ABSTRACT
National guidelines suggest that pregnant women consume 2–3 servings of fish weekly and often focus exclusively on limiting mercury exposure. We examined if meeting this recommendation in the third trimester of pregnancy was associated with differences in infant fecal microbiota composition and diversity. We used multinomial regression to analyze data from 114 infant–mother dyads. Applying 16S rRNA gene sequencing, we identified 3 infant fecal microbiota profiles: Bifidobacterium dominant, Enterobacter dominant, and Escherichia dominant. We found that 20% of mothers met the recommended fish consumption, and those infants whose mothers met the recommendation were more likely to have a Bifidobacterium-dominant profile than an Escherichia-dominant profile (RR ratio: 4.61; 95% CI: 1.40, 15.15; P = 0.01). In multivariable models, the significant association persisted (P < 0.05). Our findings support the need to expand recommendations focusing on the beneficial effects of fish consumption on the infant fecal microbiota profile.
Keywords: pregnancy, fish consumption, infant microbiota, Bifidobacterium, ω3 polyunsaturated fatty acids
Introduction
Emerging evidence suggests that infant and childhood fecal microbiota are associated with the pathogenesis of several disease states, including obesity, diabetes, respiratory infections, and asthma (1, 2). The infant microbiome is affected both prenatally and postnatally by developmental and environmental factors including delivery mode, breast or formula feeding, and medication use, as well as maternal environment and diet (3,4). However, studies investigating the effects on the infant microbiome of maternal diet during pregnancy are limited (5).
Fish is an essential food for women during pregnancy, as a primary dietary source of ω3 PUFAs, including DHA and EPA, which are necessary for fetal neurodevelopment (6–8). However, concerns about mercury seem to be a factor discouraging higher fish consumption. There are several international and national guidelines on fish consumption during pregnancy and their most often relate to mercury content targeting reduced exposure, rather than the high nutritional quality (9). The FDA and the Environmental Protection Agency have recommended that pregnant women consume 2–3 servings of fish with low levels of mercury per week, which has been linked to improved child health outcomes, including improved growth and cognition (6,7,10). Despite the clinical and research importance of fish consumption, little is known about the relation between meeting this dietary recommendation and its impact on infant fecal microbiota profiles. To address the knowledge gap, we aimed to examine the association between mothers who meet the recommended maternal fish consumption during pregnancy and infant microbiota composition.
Methods
We analyzed the data of 114 infants with fecal samples at Massachusetts General Hospital collected from November 2013 through April 2014. These children were healthy age-matched controls in a larger multicenter, prospective, observational cohort study called the MARC-43 (43th Multicenter Airway Research Collaboration, also known as the CHIME study) and coordinated by the Emergency Medicine Network (2). Study design, participants, and methodology of the larger cohort were previously reported, including monetary compensation for study participation (11–13). Inclusion criteria included age <1 y, gestational age ≥34 wk at birth, and identification of a primary care resource defined as a provider of coordinated, comprehensive, longitudinal care over a 12-mo period. Exclusion criteria included presence of comorbidities (heart–lung disease, immunodeficiency, immunosuppression, or chronic gastrointestinal disorder), fever, respiratory or gastrointestinal illness, or antibiotic treatment within the 7 d prior to enrollment (11–13). All study procedures were approved by the institutional review board at Massachusetts General Hospital, and written informed consent was obtained for all study participants from the parent or legal guardian.
We conducted structured surveys and medical record reviews at enrollment to capture maternal and child demographics, prenatal history, nutritional characteristics during pregnancy, postnatal medical history (e.g., mode of birth, gestational age, systemic antibiotic use), and environmental characteristics (e.g., daycare attendance) in infancy.
The primary exposure was whether or not the biological mother met the national recommendations for fish consumption in the third trimester of 2 to 3 servings of fish per week [4 ounce (oz)servings] (6). Fish consumption was examined by asking the mother at enrollment: “How many servings of fish (excluding shellfish and breaded fish pieces) did you usually eat?” during the third trimester of pregnancy. Those mothers eating at least 2 or more servings per week were defined as meeting the recommendation.
The primary outcome was infant fecal microbiota composition. Parents collected infant fecal samples at home using a standardized protocol and were instructed to refrigerate fecal matter–containing diapers (11,14). Within 24 h, stool was returned to study staff, divided into aliquots, and stored at − 80°C. Samples were processed and underwent 16S rRNA gene sequencing at the Alkek Center for Metagenomics and Microbiome Research at Baylor College of Medicine (14). Briefly, bacterial genomic DNA was extracted using a MO BIO PowerMag DNA isolation kit (Mo Bio Laboratories), amplified by PCR, and sequenced with the MiSeq platform (Illumina) using a 2 × 250–bp paired-end protocol. Sequencing read pairs were demultiplexed and merged using USEARCH v7.0.1090 (15). Gene sequences were clustered into operational taxonomic units (OTUs) at a similarity cutoff value of 97% using the UPARSE algorithm; taxonomies were determined by mapping OTUs to the SILVA database; and abundances were recovered by mapping the demultiplexed reads to the UPARSE OTUs.
Standard quality control practices were utilized including positive and nontemplate controls (extraction chemistries) during extraction and PCR amplification. Acceptance criteria included alignment of >99% of alignment of the sequence reads of the positive control to the expected bacterial genome, and <500 raw sequence reads in the nontemplate controls. In the current study, no amplicons were observed in the nontemplate controls and <500 reads were recovered after sequencing. All 114 fecal samples had sufficient sequence depth to obtain high degrees of sequence coverage (rarefaction cutoff, 1470 reads per sample). Sequence data are available through controlled access at NIH ImmPort (accession number, SDY1182).
We performed the microbiota analyses at the genus level because the bacterial DNA sequences in our sample were dominated by 1 OTU per genus (14). Microbiota composition was described by α diversity, more specifically the Shannon's diversity index (16). Bray–Curtis distances and the gap statistic were used to characterize the fecal microbiota profiles and the number of clusters, respectively. Microbiota data were visualized by nonmetric multidimensional scaling (NMDS), which utilized the Bray distance matrices (17). P values were adjusted for multiple testing using the Benjamini–Hochberg false-discovery rate procedure with a q-value threshold of 0.05 (18).
Associations between meeting the recommended maternal fish consumption and infant microbiota profiles were examined by using multinomial regression models. The adjusted models included predetermined confounders that may impact microbiota composition (3,5). Covariates included the following: infant age (at stool collection), infant sex, maternal supplementation with vitamin D and fish oil, delivery mode, infant lifetime history of systemic antibiotic use, mode of feeding (breastfed compared with formula fed), and daycare attendance. Given our sample size, we included ≤2 covariates per multinomial model. All models were assessed using diagnostics for assumptions of homogeneity of variance. We found no outliers that influenced significance in the full models. We conducted statistical analyses using R version 3.3. Statistical significance was determined by 2-tailed P < 0.05.
Results
Participant characteristics
Overall, the median age of infants was 3.8 mo (IQR: 2.0–4.9), 45% were female, and 53% were non-Hispanic white. Demographic characteristics did not differ significantly between the 2 fish-consumption groups (all P > 0.05), except for the infant microbiota profiles (Supplemental Table 1).
Fish consumption and infant fecal microbiota
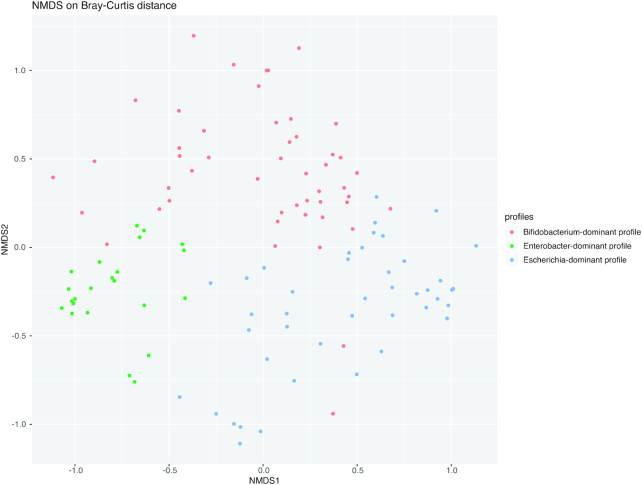
Unsupervised clustering of fecal microbiota identified 3 distinct microbiota profiles (Figure 1): 1) Bifidobacterium-dominant profile (43%), 2) Enterobacter-dominant profile (20%), and 3) Escherichia-dominant profile (37%). The Bifidobacterium-dominant profile had highest bacterial richness and α diversity (both P < 0.001; Table 1).
FIGURE 1.
Nonmetric multidimensional scaling plot of infant fecal microbiota profiles. To show the differences in fecal microbiota among 114 infants, an NMDS plot based on the Bray–Curtis distance between all subjects was generated by using the R package phyloseq. Each dot represents the overall bacterial community in each infant. Colors indicate 3 microbiota profiles: Bifidobacterium-dominant profile (green), Enterobacter-dominant profile (red), and Escherichia-dominant profile (blue). The NMDS stress value is 0.19. The NMDS plot revealed that subjects cluster together according to their microbiota profile. NMDS, nonmetric multidimensional scaling.
TABLE 1.
Richness, α diversity, and relative genera abundance by infant fecal microbiota profile1
| Bifidobacterium-dominant profile | Enterobacter-dominant profile | Escherichia-dominant profile | P value | |
|---|---|---|---|---|
| Indices | n = 49 | n = 23 | n = 42 | |
| Richness | ||||
| Number of genera | 17 (12–22) | 11 (9–15) | 14 (10–20) | 0.001 |
| α diversity | ||||
| Shannon index | 1.60 ± 0.51 | 1.15 ± 0.34 | 1.29 ± 0.51 | <0.001 |
| Relative abundance of 10 most common genera | ||||
| Bifidobacterium | 0.36 ± 0.22 | 0.09 ± 0.12 | 0.08 ± 0.09 | 0.002* |
| Enterobacter | 0.07 ± 0.11 | 0.58 ± 0.15 | 0.07 ± 0.12 | 0.002* |
| Escherichia | 0.14 ± 0.14 | 0.01 ± 0.03 | 0.44 ± 0.28 | 0.002* |
| Bacteroides | 0.03 ± 0.05 | 0.09 ± 0.16 | 0.20 ± 0.26 | 0.002* |
| Veillonella | 0.08 ± 0.15 | 0.09 ± 0.11 | 0.03 ± 0.05 | 0.29* |
| Lachnoclostridium | 0.04 ± 0.10 | 0.01 ± 0.04 | 0.02 ± 0.04 | 0.53* |
| Clostridium sensustricto 1 | 0.01 ± 0.02 | 0.04 ± 0.05 | 0.03 ± 0.09 | 0.54* |
| Streptococcus | 0.03 ± 0.05 | 0.03 ± 0.07 | 0.02 ± 0.04 | 0.75* |
| Enterococcus | 0.02 ± 0.04 | 0.03 ± 0.05 | 0.02 ± 0.04 | 0.83* |
| Haemophilus | 0.03 ± 0.09 | 0.01 ± 0.02 | 0.01 ± 0.03 | 0.60* |
Values are medians (IQRs) or means ± SDs unless otherwise indicated. *Benjamini–Hochberg adjusted P value accounting for multiple comparisons.
In the unadjusted model, infants whose mothers met the recommended maternal fish consumption were more likely to have a Bifidobacterium-dominant profile than an Escherichia-dominant profile, corresponding to an RR ratio of 4.61 (95% CI: 1.40, 15.15; P = 0.01; Supplemental Table 2). In multivariable models adjusted for a different set of confounders, the significant associations persisted (all P < 0.05; Supplemental Table 2). For example, in the model adjusted for infant age and breastfeeding status, infants whose mothers met the recommended maternal fish consumption were more likely to have a Bifidobacterium-dominant profile, with a corresponding RR ratio of 4.98 (95% CI: 1.49, 16.66; P = 0.009).
Discussion
In this prospective cohort study, we found a significant association between meeting the recommended maternal fish consumption and a higher likelihood of a Bifidobacterium-dominant microbiota profile in their offspring. This association persisted after adjustment for confounders. While there have been multiple studies that focus on the relations of mode of delivery, feeding, and exposure to antibiotics with the infant microbiome, no cohort study, to our knowledge, has examined the association of maternal third-trimester fish consumption with their infant's microbiota profile. Our observations support re-evaluation and expansion of current dietary recommendations during pregnancy.
Current recommendations for fish consumption during pregnancy vary greatly in content and complexity at national and international levels. Most often these recommendations focus on limiting mercury intake rather than the beneficial effects of fish consumption. Epidemiological studies have demonstrated that fish intake during pregnancy is associated with benefits for maternal and child health outcomes, including increased gestational length; improved cognitive, visual, motor, cardiac, and immune development for the infant; and improved maternal mood (19–22). To our knowledge, there has been 1 reported study to date examining the effects of maternal fish consumption during pregnancy on the fecal microbiota of infants (5). In this relatively small nonblinded trial from the UK, formula-fed, but not breastfed, infants of mothers assigned to the higher–salmon consumption group (two 150-gram (g) portions of salmon per week from 20 weeks of pregnancy to delivery) had lower proportions of Atopobium. No changes in Bifidobacterium were detected. The differences in the results of this study in relation to our findings might be attributable to differences in study design and setting. More specifically, Urwin et al. reported an intervention in mothers in the UK (n = 123), from 20 wk of pregnancy to birth, with no baseline stool microbiota evaluation before the intervention (5). In comparison, the study we report here is a cross-sectional analysis of an observational cohort of 114 mothers and infants within the United States. Additionally, the physiological complexity of pregnancy coupled with inter-individual variability may help explain the observed differences. Any of these factors, or a combination thereof, may have contributed to the differences in findings.
While findings from interventional studies examining the effects of maternal fish oil supplementation on child outcomes are equivocal (23), a recent randomized controlled trial demonstrated a reduced risk of persistent wheeze or asthma in the first 5 y of life among children whose mothers received supplementation in the third trimester of pregnancy (24). These health benefits are thought to be attributed to ω3 PUFAs found in fish. Recent studies have shown that ω3 PUFAs can change the intestinal microbiota composition by increasing the abundance of Bifidobacterium, a prominent genus in the stool of infants prior to the introduction of solids (4). Studies have shown that a lower abundance of Bifidobacterium is associated with atopic diseases, irritable bowel syndrome, inflammatory bowel disease, and celiac disease (25). Additionally, Bifidobacterium probiotic use is associated with preventing necrotizing enterocolitis in preterm infants with very low birth weight (26), children with antibiotic-associated diarrhea, and children with wheezing illness (27). Many studies have outlined the role of the gut microbiome in health with bifidobacteria playing a critical role (28,29). Various disease pathologies, including but not limited to enteric diseases, are associated with shifts in the proportion and diversity of bifidobacteria (30). These characteristics may contribute to microbiome instability and greater susceptibility to external factors. These diseases represent opportunities for the application of probiotic-based therapies derived from infant microbiome studies such as ours and provide focus for future interventions (29).
Our study, to our knowledge, is the first examination of the association of maternal dietary fish intake and infant microbiome composition, the investigation of which is necessary to help guide current and future recommendations. However, some limitations must be considered in the interpretation of our findings. First, the use of a self-report measure for maternal fish consumption may be subject to recall and social desirability bias. However, there are various international studies that have investigated dietary intake in pregnant women before and during pregnancy and demonstrated the validity of self-report measures in this population (31,32). Second, our sample size of 114 mother–infant dyads may be considered small, which did not allow us to adjust concurrently for all potential confounders. However, the significant association persisted after we adjusted our data for a different set of clinically relevant confounders. Third, the fecal microbiota was measured in early infancy at a single timepoint, yet fecal microbiome data quality was quite robust. Future efforts include examining the temporal change in the microbiota, as we are currently following the cohort to 6 y of age with fecal collections at multiple timepoints, and utilization of metagenomics, metatranscriptomics, and metabolomics to gain a more complete understanding of the functionalities of the fecal microbiome and how these are related to long-term health outcomes in children (33).
In conclusion, in this prospective cohort of healthy infants, meeting the national recommendations for fish consumption in the third trimester was associated with a higher likelihood of a Bifidobacterium-dominant fecal microbiota profile in the infant. Increased understanding of the impact of maternal diet on the infant microbiome and child health may lead to the development of dietary strategies to promote long-term child health outcomes and better definition of maternal dietary intake recommendations during pregnancy.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Ashley Sullivan, MS, MPH, and Janice Espinola, MPH, for their many contributions to the MARC-43 cohort. We also think the participating families; without them, none of this would be possible.
The authors’ responsibilities were as follows— LF, BR, NJA, JFP, CAC, EMT, and KH: made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; MS, SGH, CAC, LF, and KH: have drafted the article or revised it critically for important intellectual content; and all authors: read and approved the final manuscript.
Notes
This work was supported by NIH grants K24DK105989 and P30DK040561 (to EMT); UG3/UH3 OD023253 and U01 AI-087881 (to CC); R01 AI134940 and R01 AI137091 (to KH); the Agency for Healthcare Research and Quality (K12HS022986 to LF) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (1K23HD090222-01A1 to LF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Agency for Healthcare Research and Quality.
Author disclosures: NJA and JFP own shares at Diversigen Inc., a microbiome research company. All other authors report no conflicts of interest.
Supplemental Tables 1 and 2 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://cdn.nutrition.org.
Abbreviations used: NMDS, nonmetric multidimensional scaling; OTU, operational taxonomic unit.
References
- 1. Reinhardt C, Reigstad CS, Bäckhed F. Intestinal microbiota during infancy and its implications for obesity. J Pediatr Gastroenterol Nutr. 2009;48:249–56. [DOI] [PubMed] [Google Scholar]
- 2. Mansbach JM, Hasegawa K, Henke DM, Ajami NJ, Petrosino JF, Shaw CA, Piedra PA, Sullivan AF, Espinola JA, Camargo CA. Respiratory syncytial virus and rhinovirus severe bronchiolitis are associated with distinct nasopharyngeal microbiota. J Allergy Clin Immunol. 2016;137:1909–13..e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wopereis H, Oozeer R, Knipping K, Belzer C, Knol J. The first thousand days—intestinal microbiology of early life: establishing a symbiosis. Pediatr Allergy Immunol. 2014;25:428–38. [DOI] [PubMed] [Google Scholar]
- 4. Robertson RC, Kaliannan K, Strain CR, Ross RP, Stanton C, Kang JX. Maternal omega-3 fatty acids regulate offspring obesity through persistent modulation of gut microbiota. Microbiome. 2018;6:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Urwin HJ, Miles EA, Noakes PS, Kremmyda L-S, Vlachava M, Diaper ND, Godfrey KM, Calder PC, Vulevic J, Yaqoob P. Effect of salmon consumption during pregnancy on maternal and infant faecal microbiota, secretory IgA and calprotectin. Br J Nutr. 2014;111:773–84. [DOI] [PubMed] [Google Scholar]
- 6. Newberry SJ, Chung M, Booth M, Maglione MA, Tang AM, O'Hanlon CE, Wang DD, Okunogbe A, Huang C, Motala A et al.. Omega-3 fatty acids and maternal and child health: an updated systematic review. Evidence report/technology assessment. 2016;224:1–826. [DOI] [PubMed] [Google Scholar]
- 7. Nykjaer C, Higgs C, Greenwood D, Simpson N, Cade J, Alwan N. Maternal fatty fish intake prior to and during pregnancy and risks of adverse birth outcomes: findings from a British Cohort. Nutrients. 2019;11:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Groth E. Scientific foundations of fish-consumption advice for pregnant women: epidemiological evidence, benefit-risk modeling, and an integrated approach. Environ Res. 2017;152:386–406. [DOI] [PubMed] [Google Scholar]
- 9. Taylor CM, Emmett PM, Emond AM, Golding J. A review of guidance on fish consumption in pregnancy: is it fit for purpose?. Public Health Nutr. 2018;21:2149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Advice about eating fish | FDA [Internet]. US Food and Drug Administration 2019; [cited 2019 Aug 6]. Available from: https://www.fda.gov/food/consumers/advice-about-eating-fish.
- 11. Hasegawa K, Linnemann RW, Mansbach JM, Ajami NJ, Espinola JA, Fiechtner LG, Petrosino JF, Camargo CA Jr. Household siblings and nasal and fecal microbiota in infants. Pediatr Int. 2017;59:473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hasegawa K, Linnemann RW, Avadhanula V, Mansbach JM, Piedra PA, Gern JE, Camargo CA. Detection of respiratory syncytial virus and rhinovirus in healthy infants. BMC Res Notes. 2015;8:718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robinson A, Fiechtner L, Roche B, Ajami NJ, Petrosino JF, Camargo CA, Taveras EM, Hasegawa K, Hasegawa K. Association of maternal gestational weight gain with the infant fecal microbiota. J Pediatr Gastroenterol Nutr. 2017;65:509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hasegawa K, Linnemann RW, Mansbach JM, Ajami NJ, Espinola JA, Petrosino JF, Piedra PA, Stevenson MD, Sullivan AF, Thompson AD et al.. The fecal microbiota profile and bronchiolitis in infants. Pediatrics. 2016;138:e20160218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–1. [DOI] [PubMed] [Google Scholar]
- 16. Magurran AE. Measuring biological diversity. [Internet]. John Wiley & Sons; 2013; [cited 2019 Oct 7]. Available from: https://books.google.com/books?id=fIjsaxmL_S8C. [Google Scholar]
- 17. Paliy O, Shankar V. Application of multivariate statistical techniques in microbial ecology. Mol Ecol. 2016;25(5):1032–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–8. [DOI] [PubMed] [Google Scholar]
- 19. Oken E, Wright RO, Kleinman KP, Bellinger D, Amarasiriwardena CJ, Hu H, Rich-Edwards JW, Gillman MW. Maternal fish consumption, hair mercury, and infant cognition in a U.S. cohort. Environ Health Perspect. 2005;113:1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, Golding J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007;369:578–85. [DOI] [PubMed] [Google Scholar]
- 21. Prescott SL, Dunstan JA. Prenatal fatty acid status and immune development: the pathways and the evidence. Lipids. 2007;42:801–10. [DOI] [PubMed] [Google Scholar]
- 22. Golding J, Steer C, Emmett P, Davis JM, Hibbeln JR. High levels of depressive symptoms in pregnancy with low omega-3 fatty acid intake from fish. Epidemiology. 2009;20:598–603. [DOI] [PubMed] [Google Scholar]
- 23. Gould JF, Smithers LG, Makrides M. The effect of maternal omega-3 (n-3) LCPUFA supplementation during pregnancy on early childhood cognitive and visual development: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2013;97:531–44. [DOI] [PubMed] [Google Scholar]
- 24. Bisgaard H, Stokholm J, Chawes BL, Vissing NH, Bjarnadóttir E, Schoos A-MM, Wolsk HM, Pedersen TM, Vinding RK, Thorsteinsdóttir S et al.. Fish oil–derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375:2530–9. [DOI] [PubMed] [Google Scholar]
- 25. Tojo R, Suárez A, Clemente MG, de los Reyes-Gavilán CG, Margolles A, Gueimonde M, Ruas-Madiedo P. Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World J Gastroenterol. 2014;20:15163–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin H-C, Hsu C-H, Chen H-L, Chung M-Y, Hsu J-F, Lien R-I, Tsao L-Y, Chen C-H, Su B-H. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics. 2008;122:693–700. [DOI] [PubMed] [Google Scholar]
- 27. van der Aa LB, van Aalderen WMC, Heymans HSA, Henk Sillevis Smitt J, Nauta AJ, Knippels LMJ, Ben Amor K, Sprikkelman AB, Synbad Study Group. Synbiotics prevent asthma-like symptoms in infants with atopic dermatitis. Allergy. 2011;66:170–7. [DOI] [PubMed] [Google Scholar]
- 28. Yang B, Chen Y, Stanton C, Ross RP, Lee Y-K, Zhao J, Zhang H, Chen W. Bifidobacterium and lactobacillus composition at species level and gut microbiota diversity in infants before 6 weeks. Int J Mol Sci. 2019;20:3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Di Gioia D, Aloisio I, Mazzola G, Biavati B. Bifidobacteria: their impact on gut microbiota composition and their applications as probiotics in infants. Appl Microbiol Biotechnol. 2014;98:563–77. [DOI] [PubMed] [Google Scholar]
- 30. Aloisio I, Santini C, Biavati B, Dinelli G, Cencič A, Chingwaru W, Mogna L, Di Gioia D. Characterization of Bifidobacterium spp. strains for the treatment of enteric disorders in newborns. Appl Microbiol Biotechnol. 2012;96:1561–76. [DOI] [PubMed] [Google Scholar]
- 31. Forbes LE, Graham JE, Berglund C, Bell RC. Dietary change during pregnancy and women's reasons for change. Nutrients. 2018;10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fowles ER, Fowles SL. Healthy eating during pregnancy: determinants and supportive strategies. J Community Health Nurs. 2008;25(3):138–52. [DOI] [PubMed] [Google Scholar]
- 33. Martin VJ, Leonard MM, Fiechtner L, Fasano A. Transitioning from descriptive to mechanistic understanding of the microbiome: the need for a prospective longitudinal approach to predicting disease. J Pediatr. 2016;179:240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.