Abstract
Although guanosine is an endogenous nucleoside that displays antidepressant-like properties in several animal models, the mechanism underlying its antidepressant-like effects is not well characterized. The present study aimed at investigating the involvement of ERK/GSK-3β and Nrf2/HO-1 signaling pathways in the antidepressant-like effect of guanosine in the mouse tail suspension test (TST). The immobility time in the TST was taken as an indicative of antidepressant-like responses and the locomotor activity was assessed in the open-field test. Biochemical analyses were performed by Western blotting in the hippocampus and prefrontal cortex (PFC). The combined treatment with sub-effective doses of guanosine (0.01 mg/kg, p.o.) and lithium chloride (a non-selective GSK-3β inhibitor, 10 mg/kg, p.o.) or AR-A014418 (selective GSK-3β inhibitor, 0.01 μg/site, i.c.v.) produced a synergistic antidepressant-like effect in the TST. The antidepressant-like effect of guanosine (0.05 mg/kg, p.o.) was completely prevented by the treatment with MEK1/2 inhibitors U0126 (5 μg/site, i.c.v.), PD98059 (5 μg/site, i.c.v.), or zinc protoporphyrin IX (ZnPP) (HO-1 inhibitor, 10 μg/site, i.c.v). Guanosine administration (0.05 mg/kg, p.o.) increased the immunocontent of β-catenin in the nuclear fraction and Nrf2 in the cytosolic fraction in the hippocampus and PFC. The immunocontent of HO-1 was also increased in the hippocampus and PFC. Altogether, the results provide evidence that the antidepressant-like effect of guanosine in the TST involves the inhibition of GSK-3β, as well as activation of MAPK/ERK and Nrf2/HO-1 signaling pathways, highlighting the relevance of these molecular targets for antidepressant responses.
Keywords: Antidepressant, ERK, GSK-3β, Guanosine, HO-1, Nrf2
Introduction
Major depressive disorder (MDD) is currently considered the main cause of disability worldwide and is associated with high morbidity and mortality [1–3]. Despite the severity and the high prevalence of this psychiatric disorder, and the great efforts that have been done to improve its treatment, its pharmacotherapy still has several limitations. The delay for the remission of the depressive symptoms (usually 3–4 weeks after the onset of treatment), the low efficacy (almost 30% of patients do not show a complete remission of the symptoms after chronic antidepressant treatment), and the side effects associated with treatment constitute the main drawbacks of antidepressant pharmacotherapy [4, 5]. Considering these drawbacks, the investigation of novel antidepressant agents and the characterization of the molecular signaling pathways underlying their effects are needed [6].
Although the pathophysiology of MDD is not fully elucidated, it has been recognized that several signaling pathways play a significant role in the development of depressive symptoms. The nuclear factor (erythroid-derived 2)-like 2 (Nrf2)/heme oxygenase-1 (HO-1), an important antioxidant pathway, has been implicated in depressive-related behaviors and in the mechanism underlying antidepressant responses. Basically, under basal conditions, Nrf2 is associated with the repressor Kelch-like ECH associated protein 1 (Keap-1) that binds to Nrf2 and prevents its translocation to the nucleus [7, 8]. Under oxidative stress conditions, Nrf2 is phosphorylated and the Keap-1/Nrf2 complex dissociates, releasing Nrf2, which in turn moves to the nucleus, where it binds at a specific region of DNA inducing the expression of target cytoprotective genes, including HO-1 [9–12]. HO-1 is an inducible enzyme with well-established antioxidant properties contributing to defensive mechanism for neurons exposed to oxidant challenges [11].
Some signaling pathways that regulate neuroplasticity and cellular survival have been associated with Nrf2 activation such as mitogen-activated protein kinases (MAPKs), extracellular signal-regulated kinase (ERK), and phosphatididylinositol 3-kinase (PI3K)/protein kinase B (Akt) [13–15]. Other signaling pathway associated with Nrf2 activation is glycogen synthase kinase-3 (GSK-3β) [16]. The activation of this enzyme has been related with the pathogenesis of mood disorders including MDD [17–20]. On the other hand, inhibition of GSK-3β by phosphorylation at Ser9 has been implicated in the mechanism underlying the fast antidepressant responses [21]. The GSK-3β activation is involved in β-catenin stability, since GSK-3β activation leads to β-catenin degradation by ubiquitination. On the other hand, GSK-3β inhibition stabilizes β-catenin causing its accumulation in the cytosol followed by its translocation to the nucleus which results in the expression of genes related to synaptic plasticity and neurogenesis [22, 23]. β-Catenin has been implicated in MDD since the Wnt/β-catenin signaling could, at least in part, be responsible for neuronal adaptations necessary for the therapeutic action of antidepressant treatments [24].
Our group has investigated guanosine, a purine nucleoside, as a putative endogenous antidepressant. Guanosine is considered a neuromodulator that is released mainly by astrocytes under normally physiological conditions but mostly under injury conditions [25, 26]. Guanosine is able to reduce neuroinflammation, oxidative stress, and excitotoxicity, besides exerting trophic effects in neuronal and glial cells [26]. Previous studies performed by our group showed that the administration of this nucleoside causes an antidepressant-like effect in the tail suspension test (TST) and forced swimming test (FST) by activating the PI3K/mTOR signaling pathway [27], which is associated with neuroplasticity and cellular survival [13]. In addition, guanosine prevented the depressive-like behavior and hippocampal oxidative imbalance in animals subjected to acute restraint stress [28]. Considering that guanosine has antioxidant properties which may be related to its antidepressant effects, this study aimed at investigating the role of GSK-3β, MAPK/ERK, and Nrf2/HO-1 signaling pathways in the antidepressant-like effect of guanosine in the mouse TST.
Methods
Animals
Adult female Swiss mice (3 months, 30–40 g) provided by the animal facility of the University of Santa Catarina (Florianópolis, Brazil) were used. The animals were maintained at 20–22 °C with free access to water and food, under a 12:12-h light/dark cycle, with lights on at 7:00 a.m. Mice were caged in groups of 12 in a 41 × 34 × 16 cm cage. The cages were placed in the experimental room for acclimatization 24 h before the tests and manipulations were carried out between 9.00 a.m. and 5.00 p.m. All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and all experiments were performed after approval of the protocol by the Ethics Committee of the Institution (00795 and 7485180518 protocols). All efforts were made to minimize animal suffering and to reduce the number of animals used.
Drugs and treatment
The following drugs were used: guanosine, lithium chloride, AR-A014418, U0126, PD98059, and zinc protoporphyrin IX (ZnPP). All drugs were obtained from Sigma Chemical Co. (St. Louis, USA). Guanosine and lithium chloride were dissolved in distilled water and were given orally by gavage. AR-A014418, U0126, PD98059, and ZnPP were dissolved in a final concentration of 0.1% dimethyl sulfoxide in saline and were administered by intracerebroventricular (i.c.v.) route, in a volume of 5 μl per site. The i.c.v. injections were performed by employing a “free hand” method according to the procedure previously described [29, 30]. Briefly, a 0.4-mm external diameter hypodermic needle attached to a cannula, which was linked to a 25-μl Hamilton syringe, was inserted perpendicularly through the skull (no more than 2 mm into the brain of each mouse). The drugs were then administered into the left lateral ventricle. The injection was given over 30 s, and the needle remained in place for another 30 s in order to avoid the reflux of the substances injected. The injection site was 1 mm to the left from the mid-point on a line drawn through to the anterior base of the ears. I.c.v. injections were performed by an experienced investigator, and after brain dissection, the success of injection was examined, macroscopically, discarding results from mice presenting misplacement of the injection site or any sign of cerebral hemorrhage (< 5%).
Behavioral tests
Tail suspension test
The total duration of immobility induced by tail suspension was measured according to a method previously described [31]. The animals were suspended 50 cm above the floor by adhesive tape placed approximately 1 cm from the tip of the tail. Mice were considered immobile only when they hung passively and completely motionless. Immobility time was manually recorded during a 6-min period by an experienced observer. The observer was blind to the animal condition.
Open-field test
In order to investigate the effects of guanosine on locomotor and exploratory capacity, mice were submitted to the open-field test (OFT) 10 min after the TST. The parameters analyzed were number of crossings, distance traveled, speed, latency to exit the first quadrant, immobility, time and number of entries in center, number of rearings, and total time spent grooming. Tests were recorded using a digital video camera (Logitech HD webcam C525, CA, USA) and analyzed using the ANY-maze video-tracking system (Stoelting Co., Wood Dale, IL, USA), as previously described [32]. In the experiments in which guanosine and pharmacological agents were administered together, only the number of crossings in the OFT was registered, as previously described [33]. The apparatus consisted of a wooden box measuring 40 × 60 × 50 cm high. The floor of the arena was divided into 12 equal squares. The number of squares crossed with all paws (crossing) was counted during a 6-min session. The apparatus was cleaned with a solution of 10% ethanol between tests in order to hide animal clues.
Experimental design
In a first set of experiments guanosine (0.05 mg/kg, p.o.) or vehicle was administered to mice 60 min before the TST. Ten minutes after the TST, behavioral parameters were registered in the OFT, as indicated in the OFT section.
In order to investigate if the antidepressant-like effect of guanosine in the TST is mediated by the inhibition of GSK-3β activity, mice were treated with a sub-effective dose of guanosine (0.01 mg/kg, p.o.) or vehicle and immediately after, a sub-effective dose of lithium chloride (a non-selective GSK-3β inhibitor, 10 mg/kg, p.o.) or vehicle was administered. After 60 min of these treatments, the TST was carried, followed by the OFT. The dose of lithium chloride was chosen based on a study by Cunha et al. [34] which reported that it is effective in the TST when administered at a dose of 30 mg/kg, whereas a dose of 10 mg/kg produces no effect in this test.
In another set of experiments, mice were treated with a sub-effective dose of guanosine (0.01 mg/kg, p.o.) or distilled water and after 45 min, they were injected with a sub-effective dose of the selective GSK-3β inhibitor, AR-A014418 (0.01 μg/site, i.c.v.), or vehicle. After 15 min, animals were submitted to the TST and OFT. In another set of experiments, guanosine was administered (0.05 mg/kg, p.o.) and after 60 min, the hippocampus and PFC were dissected and processed for Western blotting to verify the immunocontent of β-catenin in both structures. The dose of AR-A014418 was chosen considering that a previous study showed that its administration caused a reduction in the immobility time in the TST at a dose of 1 μg/mouse, i.c.v., whereas it was not effective at 0.01 μg/mouse, i.c.v. [34].
To investigate if the antidepressant-like effect induced by guanosine is mediated by MAPK, mice were treated with an effective dose of guanosine (0.05 mg/kg, p.o.) or vehicle and 45 min after, U0126 (selective mitogen-activated protein kinase kinase (MEK1/2) inhibitor, 5 μg/site, i.c.v.) or vehicle was administered. After 15 min, the TST was carried out. In another set of experiments, mice were treated with an effective dose of guanosine (0.05 mg/kg, p.o.) or distilled water and after 45 min, they received an effective dose of PD98059 (MEK1/2 inhibitor, 5 μg/site, i.c.v.) or vehicle. After 15 min, the animals were submitted to the TST followed by OFT. Given that Nrf2 can be activated by MAPK/ERK pathway, in another set of experiments, mice were treated with guanosine (0.05 mg/kg, p.o.) and the Nrf2 immunocontent was analyzed in the hippocampus and PFC by Western blotting. This experimental protocol and the doses of U0126 and PD98059 were based on previous studies that indicate the effectivity of these inhibitors to abolish the anti-immobility effect of several compounds in the TST when administered at the same dose employed in the present study without altering locomotor activity of mice [35–37].
Finally, to evaluate the involvement of HO-1 activity in the antidepressant-like effect of guanosine in the TST, mice received an effective dose of guanosine (0.05 mg/kg, p.o.) or vehicle and after 45 min, they were treated with ZnPP (HO-1 inhibitor, 10 μg/site, i.c.v) or vehicle [36]. After 15 min, the animals were submitted to the TST followed by OFT. The experimental protocol and the dose of ZnPP were based on previous studies of our group [34, 37]. To examine if treatment with guanosine may cause HO-1 increase, the immunocontent of this protein was analyzed by Western blotting in the hippocampus and PFC 60 min after guanosine administration (0.05 mg/kg, p.o.).
Sample preparation and Western blotting analysis
Mice were decapitated for quickly dissection of PFC and hippocampus immediately after the behavioral tests, and the samples were placed in liquid nitrogen and stored at − 80 °C until use. Samples were mechanically homogenized in 400 μl of 50 mM TRIS pH 7.0, 1 mM EDTA, 100 mM NaF, 0.1 mM PMSF, 2 mM Na3VO4, 1% Triton X-100, 10% glycerol, Sigma Protease Inhibitor Cocktail (P2714). Lysates were centrifuged (10,000g for 10 min, at 4 °C) to eliminate cellular debris. The supernatants were diluted 1/1 (v/v) in 100 mM TRIS pH 6.8, 4 mM EDTA, 8% SDS and boiled for 5 min. Thereafter, sample dilution (40% glicerol, 100 mM TRIS, bromophenol blue, pH 6.8) in the ratio 25:100 (v/v) and β-mercaptoethanol (final concentration 8%) were added to the samples. Protein content was quantified using bovine serum albumin as a standard [39]. The samples (containing 70 μg protein/track) were separated by SD-PAGE using 10% gel and the proteins were transferred to nitrocellulose membranes using a semi-dry blotting apparatus (1.2 mA/cm2; 1.5 h). To verify transfer efficiency process, membranes were stained with Ponceau [40]. After the transfer process, membranes were blocked with 5% bovine serum albumin in TRIS-buffered saline for 60 min at room temperature and probed via incubation with anti-HO-1 (Santa Cruz, 1:5000; diluted in a TRIS-buffered saline solution contained 0.1% Tween 20). Next, membranes were incubated with goat anti-mouse IgG antibody, (H+L) HRP conjugate (Millipore, 1:2500) for 60 min, and the immunoreactive bands were developed using a chemiluminescence kit (Amersham ECL Prime Western Blotting Detection Reagent, GE Healthcare Life Sciences). After blocking and incubation steps, membranes were washed three times (5 min) with TRIS-buffered saline solution containing 0.1% Tween 20. The expression level of a housekeeping protein β-actin was evaluated using a mouse anti-β-actin primary antibody (Cell Signaling, 1:5000) and mouse anti-rabbit IgG-HRP: sc-2357 (Santa Cruz, 1:5000) secondary antibody. Optical density of the bands was quantified using Imagelab Software and the HO-1 immunocontent was determined based on the ratio between optical density of the HO-1 band and optical density of the β-actin band. Results are presented as percentual of control (considered 100%).
To examine whether the antidepressant-like effect of guanosine is associated with an increase in the immunocontents of β-catenin and Nrf2, mice were treated with guanosine (0.05 mg/kg, p.o.) or vehicle and after 1 h, the TST was carried out followed by OFT. Cytosolic and nuclear fractions were subsequently prepared to investigate the possible translocation of β-catenin and Nrf2 from cytosol to the nucleus. Samples were mechanically homogenized in 200 μl of buffer solution (10 mM HEPES pH 7.9, 10 mM KCl, 2 mM MgCl2, 1 mM EDTA, 2 mM Na3VO4, 1% Triton X-100, Sigma Protease Inhibitor Cocktail (P2714)) and were subsequently centrifuged (15,000g for 30 min, at 4 °C). The supernatants were removed and stored (this is the cytosolic fraction). The pellet was resuspended with buffer solution (20 mM HEPES pH 7.9, 50 mM KCl, 2 mM MgCl2, 420 mM NaCl, 1 mM EDTA, 2 mM Na3VO4, 1% Triton X-100, 25% glycerol, Sigma Protease Inhibitor Cocktail (P2714)). Samples were placed on the sonicator for 2 min and sequentially vortexed for vigorous shaking, this process was repeated three times. After extraction of the cytosolic and nuclear fractions, the samples were subjected to the same procedures described for HO-1 detection. The samples containing 50 μg protein/track were separated by SD-PAGE using 12% gel for Nrf2 immunocontent detection and 10% gel for β-catenin immunocontent detection. The incubation procedure was the same as previously described for the detection of HO-1, using anti-β-catenin (Cell Signaling, 1:1000, diluted in a TRIS-buffered saline solution contained 0.1% Tween 20) or anti-Nrf2 (Santa Cruz, 1:1000; diluted in a TRIS-buffered saline solution contained 0.1% Tween 20). Densitometric values in the nuclear fraction were normalized using anti-Histone H3 antibody produced in rabbit (Cell Signaling, 1:1000) and in the cytosolic fraction, β-actin was used mouse anti-rabbit IgG-HRP: sc-2357 (Santa Cruz, 1:5000) secondary antibody. The immunocontent of the proteins was quantified by optical density using Imagelab Software. The immunocontent of β-catenin was determined based on the ratio between optical density of the β-catenin band and optical density of the β-actin band (in cytosolic fraction) and ratio between optical density of the β-catenin band and optical density of the anti-Histone H3 antibody band (in nuclear fraction). The Nrf2 immunocontent was determined based on the ratio between optical density of the Nrf2 band and optical density of the β-actin band (in cytosolic fraction) and ratio between optical density of the Nrf2 band and optical density of the anti-Histone H3 antibody band (in nuclear fraction). Results are presented as percentual of control (considered 100%).
Statistical analysis
All the statistical analyses were performed using STATISTICA 7.0 software (StatSoft Inc., Tulsa, OK, USA). Data are expressed as mean + S.E.M. Differences among experimental groups were determined by Student’s t test or two-way ANOVA followed by Newman-Keuls post hoc test when appropriate. A value of p < 0.05 was considered significant.
Results
Behavioral responses to guanosine in the TSC and OFT
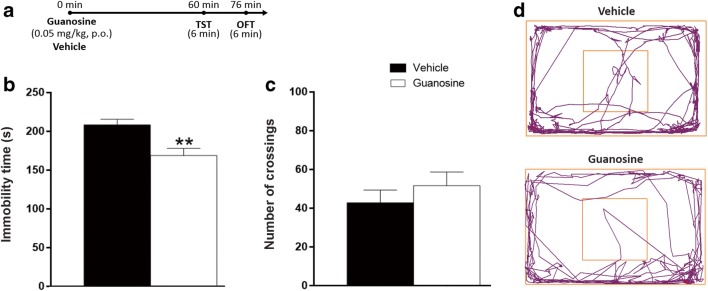
In order to confirm previous studies that show the antidepressant-like effects of guanosine in the TST in mice of either sex, guanosine was administered 1 h before the TST. Fig. 1b shows that the administration of guanosine to female mice decreased immobility time of mice in the TST 1 h after its administration, without altering the number of crossings (Fig. 1c), in agreement with the result previously shown [27]. The representative tracking images of locomotor activity from mice treated with guanosine or vehicle are shown in Fig. 1d. In addition, to reinforce the notion that the effects of guanosine in the TST are not due to any unspecific effects on overall activity which could potentially affect performance in the TST, other parameters were assessed in the OFT, as shown in Table 1. The administration of guanosine caused no alteration in distance traveled in the OFT, speed, the latency to exit the first quadrant, immobility, total time in center, number of entries in center, number of rearings, and total time of grooming.
Fig. 1.
Effect of guanosine in the TST and OFT 1 h after its administration. Timeline of experimental protocols of administrations and behavioral tests (a). Effect of guanosine treatment in the immobility time in the TST (t(12) = 3.35, p < 0.01) (b) and number of crossings in the OFT (t(12) = − 0.92, p = 0.38) (c). Representative tracking images during OFT (d). Values are expressed as mean + S.E.M. of 7 mice. **p < 0.01 compared with the vehicle-treated control group
Table 1.
Behavioral parameters analyzed in the open-field test in mice treated with guanosine (0.05 mg/kg, p.o.) or vehicle
| Vehicle | Guanosine | T values | |
|---|---|---|---|
| Distance traveled (m) | 13.80 ± 3.60 | 15.69 ± 2.73 | t(12) = − 0.42, p = 0.68 |
| Speed (mm/s) | 0.03 ± 0.01 | 0.04 ± 0.01 | t(12) = 0.22, p = 0.82 |
| Latency to exit the first quadrant (s) | 12.14 ± 1.10 | 14.00 ± 1.93 | t(12) = − 1.74, p = 0.10 |
| Immobility (s) | 195.08 ± 37.74 | 171.07 ± 43.63 | t(12) = 0.42, p = 0.68 |
| Total time in center (s) | 2.14 ± 0.91 | 3.85 ± 1.96 | t(12) = − 0.79, p = 0.44 |
| Number of entries in center | 2.28 ± 0.99 | 3.14 ± 1.39 | t(12) = − 0.50, p = 0.62 |
| Number of rearings | 6.85 ± 2.89 | 6.71 ± 1.89 | t(12) = 0.04, p = 0.97 |
| Total time of grooming (s) | 40.85 ± 6.35 | 42.57 ± 11.46 | t(12) = − 0.13, p = 0.89 |
Involvement of GSK-3β in the antidepressant-like effect of guanosine in the TST
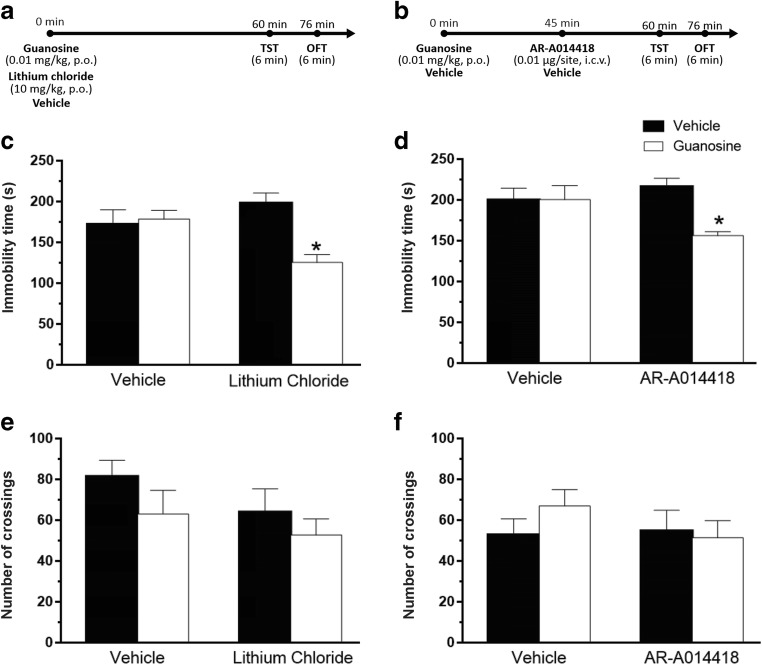
To test the hypothesis that the antidepressant-like effect of guanosine in the TST is mediated through the inhibition of GSK-3β activity, mice were administered with sub-effective doses of guanosine and lithium chloride, a non-selective GSK-3β inhibitor. Fig. 2c illustrates that the combined administration of these drugs caused a decrease in the immobility time of mice in the TST, suggesting a synergistic antidepressant-like effect. The number of crossings in the OFT was not altered by any treatment (Fig. 2e).
Fig. 2.
Involvement of GSK-3β in the antidepressant-like effect of guanosine in the TST. Timeline of experimental protocols of administrations and behavioral tests (a, b). Effect of treatment with sub-effective doses of guanosine and lithium chloride in the immobility time in the TST (guanosine treatment: F(1,24) = 6.36, p < 0.05; lithium chloride treatment: F(1,24) = 1.09, p = 0.30; guanosine treatment × lithium chloride interaction: F(1,24) = 9.15, p < 0.01) (c). Effects of treatment of mice with lithium chloride and guanosine on the locomotor activity in the OFT (guanosine treatment: F(1,24) = 2.20, p = 0.15; lithium chloride treatment: F(1,24) = 1.95, p = 0.17; guanosine × lithium chloride interaction: F(1,24) = 0.13, p = 0.72) (e). Effect of treatment with sub-effective doses of guanosine and AR-A014418 in the immobility time in the TST (guanosine treatment: F(1,28) = 5.49, p < 0.05; AR-A014418 treatment: F(1,28) = 1.22, p = 0.27; guanosine treatment × AR-A014418 interaction: F(1,28) = 5.68, p < 0.05) (d). Effects of pretreatment of mice with AR-A014418 and guanosine on the locomotor activity in the OFT (guanosine treatment: F(1,28) = 0.45, p = 0.50; AR-A014418 treatment: F(1,28) = 0.45, p = 0.41; guanosine × AR-A014418 interaction: F(1,28) = 1.14, p = 0.29) (f). Values are expressed as mean + S.E.M. of 7–8 mice. *p < 0.05 compared with the vehicle-treated control group
The inhibitor of GSK-3β AR-A014418 was used as a pharmacological tool to reinforce the notion that guanosine causes inhibition of GSK-3β. The co-administration of sub-effective doses of guanosine and AR-A014418 induced an antidepressant-like effect in TST as compared with vehicle and either drug alone (Fig. 2d). None of the treatments caused alterations in the locomotor activity in the OFT (Fig. 2f).
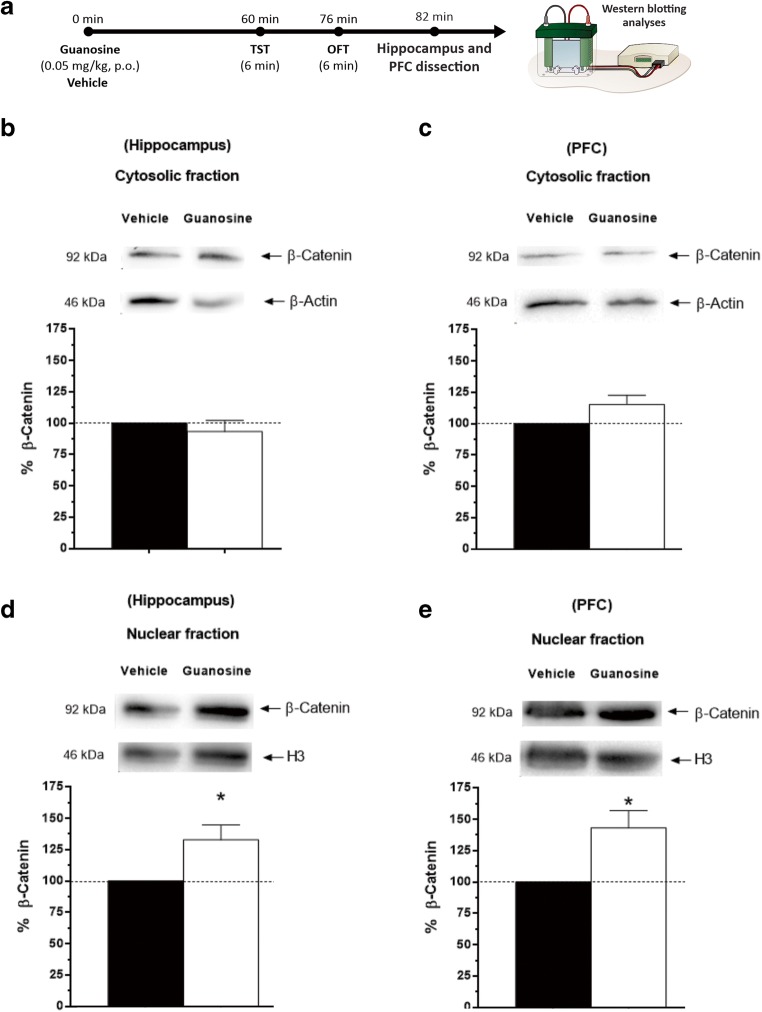
Guanosine increases β-catenin immunocontent in the hippocampus and PFC
To determine whether the administration of guanosine is able to increase the levels of β-catenin, a substrate for GSK-3β, Western blotting analyses were performed to detect this protein in the hippocampus and PFC. Guanosine was able to increase the immunocontent of β-catenin in the hippocampus and PFC in nuclear fraction without affecting immunocontent of this protein in cytosolic fraction in both structures analyzed (Fig. 3b, c). Conversely, statistical analysis revealed a significant effect of the treatment with guanosine in immunocontent of β-catenin in nuclear fraction in hippocampus and PFC when compared to the control group (Fig. 3d, e).
Fig. 3.
Guanosine increases β-catenin immunocontent in the hippocampus and PFC. Timeline of the experimental protocol (a). β-catenin immunocontent in the cytosolic fraction in the hippocampus (t(10) = 0.76, p = 0.46) (b) and PFC [t(10) = − 2.04, p = 0.06) (c). Effect of the treatment with guanosine in immunocontent of β-catenin in nuclear fraction in hippocampus (t(10) = − 3.99, p < 0.05) (d) and PFC (t(10) = 3.31, p < 0.05) (e). Results are presented as percentual of control (considered 100%) and are expressed as mean + S.E.M. (n = 6). *p < 0.05; guanosine-treated group compared with the vehicle-treated group
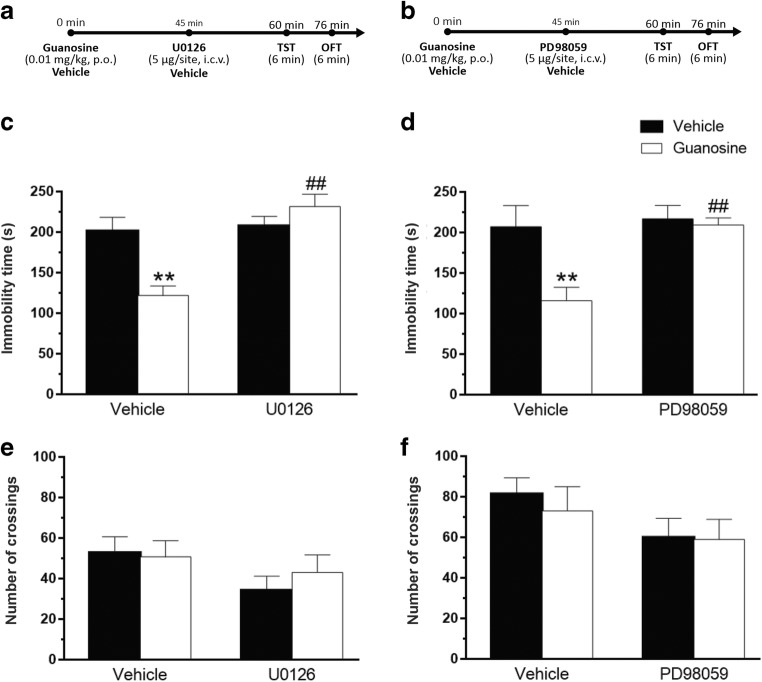
Activation of MAPK in the antidepressant-like effect of guanosine in the TST
To determine the influence of MEK1/2 inhibition on the antidepressant-like effect of guanosine in the TST, mice were treated with U0126 as shown in Fig. 4a. As illustrated in Fig. 4c, the antidepressant-like effect of guanosine was completely prevented by the treatment of animals with U0126. No differences in the locomotor activity of mice in the OFT were observed by any of the treatments (Fig. 4e). To confirm the role of MEK1/2 activation in the anti-immobility effect of guanosine in the TST, mice were treated with PD98059, another inhibitor of MEK1/2, as depicted in Fig. 4b. Figure 4d illustrates that the antidepressant-like effect of guanosine was also completely prevented by treatment of mice with PD98059. None of the treatments caused significant alterations in the locomotor activity in the OFT, as shown in Fig. 4f.
Fig. 4.
Involvement of MAPK in the antidepressant-like effect of guanosine in the TST. Timeline of experimental protocols of administrations and behavioral tests (a, b). Effects of pretreatment of mice with U0126 on the anti-immobility effect of guanosine in TST (guanosine treatment: F(1,24) = 3.86, p = 0.06; U0126 treatment: F(1,24) = 16.83, p < 0.01; guanosine treatment × U0126 interaction: F(1,24) = 13.37, p < 0.05) (c). Effects of pretreatment of mice with U0126 and guanosine on the locomotor activity in the OFT (guanosine treatment F(1,24) = 0.41, p = 0.52; U0126 treatment F(1,24) = 3.49, p = 0.07; guanosine × U0126 interaction F(1,24) = 0.79, p = 0.38) (e). Effects of pretreatment of mice with PD98059 on the anti-immobility effect of guanosine in TST (guanosine treatment: F(1,24) = 7.60, p < 0.05; PD98059 treatment: F(1,24) = 8.22, p < 0.01; guanosine treatment × PD98059 interaction: F(1,24) = 5.38, p < 0.05) (d). Effects of pretreatment of mice with PD98059 and guanosine on the locomotor activity in the OFT (guanosine treatment: F(1,24) = 0.22, p = 0.64; PD98059 treatment F(1,24) = 3.21, p = 0.09; guanosine × PD98059 interaction: F(1,24) = 0.13, p = 0.71) (f). Values are expressed as mean + S.E.M. of 7 mice. **p < 0.01 compared with the vehicle-treated control group, ##p < 0.01 compared with the group treated with guanosine + vehicle
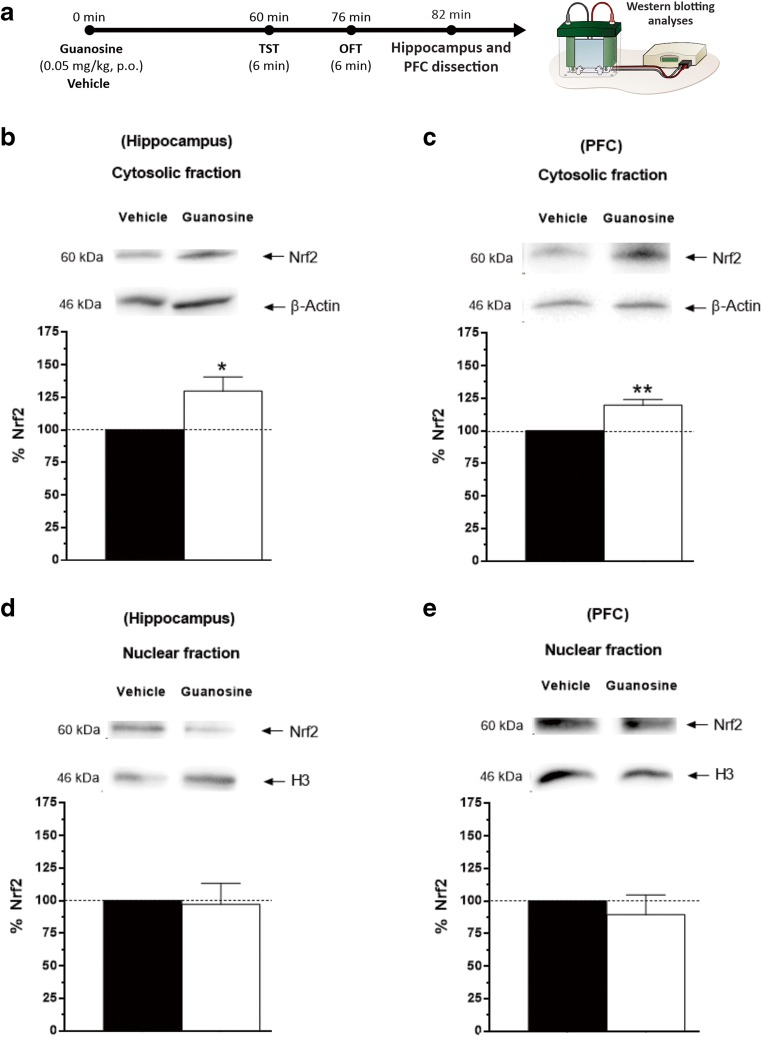
Guanosine increases Nrf2 immunocontent in the hippocampus and PFC
To test the hypothesis that Nrf2 is associated with the antidepressant responses elicited by the administration of guanosine, Western blotting analyses were performed for determining Nrf2 immunocontent in the hippocampus and PFC, as illustrated in Fig. 5a. Fig. 5b shows that the treatment with guanosine caused a significant increase in Nrf2 immunocontent in the hippocampus of mice (cytosolic fraction), when compared to the control group. A similar result was obtained in the cytosolic fraction in the PFC, as shown in Fig. 5c. Regarding nuclear fraction of Nrf2 in the hippocampus, the one-way ANOVA revealed that treatment with guanosine did not produce any alteration in this parameter in hippocampus (Fig. 5d) and in the PFC (Fig. 5e).
Fig. 5.
Guanosine increases cytosolic Nrf2 immunocontent in the hippocampus and PFC. Timeline of the experimental protocol (a). Nrf2 immunocontent in the hippocampus (t(10) = − 2.60, p < 0.05) (b) and PFC: (t(10) = − 4.62, p < 0.01) (c) of mice (cytosolic fraction). Nrf2 immunocontent in the nuclear fraction of Nrf2 in the hippocampus (t(10) = 0.18, p = 0.85) (d), and in the PFC (t(10) = 0.59, p = 0.56) (e). Results are presented as percentual of control (considered 100%) and are expressed as mean + S.E.M. (n = 6). *p < 0.05 guanosine-treated group compared with the vehicle-treated group, **p<0.01 guanosine-treated group compared with the vehicle-treated group
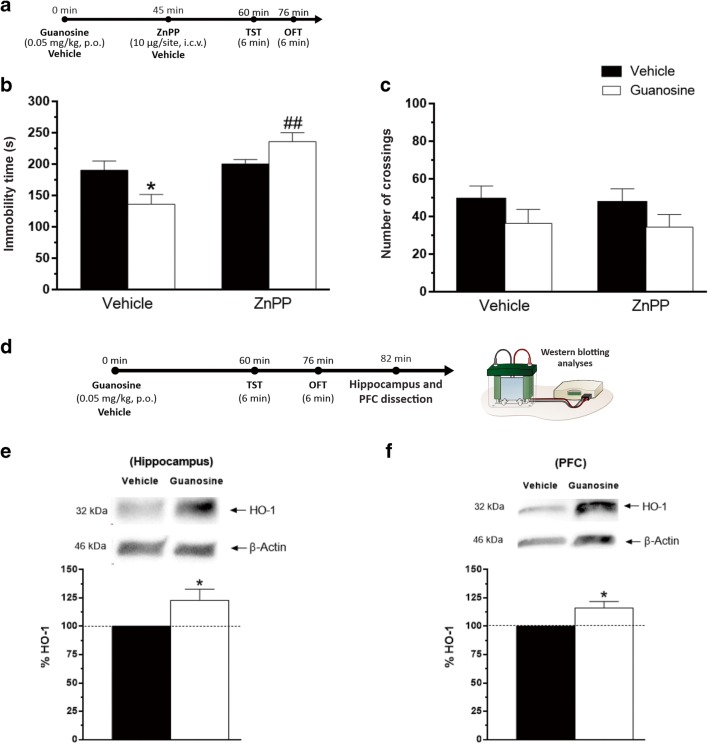
Involvement of HO-1 in the antidepressant-like effect of guanosine in the TST
Considering the elevated immunocontent of Nrf2 found in the hippocampus and PFC and the association between increased levels of Nrf2 and HO-1 activation, we decided to investigate the participation of HO-1 in the antidepressant-like effect of guanosine. The treatment with ZnPP (HO-1 inhibitor) prevented the anti-immobility effect of guanosine in the TST. As represented in Fig. 6b, post hoc analysis showed that the antidepressant-like effect of guanosine was prevented by treatment with ZnPP. The number of crossings in the OFT was not altered by treatment with ZnPP alone or in combination with guanosine (Fig. 6c).
Fig. 6.
Involvement of HO-1 in the antidepressant-like effect of guanosine in the TST. Timeline of experimental protocols of administrations and behavioral tests (a). Effects of pretreatment of mice ZnPP (HO-1 inhibitor) on the anti-immobility effect of guanosine in TST (guanosine treatment: F(1,24) = 0.50, p = 0.48; ZnPP treatment: F(1,24) = 16.84, p < 0.01; guanosine treatment × ZnPP interaction: F(1,24) = 11.19, p < 0.05) (b). Effects of treatment of mice with ZnPP and guanosine on the locomotor activity in the OFT (guanosine treatment: F(1,24) = 3.34, p = 0.07; ZnPP treatment: F(1,24) = 0.06, p = 0.79; guanosine × ZnPP interaction: F(1,24) = 0.004, p = 0.98) (c). Results are expressed as mean + SEM (n = 7). *p < 0.05 compared with the vehicle-treated control group, ##p < 0.01 compared with the group treated with guanosine + vehicle. Timeline of the experimental protocol (d). Immunocontent of HO-1 in the hippocampus (t(10) = − 2.32, p < 0.05) (e) and PFC (t(10) = − 2.76, p < 0.05) (f) of mice. Results are presented as percentual of control (considered 100%) and are expressed as mean + S.E.M. (n = 6). *p < 0.05; guanosine-treated-group compared with the vehicle-treated group
Guanosine treatment was able to increase the immunocontent of HO-1 in hippocampus and PFC of mice (Fig. 6e, f, respectively).
Discussion
The antidepressant-like effect of guanosine was previously demonstrated to be dependent on PI3K/Akt pathway activation [27]. However, the complete mechanisms regarding this effect are not totally elucidated. In this context, the present study further investigated this issue, showing that both the inhibition of GSK-3β and activation of MAPK/ERK pathway and Nrf2/HO-1 may be involved in the antidepressant-like effect of guanosine in the TST.
In a previous study, we found that guanosine has antidepressant-like effects in the TST when administered at 0.05, 0.1, and 0.5 mg/kg, p.o. [27]. For this reason, the dose of 0.05 mg/kg guanosine was chosen in the present study. In agreement with previous results [27], here, we show that the administration of guanosine by oral route at this dose elicits antidepressant-like effect in the TST in mice without altering several locomotor and exploratory parameters in the OFT. Considering that depression is more prevalent in women than in men [41], in the present study, female mice were used, whereas in the study by Bettio et al. [27], both male and female mice homogeneously distributed into the groups were used. Interestingly, it has been reported that brain levels of guanosine are increased after its oral administration [42, 43], raising the possibility that exogenous guanosine may impact the CNS, where it can act as a neuromodulator and activate different cellular targets [26].
The combined administration of sub-effective doses of guanosine and the GSK-3β inhibitors (LiCl and AR-A014418) induced a synergistic antidepressant-like effect in the TST. The inhibition of GSK-3β has been suggested to be involved in the behavioral responses of several putative antidepressants such as agmatine [44], atorvastatin [44, 45], and creatine [46]. In addition, GSK-3β inhibitors have been shown to elicit antidepressant-like effects in the TST and FST [38, 47]. Further reinforcing the notion that GSK-3β is implicated in the pathophysiology of MDD, it has been shown that GSK-3β expression is increased in the hippocampus of rodents subjected to the chronic mild stress (animal model of depression) and in patients with MDD [48, 49]. Of note, GSK-3β activation decreases β-catenin stability leading to β-catenin degradation by ubiquitination [50]. β-Catenin has been related with pathophysiology of MDD [24] probably because this protein participates on regulation of genes expression involved in synaptic plasticity and neurogenesis [22, 23], events commonly associated with antidepressants-like effects [21, 51]. When GSK-3β is active, it induces β-catenin degradation through the phosphorylation of this protein. In contrast, when GSK-3β is inactive, β-catenin accumulates in the cytosol and subsequently moves to the nucleus [52]. Our results indicate that treatment with guanosine was able to increase β-catenin immunocontent (nuclear fraction) in the hippocampus and PFC of mice. This result is similar to the one that reported an increase in β-catenin immunocontent in the hippocampus of mice treated with L803-mts, an inhibitor of GSK-3β [53]. The increase in β-catenin levels induced by guanosine administration, together with the synergistic antidepressant-like effect elicited by the combined administration of guanosine and LiCl or AR-A014418 suggests that the antidepressant-like effect of guanosine is dependent on GSK-3β inhibition.
Our results also indicate that the activation of MAPK/ERK pathway may be implicated in the anti-immobility effect of guanosine, since administration of either U0126 or PD98059 was able to abolish the antidepressant-like effect exerted by guanosine in the TST. U0126 acts directly by inhibiting the catalytic activity of the active MEK1 and blocks MEK1/2 activity, whereas PD098059 binds to MEK1 and MEK2, preventing their activation by Raf kinase and, subsequently inhibiting the activation of ERK1/2 [54, 55]. Similarly to our results, the anti-immobility effect of zinc [56] and ursolic acid [37] in the TST was also abolished by treatment with MEK1/2 inhibitors. Several studies have indicated that MAPK has the ability to stimulate the phosphorylation of Nrf2 and consequently its translocation to the nucleus [57, 58]. In addition, MAPK inhibitors were reported to abolish the effects of compounds that increase Nrf2 levels [59, 60]. Nrf2 is well-known to play an important role in defense against oxidative stress since it induces the expression of antioxidant genes [9]. Nrf2 can be activated by either MAPK/ERK pathway activation or modulation of GSK-3β/PI3K/Akt signaling pathways [57, 58].
Considering the present results that suggest that the antidepressant-like effect of guanosine is dependent on GSK-3β inhibition and MAPK/ERK activation and also taken into account the previous evidence of the participation of PI3K/Akt in the effect of guanosine in the TST [27], we hypothesized that Nrf2 may be implicated in the antidepressant responses elicited by the administration of guanosine. The relationship between Nrf2 and depression has been suggested by several evidence. Nrf2 deletion has been associated with depressive-like behavior and a reduction in serotonin and dopamine levels in the PFC [12]. In addition, chronic administration of the Nrf2 activator sulforaphane in mice was able to suppress the depressive-like behavior induced by LPS, an effect that was associated with increased levels of HO-1 in the hippocampus [12]. Here, we showed that the acute administration of guanosine increased hippocampal and cortical Nrf2 in the cytosol. This result suggests that the activation of Nrf2 pathway is likely required for the antidepressant-like effect of guanosine. However, in the present study, increased levels of Nrf2 were observed in the cytosol but not in the nucleus. Some hypothesis may be raised to account for this result. One possibility is that due to high Nrf2 turn over [61, 62], it is more difficult to detect alteration of this protein in the nucleus than in the cytosol. It is also possible that higher guanosine doses and/or time elapsed between guanosine administration and Western blotting analysis would be necessary to detect translocation of Nrf2 to the nucleus. In line with this assumption, it was reported that the fast-acting antidepressant ketamine increased nuclear translocation of Nrf2 in RAW264.7 cells in a concentration-dependent manner, so that at low ketamine concentration, a higher Nrf2 levels were found in the cytosol, whereas at higher concentrations of ketamine, this protein was detected in the nucleus [63]. Another possibility that should be considered regarding the increase in Nrf2 observed in the present study is that Nrf2 has moved to the nucleus, caused gene transcription, and was subsequently exported to the cytosol. Indeed, it has been proposed that Keap1 can enter the nucleus and escorts Nrf2 out to the cytoplasm for degradation under stress conditions [64–66].
Considering that Nrf2 activation has a critical role to increase HO-1 transcription [67] and to further investigate the involvement of Nrf2/HO-1 pathway in the behavioral effect of guanosine in the TST, we evaluated the ability of ZnPP, a widely used HO-1 inhibitor, to reverse the anti-immobility effect of guanosine in the TST. Here, we showed that that the antidepressant-like effect of guanosine was completely abrogated by ZnPP administration, suggesting that activation of HO-1 is possibly required for the antidepressant-like effect of guanosine. These data are in agreement with the reported ability of guanosine to have neuroprotective effects against different insults through activation of the HO-1 pathway [68, 69]. Further reinforcing this assumption, guanosine induced an increase in HO-1 immunocontent in the hippocampus and PFC. The activation of HO-1 has been reported to be required for the behavioral responses of several compounds with antidepressant activity such as ascorbic acid, creatine, and zinc [34, 36, 38]. In addition, a study demonstrated that a model of familial hypercholesterolemia that induces a depressive-like behavior is associated with a decrease in mRNA HO-1 in the hippocampus of mice [70]. The importance of HO-1 for the pathophysiology of depression is also indicated by clinical reports showing that patients with MDD have decreased HO-1 in red blood hemolysates or serum [71, 72]. Moreover, the severity of depressive symptoms was found to be inversely associated with serum HO-1 levels [72].
In summary, we demonstrated herein that guanosine is capable of exerting antidepressant-like effects by modulating several signaling pathways involved in neuroprotective effects.
Conclusions
In conclusion, our results demonstrated that the antidepressant-like effect induced by guanosine in the TST involves the modulation of MAPK/ERK and GSK-3β/β-catenin pathway. Additionally, the activation of NRf2/HO-1 signaling pathway may be also related to the anti-immobility effect of guanosine in the TST. The present study reinforces the notion that the antioxidant properties of guanosine may contribute to its antidepressant-like effect.
Acknowledgments
ALSR and RBL are recipients of CNPq Research Productivity Fellowship. The authors would like to thank the Multiuser Laboratory for Biological Studies (LAMEB), UFSC, for the support.
Funding information
This study was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) #449436/2014-4, #310113/2017-2, and Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES).
Compliance with ethical standards
All experiments were performed in accordance with the Guidelines of Ethic Committee on Animal Use of the Federal University of Santa Catarina (CEUA/UFSC) the guidelines laid down by the NIH (NIH Guide for the Care and Use of Laboratory Animals) in the USA. The CEUA/UFSC has approved all experimental protocols (approval numbers 00795 and 7485180518).
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Nemeroff CB. The burden of severe depression: a review of diagnostic challenges and treatment alternatives. J Psychiatr Res. 2007;41(3–4):189–206. doi: 10.1016/j.jpsychires.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Global Burden of Disease Study C Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7(2):137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 5.Agius M, Bonnici H. Antidepressants in use in clinical practice. Psychiatr Danub. 2017;29(Suppl 3):667–671. [PubMed] [Google Scholar]
- 6.Dwyer JM, Duman RS. Activation of mammalian target of rapamycin and synaptogenesis: role in the actions of rapid-acting antidepressants. Biol Psychiatry. 2013;73(12):1189–1198. doi: 10.1016/j.biopsych.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JS, Surh YJ. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett. 2005;224(2):171–184. doi: 10.1016/j.canlet.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 8.Uruno A, Motohashi H. The Keap1-Nrf2 system as an in vivo sensor for electrophiles. Nitric Oxide. 2011;25(2):153–160. doi: 10.1016/j.niox.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Niture SK, Kaspar JW, Shen J, Jaiswal AK. Nrf2 signaling and cell survival. Toxicol Appl Pharmacol. 2010;244(1):37–42. doi: 10.1016/j.taap.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002;99(18):11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neis VB, Rosa PB, Moretti M, Rodrigues ALS. Involvement of heme oxygenase-1 in neuropsychiatric and neurodegenerative diseases. Curr Pharm Des. 2018;24(20):2283–2302. doi: 10.2174/1381612824666180717160623. [DOI] [PubMed] [Google Scholar]
- 12.Martin-de-Saavedra MD, Budni J, Cunha MP, Gomez-Rangel V, Lorrio S, Del Barrio L, Lastres-Becker I, Parada E, Tordera RM, Rodrigues ALS, Cuadrado A, Lopez MG. Nrf2 participates in depressive disorders through an anti-inflammatory mechanism. Psychoneuroendocrinology. 2013;38(10):2010–2022. doi: 10.1016/j.psyneuen.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 14.Meng XB, Sun GB, Wang M, Sun J, Qin M, Sun XB. P90RSK and Nrf2 activation via MEK1/2-ERK1/2 pathways mediated by notoginsenoside R2 to prevent 6-hydroxydopamine-induced apoptotic death in SH-SY5Y cells. Evid Based Complement Alternat Med. 2013;2013:971712. doi: 10.1155/2013/971712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou W, Chen C, Zhong Y, An J, Zhang X, Yu Y, Yu Z, Fu J. PI3K/Akt pathway mediates Nrf2/ARE activation in human L02 hepatocytes exposed to low-concentration HBCDs. Environ Sci Technol. 2013;47(21):12434–12440. doi: 10.1021/es401791s. [DOI] [PubMed] [Google Scholar]
- 16.Jope RS, Roh MS. Glycogen synthase kinase-3 (GSK3) in psychiatric diseases and therapeutic interventions. Curr Drug Targets. 2006;7(11):1421–1434. doi: 10.2174/1389450110607011421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jope RS. Anti-bipolar therapy: mechanism of action of lithium. Mol Psychiatry. 1999;4(2):117–128. doi: 10.1038/sj.mp.4000494. [DOI] [PubMed] [Google Scholar]
- 18.Chen G, Huang LD, Jiang YM, Manji HK. The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. J Neurochem. 1999;72(3):1327–1330. doi: 10.1046/j.1471-4159.2000.0721327. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Bijur GN, Jope RS. Glycogen synthase kinase-3beta, mood stabilizers, and neuroprotection. Bipolar Disord. 2002;4(2):137–144. doi: 10.1034/j.1399-5618.2002.40201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costemale-Lacoste JF, Guilloux JP, Gaillard R. The role of GSK-3 in treatment-resistant depression and links with the pharmacological effects of lithium and ketamine: a review of the literature. Encephale. 2016;42(2):156–164. doi: 10.1016/j.encep.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry. 2011;16(11):1068–1070. doi: 10.1038/mp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Jope RS. Is glycogen synthase kinase-3 a central modulator in mood regulation? Neuropsychopharmacology. 2010;35(11):2143–2154. doi: 10.1038/npp.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garza JC, Guo M, Zhang W, Lu XY. Leptin restores adult hippocampal neurogenesis in a chronic unpredictable stress model of depression and reverses glucocorticoid-induced inhibition of GSK-3beta/beta-catenin signaling. Mol Psychiatry. 2012;17(8):790–808. doi: 10.1038/mp.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madsen TM, Newton SS, Eaton ME, Russell DS, Duman RS. Chronic electroconvulsive seizure up-regulates beta-catenin expression in rat hippocampus: role in adult neurogenesis. Biol Psychiatry. 2003;54(10):1006–1014. doi: 10.1016/s0006-3223(03)00700-5. [DOI] [PubMed] [Google Scholar]
- 25.Ciccarelli R, Ballerini P, Sabatino G, Rathbone MP, D'Onofrio M, Caciagli F, Di Iorio P. Involvement of astrocytes in purine-mediated reparative processes in the brain. Int J Dev Neurosci. 2001;19(4):395–414. doi: 10.1016/S0736-5748(00)00084-8. [DOI] [PubMed] [Google Scholar]
- 26.Bettio LE, Gil-Mohapel J, Rodrigues ALS. Guanosine and its role in neuropathologies. Purinergic Signal. 2016;12(3):411–426. doi: 10.1007/s11302-016-9509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bettio LE, Cunha MP, Budni J, Pazini FL, Oliveira A, Colla AR, Rodrigues ALS. Guanosine produces an antidepressant-like effect through the modulation of NMDA receptors, nitric oxide-cGMP and PI3K/mTOR pathways. Behav Brain Res. 2012;234(2):137–148. doi: 10.1016/j.bbr.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 28.Bettio LE, Freitas AE, Neis VB, Santos DB, Ribeiro CM, Rosa PB, Farina M, Rodrigues ALS. Guanosine prevents behavioral alterations in the forced swimming test and hippocampal oxidative damage induced by acute restraint stress. Pharmacol Biochem Behav. 2014;127:7–14. doi: 10.1016/j.pbb.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Kaster MP, Gadotti VM, Calixto JB, Santos AR, Rodrigues ALS. Depressive-like behavior induced by tumor necrosis factor-alpha in mice. Neuropharmacology. 2012;62(1):419–426. doi: 10.1016/j.neuropharm.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Barauna SC, Kaster MP, Heckert BT, do Nascimento KS, Rossi FM, Teixeira EH, Cavada BS, ALS R, Leal RB. Antidepressant-like effect of lectin from Canavalia brasiliensis (ConBr) administered centrally in mice. Pharmacol Biochem Behav. 2006;85(1):160–169. doi: 10.1016/j.pbb.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 31.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85(3):367–370. doi: 10.1007/bf00428203. [DOI] [PubMed] [Google Scholar]
- 32.de Paula Nascimento-Castro Cristine, Wink Ana Claudia, da Fônseca Victor Silva, Bianco Claudia Daniele, Winkelmann-Duarte Elisa C., Farina Marcelo, Rodrigues Ana Lúcia S., Gil-Mohapel Joana, de Bem Andreza Fabro, Brocardo Patricia S. Antidepressant Effects of Probucol on Early-Symptomatic YAC128 Transgenic Mice for Huntington’s Disease. Neural Plasticity. 2018;2018:1–17. doi: 10.1155/2018/4056383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neis VB, Manosso LM, Moretti M, Freitas AE, Daufenbach J, Rodrigues ALS. Depressive-like behavior induced by tumor necrosis factor-alpha is abolished by agmatine administration. Behav Brain Res. 2014;261:336–344. doi: 10.1016/j.bbr.2013.12.038. [DOI] [PubMed] [Google Scholar]
- 34.Cunha MP, Budni J, Ludka FK, Pazini FL, Rosa JM, Oliveira A, Lopes MW, Tasca CI, Leal RB, Rodrigues ALS. Involvement of PI3K/Akt signaling pathway and its downstream intracellular targets in the antidepressant-like effect of creatine. Mol Neurobiol. 2016;53(5):2954–2968. doi: 10.1007/s12035-015-9192-4. [DOI] [PubMed] [Google Scholar]
- 35.Cunha MP, Budni J, Pazini FL, Oliveira A, Rosa JM, Lopes MW, Leal RB, Rodrigues ALS. Involvement of PKA, PKC, CAMK-II and MEK1/2 in the acute antidepressant-like effect of creatine in mice. Pharmacol Rep. 2014;66(4):653–659. doi: 10.1016/j.pharep.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Manosso LM, Moretti M, Rosa JM, Cunha MP, Rodrigues ALS. Evidence for the involvement of heme oxygenase-1 in the antidepressant-like effect of zinc. Pharmacol Rep. 2017;69(3):497–503. doi: 10.1016/j.pharep.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Ramos-Hryb AB, Cunha MP, Pazini FL, Lieberknecht V, Prediger RDS, Kaster MP, Rodrigues ALS. Ursolic acid affords antidepressant-like effects in mice through the activation of PKA, PKC, CAMK-II and MEK1/2. Pharmacol Rep. 2017;69(6):1240–1246. doi: 10.1016/j.pharep.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 38.Moretti M, Budni J, Freitas AE, Rosa PB, Rodrigues ALS. Antidepressant-like effect of ascorbic acid is associated with the modulation of mammalian target of rapamycin pathway. J Psychiatr Res. 2014;48(1):16–24. doi: 10.1016/j.jpsychires.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 39.Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 40.Cordova FM, Aguiar AS, Jr, Peres TV, Lopes MW, Goncalves FM, Remor AP, Lopes SC, Pilati C, Latini AS, Prediger RD, Erikson KM, Aschner M, Leal RB. In vivo manganese exposure modulates Erk, Akt and Darpp-32 in the striatum of developing rats, and impairs their motor function. PLoS One. 2012;7(3):e33057. doi: 10.1371/journal.pone.0033057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, Mohr DC, Schatzberg AF. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. doi: 10.1038/nrdp.2016.65. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt AP, Bohmer AE, Schallenberger C, Antunes C, Tavares RG, Wofchuk ST, Elisabetsky E, Souza DO. Mechanisms involved in the antinociception induced by systemic administration of guanosine in mice. Br J Pharmacol. 2010;159(6):1247–1263. doi: 10.1111/j.1476-5381.2009.00597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vinade ER, Schmidt AP, Frizzo ME, Portela LV, Soares FA, Schwalm FD, Elisabetsky E, Izquierdo I, Souza DO. Effects of chronic administered guanosine on behavioral parameters and brain glutamate uptake in rats. J Neurosci Res. 2005;79(1–2):248–253. doi: 10.1002/jnr.20327. [DOI] [PubMed] [Google Scholar]
- 44.Neis Vivian Binder, Moretti Morgana, Bettio Luis Eduardo B., Ribeiro Camille M., Rosa Priscila Batista, Gonçalves Filipe Marques, Lopes Mark William, Leal Rodrigo Bainy, Rodrigues Ana Lúcia S. Agmatine produces antidepressant-like effects by activating AMPA receptors and mTOR signaling. European Neuropsychopharmacology. 2016;26(6):959–971. doi: 10.1016/j.euroneuro.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Ludka FK, Constantino LC, Dal-Cim T, Binder LB, Zomkowski A, Rodrigues ALS, Tasca CI. Involvement of PI3K/Akt/GSK-3beta and mTOR in the antidepressant-like effect of atorvastatin in mice. J Psychiatr Res. 2016;82:50–57. doi: 10.1016/j.jpsychires.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Rosa JM, Pazini FL, Cunha MP, Colla ARS, Manosso LM, Mancini G, Souza ACG, de Bem AF, Prediger RD, Rodrigues ALS. Antidepressant effects of creatine on amyloid beta1-40-treated mice: the role of GSK-3beta/Nrf2 pathway. Prog Neuro-Psychopharmacol Biol Psychiatry. 2018;86:270–278. doi: 10.1016/j.pnpbp.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Rosa AO, Kaster MP, Binfare RW, Morales S, Martin-Aparicio E, Navarro-Rico ML, Martinez A, Medina M, Garcia AG, Lopez MG, Rodrigues ALS. Antidepressant-like effect of the novel thiadiazolidinone NP031115 in mice. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32(6):1549–1556. doi: 10.1016/j.pnpbp.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 48.Silva R., Mesquita A.R., Bessa J., Sousa J.C., Sotiropoulos I., Leão P., Almeida O.F.X., Sousa N. Lithium blocks stress-induced changes in depressive-like behavior and hippocampal cell fate: The role of glycogen-synthase-kinase-3β. Neuroscience. 2008;152(3):656–669. doi: 10.1016/j.neuroscience.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 49.Oh DH, Park YC, Kim SH. Increased glycogen synthase kinase-3beta mRNA level in the hippocampus of patients with major depression: a study using the Stanley neuropathology consortium integrative database. Psychiatry Investig. 2010;7(3):202–207. doi: 10.4306/pi.2010.7.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4(2):68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338(6103):68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coyle JT, Duman RS. Finding the intracellular signaling pathways affected by mood disorder treatments. Neuron. 2003;38(2):157–160. doi: 10.1016/S0896-6273(03)00195-8. [DOI] [PubMed] [Google Scholar]
- 53.Kaidanovich-Beilin O, Milman A, Weizman A, Pick CG, Eldar-Finkelman H. Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampus. Biol Psychiatry. 2004;55(8):781–784. doi: 10.1016/j.biopsych.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 54.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci U S A. 1995;92(17):7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273(29):18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 56.Manosso Luana M., Moretti Morgana, Ribeiro Camille M., Gonçalves Filipe M., Leal Rodrigo B., Rodrigues Ana Lúcia S. Antidepressant-like effect of zinc is dependent on signaling pathways implicated in BDNF modulation. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2015;59:59–67. doi: 10.1016/j.pnpbp.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 57.Surh YJ, Kundu JK, Li MH, Na HK, Cha YN. Role of Nrf2-mediated heme oxygenase-1 upregulation in adaptive survival response to nitrosative stress. Arch Pharm Res. 2009;32(8):1163–1176. doi: 10.1007/s12272-009-1807-8. [DOI] [PubMed] [Google Scholar]
- 58.Qin S, Hou DX. Multiple regulations of Keap1/Nrf2 system by dietary phytochemicals. Mol Nutr Food Res. 2016;60(8):1731–1755. doi: 10.1002/mnfr.201501017. [DOI] [PubMed] [Google Scholar]
- 59.Li B, Jeong GS, Kang DG, Lee HS, Kim YC. Cytoprotective effects of lindenenyl acetate isolated from Lindera strychnifolia on mouse hippocampal HT22 cells. Eur J Pharmacol. 2009;614(1–3):58–65. doi: 10.1016/j.ejphar.2009.04.056. [DOI] [PubMed] [Google Scholar]
- 60.Zhao X, Wang R, Xiong J, Yan D, Li A, Wang S, Xu J, Zhou J. JWA antagonizes paraquat-induced neurotoxicity via activation of Nrf2. Toxicol Lett. 2017;277:32–40. doi: 10.1016/j.toxlet.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 61.Nguyen T, Sherratt PJ, Huang HC, Yang CS, Pickett CB. Increased protein stability as a mechanism that enhances Nrf2-mediated transcriptional activation of the antioxidant response element. Degradation of Nrf2 by the 26 S proteasome. J Biol Chem. 2003;278(7):4536–4541. doi: 10.1074/jbc.M207293200. [DOI] [PubMed] [Google Scholar]
- 62.Stewart D, Killeen E, Naquin R, Alam S, Alam J. Degradation of transcription factor Nrf2 via the ubiquitin-proteasome pathway and stabilization by cadmium. J Biol Chem. 2003;278(4):2396–2402. doi: 10.1074/jbc.M209195200. [DOI] [PubMed] [Google Scholar]
- 63.Tan Y, Wang Q, She Y, Bi X, Zhao B. Ketamine reduces LPS-induced HMGB1 via activation of the Nrf2/HO-1 pathway and NF-kappaB suppression. J Trauma Acute Care Surg. 2015;78(4):784–792. doi: 10.1097/TA.0000000000000588. [DOI] [PubMed] [Google Scholar]
- 64.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284(20):13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karapetian RN, Evstafieva AG, Abaeva IS, Chichkova NV, Filonov GS, Rubtsov YP, Sukhacheva EA, Melnikov SV, Schneider U, Wanker EE, Vartapetian AB. Nuclear oncoprotein prothymosin alpha is a partner of Keap1: implications for expression of oxidative stress-protecting genes. Mol Cell Biol. 2005;25(3):1089–1099. doi: 10.1128/MCB.25.3.1089-1099.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Velichkova M, Hasson T. Keap1 regulates the oxidation-sensitive shuttling of Nrf2 into and out of the nucleus via a Crm1-dependent nuclear export mechanism. Mol Cell Biol. 2005;25(11):4501–4513. doi: 10.1128/MCB.25.11.4501-4513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hsu M, Muchova L, Morioka I, Wong RJ, Schroder H, Stevenson DK. Tissue-specific effects of statins on the expression of heme oxygenase-1 in vivo. Biochem Biophys Res Commun. 2006;343(3):738–744. doi: 10.1016/j.bbrc.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 68.Quincozes-Santos André, Bobermin Larissa Daniele, Souza Débora Guerini, Bellaver Bruna, Gonçalves Carlos-Alberto, Souza Diogo Onofre. Guanosine protects C6 astroglial cells against azide-induced oxidative damage: a putative role of heme oxygenase 1. Journal of Neurochemistry. 2014;130(1):61–74. doi: 10.1111/jnc.12694. [DOI] [PubMed] [Google Scholar]
- 69.Bellaver Bruna, Souza Débora Guerini, Bobermin Larissa Daniele, Gonçalves Carlos-Alberto, Souza Diogo Onofre, Quincozes-Santos André. Guanosine inhibits LPS-induced pro-inflammatory response and oxidative stress in hippocampal astrocytes through the heme oxygenase-1 pathway. Purinergic Signalling. 2015;11(4):571–580. doi: 10.1007/s11302-015-9475-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Bem A, Engel D, de Oliveira J, Moreira EL, Neis VB, Santos DB, Lopes JB, Rodrigues ALS, Brocardo P. Hypercholesterolemia as a risk factor for depressive disorder? Free Radic Biol Med. 2014;75 Suppl 1:S28. doi: 10.1016/j.freeradbiomed.2014.10.753. [DOI] [PubMed] [Google Scholar]
- 71.Rybka J, Kedziora-Kornatowska K, Banas-Lezanska P, Majsterek I, Carvalho LA, Cattaneo A, Anacker C, Kedziora J. Interplay between the pro-oxidant and antioxidant systems and proinflammatory cytokine levels, in relation to iron metabolism and the erythron in depression. Free Radic Biol Med. 2013;63:187–194. doi: 10.1016/j.freeradbiomed.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 72.Robaczewska J, Kedziora-Kornatowska K, Kucharski R, Nowak M, Muszalik M, Kornatowski M, Kedziora J. Decreased expression of heme oxygenase is associated with depressive symptoms and may contribute to depressive and hypertensive comorbidity. Redox Rep. 2016;21(5):209–218. doi: 10.1080/13510002.2015.1101889. [DOI] [PMC free article] [PubMed] [Google Scholar]