Abstract
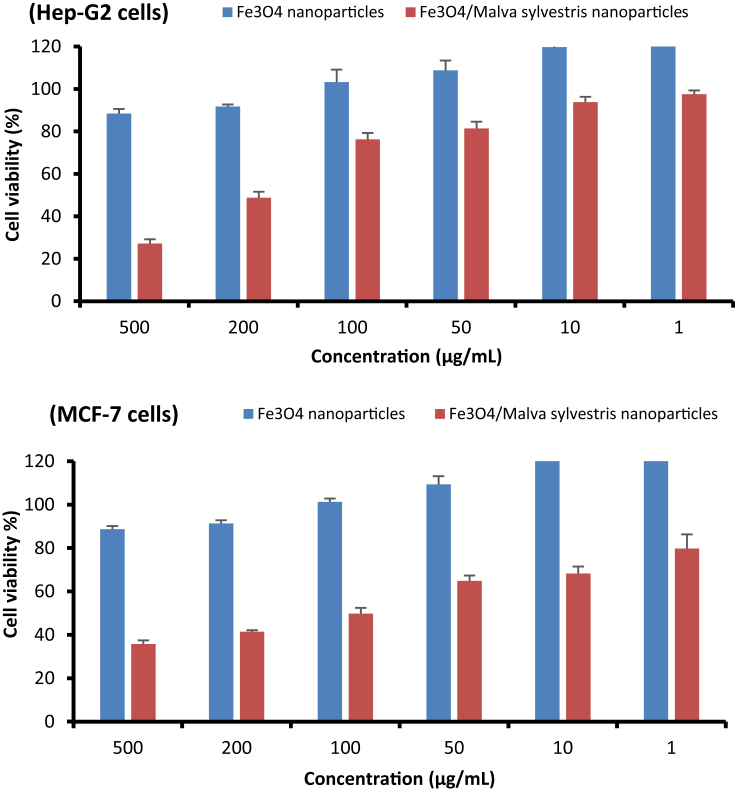
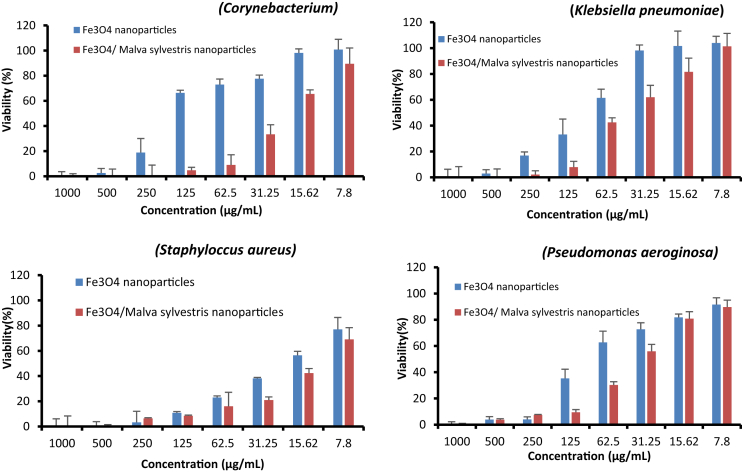
The biosynthesis of materials using medicinal plants can be a low-cost and eco-friendly approach due to their extraordinary properties. Herein, we reported a facile synthesis of Fe3O4 nanoparticles using Malva sylvestris. The surface morphology, functional groups, and elemental analysis were done to characterize the synthesized nanoparticles. The cytotoxicity performance of the synthesized nanoparticles was analyzed by exposing nanoparticles to MCF-7 and Hep-G2 cancer cell lines through MTT colorimetric assay and the IC50 value was defined as 100 μg/mL and 200 μg/mL, respectively. The antibacterial performance of synthesized nanoparticles against four different bacterial strains including Staphylococcus aureus, Corynebacterium, Pseudomonas aeruginosa, and Klebsiella pneumoniae were assessed through microdilution broth method. The synthesized Fe3O4 nanoparticles using Malva sylvestris demonstrated higher antibacterial effects against Gram-positive strains with MIC values of 62.5 μg/mL and 125 μg/mL which increase the inhibitory percentage to more than 90%.
Keywords: Malva sylvestris, Antibacterial, Fe3O4 nanoparticles, Anticancer, Cytotoxicity performance
Specifications Table
| Subject | Biology |
| Specific subject area | Developmental biology |
| Type of data | Figure and table |
| How data were acquired |
|
| Data format | Raw |
| Parameters for data collection | MIC and MBC methods for antibacterial tests and MTT assay for cytotoxic tests. |
| Description of data collection | The effects of cytotoxic and antibacterial activity of synthesized Fe3O4 nanoparticles using Malva sylvestris were evaluated on different microorganisms by MIC and MBC methods. The cytotoxic effects were determined using two different cancer cell lines. |
| Data source location | Department of Medical Nanotechnology, School of Advanced Medical Sciences and Technologies, Shiraz University of Medical Sciences, Shiraz, Iran |
| Data accessibility | Data are provided in this article. The raw data source for this article is available in the Supplementary section. |
Value of the Data
|
1. Data
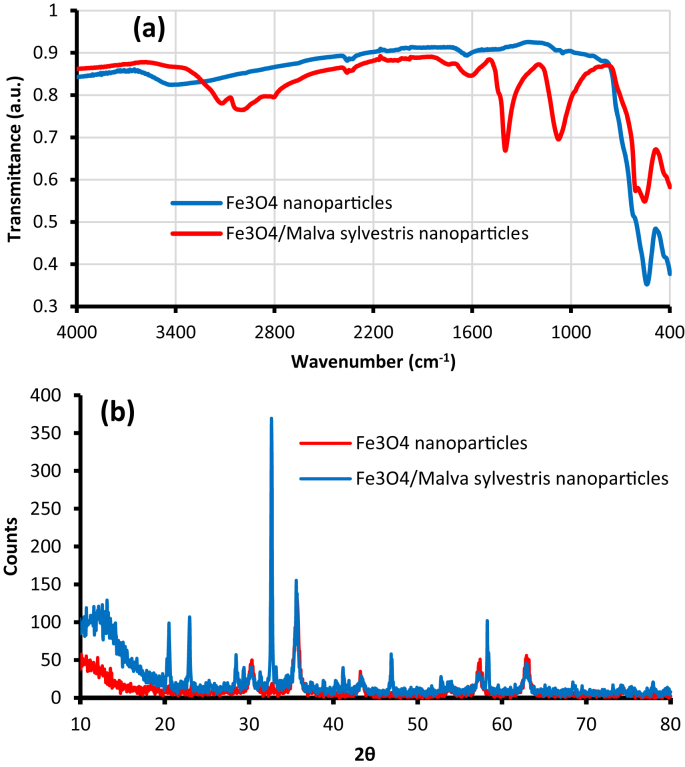
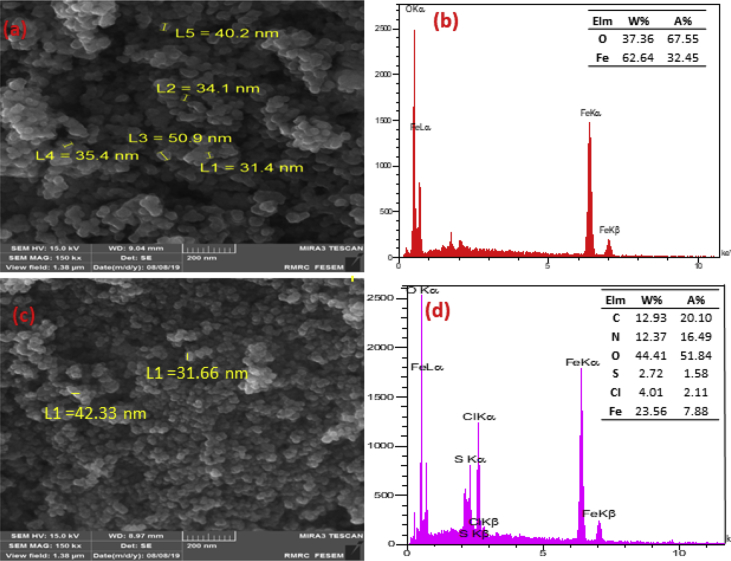
The experimental data on cytotoxic and antibacterial activity of synthesized Fe3O4 nanoparticles using Malva sylvestris are reported in this dataset. To validate the successful synthesis of Fe3O4 nanoparticles using Malva sylvestris, Fourier transform infrared spectroscopy (FTIR) was applied to determine the functional groups in the nanoparticles (Fig. 1a). The X-ray powder diffraction (XRD) analysis is depicted in Fig. 1b. SEM images and EDX analysis of Fe3O4 nanoparticles and Fe3O4/Malva sylvestris nanoparticles are presented in Fig. 2.
Fig. 1.
(a) FTIR and (b) XRD results for the Fe3O4 and Fe3O4/Malva sylvestris nanoparticles.
Fig. 2.
FESEM images and EDAX analysis of (a,b) Fe3O4 nanoparticles and (c,d) Fe3O4/Malva sylvestris nanoparticles.
The cytotoxic effects of synthesized Fe3O4 nanoparticles using Malva sylvestris against Hep-G2 and MCF-7 cell lines have demonstrated in Fig. 3. The inhibitory effect of synthesized Fe3O4 nanoparticles using Malva sylvestris against four bacterial strains was analyzed through microdilution broth assay (see Fig. 4). The performance of nanoparticles against selected microorganisms is presented in Table 1. The raw data source for this dataset is available in the Supplementary section.
Fig. 3.
Effect of nanoparticles on cell viability of MTT assay for all tested concentrations on Hep-G2 and MCF-7 cells after 24 h in comparison with control (untreated cell). Each bar represents the mean ± SD (standard deviation) of three independent tests.
Fig. 4.
Effect of nanoparticles on the viability percentages of Corynebacterium, Klebsiella pneumoniae, Staphylococcus aureus, Pseudomonas aeruginosa in tested concentrations (each bar represents the mean ± SD (standard deviation) of three independent tests).
Table 1.
Performance of nanoparticles against selected microorganisms.
| Microorganisms | Fe3O4 nanoparticles (μg/mL) |
Fe3O4/Malva sylvestris nanoparticles (μg/mL) |
||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| Staphylococcus aureus | 250 | 250 | 125 | >125 |
| Corynebacterium | 500 | 1000 | 62.5 | 125 |
| Pseudomonas aeruginosa | 250 | 500 | 125 | >125 |
| Klebsiella pneumoniae | 500 | 500 | 125 | >125 |
2. Experimental design, materials, and methods
2.1. Preparation of Fe3O4/Malva sylvestris nanoparticles
All of the materials utilized in this investigation like Ferrous(ІІ) Sulfate Heptahydrate (FeSO4.7H2O), Iron(III) Chloride Six hydrate (FeCl3.6H2O) and Ammonia solution were purchased from Merck Co. (Germany). First, 4.75 g FeCl3.6H2O, 3.89 g FeSO4.7H2O, and 320 mL deionized water were poured into a round-bottom flask and stirred for 1 h while the temperature was set to 80 °C [1,2]. Then, 0.5 g Malva sylvestris was ultrasonically mixed in 120 mL of deionized water for 30 min and then poured into the previous suspension. The obtained suspension was stirred under 80 °C for about 2 h and after that 40 mL NH3 was gradually added to the suspension [3]. After filtration, the suspension was washed and the pH scale was set on 7 and finally dried in an oven at 100 °C for 2 h.
2.2. Characterization of nanoparticles
The synthesized nanoparticles were characterized using Fourier-transform infrared spectroscopy (FTIR, Tensor ІІ FT-IR spectroscopy Bruker, Germany) in the region of 400–4000 cm−1, X-ray diffraction (XRD, Panalytical model X'Pert Pro), scanning electron microscope (SEM, Tescan model Mira III), and EDX (Tescan model S Max detector Mira III).
2.3. MTT assay
The cytotoxicity of the synthesized nanoparticles was evaluated on Hep-G2 and MCF-7 cell lines using MTT colorimetric assay. In this method, hydrogen peroxide was considered as the positive control and culture medium was the negative control. Briefly, a certain number of Hep-G2 and MCF-7 cells (10*103) were placed in each well of a sterile 96-well microplate and incubated in a humidified atmosphere of 5% CO2, 95% air at 37 °C to reach about 75–90% confluence. Then, 100 μL of the synthesized nanoparticles in a wide range of concentrations was replaced with previous media. Afterward, 25 μL of the MTT 3-(4,5 Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium stock solution (with 4 mg/mL concentration) was transferred into each well and incubated for 4 h in standard condition. The mitochondrial performance of viable cells led to formation of purple formazan crystals, where for dissolving these crystals we applied 100 μL dimethyl sulfoxide (DMSO). In the final step, the absorption of solution was recorded at 570 nm wavelength using a microplate reader (Model 50, Bio-Rad Corp, Hercules, California, USA).
2.4. Minimum inhibitory concentrations (MICs) assay
In MICs test, all of the procedures were carried out according to the standards of the Clinical and Laboratory Standards Institute (CLSI) for assessing the antibacterial susceptibility of the synthesized nanoparticles [4]. Briefly, 2-fold serial dilutions of the testing compounds (at a descending concentration from 1000μg/ml to 7.8 μg/mL) and control groups were provided with Brain heart infusion (BHI) in 96-well microplates. Afterward, the microbial concentration was adapted to match the turbidity standard of 0.5 McFarland (OD600: 0.1–0.2) in a way that the concentration of the compounds was 1000 μg/mL in the first wells. The plates were incubated for 24 h at 37 °C. Later the optical density was measured at 600 nm by a microplate reader (BioTek, Power Wave XS2). This procedure was done in triplicate.
2.5. Minimum Bactericidal Concentrations (MBCs) assay
All the selected microorganisms were cultured overnight in BHI, and then stocks with the concentration of 105–106 CFU/mL were prepared for each one. The total of 90 μL of serially diluted concentrations of compounds (from 1000 μg/mL to 7.8 μg/mL) was added to a 96-well micro-plate consisting of 90 μg/mL BHI, then 10 μg/ml of bacteria were added to each cell. Micro-plates were incubated for 24 h at 37 °C. Then, 10 μL of each bacterial suspension was added to a newly prepared BHI and incubated for another 24 hours at 37 °C to exam bactericidal performance of each compound The lowest concentration of compounds that leads no growth of bacteria was regarded as minimum bactericidal concentration (MBC). This procedure was also repeated three times.
Acknowledgments
The authors are thankful to the Shiraz University of Medical Sciences, Shiraz, Iran for funding this research (through Grant No.: SUMS-M-192).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dib.2019.104929.
Contributor Information
Seyyed Alireza Hashemi, Email: sa_hashemi@sums.ac.ir.
Bahman Ramavandi, Email: b.ramavandi@bpums.ac.ir.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hashemi S.A., Mousavi S.M., Ramakrishna S. Effective removal of mercury, arsenic and lead from aqueous media using polyaniline-Fe3O4-silver diethyldithiocarbamate nanostructures. J. Clean. Prod. 2019;239:118023. [Google Scholar]
- 2.Mousavi S.M., Hashemi S.A., Esmaeili H., Amani A.M., Mojoudi F. Synthesis of Fe3O4 nanoparticles modified by oak shell for treatment of wastewater containing Ni (II) Acta Chim. Slov. 2018;65:750–756. [PubMed] [Google Scholar]
- 3.Mousavi S.M., Zarei M., Hashemi S.A., Babapoor A., Amani A.M. A conceptual review of rhodanine: current applications of antiviral drugs, anticancer and antimicrobial activities. Artif. Cell Nanomed. B. 2019;47:1132–1148. doi: 10.1080/21691401.2019.1573824. [DOI] [PubMed] [Google Scholar]
- 4.Mousavi S.M., Hashemi S.A., Ghasemi Y., Atapour A., Amani A.M., Savar Dashtaki A., Babapoor A., Arjmand O. Green synthesis of silver nanoparticles toward bio and medical applications: review study. Artif. Cell Nanomed. B. 2018;46:S855–S872. doi: 10.1080/21691401.2018.1517769. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.