Abstract
The aim of this study was to test the application in vitro of different laser wavelengths at a low fluence in combination or not with proper photosensitizing dyes on Candida albicans biofilm with or without a synthetic killer decapeptide (KP).
Candida albicans SC5314 was grown on Sabouraud dextrose agar plates at 37°C for 24 h. Cells were suspended in RPMI 1640 buffered with MOPS and cultured directly on the flat bottom of 96-wells plates. The previously described killer decapeptide KP was used in this study.
Three different combinations of wavelengths and dyes were applied, laser irradiation has been performed at a fluence of 10 J/cm2. The effect on C. albicans biofilm was evaluated by the XTT assay. Microscopic observations were realized by fluorescence optic microscopy with calcofluor white and propidium iodide.
Compared with control, no inhibition of C. albicans biofilm viability was obtained with application of red, blue and green lasers alone or with any combination of red diode laser, toluidine blue and KP. The combined application of blue diode laser with curcumin and/or KP showed always a very significant inhibition, as curcumin alone and the combination of curcumin and KP did, while combination of blue diode laser and KP gave a less significant inhibition, the same obtained with KP alone. The combined application of green diode laser with erythrosine and/or KP showed always a very significant inhibition, as the combination of erythrosine and KP did, but no difference was observed with respect to the treatment with erythrosine alone. Again, combination of green diode laser and KP gave a significant inhibition, although paradoxically lower than the one obtained with KP alone.
Treatment with KP alone, while reducing biofilm viability did not cause C. albicans death in the adopted experimental conditions. On the contrary, combined treatment with blue laser, curcumin and KP, as well as green laser, erythrosine and KP led to death most C. albicans cells.
The combination of laser light at a fluence of 10 J/cm2 and the appropriate photosensitizing agent, together with the use of KP, proved to exert differential effects on C. albicans biofilm.
Introduction
Fungal infections, mainly those caused by Candida albicans, have an important impact on human health particularly because of the growing number of immunocompromised hosts 1). Systemic mycoses, associated to a poor outcome, can often occur in these patients, and mortality due to invasive candidosis is estimated up to 40% 2).
Recently, more advances have been made in the understanding of mechanisms by which C. albicans stress response, and metabolic adaptation 3–6).
Biofilm is the predominant growth state of many microorganisms and is identifiable as a community of adherent cells with properties that are distinct from those of free-floating or planktonic cells, particularly for the greater resistance of the cells to chemical and physical insults 7).
C. albicans produces highly structured biofilms composed of multiple cell types (i.e., unicellular round budding yeasts and oval pseudohyphae, and multicellular elongated hyphae) encased in an extracellular matrix, mainly composed of proteins and glycoproteins (55%), which plays an important role in drug resistance acting as a physical barrier to drug penetration 7). Biofilm formation initiates when planktonic yeasts adhere to a surface and begin to aggregate and form microcolonies. This first stage is immediately followed by a proliferation of yeast cells and the beginning of hyphal development 8).
The ability to form hyphae or yeast cells, i.e. C. albicans dimorphism, is important for biofilm formation because of the capability of hyphae to contribute to the mechanical and architectural stability of biofilm and to support yeast cells 7).
In patients wearing dentures, biofilm constituted by bacteria and Candida cells can be formed both on the oral mucosa and on the surface of dentures, commonly leading to acute or chronic candidosis, including denture stomatitis.
The Antimicrobial Photodynamic Therapy (APDT) is used with different types of protocols, laser and photosensitizers9). The cytotoxic effect is achieved through the local application or systemic administration (oral or intravenous) of photosensitizing agents followed by irradiation of visible light with emission spectrum appropriate to the absorption spectrum of the used photosensitizer, in the presence of oxygen 10). This induces oxidation phenomena with selective destruction of proteins, lipids, nucleic acids and other cellular components 11).
Recently the literature about C. albicans biofilms has grown with publications about the effect of APDT using different light sources and photosensitizers 12–14).
The main objective of this study was to apply different laser wavelengths at a low fluence in combination or not with proper photosensitizing dyes on in vitro C. albicans biofilm mimicking the in vivo infection. In addition, the previously described antibody-derived decapeptide KP, endowed with anticandidacial activity 15), was also used in combination with laser and dyes application.
Materials and methods
Microbial strain and culture conditions for biofilm formation
The reference strain C. albicans SC5314, selected for its ability to form biofilm, was grown on Sabouraud dextrose agar (SDA) plates at 37°C for 24 h. Cells were suspended in RPMI 1640 medium buffered with 3-(N-morpholino) propane sulfonic acid (MOPS) at a concentration of 106 cells/ml using the McFarland turbidity standard. Yeast suspensions were cultured in flat-bottomed 96-wells plates (100 µl/well).
Plates were incubated 18 h at 37°C on an orbital shaker at 180 rpm to allow biofilm formation. After 18 hours, plates were checked for the homogeneity of biofilm growth under optical microscope (Zeiss, Model Axiovert A1, Germany) at 4× and 10× magnification.
Dyes and laser sources
The study has been realized with three different laser diode prototypes, gently provided by the Department of Engineering and Architecture of the University of Parma, used with or without photosensitizing dyes coupled on the basis of colour affinity: red diode laser (650 nm) with toluidine blue, blue-violet diode laser (405 nm) with curcumin, and green diode laser (532 nm) with erythrosine. The final working concentration was 100 µM for erythrosine and curcumin, and 10 µM for toluidine blue; these concentrations were chosen on the basis of the effect obtained on C. albicans suspensions as described in a previous study 16). All the laser prototypes used have been tested with a power meter (PM-200, Thorlabs). Laser irradiation has been performed in continuous mode for the different wavelengths. On the basis of the power (30 mW) previously recorded with a power meter (Nova II, Ophir) and on the spot size (5 mm diameter), time irradiation (62 seconds) was planned for a fluence of 10 J/cm2.
Killer peptide
The previously described synthetic killer decapeptide KP 15), endowed with candidacidal activity, was used in this study. A stock solution was prepared in DMSO (20 mg/ml) and stored at 4°C until use.
Biofilm treatment
96-wells microtitre plates incubated for 18 h for biofilm growth, as described above, were washed twice with 0.1 M phosphate-buffered saline (PBS) to remove non-adherent cells before treatment. 100 µl of a KP solution in sterile water (final concentration 20 µg/ml), dye solutions or sterile water alone (control) were added into the proper wells. After 5 minutes laser irradiation was applied to the proper wells. All the plates were left at room temperature for the same time. After laser application, associated or not with dyes and KP treatment, plates where re-incubated for 2 h at 37°C on an orbital shaker at 180 rpm. Each assay was carried out in triplicate and three independent experiments were carried out.
Evaluation of biofilm viability by XTT assay
A semiquantitative measure of biofilm viability was then assessed using a 2, 3-bis (2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) reduction assay (Sigma-Aldrich, USA). Briefly, after washing 100 µl of sterile water were added to each well. Then 50 µl of the XTT reaction mixture (activation reagent and XTT reagent) prepared according to manufacturer's recommendations were added. The plates were incubated in the dark at 37°C for 2 h and the colorimetric change resulting from XTT reduction, directly related to biofilm metabolic activity, was measured in a microtitre plate reader (ELx800, Biotek Instruments, USA) at 490 nm. Percentage of inhibition in comparison to untreated controls was calculated by the following formula: [(control - treatment)/ control] × 100.
Fluorescence microscopy studies
For fluorescence microscopy studies, after laser irradiation, associated or not with dyes and KP treatment, the 96-wells plates were rinsed two times with PBS, then Calcofluor White (Invitrogen, Paisley, UK) and propidium iodide (Invitrogen,) were added at final concentrations of 25 µM and 20 µM, respectively. Plates were incubated for 30 min in the dark. Observations were performed under a fluorescent microscope (Zeiss, Model Axiovert A1, Germany) at 10 × magnification: Candida albicans cells in biofilms were stained by calcofluor white and visible in blue colour, Candida albicans dead cells were stained by propidium iodide and visible in red colour.
Pictures were taken using AxioVision 4.8 acquisition software and digital AxioCam ICc1 Rev.4 camera (Zeiss).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6 software.
Data are reported as the mean ± standard deviation (SD) from triplicate samples and were evaluated using one-way ANOVA. Multiple comparison analysis was realized with Tukey's test. A value of p < 0.05 was considered significant.
Results
Effect on C. albicans biofilm of treatment with different combinations of light, dyes and KP
The effect on C. albicans biofilm viability of the treatment with different combinations of diode laser application, dyes and KP are shown in Figures 1–4. No difference in C. albicans biofilm viability has been observed with respect to the untreated control after the application of red, blue, and green diode lasers alone.
Figure 1:
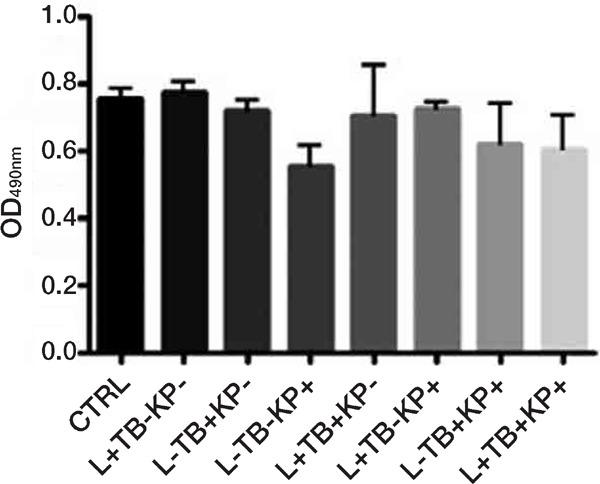
Effects on C. albicans biofilm viability, as assessed by the XTT assay, of treatment with different combinations (+ with, − without) of red diode laser (650 nm, L), toluidine blue (TB) and synthetic killer decapeptide (KP). Results are expressed as mean values and standard deviation (SD) of the optical density (OD) at 490 nm. There was no significant difference vs control (no treatment) nor among the different treatments. Data were analysed by one-way ANOVA and Tukey post hoc test. Data reported are from one representative experiment out of three experiments with comparable results.
Figure 2:
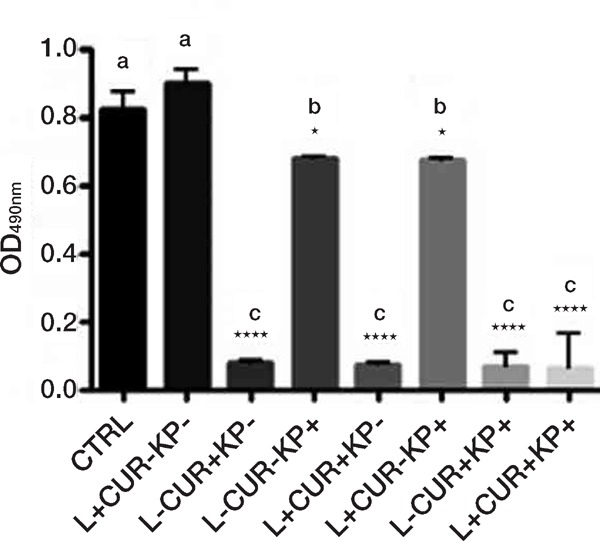
Effects on C. albicans biofilm viability, as assessed by the XTT assay, of treatment with different combinations (+ with, − without) of blue diode laser (405 nm), curcumin (CUR) and synthetic killer decapeptide (KP). Results are expressed as mean values and standard deviation (SD) of the optical density (OD) at 490 nm. Data were analysed by one-way ANOVA and Tukey post hoc test. *p < 0.05, ****p < 0.0001, significant difference vs. control (no treatment); bars with the same letter represent no significant difference. Data reported are from one representative experiment out of three experiments with comparable results.
Figure 3:
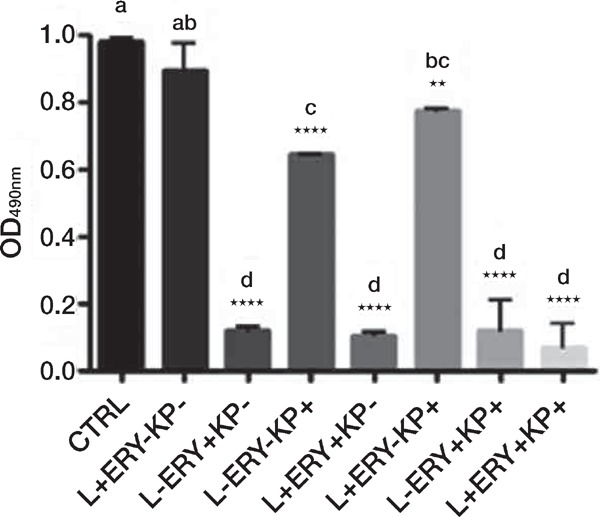
Effects on C. albicans biofilm viability, as assessed by the XTT assay, of treatment with different combinations (+ with, − without) of green diode laser (532 nm), erythrosine (ERY) and synthetic killer decapeptide (KP). Results are expressed as mean values and standard deviation (SD) of the optical density (OD) at 490 nm. Data were analysed by one-way ANOVA and Tukey post hoc test. **p < 0.01, ****p < 0.0001, significant difference vs control (no treatment); bars with the same letter represent no significant difference. Data reported are from one representative experiment out of three experiments with comparable results.
Figure 4:
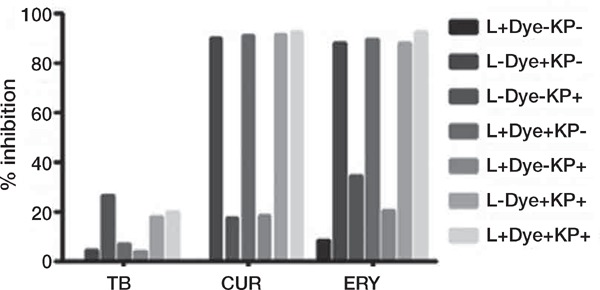
Percentage of inhibition of biofilm viability, in comparison to untreated controls, after treatment with different combinations of laser (L) and dyes (red diode laser, 650 nm, and toluidine blue, TB; blue diode laser, 405 nm, and curcumin, CUR; green diode laser, 532 nm, and erythrosine, ERY) and synthetic killer decapeptide (KP).
While treatment with curcumin and erythrosine alone at the adopted concentrations caused a very significant reduction in biofilm viability (p < 0.0001), treatment with toluidine blue alone showed no effect. Treatment with KP alone caused a reduction of biofilm viability (average inhibition of approximately 26%).
Any combined application of red diode laser with toluidine blue and/or KP gave no significant inhibition of biofilm viability (Figure 1).
In the comparison with the untreated control, the combined application of blue diode laser with curcumin and/or KP showed always a very significant inhibition, as the combination of curcumin and KP did (p < 0.0001), while combination of blue diode laser and KP gave a less significant inhibition, the same obtained with KP alone (p < 0.05). However, there was no statistically significant difference between curcumin alone and any other treatment combined with this dye, even if a light increase in % inhibition was observed when blue laser and KP were associated (90.08 vs 92.14 respectively) (Figures 2 and 4).
In the comparison with the untreated control, the combined application of green diode laser with erythrosine and/or KP showed always a very significant inhibition, as the combination of erythrosine and KP did (p < 0.0001), but no difference was observed with respect to the treatment with erythrosine alone. Again, combination of green diode laser and KP gave a significant inhibition (p < 0.01), although paradoxically lower than the one obtained with KP alone (p < 0.0001) (Figure 3).
Results obtained with XTT assay were implemented by fluorescence microscopy analysis. Staining with calcofluor white, which binds to yeast cell wall, allowed to visualizing all C. albicans cells in blue, while internalization of propidium iodide allowed to visualize in red dead C. albicans cells. Representative images are shown in Figures 5–6. Treatment with KP alone, while reducing biofilm viability, as assessed by evaluation of metabolic activity through XTT assay, did not cause C. albicans death in the adopted experimental conditions. Application of red, blue and green lasers alone did not kill C. albicans cells, nor combined treatment with toluidine blue, red laser and KP did. On the contrary, combined treatment with blue laser, curcumin and KP, as well as green laser, erythrosine and KP led to death most C. albicans cells (Figure 5–6).
Figure 5:

Fluorescence microscopy images of (A) C. albicans cells and (B) C. albicans dead cells in biofilm treated with blue laser alone; (C) C. albicans cells and (D) C. albicans dead cells in biofilm treated with Curcumin, blue laser and KP; scale bar of 250 am, magnification 10×. Panel A and C, staining with calcofluor white; panel B and D, staining with propidium iodide. The absence of candidacidal effect for blue laser alone is visible in panel B as absence of propidium iodide (red) cells.
Figure 6:
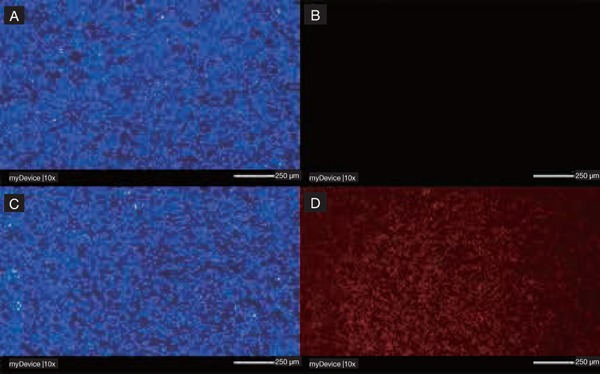
Fluorescence microscopy images of (A) C. albicans cells and (B) C. albicans dead cells in biofilm treated with green laser alone; (C) C. albicans cells and (D) C. albicans dead cells in biofilm treated with Erythrosine, green laser and KP; scale bar of 250 rm, magnification 10×. Panel A and C, staining with calcofluor white; panel B and D, staining with propidium iodide. The absence of candidacidal effect for green laser alone is visible in panel B as absence of propidium iodide (red) cells.
Discussion
A number of previously published papers reported the effect of APDT on C. albicans biofilms; in most studies high fluences, up to 350 J/cm2 17) and a long time of application up to 26 minutes 18) were used. We purposely choose a light fluence (10 J/cm2), exportable in clinical practice without danger, discomfort or damage risk for patients or oral appliances (e.g. prosthetic devices).
Evaluation of C. albicans biofilm inhibition was realized in this study by means of the XTT assay, a method for quantitative evaluation of metabolic activity that is not affected by inter-operator variability and is easier to perform than the CFU assay. Moreover, literature highlighted the limitation of CFU count for C. albicans biofilms as multicellular fungi, while presenting a large biomass compared to single yeast cells, yet form a single colony in CFU assay 19, 20).
Previous studies reported that microorganisms organized in biofilms are less susceptible to photodynamic procedure compared with those in planktonic phase because of the heterogeneity of the biofilm, the reduced growth rate of cells, the differences in gene expression, and the limited penetration of antimicrobial agents across the extracellular matrix material 21).
In this study, a very significant inhibition of C. albicans biofilm has been obtained with curcumin and erythrosine alone. Dyes concentrations were chosen on the basis of a previous study performed in vitro on Candida albicans suspensions and in vivo on a Galleria mellonella model 16). In the previous study, dyes alone gave different results. The diverse adopted conditions, i.e. C. albicans cells in suspensions compared to C. albicans in biofilm structures, may likely be responsible for the observed differences.
Martins and coworkers in 2009 22) reported that curcumin is 2.5 fold more potent than fluconazole at inhibiting the adhesion of C. albicans to buccal epithelial cells. Curcumin acts by generating reactive oxygen species, triggers an early apoptosis in C. albicans cells and affects membrane associated ATPase activity, ergosterol biosynthesis and protein secretion.
Some studies in the past suggested that curcumin alone, also at low concentrations (100 lM), may have antifungal properties that may be improved and increased in combination with laser light 23, 24).
Dovigo and coworkers 24) reported on C. albicans biofilm a reduction of about 80% with curcumin 40 M and 18 J/cm2: our results showed a 90% inhibition using a lower fluence (10 J/cm2) and a higher curcumin concentration.
Sanità and coworkers reported in 2017 the same positive effects on C. dubliniensis but using a light emitting diode (LED) source at 5.28 J/cm2 and describing better results for a longer preirradiation time (20 minutes) 25).
With regard to the combination of green 532 nm laser and erythrosine, at our knowledge there are no similar protocols in literature; available studies describe combination of erythrosine with green or blue-violet LED, requiring longer irradiation time to obtain the same fluence values 26). The results we reported in this study for erythrosine are consistent with results of a previously published study 16) on an in vivo model of Galleria mellonella where, with the same dye concentration of this study (100 M) erythrosine alone had a candidacidal effect.
At last, with reference to the combination of toluidine blue and red laser, our results are in contradiction with the study of Pupo and coworkers 27) which reported a value of viable C. albicans cells significantly reduced after PDT with methylene blue and toluidine blue regardless of the photosensitizer used, even if with a higher fluence of 53 J/cm2.
Moreover, literature describes different times of preirradiation, adfirming that killing is not preirradiation time-dependent for planktonic cultures but it must be higher in biofilms cultures 28), so a prolonged time of preirradiation may be considered to improve the efficacy of treatments in our biofilm model.
Conclusion
In our study, curcumin and erythrosine at the chosen concentrations proved to be able to significantly reduce C. albicans biofilm viability even without KP or laser association. Conversely, in these experimental conditions, no association of red laser, toluidine blue and KP was able to reduce biofilm viability. These considerations may be a key point for future studies where a reduced dye concentration or the increase of the fluence (for the red laser) could be investigated. The potential advantages of APDT in terms of reduced costs, scarce side effects, low overdose risk and unlikely resistance induction, are compatible with the optimization of APDT protocols in the future 29–35).
Conflicts of interest
“There are no conflicts to declare”.
References
- 1: Vallabhaneni S., Mody R.K., Walker T., Chiller T. The global burden of fungal diseases. Infect Dis Clin North Am. 30 (2016) 1-11. [DOI] [PubMed] [Google Scholar]
- 2: Kullberg B.J., Arendrup M.C. Invasive candidiasis. N Engl J Med. 373 (2015) 1445-56. [DOI] [PubMed] [Google Scholar]
- 3: Sardi J.C.O., Scorzoni L., Bernardi T., Fusco-Almeida A.M., Mendes Giannini M.J.S. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microb. 62 (2013) 10-24. [DOI] [PubMed] [Google Scholar]
- 4: Sellam, Whiteway M. Recent advances on Candida albicans biology and virulence. F1000Res. 5 (2016) 2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5: Nett J.E. The host's reply to Candida biofilm. Pathogens. 5 (2016) 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6: Manfredi M., Polonelli L., Aguirre-Urizar J.M., Carrozzo M., McCullough M.J. Urban legends series: oral candidosis. Oral Dis. 19 (2013) 245-61. [DOI] [PubMed] [Google Scholar]
- 7: Nobile C.J., Johnson A.D. Candida albicans biofilms and human disease. Annu Rev Microbiol. 69 (2015) 71-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8: Chevalier M., Medioni E., Precheur I. Inhibition of Candida albicans yeast-hyphal transition and biofilm formation by Solidago virgaurea water extracts. J Med Microbiol. 61 (2012) 1016-22. [DOI] [PubMed] [Google Scholar]
- 9: Rolim J.P., de Melo M.A., Guedes S.F., Albuquerque-Fihlo F.B., de Souza J.R., Nogueira N.A., Zanin I.C., Rodrigues L.K. The antimicrobial activity of photodynamic therapy against Streptococcus mutans using different photosensitizers. J Photochem Photobiol B 106 (2012) 40-6. [DOI] [PubMed] [Google Scholar]
- 10: Knopka K., Goslinski T. Photodynamic Therapy in Dentistry. J Dent Res. 86 (2007) 694-707. [DOI] [PubMed] [Google Scholar]
- 11: Valenzano M., Valenzano L. La terapia medica dei tumori epiteliali. International Journal of Migration and Transcultural Medicine. 1 (2005) 10-18. [Google Scholar]
- 12: Fumes A.C., da Silva Telles P.D., Corona S.A.M., Borsatto M.C. Effect of PDT on Streptococcus mutans and Candida albicans present in the dental biofilm: Systematic review. Photodiagnosis Photodyn Ther. 21 (2018) 363-6. [DOI] [PubMed] [Google Scholar]
- 13: Carvalho M.L., Pinto A.P., Raniero L.J., Costa M.S. Biofilm formation by Candida albicans is inhibited by photodynamic antimicrobial chemotherapy (PACT), using chlorin e6: increase in both ROS production and membrane permeability. Lasers Med Sci. 33 (2018) 647-653. 10.1007/s10103-017-2344-1. [DOI] [PubMed] [Google Scholar]
- 14: Bujdakova H. Management of Candida biofilms: state of knowledge and new options for prevention and eradication. Future Microbiol. 11 (2016) 235-251. 10.2217/fmb.15.139. [DOI] [PubMed] [Google Scholar]
- 15: Manfredi M., Merigo E., Salati A., Conti S., Savi A., Polonelli L., Bonanini M., Vescovi P. In vitro candidacidal activity of a synthetic killer decapeptide (KP) against Candida albicans cells adhered to resin acrylic discs. J Oral Pathol Med. 36 (2007) 468-471. [DOI] [PubMed] [Google Scholar]
- 16: Merigo E., Conti S., Ciociola T., Fornaini C., Polonelli L., Lagori G., Manfredi M., Vescovi P. Effect of different wavelengths and dyes on Candida albicans: In vivo study using Galleria mellonella as an experimental model. Photodiagnosis Photodyn Ther. 18 (2017) 34-8. [DOI] [PubMed] [Google Scholar]
- 17: Pereira C.A., Romeiro R.L., Costa A.C., Machado A.K., Junqueira J.C., Jorge A.O. Susceptibility of Candida albicans, Staphylococcus aureus, and Streptococcus mutans biofilms to photodynamic inactivation: an in vitro study. Lasers Med Sci. 26 (2011) 341-8. 10.1007/s10103-010-0852-3. [DOI] [PubMed] [Google Scholar]
- 18: Mima E.G., Pavarina A.C., Ribeiro D.G., Dovigo L.N., Vergani C.E., Bagnato V.S. Effectiveness of photodynamic therapy for the inactivation of Candida spp. On dentures: in vitro study. Photomed Laser Surg. 2011. December;29(12):827-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19: Chevalier M., Ranque S., Precheur I. Oral fungal-bacterial biofilm models in vitro: a review. Med Mycol. 2017. December 8. 10.1093/mmy/myx111. [DOI] [PubMed] [Google Scholar]
- 20: Falsetta M.L., Klein M.I., Colonne P.M., Scott-Anne K., Gregoire S., Pai C.H., Gonzalez-Begne M., Watson G., Krysan D.J., Bowen W.H., Koo H. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun. 82 (2014) 1968-81. 10.1128/IAI.00087-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21: Rossoni R.D., Barbosa J.O., de Oliveira F.E., de Oliveira L.D., Jorge A.O., Junqueira J.C. Biofilms of Candida albicans serotypes A and B differ in their sensitivity to photodynamic therapy. Lasers Med Sci. 29 (2014) 1679-84. [DOI] [PubMed] [Google Scholar]
- 22: Martins C.V., da Silva D.L., Neres A.T., Magalhaes T.F., Watanabe G.A., Modolo L.V., Sabino A.A., de Fatima A., de Resende M.A. Curcumin as a promising antifungal of clinical interest. J. Antimicrob Chemother. 63 (2009) 337-9. [DOI] [PubMed] [Google Scholar]
- 23: Andrade M.C., Ribeiro A.P., Dovigo L.N., Brunetti I.L., Giampaolo E.T., Bagnato V.S., Pavarina A.C. Effect of different pre-irradiation times on curcumin-mediated photodynamic therapy against planktonic cultures and biofilms of Candida spp. Arch Oral Biol. 58 (2013) 200-10. [DOI] [PubMed] [Google Scholar]
- 24: Dovigo L.N., Pavarina A.C., Carmello J.C., Machado A.L., Brunetti I.L., Bagnato V.S. Susceptibility of clinical isolates of Candida to photodynamic effects of curcumin. Lasers Surg. Med. 43 (2011) 927-34. [DOI] [PubMed] [Google Scholar]
- 25: Sanita P.V., Pavarina A.C., Dovigo L.N., Ribeiro A.P.D., Andrade M.C., Mima E.G.O. Curcumin-mediated anti-microbial photodynamic therapy against Candida dubliniensis biofilms. Lasers Med Sci. 33 (2018) 709-17. 10.1007/s10103-017-2382-8. [DOI] [PubMed] [Google Scholar]
- 26: Costa A.C., Campos V.M.R., Hashimoto E.S.S., Araujo C.F., Pereira C.A., Junqueira J.C., Jorge A.O. Effect of erythrosineand LED-mediated photodynamic therapy on buccal candidiasis infection of immunosuppressed mice and Candida albicans adherence to buccal epithelial cells. Oral Surg Oral Med Oral Pathol Oral Radiol. 114 (2012) 67-74. [DOI] [PubMed] [Google Scholar]
- 27: Pupo Y.M., Gom29 G.M., Hamblin M.R., Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 3 (2004) 436-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28: Silva M.P., dos Santos T.A., de Barros P.P., de Camargo Ribeiro F., Junqueira J.C., Jorge A.O. Action of antimicrobial photodynamic therapy on heterotypic biofilm: Candida albicans and Bacillus atrophaeus. Lasers Med Sci. 31 (2016) 605-10. [DOI] [PubMed] [Google Scholar]
- 29: Hamblin M.R., Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 3 (2004) 436-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30: Wainwright M., Crossley K.B. Photosensitizing agents — circumventing resistance and breaking down biofilms: a review. Int Biodeterior Biodegrad. 53 (2004) 119-26. [Google Scholar]
- 31: O'Riodan K., Akilov O.E., Hasan T. The potential for photodynamic therapy in the treatment of localized infections. Photodiagn Photodyn Ther. 2 (2005) 247-62. [DOI] [PubMed] [Google Scholar]
- 32: Silverman S. Oral Cancer: complications of therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 88 (1999) 122-6. [DOI] [PubMed] [Google Scholar]
- 33: Komerik N., MacRobert A.J. Photodynamic therapy as an alternative antimicrobial modality for oral infections. J Environ Pathol Toxicol Oncol. 25 (2006) 487-504. [DOI] [PubMed] [Google Scholar]
- 34: Calzavara-Pinton P.G., Venturini M., Sala R. A comprehensive overview of photodynamic therapy in the treatment of superficial fungal infections of the skin. J Photochem Photobiol B. 78 (2005) 1-6. [DOI] [PubMed] [Google Scholar]
- 35: Donnelly R.F., McCarron P.A., Tunney M.M. Antifungal photodynamic therapy. Microbiol Res. 163 (2008) 1-12. [DOI] [PubMed] [Google Scholar]


