricter time limits for treatment. Education and public enlightenment of medical professionals and the public are raised to focus the methods to determine the stroke symptoms and the actions to be taken when recognizing them, and public enlightenment is raised to take the action of emergency medical attention such as use of emergency medical service even if the symptoms disappear.69) The means for raising public awareness that have been reported to be effective include the means for assessing face, arm, speech test, and taking action for emergency medical attention, civic education through open lectures or media, such as television70) and school education.71)
The observation, care, and prehospital triage of patients are standardized to ensure that ambulance staff appropriately transport patients with a possible stroke to specialized facilities,72) hospitals where acute reperfusion therapy, including IV thrombolysis and mechanical thrombectomy, should be clarified to optimize the means for transporting patients according to the local medical resources. Ambulance staff make a triage of patients with a possible stroke and hospital selection using the prehospital stroke scale73–78) defined in a community where severity and specificity have been verified, and they inform the emergency hospital of the transportation of patients with the potential to perform reperfusion therapy as soon as possible to reduce the time to initiate treatment after the patient’s arrival at the hospital.79,80)
7. Medical history, medical examination, and laboratory testing
(Recommendations)
19. At the initial presentation, efforts should be made to consider differential diagnoses other than stroke to the extent possible (GOR A; LOE low).
20. An objective assessment of neurological severity using the National Institutes of Health Stroke Scale should be performed (GOR A; LOE low).
21. In laboratory testing, patients should be assessed for bleeding tendencies or risk factors of symptomatic intracranial hemorrhage (GOR A; LOE low).
Flow of Clinical Practice after the Patient’s Arrival at the Hospital
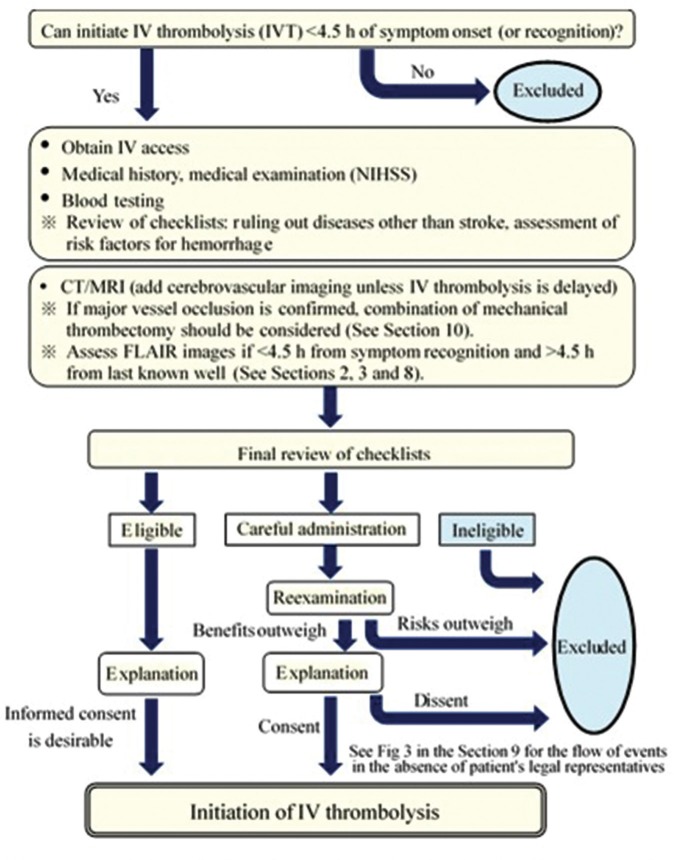
Figure 2 summarizes the flow from the patient’s arrival at the hospital to the initiation of IV thrombolysis. A delay in the diagnosis and examination may lead to a loss of treatment opportunities. For rapid diagnosis and initiation of thrombolysis, a series of medical examinations and tests to treatment after the patient’s visit should not be disrupted. An efficient clinical practice has to be established by the whole hospital, including co-medical and clerical staff, to initiate thrombolysis as early as possible while appropriately determining patient eligibility for thrombolysis. The development of critical path (manuals) suited to the situation of individual institutions or the use of case checklists listed in Table 3 may be useful.
Fig. 2.
Flow from hospital arrival to initiation of IV thrombolysis. The above medical examination, blood testing, and explanation should be performed simultaneously, if possible, and a minimum goal for each institution is <60 min of hospital arrival and initiation of IV thrombolysis (the sooner, the better).
Medical Interview and Treatment
Medical history taking and general medical and neurologic examinations will begin immediately after the patient’s arrival at the hospital. Important here is the diagnosis of stroke. Diseases known as stroke mimics that are frequently misdiagnosed as stroke include seizure without convulsion, migraine, conversion disorder (hysteria), poisoning, metabolic diseases of hypoglycemia or hepatic encephalopathy, brain tumor, chronic subdural hematoma, drug intoxication, encephalitis, Adams–Stokes syndrome, and peripheral vertigo. There have been reports that 1.4–10.4% of patients treated with IV thrombolysis received a final diagnosis of stroke mimics.81,82) There have been reports that the bleeding risk was very low when IV thrombolysis was provided for stroke mimics,82) but there have been reports that patients with seizures or those with brain tumors developed intracranial hemorrhage.83) Thus, unnecessary treatment should be avoided as much as possible.
Appropriate medical history taking, neurologic examinations, and emergency tests should be performed with a focus on stroke mimic. In addition, concurrent acute aortic dissection is defined as contraindications, and medical history taking and the medical examination should pay attention to this point. Based on the medical history, disturbance of consciousness or syncope may occur.84) However, 10–55% of patients with acute aortic dissection, in particular those with neurologic deficits, did not complain of chest and back pain.85,86) In medical examination findings, asymmetry of branchial blood pressure is important.46,47,86)
At the time of revision of the present guidelines, infective endocarditis has been changed from a condition that requires careful administration to a condition that should be considered ineligible. At the time of the medical interview and medical examination, caution should be exercised in condition taking before symptom onset, body temperature, cardiac murmur, or Janeway lesions (micro-abscess associated with palmoplantar bacterial embolus).
Stroke Scales
Neurologic examinations are essential for making a differential diagnosis of stroke, and stroke scales are useful in making an objective assessment of the severity of the stroke. The most commonly used scale is the NIHSS,87) which consists of 15 items, including those related to consciousness, visual fields, gaze, facial palsy, motor and sensory impairments, ataxia, perception, and language, and provides total scores ranging from 0 to 42 with a score of 39 representing the most severe stroke.88) In the J-ACT,1) five of six patients with sICH had an NIHSS score of ≥19. The SAMURAI rt-PA Registry found a significant independent association between NIHSS scores and the frequency of the mRS score of 0–1 at 3 months.23) A meta-analysis of nine randomized controlled trials by the Stroke Thrombolysis Trialists’ Collaborative Group showed that the severity of stroke using NIHSS was associated with an increased risk of sICH after IV thrombolysis and poor neurological outcome.35) In mild cases with the NIHSS score of ≤4 at hospital arrival, this therapy increased the frequency of sICH by 1.5% and the possibility of the mRS score of 0–1 at 90 days by 8.0%; in severe cases with the NIHSS score of ≥22, by 3.7% and 1.0%, respectively. The use of NIHSS is useful for predicting bleeding risk and outcomes. This scale provides a simple and organized medical examination to allow a rapid and reproducible scoring of the neurologic severity of patients and is thus easy to use in multidisciplinary clinical settings, resulting in the establishment of a shared awareness of patients’ signs and symptoms. However, some degree of training is required for an accurate assessment in the acute phase of stroke.87) Treating physicians should become familiar with the NIHSS in advance to ensure proper use according to specified procedures.
Laboratory Testing
Table 5 lists the clinical laboratory tests to be performed before determining patient eligibility for IV thrombolysis. Among others, the identification of bleeding tendencies or risk factors of sICH is the most important to prevent complications associated with IV alteplase. Most of the ineligibility criteria listed in Table 3 are those related to hemorrhage; therefore, patients should be assessed for all of these parameters before administration. However, alteplase can be initiated before confirmation of the platelet count in patients without history of hepatic cirrhosis or hematologic disease. If the platelet count is confirmed as not meeting eligibility criteria after administration, thrombolysis should be immediately discontinued. Also, if neurologic deficits persist after correction of abnormal blood glucose levels and patients may be diagnosed with stroke, they are considered eligible. However, hypoglycemic patients without stroke may persistent neurologic deficits; therefore, eligibility criteria should be carefully determined.
Table 5.
Clinical laboratory tests and imaging tests required before determining patient eligibility for intravenous thrombolysis
| <<Mandatory>> | |
| Assessment of the presence of thoracic aortic dissection | Either plain chest radiography, cervical ultrasonography or chest CT scan is recommended |
| Imaging test | CT or MRI scans of the brain are indispensable |
| Blood test: Blood glucose | Diagnosis of hypoglycemia or hyperglycemia (in patients with possible diagnosis of stroke after correction, it can be administered to such patients) |
| Platelet count | In patients with no medical history of hepatic cirrhosis and hematologic disease, administration may be initiated without waiting for test results |
| <<Necessary in some cases>> | |
| Blood test | In patients with possible metabolic encephalopathy; including electrolyte, ammonia, renal function, blood gas, etc. |
| In patients with possible severe liver disorders: including liver function, bilirubin | |
| In patients treated with anti-factor Xa drugs and warfarin: PT-INR of ≤1.7 | |
| In patients receiving dabigatran, heparin, etc.: aPTT of ≤40 s | |
| Chest CT scan | In patients with possible aortic dissection on plain chest CT, etc. |
| Transthoracic echocardiography | In patients with possible infective endocarditis based on medical history and medical examination findings |
Intravenous thrombolysis is contraindicated in patients with concurrent acute thoracic aortic dissection. Chest radiographs for patients with thoracic aortic dissection may show evidence of widening of the mediastinal shadow.89,90) Carotid ultrasonography is also useful for diagnosis of aortic dissection.90,91) Chest CT is continuously performed to provide a definitive diagnosis in patients with possible acute aortic dissection,91–93) and if aortic dissection cannot be ruled out, thrombolysis should be avoided.46,47)
In the third edition, patients with confirmed infective endocarditis were considered ineligible for this therapy. However, no clinical laboratory tests are available to screen for the presence of infective endocarditis rapidly and accurately during the acute phase of stroke. For patients who are strongly suspected of infective endocarditis based on medical history and medical examination findings, transthoracic echocardiography should be considered.
8. Diagnostic imaging of the brain and cervical arteries
(Recommendations)
22. Intracranial hemorrhage should be ruled out using a non-contrast CT or MRI scan, and the degree of early ischemic change be assessed immediately before IV thrombolysis (GOR A; LOE high).
23. IV thrombolysis is not recommended to patients with large early ischemic changes for the consequence of high-risk symptomatic intracranial hemorrhage (GOR D; LOE high).
24. Although cerebrovascular assessment is not mandatory prior to IV thrombolysis, it is strongly recommended to assess large vessel occlusion using CTA or MRA immediately after initiation of IV thrombolysis if mechanical thrombectomy should be considered for such patients (GOR A; LOE high).
25. Diagnostic imaging should be limited to the minimum necessary to avoid the delay of IV thrombolysis (GOR A; LOE low).
Key Points of Diagnostic Imaging
Non-contrast CT or MRI scans are necessary to distinguish ischemic or hemorrhagic stroke since it is a difficult challenge only from the basis of clinical symptoms. It is required to rule out hemorrhage and screen for the presence of early ischemic change (EIC) with certain speed and accuracy. Patient eligibility for IV thrombolysis should be determined by those who are specialized to brain image readings such as stroke specialists. Another purpose of diagnostic imaging is the confirmation of vascular lesions. A cerebrovascular assessment is not mandatory prior to IV thrombolysis although a detection of the large vessel occlusion should be done using CT angiography (CTA) or MRA to determine the eligibility for mechanical thrombectomy. Cervical ultrasonography is also useful for ruling out thoracic aortic dissection. Table 6 lists major diagnostic imaging techniques.
Table 6.
Diagnostic imaging techniques for hyperacute stroke
| CT | MRI | Ultrasonography and others | |
|---|---|---|---|
| Hemorrhage | Non-contrast CT | T2*-weighted images | |
| Ischemic lesions | Non-contrast CT CTA source images | Diffusion-weighted images | |
| Vascular assessments | CTA | MRA | Cervical ultrasonography, transcranial Doppler, transcranial color-flow imaging, cerebral angiography |
| Perfusion assessment | CT perfusion imaging | MR perfusion imaging |
Non-contrast CT Scan
Computed tomography is an important diagnostic imaging modality that is initially performed especially in diagnosing hemorrhagic lesions. Since the IV thrombolysis has begun, large interest has been given to subtle changes in the hyperacute CT scans named early CT signs.94–102) These findings include disappearance of the lentiform nucleus, loss of the insular ribbon, loss of gray-white differentiation, and effacement of cortical sulci, all of which are consistent with the presence of brain tissue ischemic lesions,94–96) and as a vessel occlusion sign, hyperdense MCA signs (occluded main trunk of the MCA)96–100) and dot signs (occluded branches of the MCA)101,102) are known to be consistent with the presence of vascular occlusions. Among these findings, EIC findings can be integrated into changes associated with slightly decreased density in the gray matter and slight edema in the cerebral cortex. The presence of at least two of the findings; disappearance of the lentiform nucleus, loss of the insular ribbon, and hypo attenuation in gray-white matter, are strongly associated with poor outcomes.100) Combinations of these three findings are reported as a predictor of vessel occlusions.103)
Accurate assessment of EIC is challenging. Thirty-one percent of EIC are detectable within 3 h,104) and 81% be detectable within 5 h of onset of the MCA occlusion,105) while it has been also reported that interrater agreement was 0.14–0.78, sensitivity was 20–87%, and specificity was 56–100%.106) For more accurate assessment, CT is required to be arranged to provide a higher level of contrast between the gray and white matters. The Middle Cerebral Artery Embolism Local Fibrinolytic Intervention Trial (MELT)-Japan107) involved the standardization of CT protocol prior to the trial. The use of standardized CT improved the accuracy of interpretation for early CT signs.108) Conditions for the optimization are shown in Table 7. Continuous interpretation training will also improve accurate early CT sign assessment.109) Online EIC interpretation training programs are available on the “Acute Stroke Imaging Standardization Group–Japan (principal investigator: Makoto Sasaki)” (ASIST-Japan; asist.umin.jp) websites.110)
Table 7.
Conditions for CT to evaluate early CT signs (Cited and modified from Acute Stroke Imaging Standardization Group-Japan110))
|
In recent years, the ASPECTS has been commonly used for the assessment of the extent of EIC.111–114) The ASPECTS is a method for the assessment of the extent of a lesion using the point deduction scoring system, where the MCA territory is divided into 10 regions of interest on two CT slices (an axial slice that passes through the lentiform nucleus and thalamus and the first axial slice where the lentiform nucleus is invisible approximately 2 cm cranial to the former slice). Generally, an ASPECTS of 7 is estimated to represent one-third of the MCA territory. The extent of EIC, as measured by ASPECTS, has been significantly correlated with the time from symptom onset to CT scanning and the residual cerebral blood flow in ischemic regions.114) Therefore, the extent of EIC and the accuracy of the assessment of ASPECTS will increase after hours passed, but often difficult to determine changes during the hyperacute phase. In recent years, automated ASPECTS Interpretation software (e.g. RAPID CT, e-ASPECTS) utilizing automated machine learning (deep learning) using artificial intelligence has been also released,115) but further assessment will be needed for its accuracy.
Computed tomography angiography source images are also commonly used for assessment of EIC findings in some countries.116,117)
CT-based Treatment Eligibility
Patients with intracranial hemorrhage and those with recent ischemic stroke (within a month) are ineligible for IV thrombolysis. The presence of extensive EIC may be a predictor for poor outcome or intracranial hemorrhage since EIC are associated with time from symptom onset or severity of stroke.
The ECASS revealed that the presence of EIC involving one-third or more of the MCA territory was associated with an increased incidence of intracranial hemorrhage, and patients with EIC involving less than one-third of the territory benefited the most from IV thrombolysis.11,118) In contrast, the NINDS rt-PA Stroke Study found that EIC involving more than one-third of the MCA territory were observed in 14% of patients with no significant association between the presence of extensive EIC and outcomes or sICH.104) A reassessment of these findings using ASPECTS indicated that patients with an ASPECTS of ≤2 had a higher incidence of sICH than those with an ASPECTS ≥3 (20% vs. 5%), and the effect of IV thrombolysis tended to be greater in patients with an ASPECTS ≥8.119) In the ECASS II, the incidence of parenchymal hemorrhage (PH 1,2) and sICH among patients with an ASPECTS ≤7 was 19-fold and fivefold higher, respectively, in the alteplase group than the placebo group, with no significant association between ASPECTS and functional outcome at 3 months.120) The J-ACT also found that lower ASPECTS were associated with an increased risk of sICH but with no significant association between ASPECTS and outcome at 3 months.121) Based on these findings, IV thrombolysis is not recommended to patients with evidence of extensive EIC because a greater extent of EIC increases the risk of sICH. However, the criterion for the term ‘‘extensive” is difficult to define. Evidence that shows “EIC changes in more than one-third of the MCA territory (i.e., an ASPECTS of <7) represents extensive EIC” is limited. It has been reported that the ischemic core on CT perfusion (cerebral blood flow <30% territory in contralateral side) of ≤50 mL, as measured by volumetric measurement software, was correlated with ASPECTS ≥6,122,123) and patients with the ischemic core on CT perfusion of ≥50 mL are ineligible for IV thrombolysis, which may be lead to unfavorable outcomes (the malignant profile).124) However, caution should be taken in defining the extensive EIC and determining eligibility for this therapy since volumetric measurement software is not popular in Japan.
Magnetic Resonance Imaging
Diffusion-weighted imaging can visualize the decreased water diffusion in cerebral ischemic tissues and reveal the EIC several minutes after the symptom onset.125) DWI are advanced in providing clear visualization of the EIC with a high sensitivity and specificity126,127) and also is excellent at visualizing the brain stem and cerebellum and cortical/subcortical microlesions, which are practically difficult in CT scans. Although FLAIR images do not provide visualization of ischemic changes as early as DWI, it has been recently suggested that a mismatch between DWI and FLAIR may be used to assume that the case is within 4.5 h of symptom onset,38,39) and efficacy of IV thrombolysis has been recently reported.20) In contrast, MRI has been considered to have poor detection of hyperacute intracranial hemorrhage. However, it has been indicated that susceptibility images, such as T2*-weighted images, provides similar or better detection than CT.128–130) The use of T2*-weighted images also identifies microbleeds which cannot be detected by CT scans. Low signal intensity changes in the cerebral artery lumen on T2*-weighted images are considered as arterial occlusion by venous thrombus and are called susceptibility vessel signs.131) Perfusion-weighted imaging (PWI) on MRI allows for a relative hemodynamic measurement. The greatest advantage of MRI, including MRA described later, is that additional information can be obtained by a single run. However, only the minimum essential imaging examination should be performed over a short period of time in determining eligibility for IV thrombolysis since it is time consuming and should not slow down thrombolysis itself.
Standardization of images is essential for the assessment of EIC based on DWI. Unlike CT and standard MRI (T1-, T2-weighted images), display conditions matters in DWI for accurate diagnosis since no reference structure for signal intensity (e.g., bone or water) is available. ASIST-Japan has proposed a standardization method using b = 0 images,132) which have been incorporated in recent MRI systems. In addition, DWI-ASPECTS have been widely used, in which originally designed for use in CT. DWI ASPECTS seems to have lower scores than CT by 0.5–0.9 points.133,134) This may be related to the clear appearance of abnormal signals with DWI compared with CT, which the detection of acute ischemic lesions in the white matter are more difficult in CT than MRI.
A reversible cerebral ischemic region that does not end to be a stroke is called an ischemic penumbra. The ischemic penumbra has been discussed as a DWI–PWI mismatch on MRI.135) In other words, when lesions of abnormal perfusion on PWI are larger than the lesions on DWI, early recanalization may not cause a stroke in the abnormal perfusion lesion. Early DWI lesions may be reversible with acute reperfusion therapy,136,137) but often coincide with the final infarcts, and cellular edema and pathological necrosis may not always coincide; therefore, it is important to compare with the clinical information. The DEFUSE study15) in patients within 3–6 h of symptom onset indicated that reperfusion after IV thrombolysis was associated with favorable outcomes in the group of patients with evidence of DWI–PWI mismatch. EPITHET in patients within 3–6 h of symptom onset17) found that IV thrombolysis tended to reduce the extension of infarcts in the presence of a DWI–PWI mismatch. As mentioned above, attempts have been made to include the presence of a DWI–PWI mismatch in the inclusion criteria to extend the therapeutic time window to 9 h in recent trials, such as the EXtending the time for Thrombolysis in Emergency Neurological Deficits (EXTEND) trial.138)
MRI-based Treatment Eligibility
In recent years, an increasing number of medical institutions in Japan have determined patient eligibility for IV thrombolysis on the basis of MRI alone without CT scanning. Since MRI has high prevalence in Japan, the eligibility based on MRI alone can be thought as a practical option.
The assessment of EIC using DWI-ASPECTS and clinical outcomes of IV thrombolysis had been sporadically reported in Japan. The SAMURAI rt-PA Registry reported that significant correlations were found between DWI-ASPECTS ≤6 and the mRS of 3–6 at 3 months, DWI-ASPECTS ≤5 and sICH, DWI-ASPECTS ≤4 and death in total of 477 patients.139) Kimura et al.140) reported that DWI-ASPECTS ≤5 were significantly correlated with NIHSS ≥20 at 7 days. Therefore, although IV thrombolysis is not recommended for patients with extensive EIC, the criterion of the definition ‘‘extensive’’ is difficult. There are techniques for calculating DWI volumes from threshold of apparent diffusion coefficient, and with an ischemic core of ≥70 mL on DWI, which is defined as malignant profile, are ineligible for IV thrombolysis in many clinical trials,124,141) However, the volumetric measurement software such as RAPID is only installed in few hospitals in Japan,142) and quantitative assessment of ischemic core is not commonly performed.
In patients who had stroke with an unknown time of onset, the time of symptom onset can be estimated based on a mismatch between DWI and FLAIR imaging. In the WAKE-UP trial conducted on the basis of this hypothesis, FLAIR imaging was conducted under the standardized conditions as shown in Table 8.20) Online imaging reading training programs are available on the THAWS trial40) in Japan (https://thaws.stroke-ncvc.jp/index.html).
It has been reported that the incidence of sICH after IV thrombolysis was doubled in patients with asymptomatic cerebral microbleeds identified on T2*-weighted images compared with those without microbleeds (5.8% vs. 2.7%), although no significant difference was found.143) It has been also reported that if the number of microbleeds was ≥11, patients have worse outcomes for IV thrombolysis, while this is a shred of the limited evidence not to perform IV thrombolysis to these patients.55,144) There are reports that susceptibilities vessel signs on T2*-weighted images found in the occluded internal carotid artery or the horizontal part of the MCA is associated with poor recanalization rate after IV thrombolysis145,146) and thought to be a predictor of poor outcome after treatment.
Although DWI–PWI mismatch is a useful indication to determine eligibility for IV thrombolysis, the additional PWI is not recommended to patients within 4.5 h of symptom onset routinely in light of the importance of initiation of IV thrombolysis as early as possible. However, this can be a useful diagnostic tool for stroke patients with unstable neurological symptoms and stroke mimics.
Nuclear Medicine Examination
A semi-qualitative assessment using brain perfusion single photon emission CT (SPECT) can be performed relatively fast and has been reported to be useful for the prediction of the severity of ischemia and the development of intracranial hemorrhage in stroke patients within 6 h of symptom onset.114,147) However, the routine use of SPECT is not recommended to IV thrombolysis eligible patients within 4.5 h of symptom onset in light of the importance of the initiation of thrombolysis as early as possible.
Cerebrovascular Assessments
Table 9 lists the diagnostic imaging modalities indicated mainly in cerebral and cervical vessels. CTA and MRA have the advantage of providing vascular assessment following the assessment of brain lesions by CT or MRI. Carotid ultrasonography, transcranial Doppler (TCD), and transcranial color-flow imaging (TC-CFI) can be used for assessments at the bedside and at emergency rooms to provide real-time monitoring in recanalization of occluded arteries. TCD and TC-CFI have been suggested to accelerate thrombolysis.148,149) To the patients suspected of acute aortic dissection, carotid ultrasonography can assess the dispersion of dissection by observing the common carotid artery.46,47) Cerebral angiography is invasive but allows to locate the occluded vessels and assess the collateral circulation and, subsequently, can be used in mechanical thrombectomy. At present, as the effect of IV thrombolysis alone is insufficient to patients with the large vessel occlusion in the anterior circulation, assessment of occluded vessels is mandatory and mechanical thrombectomy should be performed immediately. Sometimes CTA or MRA may be time consuming, but assessment of the large vessel occlusion by these modalities at the baseline in parallel with thrombolysis are highly essential in patients who can receive thrombolysis within 4.5 h of symptom onset.
Table 9.
Guidelines for management within the first 24 h after initiation of intravenous thrombolysis
|
9. Determination of patient eligibility and informed consent
(Recommendations)
26. If patients are eligible, it is desirable that the patients or their legal representatives are informed of the potential benefits and risks of IV thrombolysis and provide informed consent to the extent possible (GOR C1; LOE low).
27. If patients require careful administration, it is necessary that the patients or their legal representatives provide informed consent (GOR B; LOE low).
Determination of Patient Eligibility
Based on medical history taking, medical examinations, laboratory tests, and diagnostic imaging findings, a rapid determination should be made as to whether patients are eligible for, require careful administration, or are ineligible for IV thrombolysis. If patients are eligible or require careful administration, the patients or their legal representatives should be immediately informed of thrombolysis. IV thrombolysis is not recommended in patients who are ineligible.
Informed Consent
It is desirable that patients or their legal representatives should be informed of the potential benefits and risks of IV thrombolysis and provide informed consent before initiation of thrombolysis because thrombolysis may cause intracranial hemorrhage as a complication. However, in clinical practice, it is difficult to secure enough time to spare for providing an explanation of thrombolysis, which has its limited therapeutic time window. In addition, it is also often the case that legal representatives of patients, who often cannot understand the explanation provided or are not able to determine consent, cannot be found within the limited time window for IV thrombolysis. Guidelines in Western countries recommend that an explanation of the potential benefits and risks of treatment should be provided to the extent possible, but do not state that written informed consent is required.13,14)
A questionnaire survey performed in Japan by the Research on Bioethics in Severe Stroke group found that 15 of 19 hospitals have no institutional policy for informed consent in the absence of patients’ legal representatives in place and IV alteplase was not used in nine of 25 cases of lack of informed consent in the absence of the representatives.150) A post-hoc analysis (unpublished) of the J-MARS indicated that the mRS score of 0–1 at 3 months was achieved in as high as 45.1% of eligible patients with no conditions requiring careful administration, which was higher than previously reported in Japan and overseas, and a mortality of 5.7% was lower than previously reported. In patients requiring careful administration, corresponding values were 26.9% and 16.5%, respectively. Therefore, it is considered that the potential disadvantages experienced by eligible patients with no conditions requiring careful administration of avoiding IV thrombolysis because of the absence of their legal representatives may significantly outweigh the potential risks experienced by patients of adverse drug reactions.
Based on these findings, the Research on Bioethics in Severe Stroke group recommended the policy that it is desirable—but not mandatory—that if IV thrombolysis is to be performed in eligible patients with no conditions requiring careful administration, the patients or their legal representatives should be informed of the potential benefits and risks of the therapy to the extent possible and provide informed consent. A scenario should be avoided in which the absence of legal representatives precludes patients from receiving thrombolysis.150) The present guidelines adhere to this policy.
If patients require careful administration, it is essential that the patients or their legal representatives should be provided informed consent. In case of the absence of legal representatives, medical institutions should predefine the policy of determination of eligibility for IV thrombolysis based on their current treatment outcomes. Besides, to avoid the independent judgement of the treating physician and to make rational decisions in the absence of legal representatives, thrombolysis can be initiated after a consensual decision involving members of the medical care team only if thrombolysis in the relevant patient clearly outweighs failure to perform thrombolysis in terms of patient’s benefits. Even in this case, efforts should be made to make contact with patients’ legal representatives as soon as possible after initiation of thrombolysis and provide an explanation of the rationale for determination of patient eligibility for thrombolysis. Figure 3 shows the flow of events in the absence of patients’ legal representatives.
Fig. 3.
Flow of events in case of lack of a patient’s ability to understand explanation for judgement when the patient’s legal representative is absent.
Actual Condition of Informed Consent
In clinical practice, it is difficult to secure enough time to spare for providing explanations. Provision of explanations to patients is often started in parallel with the medical examination and tests to prevent a delay in initiation of IV thrombolysis. The use of written information for patients summarizing the main points helps patients to gain a rapid and accurate understanding. An example of representative written information for patient is shown in a table151) (omitted in this English version).
10. Endovascular therapy
(Recommendations)
28. Patients should receive mechanical thrombectomy regardless of IV thrombolysis if they meet all the following criteria: (1) the mRS scores before symptom onset of 0–1; (2) occlusion of the internal carotid artery or the segment M1 of the MCA; 3) ASPECTS ≥ 6 on brain CT or diffusion-weighted MRI scans; (4) the NIHSS score ≥6; (5) ≥18 years of age; and (6) treatment can be initiated within 6 h of symptom onset (GOR A; LOE high).
29. Patients eligible for IV thrombolysis should receive thrombolysis before initiation of mechanical thrombectomy (GOR A; LOE high).
30. The initiation of mechanical thrombectomy should not be delayed due to confirmation of efficacy for IV thrombolysis (GOR D; LOE high).
31. Local fibrinolytic therapy with urokinase within 6 h of symptom onset can improve outcomes of patients with the middle cerebral artery occlusion (GOR B; LOE high).
Mechanical Thrombectomy
Randomized controlled trials comparing the efficacy of mechanical thrombectomy to medical therapies including IV thrombolysis in patients with acute ischemic stroke due to the large vessel occlusion of the anterior circulation, i.e. Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in The Netherlands,152) Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion with Emphasis on Minimizing CT to Recanalization Times,153) EXTEND-Intra-Arterial (EXTEND-IA),154) Solitaire FR with the intention for thrombectomy as primary endovascular treatment for acute ischemic stroke,155) and randomized trial of revascularization with Solitaire FR device versus best medical therapy in the treatment of acute stroke due to anterior circulation large vessel occlusion presenting within 8 h of symptom onset,156) have clarified that thrombectomy improves the functional outcomes at 3 months after the onset. The Highly Effective Reperfusion evaluated in Multiple Endovascular Stroke Trials (HERMES) study,157) a pooled analysis of the five trials, showed that the mRS score at 90 days significantly improved in patients treated with thrombectomy (adjusted common odds ratio 2.49, 95% CI 1.76–3.53), and the proportion of independency (the mRS score of 0–2) at 90 days was significantly high (46.0% vs. 26.5%). There was no significant difference in mortality at 90 days (15.3% vs. 18.9%) and sICH within 5 days (4.4% vs. 4.3%).
If mechanical thrombectomy can be initiated as soon as possible in patients with the large vessel occlusion, the clinical benefits will increase. The secondary analysis on time between symptom onset and the initiation of this therapy (arterial puncture) and outcomes in HERMES study158) indicated that the common odds ratio was 2.79 (95% CI 1.96–3.98) after 3 h of symptom onset, 1.98 (1.30–3.00) after 6 h of symptom onset, and 1.57 (0.86–2.88) after 8 h of symptom onset, and the efficacy of thrombectomy declined with a longer time from symptom onset. In addition, among 390 patients who achieved substantial reperfusion with thrombectomy, each 1-h delay to reperfusion was associated with a decline in 19% of patients having better outcome at 3 months (OR 0.81, 95% CI 0.71–0.92). Therefore, mechanical thrombectomy should be initiated as early as possible in eligible patients who are, and should not be delayed because of confirmation of the efficacy of IV thrombolysis.
In the HERMES study, IV thrombolysis was performed in 83% of patients treated with mechanical thrombectomy and 87% of those treated with medical therapies, and there was no difference in the efficacy of mechanical thrombectomy and mortality with or without alteplase. The results of a pooled analysis of 13 studies investigating the association with the presence or absence of IV thrombolysis before mechanical thrombectomy and outcomes showed that patients treated with prior IV thrombolysis had better outcomes at 3 months (the mRS of 0–2) (OR 1.27, 95% CI 1.05–1.55), lower mortality (OR 0.71, 95% CI 0.55–0.91), and higher rate of successful recanalization (OR 1.46, 95% CI 1.09–1.96) without having increased odds of sICH (OR 1.11, 95% CI 0.69–1.77) compared with those without prior IV thrombolysis.159) Therefore, patients who are eligible for IV thrombolysis should receive thrombolysis before initiation of mechanical thrombectomy other than research purposes, because skipping thrombolysis for such patients is ethically problematic.
Mechanical thrombectomy should be performed in accordance with the Guidelines for Mechanical Thrombectomy, Third Edition.5) The details are not discussed in the present guidelines.
Local Fibrinolytic Therapies
Because the efficacy of local fibrinolytic therapy has been studied only in a limited number of patients, IV thrombolysis should be prioritized in patients who are candidates for this therapy. Local fibrinolytic therapy with intra-arterial administration of alteplase or other thrombolytics is not approved in Japan.
The Prolyse in Acute Cerebral Thromboembolism (PROACT) II study showed that patients treated with local prourokinase as the local fibrinolytic therapy without IV thrombolysis had significant higher rate of successful recanalization and rate of the mRS score of 0–2 at 3 months (40% vs. 25%) and higher incidence of sICH (10% vs. 2%) compared with the control group.160) A meta-analysis of 852 subjects, including subjects in the PROACT II study, found that the local fibrinolytic therapy group had a significantly higher incidence of favorable outcome and sICH and lower mortality, compared with the control group.161) The MELT-Japan, conducted in Japan, showed that local fibrinolytic therapy with urokinase in combination with fragmentation using microcatheters and micro guidewires for the MCA occlusion resulted in a significant increase in the rate of the mRS score of 0–1 at 3 months compared with the control group.107) For patients with the MCA occlusion within 6 hours of symptom onset and who are not candidates for IV thrombolysis, the Guidelines for the Management of Stroke7) lists local fibrinolytic therapies as grade B recommendation, while the American College of Chest Physicians162) recommends local fibrinolytic therapies with alteplase as grade C recommendation. However, the local administration of alteplase is for off-label use.
Since the late 1990s, the efficacy of local fibrinolytic therapy as an add-on therapy to IV thrombolysis, including intra-arterial alteplase, has been evaluated in randomized controlled trials and open-label studies.163–165) No conclusion of efficacy has been drawn, and no evidence is sufficient to make positive recommendations, although the results of these studies suggest that IV thrombolysis in combination with local fibrinolytic therapy may cause no increase in the incidence of sICH.
11. Management after initiation of IV thrombolysis
(Recommendations)
32. The dose of alteplase should be 0.6 mg/kg with 10% of the dose given as an initial bolus, and the remainder infused over 1 h (GOR A; LOE middle).
33. It is recommended that patients be managed in a stroke care unit or an equivalent ward for at least 24 h after initiation of treatment (GOR B; LOE high).
34. During the first 24 h of treatment, control of blood pressure and restriction of antithrombotic therapies are important. If symptoms worsen, a prompt diagnosis should be made, and neurosurgical procedures, such as hematoma evacuation by craniotomy, should be performed as soon as possible as needed (GOR B; LOE low).
Intravenous Administration of Alteplase
Intravenous thrombolysis should be initiated after obtaining informed consent. Alteplase should be reconstituted with the accompanying diluent and a dose of 0.6 mg/kg body weight (348,000 IU/kg) diluted with isotonic NaCl solution, as needed, with 10% of the dose given as an initial bolus over 1–2 min and the remainder infused over 1 h. The maximum dose should be 60 mg (34.8 million IU). The use of syringe pumps or infusion pumps is desirable, although no specific method of continuous infusion is required. Alteplase is commercially available in three package sizes in Japan: 6, 12, and 24 million IU. An example of reconstitution procedures, dose, and method of administration are shown in a table (omitted in this English version).
Management within the Initial 24 h after IV Thrombolysis
It is recommended that patients should be managed in SCUs or equivalent wards for at least 24 h after initiation of IV thrombolysis to be required for close monitoring.13) Management guidelines are shown in Table 9. The key points are control of blood pressure and restriction of antithrombotic therapies up to 24 h after thrombolysis.
Poor outcomes were associated with increased blood pressure within 24 h after IV thrombolysis,166–169) during which blood pressure should be maintained at ≤180/105 mmHg. Antihypertensive agents are mainly used intravenously. The Japanese Society of Hypertension’s Guidelines for the Management of Hypertension 2014 list the following drugs as rescue IV antihypertensive medications for hypertensive emergencies or other conditions: the vasodilators (nicardipine, diltiazem, nitroglycerin, nitroprusside, and hydralazine) and the sympatholytics (phentolamine and propranolol).170) Table 10 shows the three most commonly used drugs. The control of blood pressure after mechanical thrombectomy is in accordance with management after IV thrombolysis.
Table 10.
Most commonly used intravenous antihypertensive medications for hypertensive emergencies (Cited and modified from Eggers et al.149))
| Medications | Administration and dosage | Time to onset of effect (min) | Duration of action (min) | Side effects and other precautions |
|---|---|---|---|---|
| Nicardipine | Continuous IV infusion (0.5–6 μg/kg/min) | 5–10 | 15–30 | Tachycardia, headache, facial flushing, localized phlebitis, etc.; caution should be exercised in patients with increased intracranial pressure |
| Diltiazem | Continuous IV infusion (5–15 μg/kg/min) | <5 | 30 | Bradycardia, atrioventricular block, sinus arrest, etc. |
| Nitroglycerin | Continuous IV infusion (Protect from light) (5–100 μg/min) | 2–5 | 5–10 | Headache, vomiting, tachycardia, methemoglobinemia, tolerance may develop, etc.; protect from light |
The safety and efficacy of anticoagulants, antiplatelet agents, or thrombolytic medications have not been established when administered within 24 h after IV thrombolysis. IV aspirin administered immediately after thrombolysis increased intracranial hemorrhage, with no improvement in outcome.171) In contrast, a study of a small number of patients (n = 65) also reported that argatroban administered as a 48-h continuous IV infusion immediately after thrombolysis caused a relatively higher rate of early arterial recanalization but did not induce large intracranial hemorrhage.172) Basically, anticoagulants, antiplatelets, or thrombolytic medications should not be used within 24 h after thrombolysis. However, the use of heparin (≤10,000 units) during angiography or for the prevention of deep venous thrombosis is allowed even within 24 h after thrombolysis, in which case the risk of intracranial hemorrhage should be considered.16) The recommendation is to perform CT or MRI scans before initiating anticoagulants or antiplatelets for prevention of recurrence of stroke.13)
Most cases of sICH developed within 36 h after initiation of IV thrombolysis.173) Therefore, if worsening of neurologic deficits, heavy headache, nausea/vomiting, or acute increase in blood pressure is observed, CT or MRI should be immediately performed to determine whether or not intracranial hemorrhage is present. If patients are receiving alteplase, administration should be discontinued immediately. If sICH is identified, the primary treatment should be performed in accordance with the Guidelines for Management of Stroke in Japan (Table 9).7) If asymptomatic intracranial hemorrhage is observed, time of initiating antithrombotic therapy for prevention of the recurrence of stroke should be determined carefully.
Other reported serious adverse drug reactions included severe gastrointestinal hemorrhage, pulmonary hemorrhage, or retroperitoneal hemorrhage as well as airway obstruction associated with laryngeal edema, but seemed to be rare. Special caution should be exercised in patients receiving angiotensin converting enzyme inhibitors, because orolingual angioedema may occur. Such edema involving larynx with rapid progression poses higher risk of airway obstruction. If patients are receiving alteplase, appropriate measures, such as immediate discontinuation of administration, maintaining the airway, IV methylprednisolone, subcutaneous injection or inhalation of epinephrine in some cases, and (bronchoscope-guided) tracheal intubation, should be taken.13)
Disclosure Statements
| FY 2016–2018 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| Kazunori Toyoda | / | / | / | A | / | / | A | / | / | / | / | / |
| Masatoshi Koga | / | / | / | A | / | / | A | / | / | / | / | / |
| Yasuyuki Iguchi | / | / | / | B | / | / | A | / | / | / | / | / |
| Ryo Itabashi | / | / | / | / | / | / | / | / | / | / | / | / |
| Manabu Inoue | / | / | / | / | / | / | / | / | / | / | / | / |
| Yasushi Okada | / | / | / | A | / | / | / | / | / | / | / | / |
| Kuniaki Ogasawara | / | / | / | / | / | / | A | / | / | / | / | / |
| Akira Tsujino | / | / | / | / | / | / | / | / | / | / | / | / |
| Yasuhiro Hasegawa | / | / | / | A | / | / | / | / | / | / | / | / |
| Taketo Hatano | / | / | / | / | / | / | / | / | / | / | / | / |
| Hiroshi Yamagami | / | / | / | B | / | A | / | / | / | / | / | / |
| Toru Iwama | / | / | / | A | / | A | A | / | / | / | / | / |
| Yoshiaki Shiokawa | / | / | / | A | / | / | A | / | / | / | / | / |
| Yasuo Terayama | / | / | / | B | / | B | A | / | / | / | / | / |
| Kazuo Minematsu | / | / | / | A | / | / | / | / | / | / | / | / |
Items for COI Self-disclosure
-
Position as an officer or advisor of a company or for-profit organization, and amount of remuneration (Give amount if remuneration exceeds 1,000,000 yen or more from a single company or for-profit organization).
Amount: A: 1,000,000 yen or more B: 5,000,000 yen or more C: 10,000,000 yen or more.
-
Stock ownership and profit from stock (profit from stock for the previous year) (Give amount if annual profit from stock from a single company exceeds 1,000,000 yen, or if ownership is 5% or more of total shares).
Amount: A: 1,000,000 yen or more B: 5,000,000 yen or more C: 10,000,000 yen or more.
-
Remuneration received for patent royalties or licensing fees from companies or for-profit organizations (Give amount if royalties or licensing fees exceeds 1,000,000 yen per year for a single patent).
Amount: A: 1,000,000 yen or more B: 5,000,000 yen or more C: 10,000,000 yen or more.
-
Honoraria such as lecture fees, attending conferences (presentations, providing advice etc.) received from a company or for-profit organization for the time and labor given by the researcher (Give amount if annual honoraria from a single company or organization exceeds 500,000 yen).
Amount: A: 500,000 yen or more B: 1,000,000 yen or more C: 2,000,000 yen or more.
-
Manuscript fees received for writing articles for pamphlets, roundtable discussion articles, etc. from a single company or for-profit organization (Give amount if annual manuscript fees from a single company or organization exceeds 500,000 yen).
Amount: A: 500,000 yen or more B: 1,000,000 yen or more C: 2,000,000 yen or more.
-
Research funding provided by a company or for-profit organization (Give amount if research funding from a single company or organization allocated for medical science research (joint research, commissioned research, clinical trials etc.) exceeds 1,000,000 yen).
Amount: A: 1,000,000 yen or more B: 10,000,000 yen or more C: 20,000,000 yen or more.
-
Scholarship (incentive) donations provided by a company or for-profit organization (Give amount if scholarship (incentive) donations provided by a single company or organization to the affiliated department or division allocated for use exceeds 1,000,000 yen).
Amount: A: 1,000,000 yen or more B: 5,000,000 yen or more C: 10,000,000 yen or more.
-
Endowed departments established through donations by a company (Give amount if affiliation is with an endowed department).
A: present, B: absent.
-
Other remuneration (not directly related to research such as gifts etc.) (Give amount if annual remuneration received from a single company or organization exceeds 50,000 yen).
Amount: A: 50,000 yen or more B: 200,000 yen or more C: 500,000 yen or more.
COI disclosure for spouse, first degree relatives or any persons who share income or property assets with the declarer.
-
Position as an officer or advisor of a company or for-profit organization, and amount of remuneration (Give amount if remuneration exceeds 1,000,000 yen from a single company or organization).
Amount: A: 1,000,000 yen or more B: 5,000,000 yen or more C: 10,000,000 yen or more.
-
Stock ownership and profit from stock (profit from stock for the previous year) (Give amount if annual profit from stock from a single company exceeds 1,000,000 yen, or if ownership is 5% or more of total shares).
Amount: A: 1,000,000 yen or more B: 5,000,000 yen or more C: 10,000,000 yen or more.
-
Remuneration received for patent royalties or licensing fees from companies or for-profit organizations (Give amount if annual royalties or licensing fees exceeds 1,000,000 yen for a single patent).
Amount: A: 1,000,000 yen or more B: 5,000,000 yen or more C: 10,000,000 yen or more.
References
- 1).Yamaguchi T, Mori E, Minematsu K, et al. : Alteplase at 0.6 mg/kg for acute ischemic stroke within 3 hours of onset: Japan Alteplase Clinical Trial (J-ACT). Stroke 37: 1810–1815, 2006 [DOI] [PubMed] [Google Scholar]
- 2).Guideline Committee of the Japan Stroke Society for the intravenous rt-PA (alteplase) in acute ischemic stroke. Jpn J Stroke 27: 327–354, 2005. (Japanese) [Google Scholar]
- 3).Minematsu K, Toyoda K, Hirano T, et al. : Guidelines for the intravenous application of recombinant tissue-type plasminogen activator (alteplase), the second edition, October 2012: a guideline from the Japan Stroke Society. J Stroke Cerebrovasc Dis 22: 571–600, 2013 [DOI] [PubMed] [Google Scholar]
- 4).Guideline Committee of the Japan Stroke Society for the intravenous rt-PA (alteplase) in acute ischemic stroke. Guidelines for intravenous application of rt-PA (alteplase), October 2012, partly revised in September 2016. Jpn J Stroke 39: 43–86, 2017. (Japanese) [Google Scholar]
- 5).Guideline Committee of the Japan Stroke Society, Japan Neurosurgical Society, and Japanese Society for Endovascular Therapy. Guidelines for Mechanical Thrombectomy, the third edition, March 2018. Jpn J Stroke 40: 285–309, 2018. (Japanese) [Google Scholar]
- 6).Special Working Group in the Committee on Medical Improvement and Social Insurance, Japan Stroke Society Updated recommendations on acute reperfusion therapy for anticoagulated patients with ischemic stroke. Jpn J Stroke 40: 123–135, 2018. (Japanese) [Google Scholar]
- 7).Guideline Committee of the Japan Stroke Society for the management of stroke Japanese guidelines for the management of stroke 2015, supplement in 2017. Kyowa Kikaku, Tokyo, 2017 [Google Scholar]
- 8).Guyatt GH, Norris SL, Schulman S, et al. : Methodology for the development of antithrombotic therapy and prevention of thrombosis guidelines: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141: 53S–70S, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group : Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 333: 1581–1587, 1995 [DOI] [PubMed] [Google Scholar]
- 10).Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S: Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA 282: 2019–2026, 1999 [DOI] [PubMed] [Google Scholar]
- 11).Hacke W, Kaste M, Fieschi C, et al. : Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 274: 1017–1025, 1995 [PubMed] [Google Scholar]
- 12).Hacke W, Kaste M, Fieschi C, et al. : Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 352: 1245–1251, 1998 [DOI] [PubMed] [Google Scholar]
- 13).Albers GW, Thijs VN, Wechsler L, et al. : Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol 60: 508–517, 2006 [DOI] [PubMed] [Google Scholar]
- 14).Hacke W, Kaste M, Bluhmki E, et al. : Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 359: 1317–1329, 2008 [DOI] [PubMed] [Google Scholar]
- 15).Davis SM, Donnan GA, Parsons MW, et al. : Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol 7: 299–309, 2008 [DOI] [PubMed] [Google Scholar]
- 16).IST-3 collaborative group. Sandercock P, Wardlaw JM, Lindley RI, et al. : The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet 379: 2352–2363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Anderson CS, Robinson T, Lindley RI, et al. : Low-Dose versus Standard-Dose intravenous alteplase in acute ischemic stroke. N Engl J Med 374: 2313–2323, 2016 [DOI] [PubMed] [Google Scholar]
- 18).Thomalla G, Simonsen CZ, Boutitie F, et al. : MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset. N Engl J Med 379: 611–622, 2018 [DOI] [PubMed] [Google Scholar]
- 19).Powers WJ, Rabinstein AA, Ackerson T, et al. : 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 49: e46–e99, 2018 [DOI] [PubMed] [Google Scholar]
- 20).Ahmed N, Steiner T, Caso V, Wahlgren N, ESO-KSU session participants : Recommendations from the ESO-Karolinska Stroke Update Conference, Stockholm 13–15 November 2016. Eur Stroke J 2: 95–102, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Mori E, Minematsu K, Nakagawara J, et al. : Effects of 0.6 mg/kg intravenous alteplase on vascular and clinical outcomes in middle cerebral artery occlusion: Japan Alteplase Clinical Trial II (J-ACT II). Stroke 41: 461–465, 2010 [DOI] [PubMed] [Google Scholar]
- 22).Nakagawara J, Minematsu K, Okada Y, et al. : Thrombolysis with 0.6 mg/kg intravenous alteplase for acute ischemic stroke in routine clinical practice: the Japan post-Marketing Alteplase Registration Study (J-MARS). Stroke 41: 1984–1989, 2010 [DOI] [PubMed] [Google Scholar]
- 23).Toyoda K, Koga M, Naganuma M, et al. : Routine use of intravenous low-dose recombinant tissue plasminogen activator in Japanese patients: general outcomes and prognostic factors from the SAMURAI register. Stroke 40: 3591–3595, 2009 [DOI] [PubMed] [Google Scholar]
- 24).Aoki J, Kimura K, Sakamoto Y: Early administration of tissue-plasminogen activator improves the long-term clinical outcome at 5years after onset. J Neurol Sci 362: 33–39, 2016 [DOI] [PubMed] [Google Scholar]
- 25).Aoki J, Kimura K, Morita N, et al. : YAMATO Study (Tissue-Type Plasminogen Activator and Edaravone Combination Therapy). Stroke 48: 712–719, 2017 [DOI] [PubMed] [Google Scholar]
- 26).Wahlgren N, Ahmed N, Dávalos A, et al. : Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 369: 275–282, 2007 [DOI] [PubMed] [Google Scholar]
- 27).Multicenter Acute Stroke Trial—Europe Study Group. Hommel M, et al. : Thrombolytic therapy with streptokinase in acute ischemic stroke. N Engl J Med 335: 145–150, 1996 [DOI] [PubMed] [Google Scholar]
- 28).Mori E, Yoneda Y, Tabuchi M, et al. : Intravenous recombinant tissue plasminogen activator in acute carotid artery territory stroke. Neurology 42: 976–982, 1992 [DOI] [PubMed] [Google Scholar]
- 29).Yamaguchi T, Hayakawa T, Kikuchi H: Intravenous tissue plasminogen activator ameliorates the outcome of hyperacute embolic stroke. Cerebrovasc Dis 3: 269–272, 1993 [Google Scholar]
- 30).Kawai C, Yui Y, Hosoda S, et al. : A prospective, randomized, double-blind multicenter trial of a single bolus injection of the novel modified t-PA E6010 in the treatment of acute myocardial infarction: comparison with native t-PA. E6010 Study Group. J Am Coll Cardiol 29: 1447–1453, 1997 [DOI] [PubMed] [Google Scholar]
- 31).Logallo N, Novotny V, Assmus J, et al. : Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol 16: 781–788, 2017 [DOI] [PubMed] [Google Scholar]
- 32).Campbell BCV, Mitchell PJ, Churilov L, et al. : Tenecteplase versus Alteplase before Thrombectomy for Ischemic Stroke. N Engl J Med 378: 1573–1582, 2018 [DOI] [PubMed] [Google Scholar]
- 33).von Kummer R, Mori E, Truelsen T, et al. : Desmoteplase 3 to 9 Hours After Major Artery Occlusion Stroke: The DIAS-4 Trial (Efficacy and Safety Study of Desmoteplase to Treat Acute Ischemic Stroke). Stroke 47: 2880–2887, 2016 [DOI] [PubMed] [Google Scholar]
- 34).Emberson J, Lees KR, Lyden P, et al. : Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 384: 1929–1935, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Whiteley WN, Emberson J, Lees KR, et al. : Risk of intracerebral haemorrhage with alteplase after acute ischaemic stroke: a secondary analysis of an individual patient data meta-analysis. Lancet Neurol 15: 925–933, 2016 [DOI] [PubMed] [Google Scholar]
- 36).Xian Y, Xu H, Lytle B, et al. : Use of strategies to improve door-to-needle times with tissue-type plasminogen activator in acute ischemic stroke in clinical practice: findings from target: stroke. Circ Cardiovasc Qual Outcomes 10: e003227, 2017 [DOI] [PubMed] [Google Scholar]
- 37).Kamal N, Smith EE, Jeerakathil T, Hill MD: Thrombolysis: Improving door-to-needle times for ischemic stroke treatment - A narrative review. Int J Stroke 13: 268–276, 2018 [DOI] [PubMed] [Google Scholar]
- 38).Aoki J, Kimura K, Iguchi Y, Shibazaki K, Sakai K, Iwanaga T: FLAIR can estimate the onset time in acute ischemic stroke patients. J Neurol Sci 293: 39–44, 2010 [DOI] [PubMed] [Google Scholar]
- 39).Thomalla G, Cheng B, Ebinger M, et al. : DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4·5 h of symptom onset (PRE-FLAIR): a multicentre observational study. Lancet Neurol 10: 978–986, 2011 [DOI] [PubMed] [Google Scholar]
- 40).Koga M, Toyoda K, Kimura K, et al. : THrombolysis for Acute Wake-up and unclear-onset Strokes with alteplase at 0·6 mg/kg (THAWS) Trial. Int J Stroke 9: 1117–1124, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Graham GD: Tissue plasminogen activator for acute ischemic stroke in clinical practice: a meta-analysis of safety data. Stroke 34: 2847–2850, 2003 [DOI] [PubMed] [Google Scholar]
- 42).Selim M, Fink JN, Kumar S, et al. : Predictors of hemorrhagic transformation after intravenous recombinant tissue plasminogen activator: prognostic value of the initial apparent diffusion coefficient and diffusion-weighted lesion volume. Stroke 33: 2047–2052, 2002 [DOI] [PubMed] [Google Scholar]
- 43).Tanne D, Kasner SE, Demchuk AM, et al. : Markers of increased risk of intracerebral hemorrhage after intravenous recombinant tissue plasminogen activator therapy for acute ischemic stroke in clinical practice: the Multicenter rt-PA Stroke Survey. Circulation 105: 1679–1685, 2002 [DOI] [PubMed] [Google Scholar]
- 44).Wahlgren N, Ahmed N, Eriksson N, et al. : Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials: Safe Implementation of Thrombolysis in Stroke-MOnitoring STudy (SITS-MOST). Stroke 39: 3316–3322, 2008 [DOI] [PubMed] [Google Scholar]
- 45).Shinohara Y, Minematsu K: A caution for appropriate i.v. application of alteplase for acute ischemic stroke: dissection of the thoracic aorta. Jpn J Stroke 30: 443–444, 2008. (Japanese) [Google Scholar]
- 46).Koga M, Iguchi Y, Ohara T, Tahara Y: Acute ischemic stroke caused by Stanford type A acute aortic dissection. Jpn J Stroke 40: 432–437, 2018. (Japanese) [Google Scholar]
- 47).Koga M, Iguchi Y, Ohara T, et al. : Acute ischemic stroke as a complication of Stanford type A acute aortic dissection: a review and proposed clinical recommendations for urgent diagnosis. Gen Thorac Cardiovasc Surg 66: 439–445, 2018 [DOI] [PubMed] [Google Scholar]
- 48).Ohara T, Koga M, Tokuda N, et al. : Rapid Identification of Type A Aortic Dissection as a Cause of Acute Ischemic Stroke. J Stroke Cerebrovasc Dis 25: 1901–1906, 2016 [DOI] [PubMed] [Google Scholar]
- 49).Fuentes Fernández I, Morales Ortíz A, Sanmartín Monzó J, Jara Rubio R: Fatal outcome following thrombolysis for stroke secondary to infectious endocarditis. Neurologia 31: 421–423, 2016 [DOI] [PubMed] [Google Scholar]
- 50).Brownlee WJ, Anderson NE, Barber PA: Intravenous thrombolysis is unsafe in stroke due to infective endocarditis. Intern Med J 44: 195–197, 2014 [DOI] [PubMed] [Google Scholar]
- 51).Asaithambi G, Adil MM, Qureshi AI: Thrombolysis for ischemic stroke associated with infective endocarditis: results from the nationwide inpatient sample. Stroke 44: 2917–2919, 2013 [DOI] [PubMed] [Google Scholar]
- 52).Patel MR, Meine TJ, Lindblad L, et al. : Cardiac tamponade in the fibrinolytic era: analysis of >100,000 patients with ST-segment elevation myocardial infarction. Am Heart J 151: 316–322, 2006 [DOI] [PubMed] [Google Scholar]
- 53).De Silva DA, Manzano JJ, Chang HM, Wong MC: Reconsidering recent myocardial infarction as a contraindication for IV stroke thrombolysis. Neurology 76: 1838–1840, 2011 [DOI] [PubMed] [Google Scholar]
- 54).Akinseye OA, Shahreyar M, Heckle MR, Khouzam RN: Simultaneous acute cardio-cerebral infarction: is there a consensus for management? Ann Transl Med 6: 7, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Tsivgoulis G, Zand R, Katsanos AH, et al. : Risk of symptomatic intracerebral hemorrhage after intravenous thrombolysis in patients with acute ischemic stroke and high cerebral microbleed burden: a meta-analysis. JAMA Neurol 73: 675–683, 2016 [DOI] [PubMed] [Google Scholar]
- 56).Toyoda K, Yamagami H, Koga M: Consensus guides on stroke thrombolysis for anticoagulated patients from Japan: application to other populations. J Stroke 20: 321–331, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Suzuki K, Aoki J, Sakamoto Y, et al. : Low risk of ICH after reperfusion therapy in acute stroke patients treated with direct oral anti-coagulant. J Neurol Sci 379: 207–211, 2017 [DOI] [PubMed] [Google Scholar]
- 58).Diener HC, Bernstein R, Butcher K, et al. : Thrombolysis and thrombectomy in patients treated with dabigatran with acute ischemic stroke: Expert opinion. Int J Stroke 12: 9–12, 2017 [DOI] [PubMed] [Google Scholar]
- 59).Touzé E, Gruel Y, Gouin-Thibault I, et al. : Intravenous thrombolysis for acute ischaemic stroke in patients on direct oral anticoagulants. Eur J Neurol 25: 747–e52, 2018 [DOI] [PubMed] [Google Scholar]
- 60).Ohya Y, Makihara N, Wakisaka K, et al. : Thrombolytic therapy in severe cardioembolic stroke after reversal of dabigatran with idarucizumab: case report and literature review. J Stroke Cerebrovasc Dis 27: e128–e131, 2018 [DOI] [PubMed] [Google Scholar]
- 61).Hosoki S, Takagi M, Yamagami H, Ando D, Toyoda K, Koga M: Paradoxical elevation of plasma dabigatran after reversal with idarucizumab in stroke thrombolysis. J Neurol 265: 2451–2453, 2018 [DOI] [PubMed] [Google Scholar]
- 62).Alberts MJ, Hademenos G, Latchaw RE, et al. : Recommendations for the establishment of primary stroke centers. Brain Attack Coalition. JAMA 283: 3102–3109, 2000 [DOI] [PubMed] [Google Scholar]
- 63).Imai T, Sakurai K, Hagiwara Y, Mizukami H, Hasegawa Y: Specific needs for telestroke networks for thrombolytic therapy in Japan. J Stroke Cerebrovasc Dis 23: 811–816, 2014 [DOI] [PubMed] [Google Scholar]
- 64).Hubert GJ, Müller-Barna P, Audebert HJ: Recent advances in TeleStroke: a systematic review on applications in prehospital management and Stroke Unit treatment or TeleStroke networking in developing countries. Int J Stroke 9: 968–973, 2014 [DOI] [PubMed] [Google Scholar]
- 65).Audebert HJ, Kukla C, Vatankhah B, et al. : Comparison of tissue plasminogen activator administration management between Telestroke Network hospitals and academic stroke centers: the Telemedical Pilot Project for Integrative Stroke Care in Bavaria/Germany. Stroke 37: 1822–1827, 2006 [DOI] [PubMed] [Google Scholar]
- 66).Kageji T, Obata F, Oka H, et al. : Drip-and-ship thrombolytic therapy supported by the telestroke system for acute ischemic stroke patients living in medically under-served areas. Neurol Med Chir (Tokyo) 56: 753–758, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Langhorne P, Fearon P, Ronning OM, et al. : Stroke unit care benefits patients with intracerebral hemorrhage: systematic review and meta-analysis. Stroke 44: 3044–3049, 2013 [DOI] [PubMed] [Google Scholar]
- 68).Atsumi C, Hasegawa Y, Tsumura K, et al. : Quality assurance monitoring of a citywide transportation protocol improves clinical indicators of intravenous tissue plasminogen activator therapy: a community-based, longitudinal study. J Stroke Cerebrovasc Dis 24: 183–188, 2015 [DOI] [PubMed] [Google Scholar]
- 69).Ekundayo OJ, Saver JL, Fonarow GC, et al. : Patterns of emergency medical services use and its association with timely stroke treatment: findings from Get With the Guidelines-Stroke. Circ Cardiovasc Qual Outcomes 6: 262–269, 2013 [DOI] [PubMed] [Google Scholar]
- 70).Miyamatsu N, Kimura K, Okamura T, et al. : Effects of public education by television on knowledge of early stroke symptoms among a Japanese population aged 40 to 74 years: a controlled study. Stroke 43: 545–549, 2012 [DOI] [PubMed] [Google Scholar]
- 71).Matsuzono K, Yokota C, Takekawa H, et al. : Effects of stroke education of junior high school students on stroke knowledge of their parents: Tochigi project. Stroke 46: 572–574, 2015 [DOI] [PubMed] [Google Scholar]
- 72).Japanese Society for Emergency Medicine Standardization for observation and care of stroke by paramedics: Prehospital Stroke Life Support Giodebook 2015, Herusu Shuppan, Tokyo, 2015. (Japanese) [Google Scholar]
- 73).Kothari RU, Pancioli A, Liu T, Brott T, Broderick J: Cincinnati Prehospital Stroke Scale: reproducibility and validity. Ann Emerg Med 33: 373–378, 1999 [DOI] [PubMed] [Google Scholar]
- 74).Kimura K, Inoue T, Iguchi Y, Shibazaki K: Kurashiki prehospital stroke scale. Cerebrovasc Dis 25: 189–191, 2008 [DOI] [PubMed] [Google Scholar]
- 75).Suzuki Y, Hasegawa Y, Tsumura K, et al. : Prehospital triage for endovascular clot removal in acute stroke patients. Acute Med Surg 4: 68–74, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76).Atsumi C, Hasegawa Y, Tsumura K, et al. : Quality assurance monitoring of a citywide transportation protocol improves clinical indicators of intravenous tissue plasminogen activator therapy: a community-based, longitudinal study. J Stroke Cerebrovasc Dis 24: 183–188, 2015 [DOI] [PubMed] [Google Scholar]
- 77).Vidale S, Agostoni E: Prehospital stroke scales and large vessel occlusion: A systematic review. Acta Neurol Scand 138: 24–31, 2018 [DOI] [PubMed] [Google Scholar]
- 78).Purrucker JC, Härtig F, Richter H, et al. : Design and validation of a clinical scale for prehospital stroke recognition, severity grading and prediction of large vessel occlusion: the shortened NIH Stroke Scale for emergency medical services. BMJ Open 7: e016893, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79).McKinney JS, Mylavarapu K, Lane J, Roberts V, Ohman-Strickland P, Merlin MA: Hospital prenotification of stroke patients by emergency medical services improves stroke time targets. J Stroke Cerebrovasc Dis 22: 113–118, 2013 [DOI] [PubMed] [Google Scholar]
- 80).Patel MD, Rose KM, O’Brien EC, Rosamond WD: Prehospital notification by emergency medical services reduces delays in stroke evaluation: findings from the North Carolina stroke care collaborative. Stroke 42: 2263–2268, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81).Chen Y, Bogosavljevic V, Leys D, Jovanovic D, Beslac-Bumbasirevic L, Lucas C: Intravenous thrombolytic therapy in patients with stroke mimics: baseline characteristics and safety profile. Eur J Neurol 18: 1246–1250, 2011 [DOI] [PubMed] [Google Scholar]
- 82).Tsivgoulis G, Zand R, Katsanos AH, et al. : Safety of intravenous thrombolysis in stroke mimics: prospective 5-year study and comprehensive meta-analysis. Stroke 46: 1281–1287, 2015 [DOI] [PubMed] [Google Scholar]
- 83).Ngyyen PL, Chang JJ: Stroke mimics and acute stroke evaluation: clinical differentiation and complications after intravenous tissue plasminogen activator. J Emerg Med 49: 244–252, 2015 [DOI] [PubMed] [Google Scholar]
- 84).Gaul C, Dietrich W, Friedrich I, Sirch J, Erbguth FJ: Neurological symptoms in type A aortic dissections. Stroke 38: 292–297, 2007 [DOI] [PubMed] [Google Scholar]
- 85).Maeda K, Yasaka M, Wakugawa Y, Ogata T, Okada Y: [A case of brain infarction and thoracic aortic dissection without chest nor back pain diagnosed by carotid duplex ultrasonography]. Rinsho Shinkeigaku 49: 104–108, 2009. (Japanese) [DOI] [PubMed] [Google Scholar]
- 86).Tokuda N, Koga M, Ohara T, et al. : Urgent detection of acute type A aortic dissection in hyperacute ischemic stroke or transient ischemic attack. J Stroke Cerebrovasc Dis 27: 2112–2117, 2018 [DOI] [PubMed] [Google Scholar]
- 87).Brott T, Adams HP, Olinger CP, et al. : Measurements of acute cerebral infarction: a clinical examination scale. Stroke 20: 864–870, 1989 [DOI] [PubMed] [Google Scholar]
- 88).Lyden P: Using the National Institutes of Health Stroke Scale: A Cautionary Tale. Stroke 48: 513–519, 2017 [DOI] [PubMed] [Google Scholar]
- 89).Flemming KD, Brown RD: Acute cerebral infarction caused by aortic dissection: caution in the thrombolytic era. Stroke 30: 477–478, 1999 [DOI] [PubMed] [Google Scholar]
- 90).Iguchi Y, Kimura K, Sakai K, et al. : Hyper-acute stroke patients associated with aortic dissection. Intern Med 49: 543–547, 2010 [DOI] [PubMed] [Google Scholar]
- 91).Zirkle PK, Wheeler JR, Gregory RT, Snyder SO, Gayle RG, Sorrell K: Carotid involvement in aortic dissection diagnosed by duplex scanning. J Vasc Surg 1: 700–703, 1984 [PubMed] [Google Scholar]
- 92).Wright V, Horvath R, Baird AE: Aortic dissection presenting as acute ischemic stroke. Neurology 61: 581–582, 2003 [DOI] [PubMed] [Google Scholar]
- 93).Gaul C, Dietrich W, Friedrich I, Sirch J, Erbguth FJ: Neurological symptoms in type A aortic dissections. Stroke 38: 292–297, 2007 [DOI] [PubMed] [Google Scholar]
- 94).Tomura N, Uemura K, Inugami A, Fujita H, Higano S, Shishido F: Early CT finding in cerebral infarction: obscuration of the lentiform nucleus. Radiology 168: 463–467, 1988 [DOI] [PubMed] [Google Scholar]
- 95).Truwit CL, Barkovich AJ, Gean-Marton A, Hibri N, Norman D: Loss of the insular ribbon: another early CT sign of acute middle cerebral artery infarction. Radiology 176: 801–806, 1990 [DOI] [PubMed] [Google Scholar]
- 96).Moulin T, Cattin F, Crépin-Leblond T, et al. : Early CT signs in acute middle cerebral artery infarction: predictive value for subsequent infarct locations and outcome. Neurology 47: 366–375, 1996 [DOI] [PubMed] [Google Scholar]
- 97).Gács G, Fox AJ, Barnett HJ, Vinuela F: CT visualization of intracranial arterial thromboembolism. Stroke 14: 756–762, 1983 [DOI] [PubMed] [Google Scholar]
- 98).Pressman BD, Tourje EJ, Thompson JR: An early CT sign of ischemic infarction: increased density in a cerebral artery. AJR Am J Roentgenol 149: 583–586, 1987 [DOI] [PubMed] [Google Scholar]
- 99).Schuierer G, Huk W: The unilateral hyperdense middle cerebral artery: an early CT-sign of embolism or thrombosis. Neuroradiology 30: 120–122, 1988 [DOI] [PubMed] [Google Scholar]
- 100).Leys D, Pruvo JP, Godefroy O, Rondepierre P, Leclerc X: Prevalence and significance of hyperdense middle cerebral artery in acute stroke. Stroke 23: 317–324, 1992 [DOI] [PubMed] [Google Scholar]
- 101).Barber PA, Demchuk AM, Hudon ME, Pexman JH, Hill MD, Buchan AM: Hyperdense sylvian fissure MCA “dot” sign: A CT marker of acute ischemia. Stroke 32: 84–88, 2001 [DOI] [PubMed] [Google Scholar]
- 102).Leary MC, Kidwell CS, Villablanca JP, et al. : Validation of computed tomographic middle cerebral artery “dot” sign: an angiographic correlation study. Stroke 34: 2636–2640, 2003 [DOI] [PubMed] [Google Scholar]
- 103).Koga M, Saku Y, Toyoda K, Takaba H, Ibayashi S, Iida M: Reappraisal of early CT signs to predict the arterial occlusion site in acute embolic stroke. J Neurol Neurosurg Psychiatry 74: 649–653, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104).Patel SC, Levine SR, Tilley BC, et al. : Lack of clinical significance of early ischemic changes on computed tomography in acute stroke. JAMA 286: 2830–2838, 2001 [DOI] [PubMed] [Google Scholar]
- 105).von Kummer R, Meyding-Lamadé U, Forsting M, et al. : Sensitivity and prognostic value of early CT in occlusion of the middle cerebral artery trunk. AJNR Am J Neuroradiol 15: 9–15; discussion 16–18, 1994 [PMC free article] [PubMed] [Google Scholar]
- 106).Wardlaw JM, Mielke O: Early signs of brain infarction at CT: observer reliability and outcome after thrombolytic treatment—systematic review. Radiology 235: 444–453, 2005 [DOI] [PubMed] [Google Scholar]
- 107).Ogawa A, Mori E, Minematsu K, et al. : Randomized trial of intraarterial infusion of urokinase within 6 hours of middle cerebral artery stroke: the middle cerebral artery embolism local fibrinolytic intervention trial (MELT) Japan. Stroke 38: 2633–2639, 2007 [DOI] [PubMed] [Google Scholar]
- 108).Ogawa A. (ed). Standardization of stroke imaging and judgment for the MELT Japan. A report of the research project granted by the Ministry of Health, Labour and Welfare, Japan, Iwate Medical University, Morioka, 2002, 41–44 (Japanese) [Google Scholar]
- 109).von Kummer R: Effect of training in reading CT scans on patient selection for ECASS II. Neurology 51: S50–S52, 1998 [DOI] [PubMed] [Google Scholar]
- 110).Acute Stroke Imaging Standardization Group-Japan (ed). Practical guidelines for acute stroke imaging, Nanko-do, Tokyo, 2007. (Japanese) [Google Scholar]
- 111).Barber PA, Demchuk AM, Zhang J, Buchan AM: Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet 355: 1670–1674, 2000 [DOI] [PubMed] [Google Scholar]
- 112).Pexman JH, Barber PA, Hill MD, et al. : Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol 22: 1534–1542, 2001 [PMC free article] [PubMed] [Google Scholar]
- 113).Hill MD, Barber PA, Demchuk AM, et al. : Acute intravenous—intra-arterial revascularization therapy for severe ischemic stroke. Stroke 33: 279–282, 2002 [DOI] [PubMed] [Google Scholar]
- 114).Hirano T, Yonehara T, Inatomi Y, Hashimoto Y, Uchino M: Presence of early ischemic changes on computed tomography depends on severity and the duration of hypoperfusion: a single photon emission-computed tomographic study. Stroke 36: 2601–2608, 2005 [DOI] [PubMed] [Google Scholar]
- 115).Grunwald IQ, Ragoschke-Schumm A, Kettner M, et al. : First automated stroke imaging evaluation via electronic Alberta stroke program early CT score in a mobile stroke unit. Cerebrovasc Dis 42: 332–338, 2016 [DOI] [PubMed] [Google Scholar]
- 116).Ezzeddine MA, Lev MH, McDonald CT, et al. : CT angiography with whole brain perfused blood volume imaging: added clinical value in the assessment of acute stroke. Stroke 33: 959–966, 2002 [DOI] [PubMed] [Google Scholar]
- 117).Schramm P, Schellinger PD, Klotz E, et al. : Comparison of perfusion computed tomography and computed tomography angiography source images with perfusion-weighted imaging and diffusion-weighted imaging in patients with acute stroke of less than 6 hours’ duration. Stroke 35: 1652–1658, 2004 [DOI] [PubMed] [Google Scholar]
- 118).Larrue V, von Kummer R, del Zoppo G, Bluhmki E: Hemorrhagic transformation in acute ischemic stroke. Potential contributing factors in the European Cooperative Acute Stroke Study. Stroke 28: 957–960, 1997 [DOI] [PubMed] [Google Scholar]
- 119).Demchuk AM, Hill MD, Barber PA, Silver B, Patel SC, Levine SR: Importance of early ischemic computed tomography changes using ASPECTS in NINDS rtPA Stroke Study. Stroke 36: 2110–2115, 2005 [DOI] [PubMed] [Google Scholar]
- 120).Dzialowski I, Hill MD, Coutts SB, et al. : Extent of early ischemic changes on computed tomography (CT) before thrombolysis: prognostic value of the Alberta Stroke Program Early CT Score in ECASS II. Stroke 37: 973–978, 2006 [DOI] [PubMed] [Google Scholar]
- 121).Hirano T, Sasaki M, Tomura N, Ito Y, Kobayashi S: Low Alberta stroke program early computed tomography score within 3 hours of onset predicts subsequent symptomatic intracranial hemorrhage in patients treated with 0.6 mg/kg Alteplase. J Stroke Cerebrovasc Dis 21: 898–902, 2012 [DOI] [PubMed] [Google Scholar]
- 122).Haussen DC, Dehkharghani S, Rangaraju S, et al. : Automated CT perfusion ischemic core volume and noncontrast CT ASPECTS (Alberta Stroke Program Early CT Score): correlation and clinical outcome prediction in large vessel stroke. Stroke 47: 2318–2322, 2016 [DOI] [PubMed] [Google Scholar]
- 123).Demeestere J, Garcia-Esperon C, Garcia-Bermejo P, et al. : Evaluation of hyperacute infarct volume using ASPECTS and brain CT perfusion core volume. Neurology 88: 2248–2253, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124).Inoue M, Mlynash M, Straka M, et al. : Patients with the malignant profile within 3 hours of symptom onset have very poor outcomes after intravenous tissue-type plasminogen activator therapy. Stroke 43: 2494–2496, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125).Warach S, Gaa J, Siewert B, Wielopolski P, Edelman RR: Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol 37: 231–241, 1995 [DOI] [PubMed] [Google Scholar]
- 126).Lutsep HL, Albers GW, DeCrespigny A, Kamat GN, Marks MP, Moseley ME: Clinical utility of diffusion-weighted magnetic resonance imaging in the assessment of ischemic stroke. Ann Neurol 41: 574–580, 1997 [DOI] [PubMed] [Google Scholar]
- 127).Barber PA, Darby DG, Desmond PM, et al. : Prediction of stroke outcome with echoplanar perfusion- and diffusion-weighted MRI. Neurology 51: 418–426, 1998 [DOI] [PubMed] [Google Scholar]
- 128).Kidwell CS, Chalela JA, Saver JL, et al. : Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA 292: 1823–1830, 2004 [DOI] [PubMed] [Google Scholar]
- 129).Arnould MC, Grandin CB, Peeters A, Cosnard G, Duprez TP: Comparison of CT and three MR sequences for detecting and categorizing early (48 hours) hemorrhagic transformation in hyperacute ischemic stroke. AJNR Am J Neuroradiol 25: 939–944, 2004 [PMC free article] [PubMed] [Google Scholar]
- 130).Fiebach JB, Schellinger PD, Gass A, et al. : Stroke magnetic resonance imaging is accurate in hyperacute intracerebral hemorrhage: a multicenter study on the validity of stroke imaging. Stroke 35: 502–506, 2004 [DOI] [PubMed] [Google Scholar]
- 131).Cho KH, Kim JS, Kwon SU, Cho AH, Kang DW: Significance of susceptibility vessel sign on T2*-weighted gradient echo imaging for identification of stroke subtypes. Stroke 36: 2379–2383, 2005 [DOI] [PubMed] [Google Scholar]
- 132).Sasaki M, Ida M, Yamada K, Watanabe Y, Matsui M: Standardizing display conditions of diffusion-weighted images using concurrent b0 images: a multi-vendor multi-institutional study. Magn Reson Med Sci 6: 133–137, 2007 [DOI] [PubMed] [Google Scholar]
- 133).Barber PA, Hill MD, Eliasziw M, et al. : Imaging of the brain in acute ischaemic stroke: comparison of computed tomography and magnetic resonance diffusion-weighted imaging. J Neurol Neurosurg Psychiatry 76: 1528–1533, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134).Nezu T, Koga M, Nakagawara J, et al. : Early ischemic change on CT versus diffusion-weighted imaging for patients with stroke receiving intravenous recombinant tissue-type plasminogen activator therapy: stroke acute management with urgent risk-factor assessment and improvement (SAMURAI) rt-PA registry. Stroke 42: 2196–2200, 2011 [DOI] [PubMed] [Google Scholar]
- 135).Marks MP, Tong DC, Beaulieu C, Albers GW, de Crespigny A, Moseley ME: Evaluation of early reperfusion and i.v. tPA therapy using diffusion- and perfusion-weighted MRI. Neurology 52: 1792–1798, 1999 [DOI] [PubMed] [Google Scholar]
- 136).Kidwell CS, Saver JL, Mattiello J, et al. : Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann Neurol 47: 462–469, 2000 [PubMed] [Google Scholar]
- 137).Inoue M, Mlynash M, Christensen S, et al. : Early diffusion-weighted imaging reversal after endovascular reperfusion is typically transient in patients imaged 3 to 6 hours after onset. Stroke 45: 1024–1028, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138).Ma H, Parsons MW, Christensen S, et al. : A multicentre, randomized, double-blinded, placebo-controlled Phase III study to investigate EXtending the time for Thrombolysis in Emergency Neurological Deficits (EXTEND). Int J Stroke 7: 74–80, 2012 [DOI] [PubMed] [Google Scholar]
- 139).Nezu T, Koga M, Kimura K, et al. : Pretreatment ASPECTS on DWI predicts 3-month outcome following rt-PA: SAMURAI rt-PA Registry. Neurology 75: 555–561, 2010 [DOI] [PubMed] [Google Scholar]
- 140).Kimura K, Iguchi Y, Shibazaki K, et al. : Large ischemic lesions on diffusion-weighted imaging done before intravenous tissue plasminogen activator thrombolysis predicts a poor outcome in patients with acute stroke. Stroke 39: 2388–2391, 2008 [DOI] [PubMed] [Google Scholar]
- 141).Mlynash M, Lansberg MG, De Silva DA, et al. : Refining the definition of the malignant profile: insights from the DEFUSE-EPITHET pooled data set. Stroke 42: 1270–1275, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142).Straka M, Albers GW, Bammer R: Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging 32: 1024–1037, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143).Fiehler J, Albers GW, Boulanger JM, et al. : Bleeding risk analysis in stroke imaging before thromboLysis (BRASIL): pooled analysis of T2*-weighted magnetic resonance imaging data from 570 patients. Stroke 38: 2738–2744, 2007 [DOI] [PubMed] [Google Scholar]
- 144).Charidimou A, Shoamanesh A: Clinical relevance of microbleeds in acute stroke thrombolysis: comprehensive meta-analysis. Neurology 87: 1534–1541, 2016 [DOI] [PubMed] [Google Scholar]
- 145).Kimura K, Iguchi Y, Shibazaki K, Watanabe M, Iwanaga T, Aoki J: M1 susceptibility vessel sign on T2* as a strong predictor for no early recanalization after IV-t-PA in acute ischemic stroke. Stroke 40: 3130–3132, 2009 [DOI] [PubMed] [Google Scholar]
- 146).Kimura K, Sakamoto Y, Aoki J, Iguchi Y, Shibazaki K, Inoue T: Clinical and MRI predictors of no early recanalization within 1 hour after tissue-type plasminogen activator administration. Stroke 42: 3150–3155, 2011 [DOI] [PubMed] [Google Scholar]
- 147).Ueda T, Hatakeyama T, Kumon Y, Sakaki S, Uraoka T: Evaluation of risk of hemorrhagic transformation in local intra-arterial thrombolysis in acute ischemic stroke by initial SPECT. Stroke 25: 298–303, 1994 [DOI] [PubMed] [Google Scholar]
- 148).Alexandrov AV, Molina CA, Grotta JC, et al. : Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med 351: 2170–2178, 2004 [DOI] [PubMed] [Google Scholar]
- 149).Eggers J, König IR, Koch B, Händler G, Seidel G: Sonothrombolysis with transcranial color-coded sonography and recombinant tissue-type plasminogen activator in acute middle cerebral artery main stem occlusion: results from a randomized study. Stroke 39: 1470–1475, 2008 [DOI] [PubMed] [Google Scholar]
- 150).Miyamoto S. A report from the Research on Bioethics in Severe Stroke research group, 2012. (Japanese)
- 151).Lees KR, Emberson J, Blackwell L, et al. : Effects of alteplase for acute stroke on the distribution of functional outcomes: a pooled analysis of 9 trials. Stroke 47: 2373–2379, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152).Berkhemer OA, Fransen PS, Beumer D, et al. : A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 372: 11–20, 2015 [DOI] [PubMed] [Google Scholar]
- 153).Goyal M, Demchuk AM, Menon BK, et al. : Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 372: 1019–1030, 2015 [DOI] [PubMed] [Google Scholar]
- 154).Campbell BC, Mitchell PJ, Kleinig TJ, et al. : Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 372: 1009–1018, 2015 [DOI] [PubMed] [Google Scholar]
- 155).Saver JL, Goyal M, Bonafe A, et al. : Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 372: 2285–2295, 2015 [DOI] [PubMed] [Google Scholar]
- 156).Jovin TG, Chamorro A, Cobo E, et al. : Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 372: 2296–2306, 2015 [DOI] [PubMed] [Google Scholar]
- 157).Goyal M, Menon BK, van Zwam WH, et al. : Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 387: 1723–1731, 2016 [DOI] [PubMed] [Google Scholar]
- 158).Saver JL, Goyal M, van der Lugt A, et al. : Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 316: 1279–1288, 2016 [DOI] [PubMed] [Google Scholar]
- 159).Mistry EA, Mistry AM, Nakawah MO, et al. : Mechanical thrombectomy outcomes with and without intravenous thrombolysis in stroke patients: a meta-analysis. Stroke 48: 2450–2456, 2017 [DOI] [PubMed] [Google Scholar]
- 160).Furlan A, Higashida R, Wechsler L, et al. : Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in acute cerebral thromboembolism. JAMA 282: 2003–2011, 1999 [DOI] [PubMed] [Google Scholar]
- 161).Lisboa RC, Jovanovic BD, Alberts MJ: Analysis of the safety and efficacy of intra-arterial thrombolytic therapy in ischemic stroke. Stroke 33: 2866–2871, 2002 [DOI] [PubMed] [Google Scholar]
- 162).Lansberg MG, O’Donnell MJ, Khatri P, et al. : Antithrombotic and thrombolytic therapy for ischemic stroke: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141: e601S–e636S, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163).Lewandowski CA, Frankel M, Tomsick TA, et al. : Combined intravenous and intra-arterial r-TPA versus intra-arterial therapy of acute ischemic stroke: Emergency Management of Stroke (EMS) bridging trial. Stroke 30: 2598–2605, 1999 [DOI] [PubMed] [Google Scholar]
- 164).IMS Study Investigators : Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke 35: 904–911, 2004 [DOI] [PubMed] [Google Scholar]
- 165).IMS II Trial Investigators : The Interventional Management of Stroke (IMS) II Study. Stroke 38: 2127–2135, 2007 [DOI] [PubMed] [Google Scholar]
- 166).Yong M, Kaste M: Association of characteristics of blood pressure profiles and stroke outcomes in the ECASS-II trial. Stroke 39: 366–372, 2008 [DOI] [PubMed] [Google Scholar]
- 167).Ahmed N, Wahlgren N, Brainin M, et al. : Relationship of blood pressure, antihypertensive therapy, and outcome in ischemic stroke treated with intravenous thrombolysis: retrospective analysis from Safe Implementation of Thrombolysis in Stroke-International Stroke Thrombolysis Register (SITS-ISTR). Stroke 40: 2442–2449, 2009 [DOI] [PubMed] [Google Scholar]
- 168).Tomii Y, Toyoda K, Nakashima T, et al. : Effects of hyperacute blood pressure and heart rate on stroke outcomes after intravenous tissue plasminogen activator. J Hypertens 29: 1980–1987, 2011 [DOI] [PubMed] [Google Scholar]
- 169).Endo K, Kario K, Koga M, et al. : Impact of early blood pressure variability on stroke outcomes after thrombolysis: the SAMURAI rt-PA Registry. Stroke 44: 816–818, 2013 [DOI] [PubMed] [Google Scholar]
- 170).Shimamoto K, Ando K, Fujita T, et al. : The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res 37: 253–390, 2014 [DOI] [PubMed] [Google Scholar]
- 171).Zinkstok SM, Roos YB, ARTIS investigators : Early administration of aspirin in patients treated with alteplase for acute ischaemic stroke: a randomised controlled trial. Lancet 380: 731–737, 2012 [DOI] [PubMed] [Google Scholar]
- 172).Barreto AD, Alexandrov AV, Lyden P, et al. : The argatroban and tissue-type plasminogen activator stroke study: final results of a pilot safety study. Stroke 43: 770–775, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173).Lopez-Yunez AM, Bruno A, Williams LS, Yilmaz E, Zurrú C, Biller J: Protocol violations in community-based rTPA stroke treatment are associated with symptomatic intracerebral hemorrhage. Stroke 32: 12–16, 2001 [DOI] [PubMed] [Google Scholar]