Abstract
The mechanisms by which taste and odor are combined in determining food choice behavior are poorly understood. Previous work in human subjects has yielded mixed results, potentially due to differences in task context across studies, and a lack of control over flavor experience. Here, we used rats as a model system to systematically investigate the role of experience and unisensory component liking in the multisensory interactions underlying consumption behavior. We demonstrate that taste–smell mixture consumption is best explained by a linear average of component liking. The observed pattern of results was not dependent on prior experience with specific taste–smell combinations, and unique for multisensory as opposed to unisensory mixture consumption. The results are discussed with respect to existing models of flavor integration, and a maximum-likelihood integration model previously described for multisensory judgments in other systems.
Keywords: crossmodal, maximum-likelihood integration, mixture, retronasal, smell, taste
Introduction
Food is perceived through its flavor: an amalgamation of sensory qualities, including taste and smell, among others (Prescott 1999; Auvray and Spence 2008; van Stokkom et al. 2018; Spence 2019). The multisensory experience of flavor is responsible for our enjoyment of food, and as such is a major determinant of food choice (Prescott 2015). Previous work in humans has provided much insight into the multisensory interactions underlying perceptual judgments of flavor stimuli, but a thorough understanding of the integrative mechanisms underlying food choice behavior is still lacking.
The extant literature on the human psychophysics of flavor perception has convincingly demonstrated that both taste and smell qualities contribute to perceptual judgments of multisensory flavor stimuli (Frank and Byram 1988; Prescott 1999; Small and Prescott 2005). However, different task contexts have yielded different results with regard to the nature of the integrative process. For example, oral detection of taste–smell mixtures is faster and more accurate than can be predicted on the basis of independent unisensory processing channels (Veldhuizen et al. 2010; Marks et al. 2012; Shepard et al. 2015), whereas intensity judgments of taste–smell mixtures do not reflect their integration, but a linear sum of their component stimulus judgments (Murphy et al. 1977; Murphy and Cain 1980; Garcia-Medina 1981; Hornung and Enns 1986).
Another factor besides task context that may affect how taste and smell are integrated is flavor “congruency”: the implicit association between taste and smell components formed through past eating experiences. For example, enhanced performance on detection tasks has reliably been shown to occur only for congruent taste–smell mixtures (Dalton et al. 2000; Veldhuizen et al. 2010; Shepard et al. 2015), whereas multisensory intensity judgments appear to be independent of congruency (Murphy and Cain 1980). Work on flavor “pleasantness” judgments has yielded both positive and negative effects of congruency: some studies report that congruency enhances pleasantness ratings (Schifferstein and Verlegh 1996; Amsellem and Ohla 2016; Fondberg et al. 2018); others show no effect (Small et al. 2004) or decreases (Seo et al. 2013) in pleasantness.
Thus, findings from human psychophysics suggest that the way in which flavor components are integrated depends on task context and experience. However, it is unclear how perceptual judgments such as detection, pleasantness, and intensity relate to food choice behavior (Amsellem and Ohla 2016). Moreover, work in human subjects complicates meaningful control over flavor experience (however, see Stevenson et al. 1995, 1999; Prescott et al. 1998; Small et al. 2004; Lim et al. 2014; Amsellem and Ohla 2016; Fondberg et al. 2018). From birth, human subjects presumably form taste–odor associations through consumption, thereby continuously creating highly subjective congruent (and incongruent) flavor combinations (Prescott 2015).
Here, we directly address how taste and smell components of flavor interact to inform consumption behavior in rats. The use of rats as a model system allowed us to control flavor experience by exposing animals to specific taste–smell mixtures during an extensive “training” period, and directly measure flavor liking via consumption in a subsequent series of one-bottle tests. Specifically, we tested how taste–smell mixture consumption differs from unisensory component consumption, and how any observed effects may be influenced by flavor experience. The results demonstrate that mixture consumption is best explained by a weighted average of component consumption and that this integrative process is independent of experience.
Methods
Subjects
A total of 34 Long-Evans rats (17 females, 17 males) were used for this study. Pregnant dams were obtained from www.criver.com, and litters were kept together until weaning on postnatal day (PND) 21. All animals were kept on a 12-h light cycle (6 AM–6 PM) and had ad lib access to food and water, unless otherwise indicated. All procedures took place in animals’ housing facilities. Animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals, and all procedures were approved by the Institutional Animal Care and Use Committee of Wake Forest School of Medicine.
Stimuli
Stimuli consisted of aqueous solutions of taste and/or smell compounds (obtained from www.fischersci.com and www.sigmaaldrich.com; >98% purity; dissolved in distilled water). Tastants: 10 mM saccharin, 58 mM sucrose, and 50 mM sodium chloride. Odorants: n-amyl acetate and 2-hexanone (0.025% weight/volume).
Procedures
Training
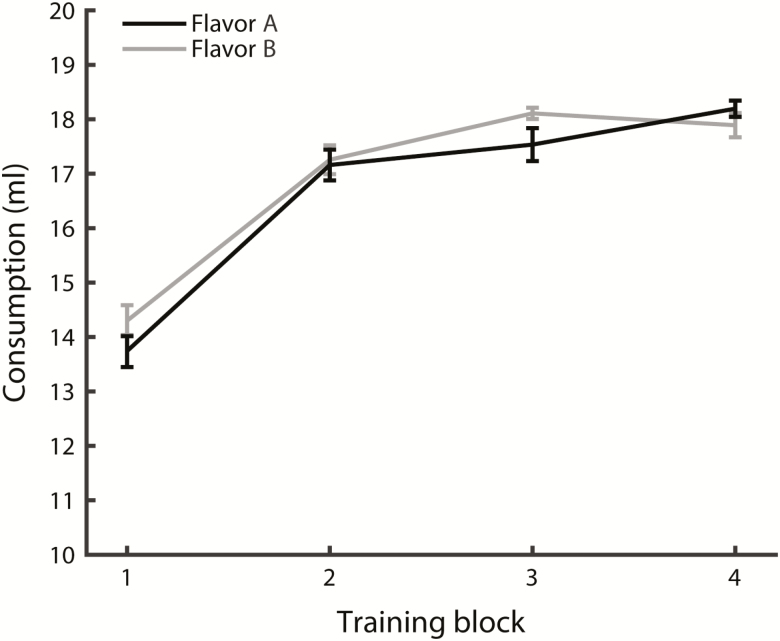
Training occurred over a period of 4 weeks, from PND 21–23 to PND 47–49. Each week, animals were exposed to taste–smell mixtures for 6 consecutive training days. Each animal was exposed to 2 unique bimodal mixtures consisting of one tastant and one odorant (TA+OA, TB+OB). On each training day, animals received 20 ml of a single mixture for 16 h (from 5–6 PM to 9–10 AM). Mixture identity alternated each training day (A-B-A-B-A-B), resulting in 3 exposures per week (12 total over the course of training) to mixtures A and B each. During mixture exposure, animals were single-housed; in between exposures (from 9–10 AM to 5–6 PM), animals were pair-housed with a littermate and received ad lib water. Each block of 6 training days was followed by one day of ad lib access to plain water. For all animals, the taste component of one of the training mixtures was saccharin (mixture A); mixture B contained either sodium chloride (n = 22 animals) or sucrose (n = 12 animals). Odor components were identical for each animal; identity of the odor component paired with saccharin was counterbalanced between animals. To ensure equal exposure to mixtures A and B, we monitored consumption throughout the training period. Figure 1 shows amount consumed for mixtures A and B, averaged over animals and days for each week of training. Two-way analysis of variance (ANOVA) with factors Flavor (A, B) and Training Week (1–4) on training consumption showed no effect of Flavor (F(1,264) = 1.73, P = 0.19), indicating that animals received balanced exposure to mixtures. A significant effect of Training Week (F(3,264) = 113.51, P < 0.001) reflects increased overall consumption with age. No significant interaction was observed (F(3,264) = 1.43, P = 0.23).
Figure 1.
Consumption of multisensory mixtures across each of the 4 training blocks averaged (±SEM) over all animals (n = 34). Mixture A always contained saccharin as the taste component; mixture B contained sodium chloride (n = 22 animals) or sucrose (n = 12 animals). Odor pairings were counterbalanced for each taste (i.e., mixture A/B contained AA/2H as the odor component in 50% of the animals).
Testing
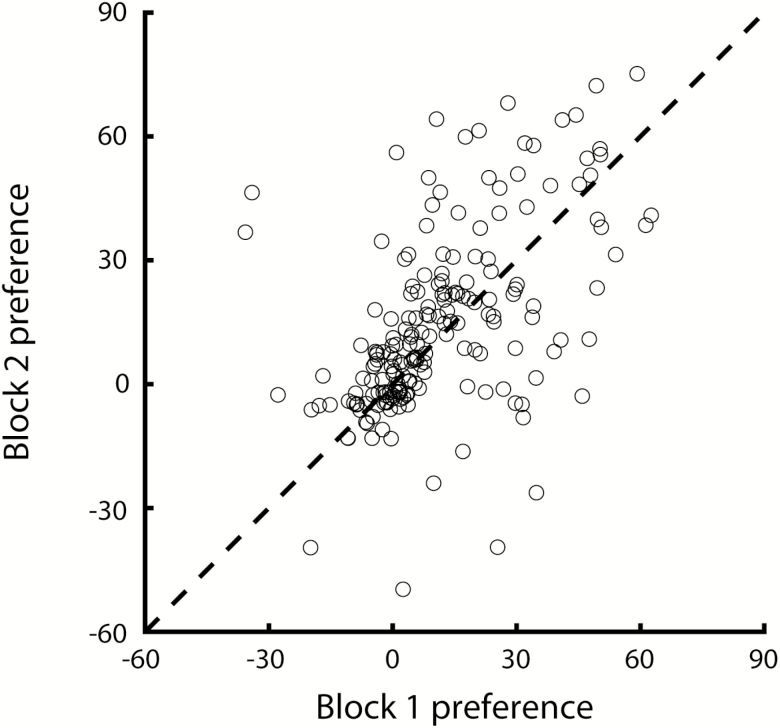
Testing occurred over a period of 18 consecutive days, starting 2 days after the last training day, from PND 49–51 to 66–68. Each testing day, animals received a single solution for 16 h (from 5–6 PM to 9–10 AM), consisting of 1) a congruent mixture of taste and odor (TA + OA, TB + OB); 2) an incongruent mixture (TA + OB, TB + OA); 3) a unimodal taste or odor component (TA, TB, OA, OB); and 4) plain water. This resulted in a total of 9 conditions. In between each testing exposure, animals were given ad lib access to water. In 24 animals, 2 blocks of testing were conducted, with each condition given once, randomly ordered, within each block. In a second group of animals (n = 10; trained with saccharin and sodium chloride), 2 additional conditions were tested: 1) a mixture of taste components (TA + TB) and 2) a mixture of odor components (OA + OB). For this group, conditions were tested once, over a period of 11 consecutive days. To demonstrate that the testing procedure reliably measures solution liking, we compared preference (i.e., consumption relative to water) in each condition between the 2 blocks of testing for all animals that underwent 2 testing blocks. Consumption was significantly correlated between blocks (Figure 2; r = 0.53, P < 0.001; correlations for individual conditions ranged from 0.10 to 0.83), and preferences did not differ between testing blocks (2-way ANOVA with factors Block and Condition: Block: F(1,362) = 1.88, P = 0.17; Condition: F(10,362) = 10.43, P < 0.001; interaction: F(10,362) = 0.22, P = 0.99), indicating stable preference throughout the testing phase.
Figure 2.
Preference (consumption relative to water) during the first versus the second testing block in each condition for animals that were tested twice on each condition (n = 192).
Data analysis
Consumption was measured by weighing bottles before and after training/testing sessions (Consumption = WeightBefore − WeightAfter). For conditions that were tested twice, all analyses were based on the average consumption across both days.
To explain consumption in multisensory conditions, we tested several models relating unisensory consumption to multisensory consumption. The additive model assumes that mixture consumption is based on the sum of component consumption relative to water: Mixture consumption = (Taste consumption + Odor consumption) − Plain water consumption. The averaging model assumes that taste and odor both receive 50% of the weight in consumption decisions: Mixture consumption = (Taste consumption + Odor consumption)/2. Finally, we tested a nonintegration model in which multisensory consumption was simply determined by the combined distribution of taste and odor component consumption. This was accomplished by adopting a bootstrap analysis based on randomly drawing 3 consumption values: one from the distribution of taste only conditions; one from the distribution of odor only conditions; and one from the combined distribution of taste and odor only conditions, serving as surrogate “taste,” “odor,” and “mixture” consumption values, respectively. Triplets were drawn from within a given training group (i.e., saccharin/sodium chloride, saccharin/sucrose) to match experimental conditions, and the number of random triplets in a simulation was equal to the number of multisensory conditions in that training group. From each simulation, we then calculated the probability that “multisensory” values were intermediate to “taste” and “odor” values. This procedure was repeated 1000 times for each training condition. Confidence intervals of the resulting distribution of probabilities were used to statistically evaluate the observed data.
Results
The present study investigated the impact of multisensory flavor experience and unisensory component liking on consumption of taste–smell mixtures in rats. During a training period, animals were first exposed to specific bimodal mixtures consisting of a single taste and a single smell component, thereby creating congruent and incongruent multisensory stimulus combinations (i.e., combinations of taste and smell stimuli that had been experienced together during training, and combinations of taste and smell that had been experienced in different pairings during training, respectively). During a subsequent testing period, we assessed animals’ liking for multisensory stimulus combinations and their unisensory components via consumption in a series of one-bottle tests.
Rats combine taste and smell to inform consumption behavior regardless of congruency
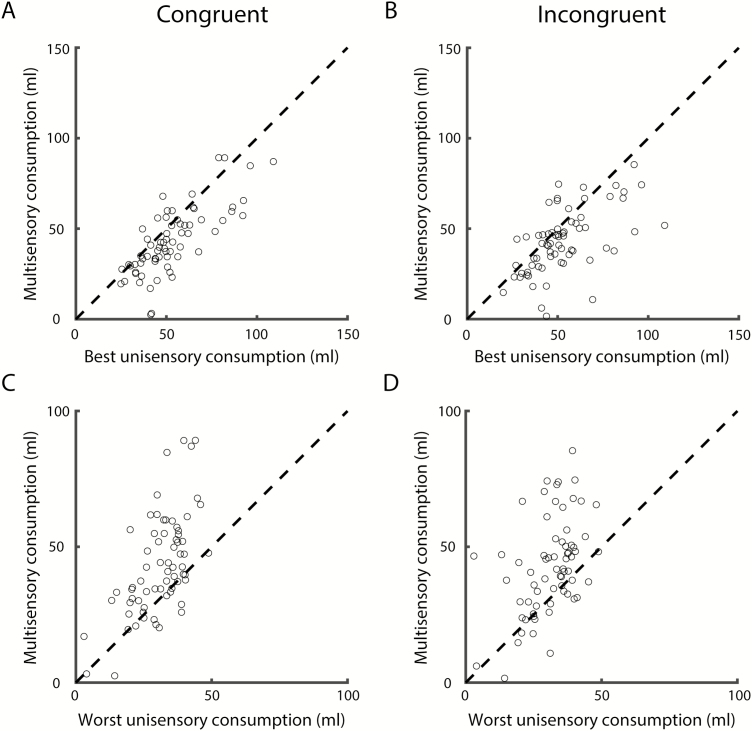
We first tested whether multisensory mixtures are treated differently from their unisensory components. For each multisensory condition in each animal (n = 4 conditions × n = 34 animals, resulting in a total of n = 136 conditions), we determined what the most preferred unisensory component was (“best unisensory”) and directly compared consumption in these 2 conditions (Congruent, Figure 3A; Incongruent, Figure 3B). Taste components were preferred over odor components in most cases (n = 121 of 136 cases, 89.0%). Statistical comparison revealed that mixture consumption was less than best unisensory consumption (congruent: t(67) = 6.35, P < 0.001; incongruent: t(67) = 4.91, P < 0.001). Moreover, mixture consumption was significantly greater than the least preferred component solution (congruent: t(67) = −6.83, P < 0.001; incongruent: t(67) = −6.16, P < 0.001; Figure 3C,D, respectively). Comparison of consumption patterns between congruent and incongruent conditions further shows that mixture consumption does not differ between congruent and incongruent conditions (t-test comparing congruent mixture minus best vs. incongruent mixture minus best: t(134) = 0.02, P = 0.98; congruent mixture minus worst vs. incongruent mixture minus worst: t(134) = 0.19, P = 0.85). Bootstrap analysis (see Methods) further demonstrated that the observed pattern of results cannot be explained by a nonintegration model in which mixture consumption is simply determined by the combined distribution of unisensory taste and odor component consumption (P < 0.01). Thus, rats integrate taste and smell cues to inform consumption decisions, regardless of the configuration in which these components were previously experienced. However, it remains unclear how taste and smell components are combined to inform mixture consumption.
Figure 3.
Congruent (n = 68; A, C) and incongruent (n = 68; B, D) mixture consumption versus most (A, B) and least (C, D) preferred component consumption.
Mixture consumption is best predicted by a weighted average of taste and odor consumption
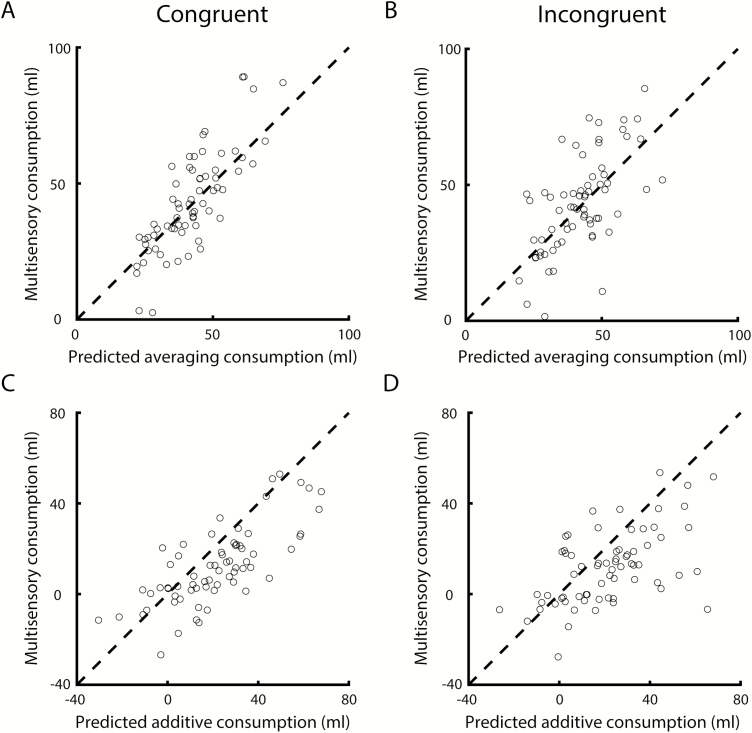
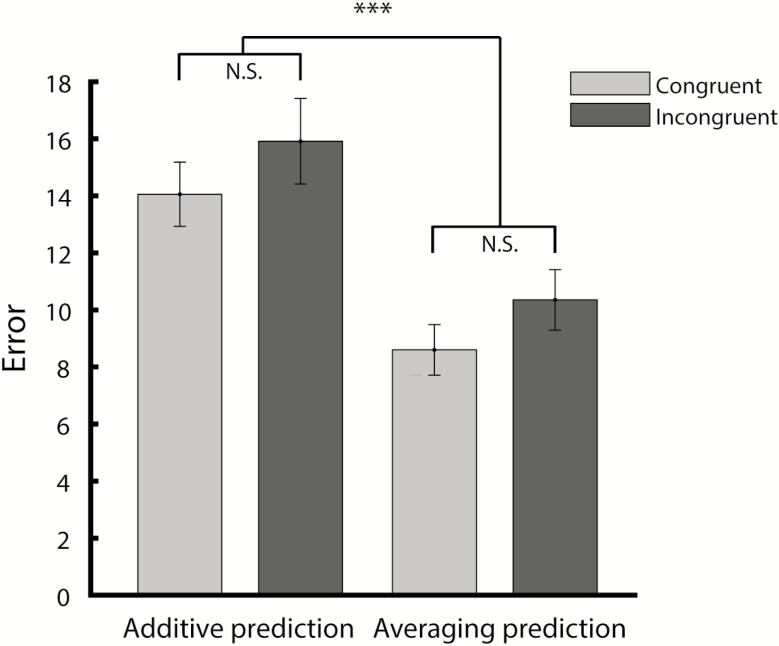
Based on the findings described above as well as previous studies on multisensory cue combination, we tested the validity of averaging and additive models to explain mixture consumption (see Methods). Under the averaging model, mixture consumption is explained by an average of the components. The idea is that a judgment of a multisensory stimulus (in this case: how much do I like this mixture solution?) is computed by weighing the information provided by the components (Ernst and Banks 2002; Adams et al. 2004). Under the additive model, mixture consumption is informed by adding component liking. Note that both of these models in principle could explain our finding that mixture consumption is typically intermediate to unisensory component consumption. Under the additive model, the pattern of results observed here requires that one of the components is unpalatable (i.e., has “negative” liking, relative to plain water) and the other palatable. Note that solution liking/palatability was not directly measured in the current study, but inferred from our measure of consumption (see Discussion for potential additional contributions to consumption). This was true for n = 50 (36.8%) of the cases (n = 47 odor unpalatable and taste palatable, n = 3 taste unpalatable and odor palatable). Note that a superadditive model, in which mixture consumption exceeds best unisensory component consumption, is not compatible with the pattern of results observed here and will therefore not be considered any further. Figure 4 shows mixture consumption versus consumption predicted by the averaging and additive models. Model fit was determined by computing the summed square error, shown in Figure 5. Two-way ANOVA with factors Model (averaging, additive) and Experience (congruent, incongruent) on summed square error revealed a significant main effect of Model, indicating that the averaging model was a better predictor for mixture consumption than the additive model. No significant effect of Experience or interaction was observed, indicating that the integrative operation is not dependent on experience with specific flavors (Model: F(1,268) = 22.34, P < 0.001; Experience: F(1,268) = 2.40, P = 0.12; interaction: F(1,268) < 0.01, P = 0.96). Further testing for potential effects of a variety of experimental variables (i.e., Taste Identity [saccharin, sucrose, sodium chloride], Postingestive Effect [saccharin, sucrose], Odor Identity [2H, AA], and Sex [male, female]) on the observed pattern of results reveals the generalizability of the observed integrative patterns: 3-way ANOVA on summed square error did not yield significant 2-way interactions between any of the stimulus- or animal-specific factors listed above, and the factor Model (additive, averaging), or 3-way interactions with factors Model and Experience (congruent, incongruent). Moreover,s integration patterns did not differ between testing blocks, indicating that integrative patterns were stable across testing sessions.
Figure 4.
Congruent (n = 68; A, C) and incongruent (n = 68; B, D) mixture consumption versus average (A, B) and additive (C, D) model predictions.
Figure 5.
Summed square error (mean ± SEM) for average and additive model predictions of congruent (n = 68) and incongruent (n = 68) mixture consumption. ***P < 0.001; NS, not significant.
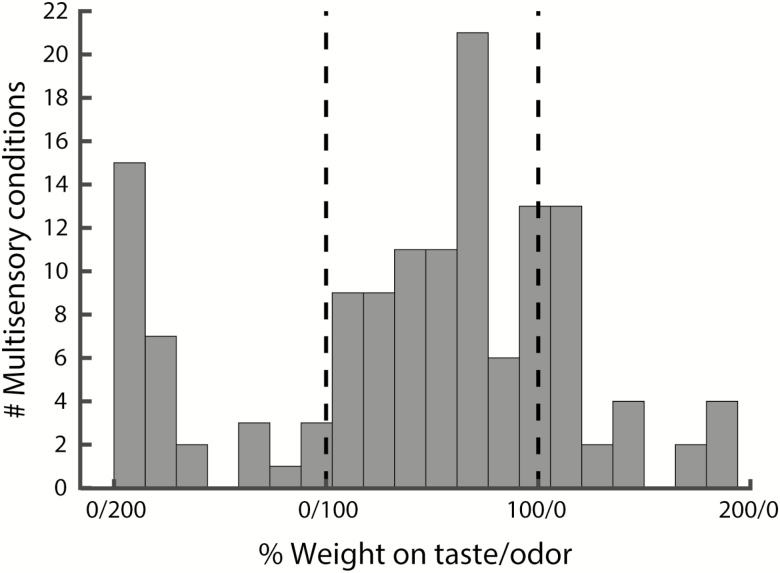
The averaging model that was favored by our data assumed equal weight on taste and smell components (i.e., a 50/50% weight ratio), but it is possible that weights are not equal. To gain more detailed insight into the exact averaging operation performed by the animals in our study, we calculated (for each mixture condition in each animal) the weight on taste/odor that best explains mixture consumption. A 100/0 (0/100) weight ratio would indicate that mixture consumption is identical to taste (odor) consumption. A weight greater than 100 would indicate that mixture consumption is greater than best unisensory component consumption (200% indicating double the amount), or smaller than worst unisensory component consumption (in this case 200% would indicate half the amount). Figure 6 shows all weight ratios, revealing a complex distribution. The main portion of the distribution contains the majority of ratios (n = 77, 56.6%), and appears to have a slight bias toward weight on taste. Secondary peaks can be seen on either side of the main peak (representing n = 59 conditions, 43.4%).
Figure 6.
Distribution of taste/odor weight ratios for all multisensory conditions (n = 136). Dashed lines indicate 100% weight on odor (0/100) and taste (100/0). Component weights with values > 100 indicate cases where mixture consumption was greater than best unisensory component consumption, or smaller than worst unisensory component consumption.
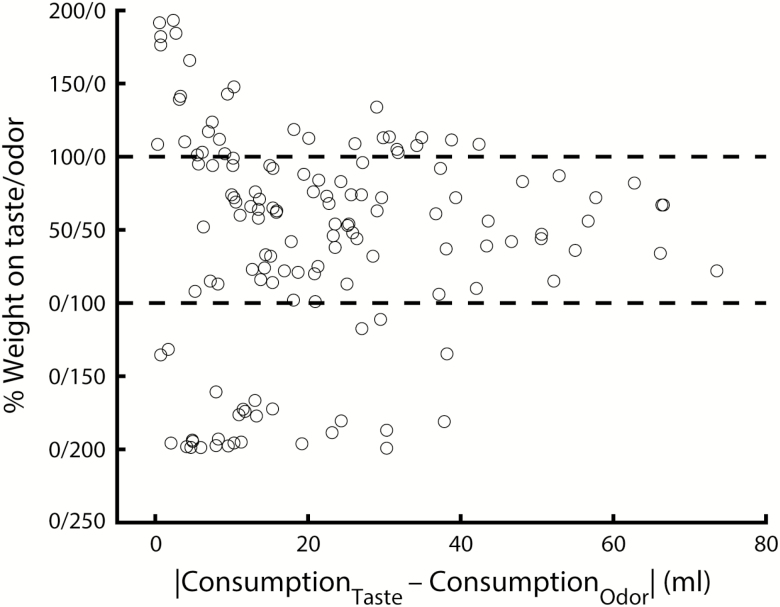
To gain insight into the factors that may underlie the observed variability in weight ratios, we tested potential involvement of several experimental variables. First, it is possible that component weight varies with component palatability: components that are liked more are weighed more. Figure 7 shows component weight as a function of (relative) component consumption. Overall, no relation between weight and (relative) palatability was observed (R2 = 0.01, P = 0.23), indicating that component weight cannot simply be explained by component palatability. Moreover, the results shown in Figure 7 also shed light on the secondary peaks observed in Figure 6: component weights larger than 100 or less than 0 are observed when differences in component consumption are close to zero (i.e., when both components are equally liked by the animal). Second, it is possible that component weight varies with stimulus-specific factors. To test this possibility, we performed a series of one-way ANOVAs on component weight with factors Taste Identity, Odor Identity, and Congruency, yielding no significant effects (respectively, F(2,133) = 0.85, P = 0.43; F(1,134) < 0.01, P = 0.97; F(1,134) = 1.24, P = 0.27). For this analysis, component weight was expressed as a single variable by converting all cases with a weight on taste of zero to negative numbers using the formula: (odor weight × −1) + 100. Finally, we tested the possibility that component weight varies with internal (i.e., animal-specific) factors. One-way ANOVA on component weight with factors Animal Identity and Sex revealed a significant effect of Animal Identity (F(33,102) = 1.98, P < 0.01; F(1,134) = 1.26, P = 0.26), suggesting that the amount of weight carried by taste and smell components of a mixture varies from animal to animal.
Figure 7.
Weight ratio versus relative component preference (taste minus odor consumption) for all multisensory conditions (n = 136).
Component averaging is unique to multisensory mixtures
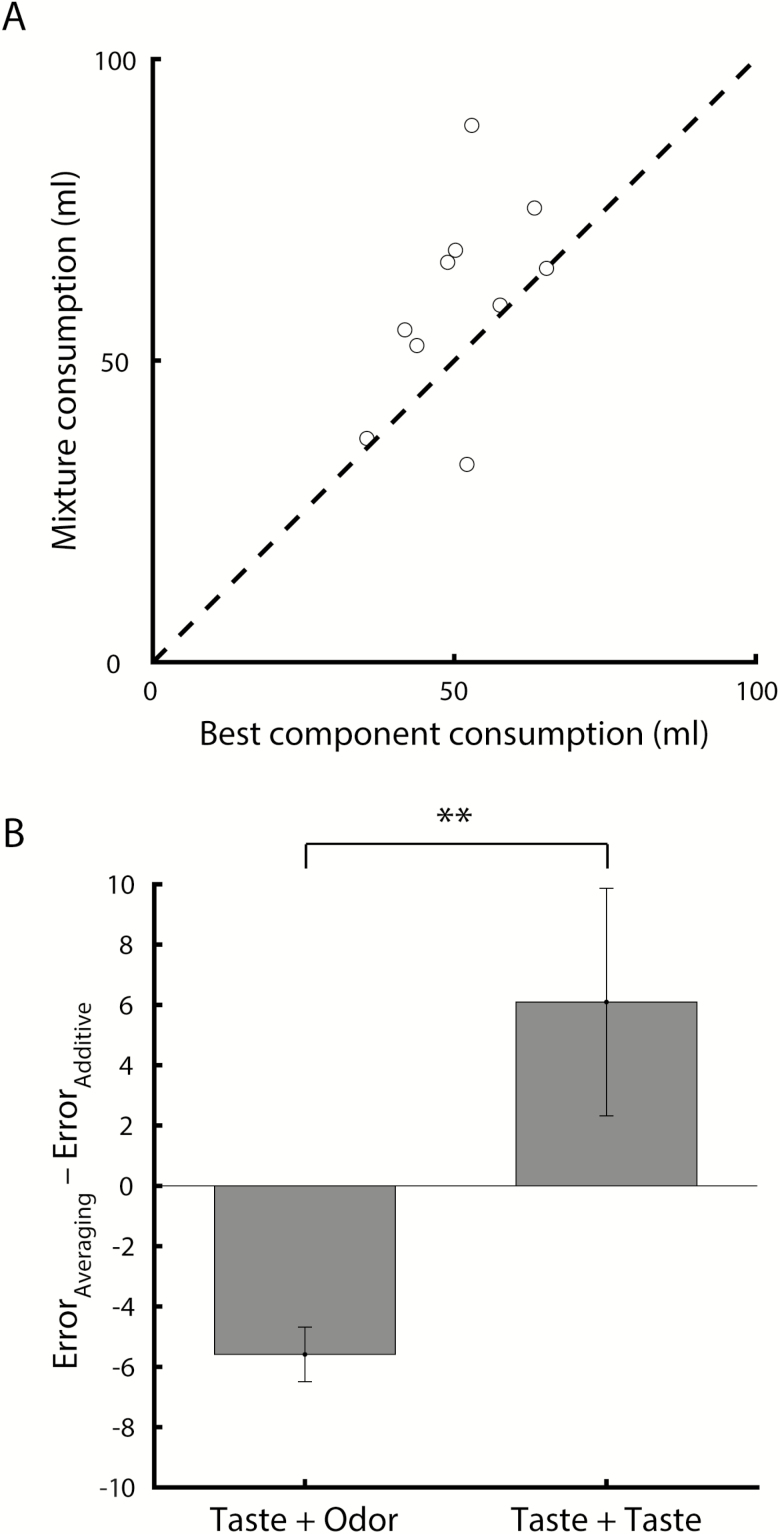
The findings presented above demonstrate that when making a consumption decision, animals judge a taste–smell mixture by weighing palatability of the components. However, it is unknown whether the observed averaging operation is unique to multisensory mixtures or applies more generally in the context of consumption. To test this, a subset of animals (n = 10) were tested on unimodal (i.e., taste–taste) mixtures in addition to the taste–smell mixtures discussed above. Figure 8A shows mixture consumption relative to component consumption for unimodal mixture conditions. Compared with Figure 3, the opposite pattern was observed: mixture consumption was greater than consumption of the best component. Comparing averaging and additive model predictions revealed a better fit of the additive model, a pattern that differed significantly from the one observed for multisensory mixtures (Figure 8B; t-test comparing averaging minus additive error for Taste + Taste conditions versus Taste + Odor conditions: t(144) = −3.31, P < 0.01). Further investigation of optimal weight ratios revealed that the majority of the weight was placed on the most preferred component (on average: 115.65 ± 18.02% weight on sodium chloride when sodium chloride was the preferred component [n = 7]; 108.75 ± 5.13% weight on saccharin when saccharin was the preferred component [n = 3]; t-test comparing weight on sodium chloride when sodium chloride was preferred versus weight on saccharin when saccharin was preferred: t(8) = −0.24, P = 0.82). Thus, an averaging operation as a model for explaining mixture consumption appears to be unique to multisensory mixtures.
Figure 8.
(A) Taste–taste mixture consumption versus most preferred component consumption (n = 10). (B) Average (±SEM) difference in prediction error for the averaging and additive models, for taste–odor (n = 136) and taste–taste (n = 10) mixtures. **P < 0.01.
Discussion
In this article, we investigated if and how rats combine taste and odor information to inform flavor liking. Using a unique experimental design in which we experimentally manipulate flavor experience and directly measure consumption as a behavioral read out, we demonstrate that flavor liking is best explained by a weighted average of component liking. Moreover, this integrative operation appears to be independent of experience with specific taste–smell combinations.
At a general level, our findings are consistent with previous work in humans, demonstrating that taste and smell interact to inform multisensory flavor judgments. Most of these previous studies revealed additive or superadditive interactions, where the individual components combine to enhance the judgment of the multisensory stimulus (Dalton et al. 2000; White and Prescott 2007; Welge-Lussen et al. 2009; Shepard et al. 2015). This is an adaptive computation in the context of signal detection because it effectively boosts stimulus intensity, thereby increasing detectability (Stein et al. 1989, 1996, 2014; McDonald et al. 2000; Lippert et al. 2007). However, further perceptual evaluation of a flavor may require different computations (Fetsch et al. 2013), but the specific relation between taste–smell interactions and food choice has not been addressed in humans, and it is unclear how sweetness (Schifferstein and Verlegh 1996; Stevenson et al. 1999; Labbe et al. 2007) and pleasantness (Schifferstein and Verlegh 1996; Seo et al. 2013; Amsellem and Ohla 2016; Fondberg et al. 2018) ratings relate to consumption decisions. Different perceptual strategies may affect the multisensory operation performed by the subject. Work on flavor perception in animal models, on the other hand, typically uses consumption as a behavioral read out, but has mostly focused on taste–smell interactions in the context of classical conditioning (Holman 1975; Rescorla and Cunningham 1978; Rusiniak et al. 1979; Palmerino et al. 1980; Rescorla 1980; Rescorla and Durlach 1981; Sclafani and Ackroff 1994; McBride and Slotnick 1997; Slotnick et al. 1997; Harris and Thein 2005; Dwyer et al. 2011). These studies have investigated plastic changes in the perceived palatability of individual flavor components after exposure to mixtures (the influence of this type of leaning is further discussed below), but did not systematically investigate how taste and smell components of a mixture interact to inform an instantaneous judgment.
A parallel with the present findings can be found in the extant literature on multisensory judgments in other systems. For example, work on visuo-haptic integration has demonstrated that when judging-specific properties of a stimulus, multisensory sources of information may be optimally combined by linear weighing of the components (i.e., maximum-likelihood integration; Hillis et al. 2002; Ernst and Banks 2002; Adams et al. 2004; Xu et al. 2017). The present findings are generally consistent with this model. However, the current experiments were not designed to test 2 of its key predictions, namely that the weight carried by the components should be inversely proportional to the reliability of the components and that the reliability of the multisensory judgments should exceed the reliability of the components. In the context of flavor perception, reliability could potentially be influenced by stimulus-specific and animal-specific factors. The effect of animal identity on component weight ratio observed in the present study argues for a role of the latter possibility, and may, for example, reflect individual variation in taste/odor sensitivity. Future studies experimentally manipulating taste and smell reliability will further investigate to what extent flavor judgments are consistent with maximum-likelihood integration.
The multisensory operation observed here differed from the operation performed on within-modality taste–taste mixtures: whereas taste–odor consumption clearly favored the averaging model, taste–taste consumption did not favor either averaging or additive models. Instead, we observed a pattern that is in line with previous studies on taste-taste mixtures whereby the stronger component suppresses the weaker component (i.e., “mixture suppression”; Moskowitz 1972; Gillan 1983; Oram et al. 2001; Green et al. 2010; Maier and Katz 2013). One factor that may contribute to the discrepancy between multisensory versus unisensory mixtures observed in the present study may lie in the fact that taste-taste mixture suppression is thought to be the result of interactions at the periphery (Formaker et al. 1997; Breza and Contreras 2012). Since taste and odor signals are sourced from distinct sensory epithelia, no peripheral suppression occurs. Similarly, judgments of combined, noncompeting, within-modality visual cues follow maximum-likelihood integration (Hillis et al. 2002; Ernst and Bulthoff 2004).
We did not observe an effect of experience on multisensory integration of flavor components in the present study. This is in contrast with previous findings regarding taste–smell integration (Schifferstein and Verlegh 1996; Stevenson et al. 1999; Labbe et al. 2007; Small et al. 2004; Seo et al. 2013; Fondberg et al. 2018). Several factors may contribute to the discrepancy between the results observed here and the human literature. As mentioned already above, task context may influence how taste and smell components of a flavor are integrated and may also affect the role of experience. Whereas flavor detection, and other perceptual judgments such as identification and intensity and sweetness ratings may rely on a feedforward computation performed on hard-wired, experience-driven convergence of taste and smell signals (Rowland and Stein 2007, 2008), consumption decisions are imperative, even when no explicit memory of a specific flavor combination exists. With respect to task context, it is also important to note that consumption in a one-bottle context is driven by factors other than palatability of the stimulus components. Although animals in the present study were never deprived of fluid during testing sessions (and testing sessions were performed at the same time each day)—allowing for a sensitive measure of flavor liking—different conditions were tested on different days, and comparisons are therefore indirect. Future work using a 2-bottle task may provide a more direct comparison between congruent and incongruent flavor palatability, for example. Further experimentation is also needed to test the generalizability of our findings to a larger stimulus space, including a range of component concentrations and palatability. In addition, it remains unclear how stimulus liking interacts with physiological factors such as thirst, satiety, or postingestive effects to dynamically affect consumption behavior. Future work will investigate how multisensory interactions in flavor consumption may vary with different time scales of testing. The exact integrative operation and/or the role of experience may depend on the perceptual strategy used by the subject. Previous work has shown that human subjects integrate taste and smell components of a mixture differently depending on the instructions they receive: instructing subjects to pay attention to the individual components of a taste–smell mixture minimized effects of congruency (Murphy and Samantha 2009). It is possible that rats and humans employ different default strategies when evaluating taste-smell mixtures. Finally, studies in human subjects often lack control over (and even knowledge of) flavor experience (but see Stevenson et al. 1995, 1999; Prescott et al. 1998; Small et al. 2004; Lim et al. 2014; Amsellem and Ohla 2016; Fondberg et al. 2018), and choice of (in)congruent stimulus combinations is sometimes poorly justified (e.g., it is not obvious why almond-saccharin and ham-sucrose are congruent and incongruent, respectively; Schifferstein and Verlegh 1996; Dalton et al. 2000). Here, we explicitly define—and experimentally control—congruency in terms of experience. However, it is possible that the training paradigm used in the present study was not effective in establishing taste–smell congruency. Rats were exposed to specific taste–smell combinations from weaning to early adulthood, covering an extensive period during which animals are known to learn about the sensory qualities of food (Fanselow and Birk 1982; Sclafani and Ackroff 1994; Stevenson 2001; Gautam and Verhagen 2010; Maier et al. 2014; Blankenship et al. 2019). Nonetheless, the establishment of flavor correspondences may occur during a critical period outside of the training period used here, or require more extensive exposure. Finally, even though experience with specific flavor combinations is a key component of most definitions of congruency—both implicit (Murphy and Cain 1980; Schifferstein and Verlegh 1996; Fondberg et al. 2018) and explicit (Lim et al. 2014)—it is possible that innate correspondences between certain taste and smell components exist and that these correspondences affect the multisensory computations underlying consumption decisions. For example, certain volatiles found in fruits are known to selectively enhance sweetness (Baldwin et al. 2008; Tieman et al. 2012; Schwieterman et al. 2014) and may be innately congruent with sweet taste. Future work, employing different training protocols or similar protocols in the context of different tasks, or using putative innately congruent flavor combinations, will test the role of these factors in informing flavor consumption decisions.
Our finding that experience does not affect instantaneous decisions about taste–smell mixtures relative to their components does not imply that experience has no effect on perception of the components. Although we did not compare component consumption before and after training, it is possible, and perhaps even likely, that our training procedure changed the palatability of components through associative learning (Holman 1975; Rescorla and Cunningham 1978; Rescorla 1980; Fanselow and Birk 1982; Holder 1991; Capaldi et al. 1994; Yeomans et al. 2006; Blankenship et al. 2019). It is also possible that mere exposure to the individual components (regardless of the exact pairings) is necessary for their integration during subsequent testing. Future experiments that involve comparison to a naive control group will test for this possibility.
In summary, the present findings provide novel insight into the mechanisms underlying multisensory flavor judgments. When judging a taste–smell mixture in the context of consumption, animals linearly weigh the information provided by the components. A similar pattern has been observed in visuo-haptic (Ernst and Banks 2002), auditory-haptic (Bresciani et al. 2005), and visuo-vestibular (Butler et al. 2011; Frissen et al. 2011) integration, suggesting an evolutionarily conserved underlying neural computation. However, the specific mechanisms that underlie taste–smell integration appear to be unique in that they are not determined by experience with specific multisensory stimulus combinations, consistent with the flavor system’s unique computational goal of evaluating a virtually unlimited set of behaviorally relevant multisensory stimulus combinations.
Funding
This work was supported by the National Institute of Deafness and Other Communications Disorders of the National Institutes of Health (R01 DC016063 to J.X.M.).
Acknowledgments
The authors thank Chad Collins for help with data collection and stimulus preparation and to Ben Rowland and Emilio Salinas for helpful comments on an earlier draft of this manuscript.
Author contributions
J.X.M. and V.E.E. conceived of and designed the research; V.E.E. performed the experiments; V.E.E. analyzed the data; V.E.E. and J.X.M. interpreted the results and drafted the manuscript.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- Adams WJ, Graf EW, Ernst MO. 2004. Experience can change the ‘light-from-above’ prior. Nat Neurosci. 7:1057–1058. [DOI] [PubMed] [Google Scholar]
- Amsellem S, Ohla K. 2016. Perceived odor–taste congruence influences intensity and pleasantness differently. Chem Senses. 41:677–684. [DOI] [PubMed] [Google Scholar]
- Auvray M, Spence C. 2008. The multisensory perception of flavor. Conscious Cogn. 17:1016–1031. [DOI] [PubMed] [Google Scholar]
- Baldwin EA, Goodner K, Plotto A. 2008. Interaction of volatiles, sugars, and acids on perception of tomato aroma and flavor descriptors. J Food Sci. 73:S294–S307. [DOI] [PubMed] [Google Scholar]
- Blankenship ML, Grigorova M, Katz DB, Maier JX. 2019. Retronasal odor perception requires taste cortex, but orthonasal does not. Curr Biol. 29:62–69.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresciani JP, Ernst MO, Drewing K, Bouyer G, Maury V, Kheddar A. 2005. Feeling what you hear: auditory signals can modulate tactile tap perception. Exp Brain Res. 162:172–180. [DOI] [PubMed] [Google Scholar]
- Breza JM, Contreras RJ. 2012. Acetic acid modulates spike rate and spike latency to salt in peripheral gustatory neurons of rats. J Neurophysiol. 108:2405–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JS, Campos JL, Bülthoff HH, Smith ST. 2011. The role of stereo vision in visual-vestibular integration. Seeing Perceiv. 24:453–470. [DOI] [PubMed] [Google Scholar]
- Capaldi ED, Owens J, Palmer KA. 1994. Effects of food deprivation on learning and expression of flavor preferences conditioned by saccharin or sucrose. Anim Learn Behav. 22:173–180. [Google Scholar]
- Dalton P, Doolittle N, Nagata H, Breslin PA. 2000. The merging of the senses: integration of subthreshold taste and smell. Nat Neurosci. 3:431–432. [DOI] [PubMed] [Google Scholar]
- Dwyer DM, Haselgrove M, Jones PM. 2011. Cue interactions in flavor preference learning: a configural analysis. J Exp Psychol Anim Behav Process. 37:41–57. [DOI] [PubMed] [Google Scholar]
- Ernst MO, Banks MS. 2002. Humans integrate visual and haptic information in a statistically optimal fashion. Nature. 415:429–433. [DOI] [PubMed] [Google Scholar]
- Ernst MO, Bülthoff HH. 2004. Merging the senses into a robust percept. Trends Cogn Sci. 8:162–169. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Birk J. 1982. Flavor-flavor associations induce hedonic shifts in taste preference. Anim Learn Behav. 10:223–228. [Google Scholar]
- Fetsch CR, DeAngelis GC, Angelaki DE. 2013. Bridging the gap between theories of sensory cue integration and the physiology of multisensory neurons. Nat Rev Neurosci. 14:429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondberg R, Lundström JN, Blöchl M, Olsson MJ, Seubert J. 2018. Multisensory flavor perception: the relationship between congruency, pleasantness, and odor referral to the mouth. Appetite. 125:244–252. [DOI] [PubMed] [Google Scholar]
- Formaker BK, MacKinnon BI, Hettinger TP, Frank ME. 1997. Opponent effects of quinine and sucrose on single fiber taste responses of the chorda tympani nerve. Brain Res. 772:239–242. [DOI] [PubMed] [Google Scholar]
- Frank RA, Byram J. 1988. Taste–smell interactions are tastant and odorant dependent. Chem Senses. 13:445–455. [Google Scholar]
- Frissen I, Campos JL, Souman JL, Ernst MO. 2011. Integration of vestibular and proprioceptive signals for spatial updating. Exp Brain Res. 212:163–176. [DOI] [PubMed] [Google Scholar]
- Garcia-Medina MR. 1981. Flavor-odor taste interactions in solutions of acetic acid and coffee. Chem Senses. 6:13–22. [Google Scholar]
- Gautam SH, Verhagen JV. 2010. Evidence that the sweetness of odors depends on experience in rats. Chem Senses. 35:767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillan DJ. 1983. Taste-taste, odor-odor, and taste-odor mixtures: greater suppression within than between modalities. Percept Psychophys. 33:183–185. [DOI] [PubMed] [Google Scholar]
- Green BG, Lim J, Osterhoff F, Blacher K, Nachtigal D. 2010. Taste mixture interactions: suppression, additivity, and the predominance of sweetness. Physiol Behav. 101:731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Thein T. 2005. Interactions between conditioned and unconditioned flavor preferences. J Exp Psychol Anim Behav Process. 31:407–417. [DOI] [PubMed] [Google Scholar]
- Hillis JM, Ernst MO, Banks MS, Landy MS. 2002. Combining sensory information: mandatory fusion within, but not between, senses. Science. 298:1627–1630. [DOI] [PubMed] [Google Scholar]
- Holder MD. 1991. Conditioned preferences for the taste and odor components of flavors: blocking but not overshadowing. Appetite. 17:29–45. [DOI] [PubMed] [Google Scholar]
- Holman EW. 1975. Immediate and delayed reinforcers for flavor preferences in rats. Learn Motiv. 6:91–100. [Google Scholar]
- Hornung DE, Enns MP. 1986. The contributions of smell and taste to overall intensity: a model. Percept Psychophys. 39:385–391. [DOI] [PubMed] [Google Scholar]
- Labbe D, Rytz A, Morgenegg C, Ali S, Martin N. 2007. Subthreshold olfactory stimulation can enhance sweetness. Chem Senses. 32:205–214. [DOI] [PubMed] [Google Scholar]
- Lim J, Fujimaru T, Linscott TD. 2014. The role of congruency in taste–odor interactions. Food Qual Prefer. 34:5–14. [Google Scholar]
- Lippert M, Logothetis NK, Kayser C. 2007. Improvement of visual contrast detection by a simultaneous sound. Brain Res. 1173:102–109. [DOI] [PubMed] [Google Scholar]
- Maier JX, Blankenship ML, Barry NC, Richards SE, Katz DB. 2014. Stability and flexibility of the message carried by semiochemical stimuli, as revealed by devaluation of carbon disulfide followed by social transmission of food preference. Behav Neurosci. 128:413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier JX, Katz DB. 2013. Neural dynamics in response to binary taste mixtures. J Neurophysiol. 109:2108–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks LE, Veldhuizen MG, Shepard TG, Shavit AY. 2012. Detecting gustatory-olfactory flavor mixtures: models of probability summation. Chem Senses. 37:263–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride SA, Slotnick B. 1997. The olfactory thalamocortical system and odor reversal learning examined using an asymmetrical lesion paradigm in rats. Behav Neurosci. 111:1273–1284. [DOI] [PubMed] [Google Scholar]
- McDonald JJ, Teder-Sälejärvi WA, Hillyard SA. 2000. Involuntary orienting to sound improves visual perception. Nature. 407:906–908. [DOI] [PubMed] [Google Scholar]
- Moskowitz HR. 1972. Perceptual changes in taste mixtures. Percept Psychophys. 11:257–262. [Google Scholar]
- Murphy C, Cain WS. 1980. Taste and olfaction: independence vs interaction. Physiol Behav. 24:601–605. [DOI] [PubMed] [Google Scholar]
- Murphy C, Cain WS, Bartoshuk LM. 1977. Mutual action of taste and olfaction. Sens Process. 1:204–211. [PubMed] [Google Scholar]
- Murphy JP, Samantha. 2009. Inhibition of evaluative and perceptual odour–taste learning by attention to the stimulus elements. Q J Exp Psychol. 62:2133–2140. [DOI] [PubMed] [Google Scholar]
- Oram N, Laing DG, Freeman MH, Hutchinson I. 2001. Analysis of taste mixtures by adults and children. Dev Psychobiol. 38:67–77. [DOI] [PubMed] [Google Scholar]
- Palmerino CC, Rusiniak KW, Garcia J. 1980. Flavor-illness aversions: the peculiar roles of odor and taste in memory for poison. Science. 208:753–755. [DOI] [PubMed] [Google Scholar]
- Prescott J. 1999. Flavour as a psychological construct: implications for perceiving and measuring the sensory qualities of foods. Food Qual Prefer. 10:349–356. [Google Scholar]
- Prescott J. 2015. Multisensory processes in flavour perception and their influence on food choice. Curr Opin Food Sci. 3:47–52. [Google Scholar]
- Prescott RJS, Robert AB, John. 1998. Changes in odor sweetness resulting from implicit learning of a simultaneous odor–sweetness association: an example of learned synesthesia. Learn Motiv. 29:113–132. [Google Scholar]
- Rescorla RA, Cunningham CL. 1978. Within-compound flavor associations. J Exp Psychol Anim Behav Process. 4:267–275. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Durlach PJ. 1981. Within-event learning in Pavlovian conditioning. In: Spear NE, Miller RR (eds.), Information processing in animals, memory mechanisms. Hillsdale (NJ): Erlbaum; p. 81–112. [Google Scholar]
- Rescorla RA. 1980. Simultaneous and successive associations in sensory preconditioning. J Exp Psychol Anim Behav Process. 6:207–216. [DOI] [PubMed] [Google Scholar]
- Rowland BA, Stein BE. 2007. Multisensory integration produces an initial response enhancement. Front Integr Neurosci. 1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland BA, Stein BE. 2008. Temporal profiles of response enhancement in multisensory integration. Front Neurosci. 2:218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusiniak KW, Hankins WG, Garcia J, Brett LP. 1979. Flavor-illness aversions: potentiation of odor by taste in rats. Behav Neural Biol. 25:1–17. [DOI] [PubMed] [Google Scholar]
- Schifferstein HN, Verlegh PW. 1996. The role of congruency and pleasantness in odor-induced taste enhancement. Acta Psychol (Amst). 94:87–105. [DOI] [PubMed] [Google Scholar]
- Schwieterman ML, Colquhoun TA, Jaworski EA, Bartoshuk LM, Gilbert JL, Tieman DM, Odabasi AZ, Moskowitz HR, Folta KM, Klee HJ, et al. 2014. Strawberry flavor: diverse chemical compositions, a seasonal influence, and effects on sensory perception. PLoS One. 9:e88446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. 1994. Glucose- and fructose-conditioned flavor preferences in rats: taste versus postingestive conditioning. Physiol Behav. 56:399–405. [DOI] [PubMed] [Google Scholar]
- Seo HS, Iannilli E, Hummel C, Okazaki Y, Buschhüter D, Gerber J, Krammer GE, van Lengerich B, Hummel T. 2013. A salty-congruent odor enhances saltiness: functional magnetic resonance imaging study. Hum Brain Mapp. 34:62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard TG, Veldhuizen MG, Marks LE. 2015. Response times to gustatory-olfactory flavor mixtures: role of congruence. Chem Senses. 40:565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick BM, Westbrook F, Darling FMC. 1997. What the rat’s nose tells the rat’s mouth: long delay aversion conditioning with aqueous odors and potentiation of taste by odors. Anim Learn Behav. 25:357–369. [Google Scholar]
- Small DM, Prescott J. 2005. Odor/taste integration and the perception of flavor. Exp Brain Res. 166:345–357. [DOI] [PubMed] [Google Scholar]
- Small DM, Voss J, Mak YE, Simmons KB, Parrish T, Gitelman D. 2004. Experience-dependent neural integration of taste and smell in the human brain. J Neurophysiol. 92:1892–1903. [DOI] [PubMed] [Google Scholar]
- Spence C. 2019. On the relationship(s) between color and taste/flavor. Exp Psychol. 66:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BE, London N, Wilkinson LK, Price DD. 1996. Enhancement of perceived visual intensity by auditory stimuli: a psychophysical analysis. J Cogn Neurosci. 8:497–506. [DOI] [PubMed] [Google Scholar]
- Stein BE, Meredith MA, Huneycutt WS, McDade L. 1989. Behavioral indices of multisensory integration: orientation to visual cues is affected by auditory stimuli. J Cogn Neurosci. 1:12–24. [DOI] [PubMed] [Google Scholar]
- Stein BE, Stanford TR, Rowland BA. 2014. Development of multisensory integration from the perspective of the individual neuron. Nat Rev Neurosci. 15:520–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RJ. 2001. Is sweetness taste enhancement cognitively impenetrable? Effects of exposure, training and knowledge. Appetite. 36:241–242. [DOI] [PubMed] [Google Scholar]
- Stevenson RJ, Prescott J, Boakes RA. 1995. The acquisition of taste properties by odors. Learn Motiv. 26:433–455. [Google Scholar]
- Stevenson RJ, Prescott J, Boakes RA. 1999. Confusing tastes and smells: how odours can influence the perception of sweet and sour tastes. Chem Senses. 24:627–635. [DOI] [PubMed] [Google Scholar]
- Tieman D, Bliss P, McIntyre LM, Blandon-Ubeda A, Bies D, Odabasi AZ, Rodríguez GR, van der Knaap E, Taylor MG, Goulet C, et al. 2012. The chemical interactions underlying tomato flavor preferences. Curr Biol. 22:1035–1039. [DOI] [PubMed] [Google Scholar]
- van Stokkom VL, Blok AE, van Kooten O, de Graaf C, Stieger M. 2018. The role of smell, taste, flavour and texture cues in the identification of vegetables. Appetite. 121:69–76. [DOI] [PubMed] [Google Scholar]
- Veldhuizen MG, Shepard TG, Wang MF, Marks LE. 2010. Coactivation of gustatory and olfactory signals in flavor perception. Chem Senses. 35:121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welge-Lussen A, Husner A, Wolfensberger M, Hummel T. 2009. Influence of simultaneous gustatory stimuli on orthonasal and retronasal olfaction. Neurosci Lett. 454:124–128. [DOI] [PubMed] [Google Scholar]
- White TL, Prescott J. 2007. Chemosensory cross-modal stroop effects: congruent odors facilitate taste identification. Chem Senses. 32:337–341. [DOI] [PubMed] [Google Scholar]
- Xu Y, Regier T, Newcombe NS. 2017. An adaptive cue combination model of human spatial reorientation. Cognition. 163:56–66. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Mobini S, Elliman TD, Walker HC, Stevenson RJ. 2006. Hedonic and sensory characteristics of odors conditioned by pairing with tastants in humans. J Exp Psychol Anim Behav Process. 32:215–228. [DOI] [PubMed] [Google Scholar]