Abstract
The aim of this work was to evaluate the influence of Arthrospira platensis F&M-C256 (spirulina) incorporation on the nutritional and functional properties of “crostini”, a leavened bakery product largely consumed in Italy and Europe. Sourdough was used as leavening and fermentation agent and three concentrations of A. platensis F&M-C256 were tested: 2%, 6% and 10% (w/w). Despite a lower volume increase compared to the control, the A. platensis F&M-C256 “crostini” doughs reached a technological appropriate volume after fermentation. At the end of fermentation, no significant differences in microorganisms concentrations were observed. A. platensis F&M-C256 “crostini” showed higher protein content compared to the control. Considering the European Commission Regulation on nutritional claims, “crostini” incorporated with 6% and 10% biomass can be claimed to be a “source of protein”. Six and ten percent A. platensis “crostini” also presented significantly higher antioxidant capacity and phenolics. A significantly lower value of in vitro dry matter and protein digestibility between A. platensis F&M-C256 “crostini” and the control was found. The overall acceptability decreased with increasing A. platensis F&M-C256 addition. The combination of spirulina biomass addition and the sourdough technology led to the development of a novel microalgae-based bakery product with nutritional and functional features.
Subject terms: Applied microbiology, Industrial microbiology
Introduction
Bakery products are a staple presence of the diet worldwide. This category includes different food products such as bread, cakes, pastries, biscuits and breakfast cereals. In Italy, many bakery goods are obtained through sourdough technique that is used both for artisanal and industrial productions1–4. Sourdough is typically defined as a mixture of water and flour fermented by lactic acid bacteria (LAB), primarily responsible for acidification, and yeasts, that promote the leavening process5,6. Sourdough LAB are mainly heterofermentative species belonging to Lactobacillus, while typical yeasts associated with this food matrix are Saccharomyces cerevisiae, Candida milleri and Issatchenkia orientalis5. The microbial populations are kept stable, with a LAB/yeast ratio between 1:10 and 1:100, and active through daily refreshments2,4,7. In addition to traditional products (e.g. typical breads and sweet leavened cakes), sourdough is being used for different baked goods: snacks, crackers, “grissini”, leading to the development of novel products. Indeed, the use of sourdough is recognized to improve rheological, sensory, technological and nutritional features of the final products, conferring peculiar and distinctive characteristics8. Sourdough fermentation lowers the glycaemic index by reducing starch digestibility, increases the bioavailability of mineral salts by reducing phytate content, and promotes proteolysis by activating endogenous cereal proteases and through LAB peptidases9–13. Some LAB strains can also produce bioactive molecules with nutraceutical properties such as amino acid derivatives (e.g. γ-amino butyric acid)14, bioactive peptides15–17, or exopolysaccharides with prebiotic potentialities18,19.
In order to further enhance bakery products overall quality, the addition of functional ingredients has been proposed: pseudo-cereals, legumes, sprouted cereals, wheat germ, fibers, microalgae, spices, herbs, etc.14,20–24. Indeed, new functional foods with a high nutritional value, consumable on a daily basis, are increasingly requested25.
The cyanobacterium Arthrospira platensis, often incorrectly reported as Spirulina platensis26 and known commercially as “spirulina”, can be used as food ingredient for the production of functional bakery products such as biscuits20 and bread21. Cyanobacteria, such as A. platensis, Aphanizomenon flos-aquae from Klamath Lake and Nostoc sphaeroides are rich in high biological value proteins, polyunsaturated fatty acids, carotenoids, vitamins, minerals, phenolic compounds and bioactive molecules27. In particular, A. platensis is considered a source of proteins (up to 70% of dry weight), iron, γ-linolenic acid, sulphated polysaccharides and phycocyanin28. A. platensis also shows various activities of pharmacological interest, such as antioxidant, immunomodulatory, hypolipidemic and anti-inflammatory activity29–31. An in vitro trial showed an absence of toxicity of A. platensis F&M-C256 aqueous and methanolic extracts against Artemia salina and a low toxicity level for the same aqueous extract against human dermal fibroblasts32.
A. platensis is considered a “safe food source” in several countries (e.g. Europe, Australia, the USA) and, having been used as food before May 1997, it is authorized in Europe for food consumption33. The safety of A. platensis for human consumption is supported by its long history of use as food component34. In Africa A. platensis is integrated into traditional preparations, called Dih’e34,35, and consumed from 10 to 40 g per person per day36. Despite the limited acceptance by consumers, several companies are starting to pay attention to the microalgae sector and the algae products market is expected to reach US$ 44.7 billion by 202337. Examples of large companies that are investing in innovative algae-based food products are Corbion (https://www.corbion.com/algae-portfolio) and Dulcesol Group (http://en.dulcesol.com/). Although the emergence of spirulina-derived food products is increasing in the market, the launch of these products is not necessarily accompanied by thorough product development studies. A deeper comprehension of the complex interactions which take place in the different food matrixes and technological processes, as well as the deriving positive and negative effects on human health, should be considered in order to support a successful integration of this innovative ingredient in the diet, and the consumer acceptance in the long-term. Previous studies have shown that the addition of A. platensis biomass can promote significant impacts on a food product microstructure, nutritional and functional properties, namely in bakery products such as biscuits20 and pasta21. When dealing with fermented products it is also expected that the introduction of cyanobacterial biomass might impact the microorganisms performance and the final products properties. Some studies on spirulina-enriched bread have been published38,39, but to the best of our knowledge, no literature data on spirulina-based bakery product fermented by sourdough are available.
“Crostini” is a typical Italian leavened product, obtained by cooking and successive toasting to reach a humidity less than 10% on final product weight40. The aim of this work was to evaluate the influence of incorporating A. platensis F&M-C256 biomass on the nutritional and functional properties of “crostini” made by using sourdough. The effects of A. platensis F&M-C256 addition (2, 6 and 10% w/w) in the dough were evaluated through physicochemical, microbiological and sensory analyses.
Materials and Methods
A. platensis F&M-C256 biomass production
Biomass of Arthrospira platensis F&M-C256, a strain belonging to the culture collection of Fotosintetica & Microbiologica S.r.l. (F&M), Italy, was produced at Azienda Agricola Serenissima S.S. (Conche di Codevigo, Padova, Italy). The cyanobacterium was cultivated in Zarrouk medium41 in GWP®-II photobioreactors42 in semi-batch mode, harvested by filtration, and the biomass was washed with tap water to remove excess bicarbonate. The biomass was then dried at low temperature (33 °C) (North West Technology, Cuneo, Italy) for 20 h and the obtained flakes were stored at −20 °C until use. The biochemical composition of the biomass was determined as Abiusi et al.43, moisture and ash were analysed following ISTISAN protocols44 (Table 1).
Table 1.
Biochemical composition of A. platensis F&M-C256 biomass used in the experiments. Data are expressed as % (w/w) of algal powder. Results are expressed as average ± standard deviation (n = 3).
| Moisture | Ash | Lipid | Protein | Carbohydrate | |
|---|---|---|---|---|---|
| A. platensis F&M-C256 | 7.7 ± 0.1 | 5.8 ± 0.1 | 6.7 ± 0.002 | 67.3 ± 0.01 | 12.5 ± 0.2 |
Lactic acid bacteria and yeast growth conditions
Lactobacillus farciminis DZB19 and Saccharomyces cerevisiae LV8 strains isolated from Italian sourdoughs and belonging to the collection of the Department of Agriculture, Food, Environment and Forestry of the University of Florence (Italy), were used in this study. L. farciminis was routinely propagated for 24 h at 30 °C in MR3i medium45, S. cerevisiae LV8 strain was aerobically cultured at 30 °C in MYPG, a medium containing (in g/L): malt extract 5, yeast extract 3, meat extract 5 and glucose 10. Cells were recovered by centrifugation (5,000 × g for 20 min), successively washed in physiological solution and used for the preparation of the sourdoughs.
“Crostini” preparation
Sourdough was prepared inoculating L. farciminis DZB19 at the initial concentration of 7 log CFU/g and S. cerevisiae at 6 log CFU/g in a dough made with 190 g Type “00” wheat flour and 110 mL water, with a dough yield [DY = (amount of flour + amount of water) x100/(amount of flour]) of 158. The sourdough was fermented for 18 h at 30 °C in a proofing room, before being used for “crostini” preparation.
The recipe of “crostini” is reported in Table 2. A. platensis F&M-C256 biomass was added at different percentages by replacing total flour: 2% (Ap 2 “crostini”), 6% (Ap 6 “crostini”) and 10% (Ap 10 “crostini”). A control, without microalgae incorporation was also prepared and analysed. The ingredients were mixed in a twin arm mixer (Bernardi, Italy) at room temperature for 10 min at 50 rpm mixing rate. After 25 min of proofing at 25 °C, the doughs were divided into batches of 300 g, to obtain molds of 25 cm length and 1.8 cm diameter. Afterwards, the molded doughs were fermented at 30 °C for 2 h and baked at 160 °C for 11 min. Finally, the “crostini”, were obtained by cutting the molds in pieces of 1.1 cm thickness and then toasted at 140 °C for 14 min. After cooling, sample “crostini” were stored at room temperature in hermetic containers, protected from light. Some of the “crostini” batches were immediately crushed to powder and frozen to be used for chemical analysis, antioxidant capacity, and in vitro digestibility.
Table 2.
“Crostini” recipes (%, w/w). Control (without A. platensis F&M-C256 incorporation) (C) and “crostini” enriched with 2% (Ap 2), 6% (Ap 6) and 10% (Ap 10) (w/w) A. platensis F&M-C256 biomass.
| Ingredients | C | Ap 2 | Ap 6 | Ap 10 |
|---|---|---|---|---|
| Sourdough | 25 | 25 | 25 | 25 |
| Wheat flour | 47 | 45 | 41 | 37 |
| Water | 17 | 17 | 17 | 17 |
| Extra virgin olive oil | 10 | 10 | 10 | 10 |
| Salt | 1 | 1 | 1 | 1 |
| A. platensis F&M-C256 | 0 | 2 | 6 | 10 |
Dough analyses
Microbiological analyses
Ten grams of doughs at the end of fermentation time were transferred into 90 mL of sterile physiological solution and homogenized for 2 min in a Stomacher Lab Blender 400 (Seward Ltd, Worthing, West Sussex, UK). Afterwards, decimal dilutions were performed and 100 µL of appropriate dilutions were plated onto MR3i medium for enumeration of LAB and onto MYPG agar containing sodium propionate (2 g/L) for enumeration of S. cerevisiae. LAB colonies were counted after incubation for 48–72 h at 30 °C under anaerobic conditions and yeast colonies after incubation for 48 h at 30 °C under aerobic conditions. Plate counts were performed in duplicate.
Physico-chemical analyses
The pH and the total titratable acidity (TTA) of the doughs were determined at the beginning and at the end of the fermentation time. The pH values were measured by a pH-meter (Metrohm Italiana Srl, Varese, Italy).
TTA was determined on doughs and A. platensis F&M-C256 powder by using 10 g of samples, homogenized with 90 mL of distilled water for 3 min and expressed as the amount (mL) of 0.1 N NaOH needed to reach a pH value of 8.5.
The increase in dough volume was assessed by placing 100 g of each dough in a graduated cylinder (1 L). After 2 h of fermentation at 30 °C the volume of the doughs (in mL) was recorded. The volume increase was calculated using the following formula: (∆V/V0) x 100, where ∆V was the difference between the volume after the 2 h of fermentation and the initial volume (V0)46.
“Crostini” analyses
HPLC analysis of organic acids
“Crostini” and A. platensis F&M-C256 samples were crushed to powder, diluted ten times and filtered by Amicon® Ultra-4 Centrifugal Filters (3.000 Da NMWL) (Merck Millipore) before the injection. Lactic and acetic acid were determined by High-Performance Liquid Chromatography (HPLC) analysis (Varian Inc, Palo Alto, CA, USA).
Free amino acid determination
For the free amino acid (FAA) quantification, water/salt soluble extracts (WSE) of “crostini” were prepared as follows. Five grams of the sample were diluted with 20 mL of 50 mM Tris-HCl (pH 8.8), held at 4 °C for 1 h, vortexed at 15-min intervals, and centrifuged at 20,000 × g for 20 min15. The supernatants were used for the analysis. Before injection on HPLC for FAA quantification, the sample extracts were prepared according to Galli et al.47. Separation was obtained with a Kinetex 5 µm C18 100 Å column (150 × 4.60 mm; Phenomenex, Bologna, Italy) connected to fluorimetric detector (Jasco Europe, Lecco, Italy) with wavelengths set at 293 nm (Ex) and 492 nm (Em) under the conditions reported by Tuberoso et al.48. The quantitative analysis was performed using calibration graphs constructed according to the internal standard method.
Proximate chemical composition and colour analyses
The “crostini” biochemical composition was determined as in Abiusi et al.43. Moisture and ash were analysed following ISTISAN protocols44. The total energetic value was calculated following the conversion factors designated in the annex XIV of Regulation (EU) N° 1169/201149.
The colour of “crostini” samples was measured instrumentally using a Minolta CR-400 (Japan) colorimeter with standard illuminant D65 and a visual angle of 2°. The results were expressed in terms of L*, lightness (values increase from 0 to 100%); a*, redness to greenness (+60 to −60 positive to negative values, respectively); b*, yellowness to blueness (+60 to −60 positive to negative values, respectively), according to the CIEL*a*b* system. Chroma, C*ab (saturation), and hue angle, h°ab, were also calculated, as defined by: C*ab = [(a*2 + b*2)]1/2; h°ab = arctan (b*/a*). The measurements were conducted under the same light conditions, using a white standard (L* = 94.61, a* = −0.53, b* = 3.62), under artificial fluorescent light at room temperature, replicated twelve times for each formulation sample (one measurement per “crostino”).
Phycocyanin, phenolics and antioxidant capacity determination
Phycocyanin content in A. platensis F&M-C256 lyophilised biomass and in powdered “crostini” was determined according to Herrera et al.50. Phycocyanin was extracted suspending freeze dried A. platensis F&M-C256 powder or “crostini” powders in a 1% (w/v) CaCl2 solution at pH 6.8 (typically 1 g powder in 20 mL of extraction solution). The extracted phycocyanin was spectrophotometrically quantified and purity was assessed at 620 nm and 280 nm using a UV-Vis spectrophotometric reader (Cary 60 UV-Vis, Agilent Technologies, California, USA).
The total phenolic content assay was carried out according to Rajauria et al.51 using the Folin Ciocalteu assay. Samples of 0.1 g of lyophilised “crostini” were dissolved in 6 mL of deionised water and sonicated for 30 min at the maximum power (frequency 20 kHz, power 130 W) maintaining the temperature below 30 °C by immerging the sample flask in an ice bath (MicrosonTM XL2000, Misonix Inc., Farmingdale, New York, USA). To 100 µL aliquots of each sample, 2 mL of 2% sodium carbonate (Sigma-Aldrich) in water were added. After 2 min, 100 μL of 50% Folin Ciocalteu reagent (Sigma-Aldrich) were added. The reaction mixture was incubated in darkness at 25 °C for 30 min. The absorbance of each sample was measured at 720 nm using a UV-Vis spectrophotometric reader (Cary 60 UV-Vis, Agilent Technologies, California, USA). Results were expressed in gallic acid equivalents (mg GAE g−1) through a calibration curve with gallic acid (0 to 150 μg mL−1, R2 = 0.9907) (Sigma-Aldrich).
To evaluate the radical scavenging capacity of the “crostini” samples, the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay was carried out according to Rajauria et al.51. Briefly, the assay was performed by reaction of 1 mL of DPPH radical solution (165 μM, in methanol, Sigma-Aldrich) with 1 mL of sample (0.2 g of lyophilised “crostini” extracted for 30 min in 5 mL of a 1:5 methanol/:water solution). The reaction mixtures were incubated in darkness at 30 °C for 30 min and the absorbances were measured at 517 nm using a UV-Vis spectrophotometric reader (Cary 60 UV-Vis, Agilent Technologies, California, USA). The antioxidant capacity of the samples was expressed as Radical Scavenging Activity (% RSA). Samples RSA capacity was also converted in µg of Vitamin C Equivalent Antioxidant Capacity (VCEAC) per milligram of sample through a calibration curve (ascorbic acid: 0 to 10 µg mL−1, R2 = 0.9918).
In vitro digestibility tests
The in vitro digestibility was evaluated by the method of Boisen & Fernández52, modified by Niccolai et al.32, on lyophilised “crostini”. The method reproduces the chemical-enzymatic digestion (by gastric and pancreatic juices) that occurs in the proximal tract of the monogastric digestive system. One-gram samples (particle size ≤ 1 mm) were weighed and transferred to 250 mL conical flasks. The analysis comprised two steps of enzymatic digestion: the first performed with porcine pepsin (Applichem, Germany) and the second with porcine pancreatin (Applichem)20. The dry matter (IVD) and protein (IVPD) in vitro digestibility (%) of A. platensis F&M-C256 biomass and of “crostini” samples were calculated from the difference between the initial biomass and the undigested biomass (after correction for the blank assay) divided by the initial biomass and multiplied by 100. Casein (Sigma Aldrich Corp., St. Louis, USA) was utilised as the reference material with 100% digestibility.
Sensory analysis
An untrained panel of 35 people, 17 males and 18 females, aged from 18 to 60, evaluated the “crostini” in terms of colour, smell, taste, texture, global appreciation (6 levels from “very pleasant” to “very unpleasant”). The buying intention was also assessed, from “would certainly buy” to “certainly would not buy” (5 levels). The assays were conducted in a standardized sensory analysis room, according to the standard EN ISO 8589 (2010).
Statistical analysis
Statistical analysis of the experimental data was performed using STATISTICA from StatSoft (version 8.0) and using Statgraphics Centurion XV from StatPoint Technologies Inc., through variance analysis (one-way ANOVA), by the Scheffé test – Post Hoc Comparison (significance level of 95%, p < 0.05) and through ANOVA followed by multiple range tests to determine the least significant differences (LSD) (significance level of 95%, p < 0.05). All analyses were conducted at least in triplicate and presented as average ± standard deviation.
Results and Discussion
Fermentation parameters of dough and chemical analysis of “crostini” and A. platensis F&M-C256
Two common parameters used to characterize the sourdough, Total Titratable Acidity (TTA) and organic acids concentrations, were determined also on A. platensis F&M-C256 biomass to evaluate its influence on sourdough characteristics. No data concerning TTA in microalgae biomasses are available in the literature, our results showed a TTA value of 15 mL and lactic acid and acetic acid concentrations of 0.84 g/100 g and 0.42 g/100 g, respectively. Consequently, microalgae addition affected the acidification parameters of doughs and “crostini” as discussed below. Table 3 shows both the values of the principal technological parameters and the cell densities of doughs at the end of the fermentation, and the organic acids and free amino acid (FAA) content in the “crostini”.
Table 3.
Acidification parameters (pH and Total Titratable Acidity, TTA), volume increase (%, ∆V/V0), microorganism concentrations (LAB and yeast) of the doughs at the end of fermentation and organic acids and free amino acid (FAA) content of “crostini”.
| Doughs | “Crostini” | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Final pH | -∆ pH | Final TTA (mL) |
∆ TTA (mL) |
ΔV/V0 x100 | LAB (CFU/g) |
Yeasts (CFU/g) |
Lactic acid (g/100 g) |
Acetic acid (g/100 g) |
FAA g/100 g dry weight |
|
| C | 4.66 ± 0.09a | 0.36 ± 0.05b | 3.13 ± 0.12a | 0.70 ± 0.00a | 196 ± 15b | (2.6 ± 0.21) × 108a | (2.7 ± 0.78) × 107a | 0.27 ± 0.05a | 0.01 ± 0.01a | 0.10 ± 0.02a |
| Ap 2 | 4.85 ± 0.09ab | 0.31 ± 0.11ab | 3.87 ± 0.23b | 1.70 ± 0.21ab | 163 ± 21ab | (2.6 ± 1.22) × 108a | (3.3 ± 1.37) × 107a | 0.29 ± 0.06a | 0.02 ± 0.01a | 0.14 ± 0.03a |
| Ap 6 | 4.90 ± 0.00ab | 0.17 ± 0.04a | 5.45 ± 0.21c | 1.20 ± 0.28ab | 161 ± 25ab | (3.5 ± 0.12) × 108a | (3.9 ± 0.45) × 107a | 0.37 ± 0.19a | 0.05 ± 0.03b | 0.37 ± 0.01b |
| Ap 10 | 5.04 ± 0.06b | 0.17 ± 0.04a | 6.85 ± 0.21d | 1.85 ± 0.78b | 144 ± 15a | (3.3 ± 0.47) × 108a | (3.1 ± 0.95) × 107a | 0.34 ± 0.11a | 0.06 ± 0.02b | 0.45 ± 0.02c |
(C = control without A. platensis F&M-C256 incorporation; Ap 2, Ap 6 and Ap 10 indicate doughs and “crostini” enriched with 2, 6 and 10% (w/w) A. platensis F&M-C256 biomass; ∆: difference between the final and the initial value). Results are expressed as average ± standard deviation (n = 3). Different letters in the same column correspond to significant differences (p < 0.05).
The control displayed the lowest ∆TTA, 0.70 ± 0.00 mL, and the highest ∆pH, 0.36 ± 0.05 at the end of the fermentation process; the opposite trend was observed for Ap 10, that was characterized by the highest ∆TTA and the lowest ∆pH among the samples. In addition to the acidifying effect, A. platensis F&M-C256 acted also as buffering agent, probably due to residual bicarbonates of the growth medium, hence to a higher ∆TTA did not correspond a greater pH decrease. All the samples at least doubled their volume at the end of fermentation time, although the volume increase of Ap 10 was lower compared to the control, indicating that a high percentage of A. platensis F&M-C256 negatively affects the gluten network development of the dough. Microorganism concentrations were not affected by A. platensis F&M-C256 supplementation, and showed final values of about 8 log CFU/g for L. farciminis and 7 log CFU/g for S. cerevisiae consistent with those reported for stable sourdoughs2. Organic acids concentrations, determined in the “crostini” after cooking, were affected by their content in A. platensis F&M-C256 powder rather than by different LAB activity. The amount of lactic acid did not show significant differences among the samples, although a higher lactic acid content was found with the increase of A. platensis F&M-C256 concentrations. For acetic acid concentration, a statistically significant (p < 0.05) increase was observed for higher A. platensis F&M-C256 incorporations (Ap 6 and Ap 10).
FAA content increased with the increasing percentage of A. platensis F&M-C256. The addition of 6% or more of microalgal biomass in the recipe significantly (p < 0.05) increased the amino acid content of “crostini”.
Table 4 presents the proximate chemical composition of “crostini” incorporated with A. platensis F&M-C256 biomass. “Crostini” samples presented 7.4 to 9.3% of moisture, which is in the range of allowed moisture values for this type of dried bakery foods40. For Ap 2 “crostini” there is no significant difference in comparison to the control. At or above 6% A. platensis F&M-C256 biomass addition there is a significant increase in “crostini” moisture content which might be related to a significantly (p < 0.05) higher water absorption capacity of A. platensis F&M-C256 biomass in comparison to wheat flour (approximately + 150%)53. A significant increase (p < 0.05) in ash content was observed for Ap 6 and Ap 10 “crostini” compared to the control (+50% and + 61%, respectively). The higher ash content could be due to the CO32− and HCO3− residual in the cyanobacterial biomass. As expected, a higher amount of proteins corresponds to a higher amount of A. platensis F&M-C256 biomass in the product. A 44% and 69% increase of protein content between A. platensis “crostini” (Ap 6 and Ap 10, respectively) and the control was found. These values were comparable with those found in bread enriched with spirulina38,39 and were coherent with theoretical calculation, as also reported by Batista et al.20 and Bolanho et al.54 on cookies with 2 and 5% A. platensis biomass. As expected, in terms of lipids content, no significant variation (p > 0.05) between A. platensis F&M-C256 “crostini” (16.5–18.2%) and the control (16.6%) was found. Carbohydrate content of A. platensis F&M-C256 “crostini”, in agreement with the cyanobacterial biomass composition, ranged from 57.8 to 59.7% and was not significantly different from the control.
Table 4.
Proximate chemical composition (g/100 g) of control (without A. platensis F&M-C256 incorporation) (C) and of “crostini” enriched with 2% (Ap 2), 6% (Ap 6) and 10% (Ap 10) (w/w) A. platensis F&M-C256 biomass.
| Moisture | Ash | Lipid | Protein | Carbohydrate | Energetic value (kcal/100 g) | |
|---|---|---|---|---|---|---|
| C | 7.7 ± 2.0a | 1.8 ± 0.3a | 16.6 ± 0.7a | 9.9 ± 2.2a | 61.0 ± 6.7b | 433.2 |
| Ap 2 | 7.4 ± 0.4a | 1.9 ± 0.04a | 16.5 ± 0.8a | 10.4 ± 2.4a | 59.7 ± 4.0a,b | 429.0 |
| Ap 6 | 9.1 ± 1.0b | 2.7 ± 0.1b | 18.2 ± 0.9a | 14.3 ± 2.2b | 57.8 ± 3.8a,b | 450.5 |
| Ap 10 | 9.3 ± 0.4b | 2.9 ± 0.6b | 16.8 ± 0.8a | 16.7 ± 1.9c | 57.8 ± 2.4a,b | 449.0 |
Results are expressed as average ± standard deviation (n = 9). Different letters in the same column correspond to significant differences (p < 0.05).
Therefore, the newly developed “spirulina crostini” could be regarded as a very interesting protein-fortified bakery product, reaching values as high as 14–17% protein. In fact, for Ap 6 and Ap 10 “crostini”, the protein content corresponds to 12.7 and 14.9%, respectively, of the total energy content of the sample. Therefore, these products can be claimed as “source of protein” considering the European Commission Regulation on nutritional claims which states that “a claim that a food is a source of protein may only be made where at least 12% of the energy value of the food is provided by protein”55.
In terms of energetic content, the values ranged from 429 to 450 kcal/100 g.
“Crostini” bioactive compounds and antioxidant capacity
The presence of bioactive compounds in Arthrospira platensis biomass is associated with its antioxidant potential, immunomodulatory, anti-inflammatory and other activities56. Among the main bioactives, A. platensis contains phycocyanin, a blue proteic pigment composed of an open chain tetrapyrrole chromophore (phycocyanobilin) covalently attached to an apoprotein, that presents nutraceutical properties and health benefits, principally attributed to its antioxidant activity57,58.
Figure 1 shows the linear regression between phycocyanin content of “crostini” after cooking and level of incorporation of A. platensis F&M-C256 biomass. The control “crostini” did not contain phycocyanin. As expected, an increase in the A. platensis F&M-C256 biomass content within the “crostini” corresponded to an increase in phycocyanin content. Ap 2, Ap 6 and Ap 10 “crostini” contained 0.1%, 0.5% and 0.9% phycocyanin, respectively. Thus, surpisingly, about all the pigment from the microalgal biomass (which contains 8.2% phycocyanin w/w; results not shown) was still present in the cooked “crostini”. Aliquots of A. platensis F&M-C256 biomass (both lyophilised and moistened with 30% water), were heated to simulate the “crostini” baking conditions. The test highlighted an important phycocyanin loss that increased with temperature and exposure time (results not shown). Since phycocyanin reduction during “crostini” baking was much lower compared to that of biomass alone, we hypothesize that some dough components might act as protecting agents against phycocyanin degradation. Further investigation to fully clarify this protective action of dough is necessary.
Figure 1.
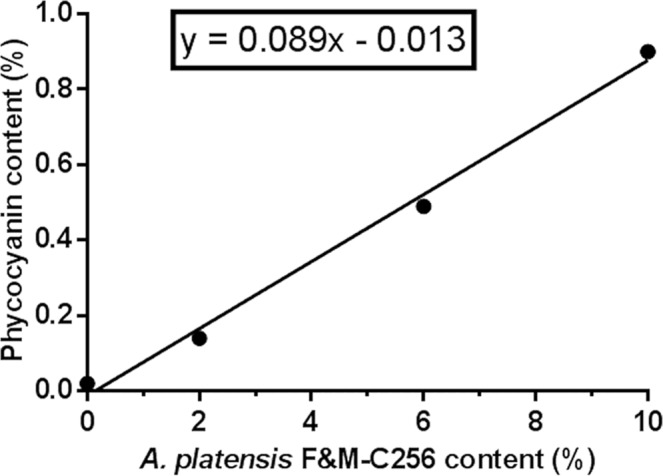
Linear regression between phycocyanin content (%, w/w) of control (without A. platensis F&M-C256 incorporation) (0%) and of “crostini” enriched with 2%, 6% and 10% (w/w) A. platensis F&M-C256 biomass. Results are expressed as average ± standard deviation (n = 9).
Phenolic compounds, synthesized as secondary metabolites by plants, but also by cyanobacteria, are considered one of the most important classes of natural antioxidants59. Phenolics, that include simple phenols, flavonoids, phenylpropanoids, tannins, lignins, phenolic acids, and their derivatives59, are receiving increasing interest from food manufacturers and consumers for their health benefits60. Figure 2 shows the total phenolic content of “crostini”. A value of 2.3 mg GAE g−1 was found in the control “crostini”, very likely supplied by the extra-virgin olive oil used in the dough preparation61. The addition of A. platensis F&M-C256 biomass, with a total phenolic content of 10 mg GAE g−1 ± 0.1, resulted in a significant (p < 0.05) supplementation of phenolic compounds to the “crostini”. Ap 10 “crostini” presented the highest phenolic content (3.4 mg GAE g−1). Other studies reported a higher total phenolic content of A. platensis biomass62, as well as a correlation with its antioxidant activity20. Bolanho et al.54 reported a total phenolic content for A. platensis biomass of 12 mg GAE g−1, similar to that found in the present study. They also showed an increase of about 65% (from 1.4 to 2.3 mg GAE g−1) in total phenolic content in 5% A. platensis cookies when compared to the control, which is higher than the increase found in this study for Ap 6 “crostini” (26%).
Figure 2.
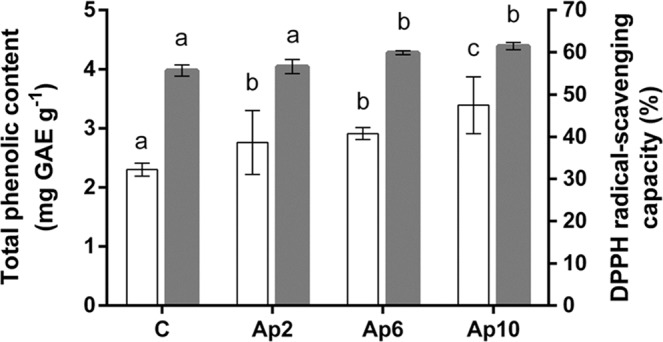
Total phenolic content (expressed as gallic acid equivalents mg g−1 dry weight) (white columns) and radical-scavenging capacity (%) (grey columns) of control (without A. platensis F&M-C256 incorporation) (C) and of “crostini” enriched with 2% (Ap2), 6% (Ap6) and 10% (Ap10) (w/w) A. platensis F&M-C256 biomass. Results are expressed as average ± standard deviation (n = 9). Different letters correspond to significant differences (p < 0.05).
The radical-scavenging capacity of A. platensis F&M-C256-enriched “crostini” was tested by the DPPH method. The incorporation of A. platensis F&M-C256 biomass led to a significant (p < 0.05) increase in the radical-scavenging capacity for 6 and 10% A. platensis F&M-C256 “crostini” compared to the control (Fig. 2). Overall, DPPH radical scavenging capacity ranged from 57% to 61%, corresponding to a VCEAC of 0.60 to 0.64 µg per mg of “crostini” (from Ap 2 to Ap 10 “crostini”, respectively) (Fig. 2). The control showed a radical scavenging capacity of about 55%, indicating that ingredients in the sourdough preparation already confer a strong antioxidant capacity, that is only slightly increased by A. platensis F&M-C256 biomass addition. The results of this work are in agreement with the study conducted by Siriwardhana et al.63 which reported a high correlation between DPPH radical scavenging activities and total phenolic content. Other authors64,65 have studied the antioxidant capacity of A. platensis enriched bakery products (principally cookies), attributing this property to the phycobiliproteins provided by this microorganism. El Baky et al.64 observed increasing antioxidant activity for biscuits containing A. platensis biomass (from 0.3 to 0.9%). Singh et al.65 found a linear positive correlation between A. platensis concentration (from 1.6 to 8.4%) in biscuits and antioxidant activity. Our results are in agreement with the findings of El Baky et al.64 and Singh et al.65, considering that the antioxidant capacity increases with the increasing levels of A. platensis F&M-C256 in “crostini”.
The extra-virgin olive oil used in the dough preparation presumably contributed to the high antioxidant capacity of control “crostini”. The addition of A. platensis F&M-C256 biomass, replacing wheat flour in the doughs, weakly increased the radical-scavenging capacity, with a more moderate effect than observed for phenols. After baking, all A. platensis F&M-C256 “crostini” still presented a high content of phycocyanin, possibly responsible, together with phenolics, for the observed antioxidant capacity.
“Crostini” in vitro digestibility
The in vitro digestibility analysis reproduces the chemical-enzymatic attack that occurs in the proximal tract of the monogastric digestive system through a double enzymatic incubation with pepsin and pancreatin52. Most of the literature on algae digestibility focuses on seaweeds66–68 and only few studies deal with microalgae69,70. This is the first work, to our knowledge, on in vitro digestibility of cyanobacteria-enriched “crostini”.
The in vitro dry matter (IVD) and protein digestibility (IVPD) results are presented in Fig. 3. A. platensis F&M-C256 “crostini” showed lower IVD values than the control, but still above 85%. The reason could be that A. platensis F&M-C256 biomass is not completely digestible (78% ± 2.6). The dry matter digestibility of Ap 2 and Ap 6 “crostini” (88.4% and 86.8%, respectively) (Fig. 3) was slightly lower compared to the values found for 2 and 6% A. platensis F&M-C256-based cookies (94.6% and 92.1%, respectively) by Batista et al.20, but still within the IVD range of commercial wheat crackers (78.3–93%)71.
Figure 3.
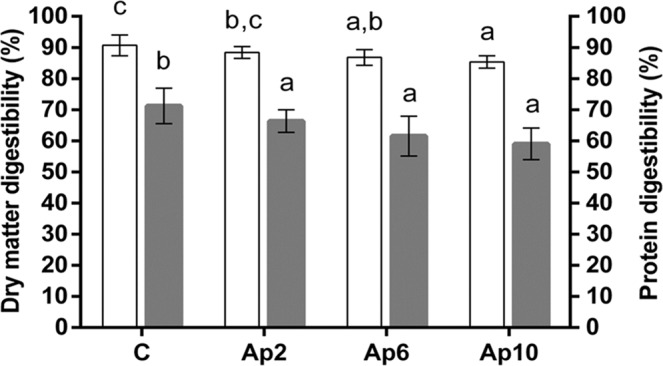
In vitro dry matter digestibility (IVD, %) (white columns) and in vitro protein digestibility (IVPD, %) (grey columns) of control (without A. platensis F&M-C256 incorporation) (C) and of “crostini” enriched with 2% (Ap2), 6% (Ap6) and 10% (Ap10) (w/w) A. platensis F&M-C256 biomass. Results are expressed as average ± standard deviation (n = 9). Different letters correspond to significant differences (p < 0.05).
IVPD values reflect those of dry matter digestibility (Fig. 3). Protein digestibility is an important factor to estimate the availability of protein for intestinal absorption, which influences the efficiency of protein utilization72. Many authors reported very diverse protein digestibility values (from 70 to 84%) for different species of Arthrospira73–75. In our study, an IVPD value of 81% ± 3.0 for A. platensis F&M-C256 biomass was found. A. platensis F&M-C256 “crostini” showed significantly (p < 0.05) lower IVPD values than the control (Fig. 3). This is in accordance with De Marco et al.21, which evaluated the protein digestibility of A. platensis enriched dried pasta (from 5 to 20%). The IVPD reduction at the increasing percentage of A. platensis F&M-C256 biomass addition could be due to the content of algae minor compounds (i.e. phenols or polysaccharides) that may react with gluten, forming insoluble complexes and inhibiting the activity of pepsin and pancreatin enzymes76,77. However, it should be noted that the control “crostini” has 9.9% total protein (Table 4), with 71% IVPD, which should result in approximately 7 g digestible protein/100 g. On the other hand, the Ap 10 “crostini” has a much higher protein content (16.7%, Table 4), so even with a lower IVPD (59%), there is still a higher digestible protein content, of approximately 10 g/100 g.
“Crostini” colour and appearance
A. platensis F&M-C256 “crostini” presented unusual and innovative tonalities. The different samples showed very distinct colours, as evident by visual observation (Fig. 4) and through the instrumental colour parameters analysis represented in Table 5.
Figure 4.
Control “crostini” (without A. platensis F&M-C256 incorporation) (C) and “crostini” enriched with 2% (Ap 2), 6% (Ap 6) and 10% (Ap 10) (w/w) A. platensis F&M-C256 biomass.
Table 5.
Instrumental color parameters of control (without A. platensis F&M-C256 incorporation) (C) and of “crostini” enriched with 2% (Ap 2), 6% (Ap 6) and 10% (Ap 10) (w/w) A. platensis F&M-C256 biomass.
| L* | a* | b* | C* | h° | |
|---|---|---|---|---|---|
| C | 69.0 ± 1.0d | −1.1 ± 0.1d | 16.8 ± 0.7d | 16.8 ± 0.7d | 93.8 ± 0.2c |
| Ap 2 | 35.9 ± 0.4c | 0.7 ± 0.2c | 12.5 ± 0.6c | 12.5 ± 0.6c | 86.6 ± 0.9b |
| Ap 6 | 28.9 ± 0.8b | 0.4 ± 0.1b | 3.7 ± 0.7b | 3.7 ± 0.7b | 83.7 ± 1.9b |
| Ap 10 | 26.7 ± 0.6a | 0.3 ± 0.1a | 1.2 ± 0.4a | 1.2 ± 0.4a | 76.7 ± 4.3a |
Results are expressed as average ± standard deviation (n = 12). Different letters in the same column correspond to significant differences (p < 0.05).
Regarding luminosity (L*), there was a drastic significant (p < 0.05) decrease from the light control sample (69%) to the very dark A. platensis F&M-C256 “crostini” samples (36 to 27%). These results are in agreement with previous studies, namely A. platensis F&M-C256 cookies20 and pasta78. In terms of chromaticity (C*), which results from the combination of a* and b* coordinates, it is clear that b* (blue/yellow intensity) parameter is dominant over a* (red/green intensity). In fact, a* values are very close to zero (≤1), which means that green/red colour intensity is very low. On the other hand, the control sample has a b* value of 17, i.e. on the yellow range. By increasing A. platensis F&M-C256 concentration, a significant decrease in b* is observed, reaching values close to 1 for the Ap 10 “crostini”. Batista et al.20 observed a similar behaviour for A. platensis F&M-C256 cookies, although with much higher a* and b* values and constant hue angle for cookies with 2% and 6% A. platensis F&M-C256 biomass incorporation (h = 117°). In the present study, the hue angle (h°) significantly (p < 0.05) decreases with A. platensis F&M-C256 concentration. The control sample presents an h° of 94°, which is very close to a pure yellow tonality (90°) while Ap 2 and Ap 6 “crostini” show lower values around 84–87° and Ap 10 “crostini” goes down to 77°. From these results, it seems that A. platensis F&M-C256 incorporation is not contributing to a vivid coloration but mainly to a darkening effect. Probably A. platensis F&M-C256 pigments, namely green chlorophylls, are highly degraded during the “crostini” high temperature baking and toasting procedures. As will be detailed below (section 3.5) these colour characteristics of the A. platensis “crostini” are not well appreciated by the sensory panelists.
The “crostini” characteristic dimensions were also measured (using a digital caliper) and it was observed that the surface area of the “crostini” decreased linearly (R2 = 0.96) when increasing A. platensis F&M-C256 content (results not shown). In fact, the control “crostini” had a surface area of 10.0 ± 1.6 cm2, while, Ap 2, Ap 6 and Ap 10 “crostini” showed surface areas of 8.0 ± 1.4 cm2, 5.4 ± 1.1 cm2 and 3.9 ± 0.7 cm2. This is in agreement with the lower volume increase observed for A. platensis F&M-C256 doughs (Table 3). Graça et al.22 have also reported lower dough volume expansion and lower surface areas on bread enriched with Chlorella vulgaris.
“Crostini” sensory evaluation
Sensory analysis assays were carried out with A. platensis F&M-C256 “crostini”, at 2, 6 and 10% incorporation level. Figure 5 represents the average scores of the sensorial parameters as evaluated by the panel. From the graph it is clear that the less appreciated samples were Ap 6 and Ap 10 “crostini” (average score 2.5 for global appreciation, “slightly unpleasant”) The global sensory appreciation of Ap 2 “crostini” was considered “pleasant” (average score 3.9), similar to the control sample (average score 3.7). In fact, no significant differences (p > 0.05) were found between Ap 2 and control “crostini”, regarding taste, smell or texture attributes, while colour was significantly (p < 0.05) less appreciated (average scores 2.6 and 4.0, respectively). Many authors reported the results of sensory analyses of microalgae-based food products such as bread38,39,79,80, pancakes81, croissants82, cookies20,54,64,65 and pasta83,84 finding that these products reported different appreciation in terms of sensorial acceptance. Bolanho et al.54 found that the addition of A. platensis biomass (2 and 5%) in the cookies decreased the sensorial acceptance when compared to the control cookie. Singh et al.65 also reported that the addition of A. platensis (>7% incorporation level) to cookies prepared from sorghum and whole wheat flour negatively affected the textural and sensory attributes of flavour. On the contrary, El-Baky et al.64 found that cookies supplemented with different levels of A. platensis biomass (0.3, 0.6 and 0.9% incorporation level) were significantly acceptable for colour, odour, flavour, texture and global appreciation.
Figure 5.
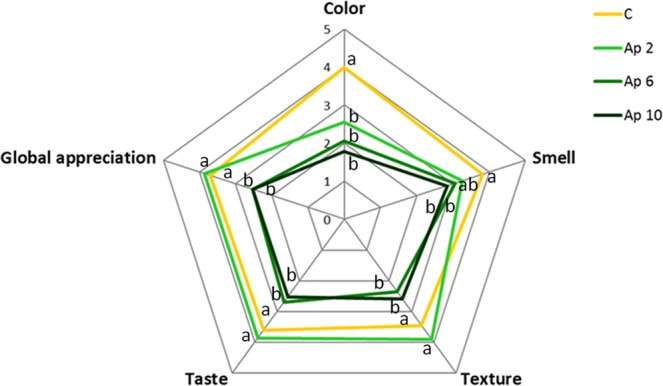
Responses of the sensory analysis panel tasters (n = 35) of control (without A. platensis F&M-C256 incorporation) (C) and of “crostini” enriched with 2% (Ap 2), 6% (Ap 6) and 10% (Ap 10) (w/w) A. platensis F&M-C256 biomass. 0 – “very unpleasant”; 1 – “unpleasant”; 2 – “slightly unpleasant”; 3 – “slightly pleasant”; 4 – “pleasant”; 5 – “very pleasant”. Different letters correspond to significant differences (p < 0.05).
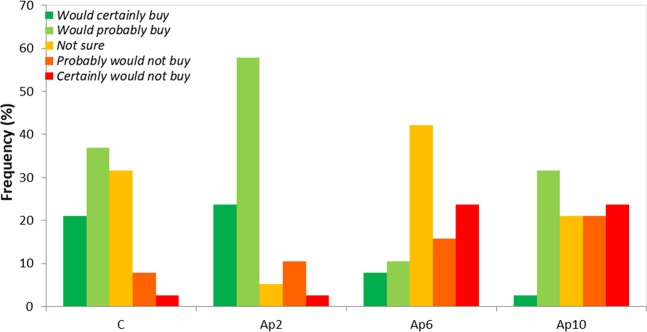
Figure 6 presents the answers given by the panelists in relation to the buying intention. Around 60% of the tasters “would probably buy” and 22% “would certainly buy” the Ap 2 “crostini”, the most appreciated product. While, Ap 6 and Ap 10 “crostini” presented a high variability in terms of buying intention. Around 35% of panel tasters would probably buy the Ap 10 “crostini” and less than 15% gave the same response for Ap 6 “crostini”. Maybe Ap 6 “crostini” did not show any distinctive character neither in terms of strong taste nor in terms of delicate taste, as confirmed by insecure tasters (“not sure”, 42%). Around 25% “certainly would not buy” the Ap 6 and Ap 10 “crostini”, principally due to taste and texture (Fig. 6).
Figure 6.
Responses of the sensory analysis panel tasters (n = 35) of control (without A. platensis F&M-C256 incorporation) (C) and of “crostini” enriched with 2% (Ap2), 6% (Ap6) and 10% (Ap10) (w/w) A. platensis F&M-C256 biomass intention of buying.
Conclusion
The combination of Arthrospira platensis (spirulina) biomass addition and the sourdough technology led to the development of a novel microalgae-based bakery product. The addition of Arthrospira platensis F&M-C256 biomass as ingredient resulted in sourdough “crostini” with an innovative green colour. Increasing microalgae content from 2% to 10% (w/w) resulted in a significant (p < 0.05) increase in the “crostini” protein, phycocyanin and total phenolic content. Considering the European Commission Regulation on nutritional claims, “crostini” incorporated with 6% and 10% A. platensis F&M-C256 can be claimed as “source of protein”. A lower value of in vitro dry matter and protein digestibility between A. platensis F&M-C256 “crostini” and the control was found, although IVD values are always above 85%. Two percent A. platensis F&M-C256 “crostini” presented the highest sensory scores and resulted the most appreciated product, even more than the control without cyanobacterial biomass. To improve the acceptance by consumers of 6% and 10% A. platensis F&M-C256 “crostini”, educational marketing strategies and formulation enhancements should be considered.
This study suggests that A. platensis F&M-C256-based sourdough “crostini” represent original functional food products, with antioxidant properties (also due to the extra virgin olive oil utilization) and containing alternative source of digestible proteins. Moreover, the use of sourdough fermentation in producing A. platensis F&M-C256 “crostini” can provide an additional health value considering the enhancing of nutritional properties associated to this biotechnology process. This new product could become widely consumed by particular categories of people such as sportsmen, vegetarians, vegans and the elderly, but also by consumers interested in a product with an innovative taste and colour.
Acknowledgements
The authors thank the REGIONE TOSCANA (PAR FAS 2007–2013 projects) for financial support to Centro Competenza VALORE (Florence, Italy) where part of the biochemical analyses have been carried out. The authors also wish to thank Patricia Fradinho for technical assistance in sensory analyses and all the panelists of the FCT Research unit UID/AGR/04129/2013 (LEAF).
Author contributions
A.N., M.V., V.G., N.P., L.G., M.R.T.: study conception and design; M.V., V.G., N.P., A.N.: “crostini” preparation and analyses; M.D.: “crostini” biochemical composition; A.N.: “crostini” phycocyanin, phenolics, antioxidant and in vitro digestibility determination, figure 4 photograph creator; A.P.B., A.R.: “crostini” colour determination and sensory analyses; all the authors participated in data analyses, discussion of results and revising the manuscript.
Competing interests
M.R. Tredici and L. Rodolfi have financial interest in F&M S.r.l. M. Venturi has financial interest in FoodMicroTeam S.r.l. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Foschino R, Gallina S, Andrighetto C, Rossetti L, Galli A. Comparison of cultural methods for the identification and molecular investigation of yeasts from sourdoughs for Italian sweet baked products. FEMS Yeast Res. 2004;4:609–618. doi: 10.1016/j.femsyr.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Lattanzi A, et al. The lactic acid bacteria and yeast microbiota of eighteen sourdoughs used for the manufacture of traditional Italian sweet leavened baked goods. Int. J. Food Microbiol. 2013;163:71–79. doi: 10.1016/j.ijfoodmicro.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Minervini F, et al. Lactic acid bacterium and yeast microbiotas of 19 sourdoughs used for traditional/typical Italian breads: interactions between ingredients and microbial species diversity. Appl. Environ. Microb. 2012;78:1251–1264. doi: 10.1128/AEM.07721-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venturi M, Guerrini S, Vincenzini M. Stable and non-competitive association of Saccharomyces cerevisiae, Candida milleri and Lactobacillus sanfranciscensis during manufacture of two traditional sourdough baked goods. Food Microbiol. 2012;31:107–115. doi: 10.1016/j.fm.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 5.De Vuyst L, Neysens P. The sourdough microflora: biodiversity and metabolic interactions. Trends Food Sci. Technol. 2005;16:43–56. doi: 10.1016/j.tifs.2004.02.012. [DOI] [Google Scholar]
- 6.Corsetti A, Settanni L. Lactobacilli in sourdough fermentation. Food Res. Int. 2007;40:539–558. doi: 10.1016/j.foodres.2006.11.001. [DOI] [Google Scholar]
- 7.Van Kerrebroeck S, Maes D, De Vuyst L. Sourdoughs as a function of their species diversity and process condtomasitions, a meta-analysis. Trends Food Sci. Technol. 2017;68:152–159. doi: 10.1016/j.tifs.2017.08.016. [DOI] [Google Scholar]
- 8.Chavan RS, Chavan SR. Sourdough technology—a traditional way for wholesome foods: a review. Compr. Rev. Food Sci. F. 2011;10:169–182. doi: 10.1111/j.1541-4337.2011.00148.x. [DOI] [Google Scholar]
- 9.De Angelis M, et al. Phytase activity in sourdough lactic acid bacteria: purification and characterization of a phytase from Lactobacillus sanfranciscensis CB1. Int. J. Food Microbiol. 2003;87:259–270. doi: 10.1016/S0168-1605(03)00072-2. [DOI] [PubMed] [Google Scholar]
- 10.De Angelis M, et al. Sourdough fermentation as a tool for the manufacture of low-glycemic index white wheat bread enriched in dietary fibre. Eur. Food Res. Technol. 2009;229:593–601. doi: 10.1007/s00217-009-1085-1. [DOI] [Google Scholar]
- 11.Gänzle MG, Loponen J, Gobbetti M. Proteolysis in sourdough fermentations: mechanisms and potential for improved bread quality. Trends Food Sci. Technol. 2008;19:513–521. doi: 10.1016/j.tifs.2008.04.002. [DOI] [Google Scholar]
- 12.Gobbetti M, Rizzello CG, Di Cagno R, De Angelis M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014;7:30–40. doi: 10.1016/j.fm.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Gobbetti M, et al. How to improve the gluten-free diet: The state of the art from a food science perspective. Food Res. Int. 2018;110:22–32. doi: 10.1016/j.foodres.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Coda R, Rizzello CG, Gobbetti M. Use of sourdough fermentation and pseudo-cereals and leguminous flours for the making of a functional bread enriched of γ-aminobutyric acid (GABA) Int. J. Food Microbial. 2010;137:236–245. doi: 10.1016/j.ijfoodmicro.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Coda R, Rizzello CG, Pinto D, Gobbetti M. Selected lactic acid bacteria synthesize antioxidant peptides during sourdough fermentation of cereal flours. Appl. Env. Microbiol. 2012;78:1087–1096. doi: 10.1128/AEM.06837-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ortiz-Martinez M, Winkler R, Garcia-Lara S. Preventive and therapeutic potential of peptides from cereals against cancer. J. Proteomics. 2014;111:165–183. doi: 10.1016/j.jprot.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 17.Rizzello CG, et al. Bioactive peptides from vegetable food matrices: Research trends and novel biotechnologies for synthesis and recovery. J. Funct. Food. 2016;27:549–569. doi: 10.1016/j.jff.2016.09.023. [DOI] [Google Scholar]
- 18.Caggianiello G, Kleerebezem M, Spano G. Exopolysaccharides produced by lactic acid bacteria: from health-promoting benefits to stress tolerance mechanisms. Appl. Microbiol. Biot. 2016;100:3877–3886. doi: 10.1007/s00253-016-7471-2. [DOI] [PubMed] [Google Scholar]
- 19.Zannini E, Waters DM, Coffey A, Arendt EK. Production, properties, and industrial food application of lactic acid bacteria-derived exopolysaccharides. Appl. Microbiol. Biot. 2016;100:1121–1135. doi: 10.1007/s00253-015-7172-2. [DOI] [PubMed] [Google Scholar]
- 20.Batista AP, et al. Microalgae biomass as an alternative ingredient in cookies: sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017;26:161–171. doi: 10.1016/j.algal.2017.07.017. [DOI] [Google Scholar]
- 21.De Marco ER, Steffolani ME, Martínez CS, León AE. Effects of spirulina biomass on the technological and nutritional quality of bread wheat pasta. LWT-Food Sci. Technol. 2014;58:102–108. doi: 10.1016/j.lwt.2014.02.054. [DOI] [Google Scholar]
- 22.Graça C, Fradinho P, Sousa I, Raymundo A. Impact of Chlorella vulgaris on the rheology of wheat flour dough and bread texture. LWT-Food Sci. Technol. 2018;89:466–474. doi: 10.1016/j.lwt.2017.11.024. [DOI] [Google Scholar]
- 23.Rizzello CG, Cassone A, Coda R, Gobbetti M. Antifungal activity of sourdough fermented wheat germ used as an ingredient for bread making. Food Chem. 2011;127:952–959. doi: 10.1016/j.foodchem.2011.01.063. [DOI] [PubMed] [Google Scholar]
- 24.Ragaee S, Guzar I, Dhull N, Seetharaman K. Effects of fiber addition on antioxidant capacity and nutritional quality of wheat bread. LWT-Food Sci. Technol. 2011;44:2147–2153. doi: 10.1016/j.lwt.2011.06.016. [DOI] [Google Scholar]
- 25.Di Cagno R, Coda R, De Angelis M, Gobbetti M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013;33:1–10. doi: 10.1016/j.fm.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Tomaselli, L., Palandri, M. R. & Tredici, M. R. On the correct use of the Spirulina designation. Algological Studies/Archiv für Hydrobiologie, Supplement Volumes, 539–548 (1996).
- 27.Plaza M, Herrero M, Cifuentes A, Ibanez E. Innovative natural functional ingredients from microalgae. J. Agr. Food Chem. 2009;57:7159–7170. doi: 10.1021/jf901070g. [DOI] [PubMed] [Google Scholar]
- 28.Kulshreshtha A, Jarouliya U, Bhadauriya P, Prasad GBKS, Bisen PS. Spirulina in health care management. Curr. Pharm. Biotechno. 2008;9:400–405. doi: 10.2174/138920108785915111. [DOI] [PubMed] [Google Scholar]
- 29.Mao TK, Van de Water J, Gershwin ME. Effect of Spirulina on the secretion of cytokines from peripheral blood mononuclear cells. J. Med. Food. 2000;3:135–140. doi: 10.1089/jmf.2000.3.135. [DOI] [PubMed] [Google Scholar]
- 30.Gutiérrez-Salmean G, Fabila-Castillo L, Chamorro-Cevallos G. Nutritional and toxicological aspects of Spirulina (Arthrospira) Nutr. Hosp. 2015;32:34–40. doi: 10.3305/nh.2015.32.1.9001. [DOI] [PubMed] [Google Scholar]
- 31.Bigagli E, et al. Safety evaluations and lipid-lowering activity of an Arthrospira platensis enriched diet: A 1-month study in rats. Food Res. Int. 2017;102:380–386. doi: 10.1016/j.foodres.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Niccolai A, et al. In vitro toxicity of microalgal and cyanobacterial strains of interest as food source. J. Appl. Phycol. 2017;29:199–209. doi: 10.1007/s10811-016-0924-2. [DOI] [Google Scholar]
- 33.European Union, Novel Food catalogue, http://ec.europa.eu/food/safety/novel_food/catalogue/search/public/index.cfm.
- 34.Abdulqader G, Barsanti L, Tredici MR. Harvest of Arthrospira platensis from Lake Kossorom (Chad) and its household usage among the Kanembu. J. Appl. Phycol. 2000;12:493–498. doi: 10.1023/A:1008177925799. [DOI] [Google Scholar]
- 35.Grobbelaar JU. From laboratory to commercial production: a case study of a Spirulina (Arthrospira) facility in Musina, South Africa. J. Appl. Phycol. 2009;21:523–527. doi: 10.1007/s10811-008-9378-5. [DOI] [Google Scholar]
- 36.Marles RJ, et al. United States pharmacopeia safety evaluation of Spirulina. Crit. Rev. Food Sci. Nutr. 2011;51:593–604. doi: 10.1080/10408391003721719. [DOI] [PubMed] [Google Scholar]
- 37.Credence Research, Algae Products Market by Application (Nutraceuticals, Food & Feed Supplements, Pharmaceuticals, Paints & Colorants, Pollution Control, Others) - Growth, Future Prospects, Competitive Analysis, and Forecast 2016–2023, http://www.credenceresearch.com/report/algae-products-market (2016).
- 38.Ak B, et al. Nutritional and physicochemical characteristics of bread enriched with microalgae Spirulina platensis. Int. J. Eng. Res. Appl. 2016;6:30–38. [Google Scholar]
- 39.Saharan V, Jood S. Vitamins, minerals, protein digestibility and antioxidant activity of bread enriched with spirulina platensis powder. J. Agr. Sci. 2017;7:1292–1297. [Google Scholar]
- 40.D.P.R. 23 June 1993, n. 28, Regolamento relativo alle denominazioni legali di alcuni prodotti da forno.
- 41.Zarrouk, C. Contribution à l’étude d’une cyanophycée, Influence de divers facteurs physiques et chimiques sur la croissance et la photosynthèse de Spirulina maxima (Setch et Gardner) Geitler. Ph.D. thesis, Université de Paris, Paris (1966).
- 42.Tredici MR, Rodolfi L, Biondi N, Bassi N, Sampietro G. Techno-economic analysis of microalgal biomass production in a 1-ha Green Wall Panel (GWP®) plant. Algal Res. 2016;19:253–263. doi: 10.1016/j.algal.2016.09.005. [DOI] [Google Scholar]
- 43.Abiusi F, et al. Growth, photosynthetic efficiency, and biochemical composition of Tetraselmis suecica F&M‐M33 grown with LEDs of different colors. Biotechnol. Bioeng. 2014;111:956–964. doi: 10.1002/bit.25014. [DOI] [PubMed] [Google Scholar]
- 44.ISTISAN (Istituto Superiore di Sanità) ISSN 1123-3117 Rapporti ISTISAN 96/34, Metodi di analisi utilizzati per il controllo chimico degli alimenti, 265 (1996).
- 45.Galli V, et al. Liquid and firm sourdough fermentation: microbial robustness and interactions during consecutive backsloppings. LWT-Food Sci. Technol. 2019;105:9–15. doi: 10.1016/j.lwt.2019.02.004. [DOI] [Google Scholar]
- 46.Zannini E, et al. Microbiological and technological characterization of sourdoughs destined for bread-making with barley flour. Food Microbiol. 2009;26:744–753. doi: 10.1016/j.fm.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 47.Galli, V., Venturi, M., Pini, N., Guerrini, S. & Granchi, L. Exploitation of sourdough lactic acid bacteria to reduce raffinose family oligosaccharides (RFOs) content in breads enriched with chickpea flour. Eur. Food Res. Technol. 1–11 (2019).
- 48.Tuberoso CIG, Congiu F, Serreli G, Mameli S. Determination of dansylated amino acids and biogenic amines in Cannonau and Vermentino wines by HPLC-FLD. Food Chem. 2015;175:29–35. doi: 10.1016/j.foodchem.2014.11.120. [DOI] [PubMed] [Google Scholar]
- 49.European Union. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers. Official Journal of the European Union L 304/18, 22.11.2011 (2011).
- 50.Herrera A, Boussiba S, Napoleone V, Hohlberg A. Recovery of c-phycocyanin from the cyanobacterium Spirulina maxima. J. Appl. Phycol. 1989;1:325–331. doi: 10.1007/BF00003469. [DOI] [Google Scholar]
- 51.Rajauria G, Jaiswal AK, Abu-Ghannam N, Gupta S. Antimicrobial, antioxidant and free radical‐scavenging capacity of brown seaweed Himanthalia elongata from western coast of Ireland. J. Food Biochem. 2013;37:322–335. doi: 10.1111/j.1745-4514.2012.00663.x. [DOI] [Google Scholar]
- 52.Boisen S, Fernández JA. Prediction of the total tract digestibility of energy in feedstuffs and pig diets by in vitro analyses. Anim. Feed Sci. Tech. 1997;68:277–286. doi: 10.1016/S0377-8401(97)00058-8. [DOI] [Google Scholar]
- 53.Batista, A. P. et al. Rheological characterization of Spirulina gluten-free cookie doughs in The Multidisciplinary Science of Rheology – towards a healthy and sustainable development (eds Hernandez, M. J., Sanz, T., Salvador, A., Rubio-Hernandez, F.J., Steinbrüggen, R.) 20–23 (ISBN: 978-84-697-5123-7) (IBERIO, 2017).
- 54.Bolanho BC, et al. Antioxidant and nutritional potential of cookies enriched with Spirulina platensis and sources of fibre. J. Food Nutr. Res. 2014;53:171–179. [Google Scholar]
- 55.Reg (EC) N. 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutritions and health claims made on foods (OJ L 404, 30.12.2006, p, 9).
- 56.Wu Q, et al. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: an overview. Arch. Toxicol. 2016;90:1817–1840. doi: 10.1007/s00204-016-1744-5. [DOI] [PubMed] [Google Scholar]
- 57.Liu, Q., Huang, Y., Zhang, R., Cai, T., & Cai, Y. Medical application of Spirulina platensis derived C-phycocyanin. Evid.-Based Compl. Alt. (2016). [DOI] [PMC free article] [PubMed]
- 58.Bhat VB, Madyastha KM. C-phycocyanin: a potent peroxyl radical scavenger in vivo and in vitro. Biochem. Biophys. Res. Comm. 2000;275:20–25. doi: 10.1006/bbrc.2000.3270. [DOI] [PubMed] [Google Scholar]
- 59.El-Baky, H. A., El Baz, F. K. & El-Baroty, G. S. Production of phenolic compounds from Spirulina maxima microalgae and its protective effects. Afr. J. Biotechnol. 8 (2009).
- 60.Machu L, et al. Phenolic content and antioxidant capacity in algal food products. Molecules. 2015;20:1118–1133. doi: 10.3390/molecules20011118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luceri C, Bigagli E, Pitozzi V, Giovannelli L. A nutrigenomics approach for the study of anti-aging interventions: olive oil phenols and the modulation of gene and microRNA expression profiles in mouse brain. European journal of nutrition. 2017;56:865–877. doi: 10.1007/s00394-015-1134-4. [DOI] [PubMed] [Google Scholar]
- 62.Kepekçi RA, Saygideger SD. Enhancement of phenolic compound production in Spirulina platensis by two-step batch mode cultivation. J. Appl. Phycol. 2012;24:897–905. doi: 10.1007/s10811-011-9710-3. [DOI] [Google Scholar]
- 63.Siriwardhana N, Lee KW, Jeon YJ, Kim SH, Haw JW. Antioxidant activity of Hizikia fusiformis on reactive oxygen species scavenging and lipid peroxidation inhibition. Food Sci. Technol. Int. 2003;9:339–346. doi: 10.1177/1082013203039014. [DOI] [Google Scholar]
- 64.El Baky HHA, El Baroty GS, Ibrahem EA. Functional characters evaluation of biscuits sublimated with pure phycocyanin isolated from Spirulina and Spirulina biomass. Nutr. Hosp. 2015;32:231–241. doi: 10.3305/nh.2015.32.1.8804. [DOI] [PubMed] [Google Scholar]
- 65.Singh P, Singh R, Jha A, Rasane P, Gautam AK. Optimization of a process for high fibre and high protein biscuit. J. Food Sci. Technol. 2015;52:1394–1403. doi: 10.1007/s13197-013-1139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fleurence J. Seaweed proteins: biochemical, nutritional aspects and potential uses. Trends Food Sci. Tech. 1999;10:25–28. doi: 10.1016/S0924-2244(99)00015-1. [DOI] [Google Scholar]
- 67.Paiva L, Lima E, Patarra RF, Neto AI, Baptista J. Edible Azorean macroalgae as source of rich nutrients with impact on human health. Food Chem. 2014;164:128–135. doi: 10.1016/j.foodchem.2014.04.119. [DOI] [PubMed] [Google Scholar]
- 68.Tibbetts SM, Milley JE, Lall SP. Nutritional quality of some wild and cultivated seaweeds: Nutrient composition, total phenolic content and in vitro digestibility. J. Appl. Phycol. 2016;28:3575–3585. doi: 10.1007/s10811-016-0863-y. [DOI] [Google Scholar]
- 69.Machů L, Mišurcová L, Samek D, Hrabě J, Fišera M. In vitro digestibility of different commercial edible algae products. J. Aquat. Food Prod. T. 2014;23:423–435. doi: 10.1080/10498850.2012.721873. [DOI] [Google Scholar]
- 70.Tibbetts SM, MacPherson T, McGinn PJ, Fredeen AH. In vitro digestion of microalgal biomass from freshwater species isolated in Alberta, Canada for monogastric and ruminant animal feed applications. Algal Res. 2016;19:324–332. doi: 10.1016/j.algal.2016.01.016. [DOI] [Google Scholar]
- 71.Batista, A. P. et al. Microalgae biomass incorporation in salted cookies (“crackers”) increases their functional properties. 4th EABA and EC Contractors’ Conference and the 11th International Algae Congress. Berlin, Germany, 05–07 December 2017 (2017).
- 72.FAO/WHO Protein and amino acid requirements in human nutrition, World Health Organization technical report series, Paper No. 935, Geneva (2007). [PubMed]
- 73.Devi MA, Subbulakshmi G, Devi KM, Venkataraman LV. Studies on the proteins of mass-cultivated, blue-green alga (Spirulina platensis) J. Agr. Food Chem. 1981;29:522–525. doi: 10.1021/jf00105a022. [DOI] [PubMed] [Google Scholar]
- 74.Becker, W. Microalgae in human and animal nutrition in Handbook of Microalgal Culture: Biotechnology and Applied Phycology (ed. Richmond A.) 312–351 (Blackwell Publishing Ltd, 2004).
- 75.Wild KJ, Steingaß H, Rodehutscord M. Variability in nutrient composition and in vitro crude protein digestibility of 16 microalgae products. J. Anim. Physiol. An. N. 2018;102:1306–1319. doi: 10.1111/jpn.12953. [DOI] [PubMed] [Google Scholar]
- 76.Hurrell, R. F. & Finot, P. A. Effects of food processing on protein digestibility and amino acid availability in Digestibility and amino acid availability in cereals and oilseeds (eds Finely, J. W., Hopkins, D. T.) 233–246 (1985).
- 77.Shahidi F, Naczk M, Griffiths W. Food phenolics: Sources, chemistry, effects, applications. Trends Food Sci. Tech. 1996;7:243. [Google Scholar]
- 78.Fradique M, et al. Incorporation of Chlorella vulgaris and Spirulina maxima biomass in pasta products. Part 1: Preparation and evaluation. J. Sci. Food Agr. 2010;90:1656–1664. doi: 10.1002/jsfa.3999. [DOI] [PubMed] [Google Scholar]
- 79.Constantinescu G, Dinu M, Buculei AS. Spirulina platensis effect as protein supplement on rheological properties of dough and nutritional qualities of hot-dog rolls. J. Agroal. Process. Technol. 2014;20:171–177. [Google Scholar]
- 80.Achour HY, Doumandji A, Sadi S, Saadi S. Evaluation of nutritional and sensory properties of bread enriched with Spirulina. Ann. Food Sci. Technol. 2014;15:270–275. [Google Scholar]
- 81.Kumar D. Nutritional and organoleptic attributes of novel pancake fortified with spirulina (Arthrospira platensis) Int. J. App. Home Sci. 2017;4:785–789. [Google Scholar]
- 82.Massoud R, Khosravi-Darani K, Nakhsaz F, Varga L. Evaluation of physicochemical, microbiological and sensory properties of croissants fortified with Arthrospira platensis (Spirulina) Czech J. Food Sci. 2016;34:350–355. doi: 10.17221/289/2015-CJFS. [DOI] [Google Scholar]
- 83.Fradique M, et al. Isochrysis galbana and Diacronema vlkianum biomass incorporation in pasta products as PUFA’s source. LWT-Food Sci. Technol. 2013;50:312–319. doi: 10.1016/j.lwt.2012.05.006. [DOI] [Google Scholar]
- 84.Zouari N, et al. Blue-green algae (Arthrospira platensis) as an ingredient in pasta: free radical scavenging activity, sensory and cooking characteristics evaluation. Int. J. Food Sci. Nutr. 2011;62:811–813. doi: 10.3109/09637486.2011.582461. [DOI] [PubMed] [Google Scholar]