Abstract
Sparganosis is an infestation caused by a tapeworm belonging to the genus Spirometra. The typical radiologic findings of sparganosis describe elongated, folded, band or tubular, hypoechoic structures with surrounding increased echogenicity in ultrasonography. These imaging features have been highly consistent with pathology results. Here, we report an interesting case of axillary sparganosis that manifested changes on ultrasound images over a period of 6 months.
Keywords: Sparganosis, Ultrasonography, Axilla
Introduction
Sparganosis is a parasitic infestation that is caused by the sparganum, a plerocercoid larva of the tapeworm belonging to the genus Spirometra. In humans, it is acquired by ingestion of larvae-containing water or by eating raw snakes or frogs. Once ingested, the larvae are commonly found in the abdomen, urogenital organs, extremities, central nervous system, chest, and orbital region [1]. The most common clinical manifestation of sparganosis is a migrating subcutaneous mass. In most cases, it is difficult to diagnose preoperatively because it does not have characteristic clinical features. However, ultrasound sonography findings are useful because they show some morphological features. There are reports describing the ultrasound findings observed in sparganosis. However, to the best of our knowledge, there is no report of sparganosis with changes on ultrasound images over a period of 6 months, as detailed in this report.
Case report
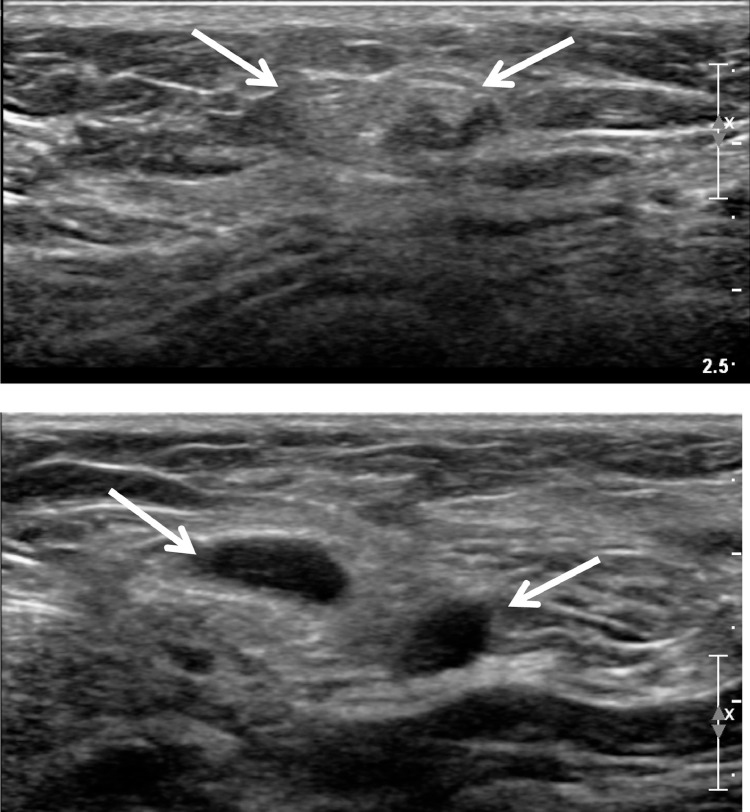
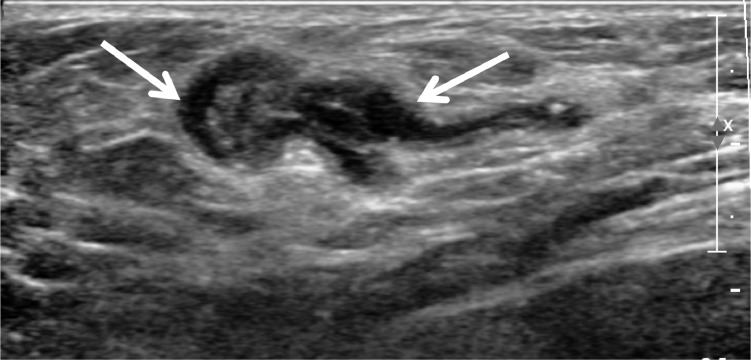
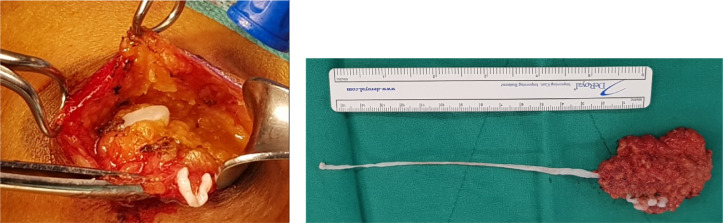
A 63-year-old woman visited our hospital for evaluation of a left-sided axillary mass and pain for 1 month prior to presentation. Initial ultrasonography revealed a relatively well-circumscribed, heterogenous, high echoic mass measuring 1.5 × 0.6 cm without internal hypoechoic tubular or elongated structures (Fig. 1A). No blood flow was seen on color Doppler sonography. In addition, several round anechoic-to-hypoechoic masses were noted around the previously mentioned high echoic mass (Fig. 1B). Ultrasonography-guided, 23-gauge fine needle aspiration was performed, and the pathology results revealed a cluster of aggregates of yeast-like or eggshell appearance material. Pathologically, a cluster is suspected of being either a yeast-like or eggshell-shaped organism. However, a pathologist noted the importance of identifying tissue artifacts that may occur during slide production to distinguish between fungal and parasitic organisms (Fig. 2). Seven months after her initial presentation, the patient complained of discomfort in the left side of the axilla. The ultrasound examination was repeated and showed multiple well-defined, hypoechoic, tubular masses with folded band-like tracts and a tubule-in-tubule appearance as well as surrounding increased echogenicity in the subcutaneous fat layer. These masses were different sizes, and the longest tubular structure measured 2.9 × 0.7 × 3.3 cm. There was no internal motion within the masses and they had no internal vascularity on color Doppler sonography (Fig. 3). The previously noted round anechoic-to-hypoechoic masses were not observed. Sparganosis was strongly suspected based on the ultrasound examination, and surgical excision was performed. Two ivory-white, opaque, ribbon-like sparganum worms (length: 17cm) were extracted during surgery, the reddish material is considered to be the blood of surrounding tissue at the time of surgery, and examination of the surgical specimens revealed the presence of spargana (Fig. 4). There was no larva. The pathology result was consistent with sparganosis parasite infection (Fig. 5).
Fig. 1.
Initial ultrasound shows a relatively well-circumscribed heterogenous high echoic mass (A) and oval cystic lesions (B) in the subcutaneous fat layer in left axilla.
Fig. 2.
FNA shows granulation tissue-like loose matrix with calcareous corpuscles. (Papanicolaou, x200).
Fig. 3.
After 6 months, ultrasound shows a well-defined hypoechoic, tubular mass with internal heterogeneous echogenicity, and tubule-in-tubule appearance in the subcutaneous fat layer.
Fig. 4.
Sparganum were obtained by surgical removal.
Fig 5.
A thread-like worm composed of eosinophilic tegument (long arrow), smooth muscle fibers (short arrow), and basophilic calcareous bodies. (H-E, x100).
Discussion
Sparganosis is a rare parasitic infestation that is endemic in East Asian countries, including Korea, China, and Japan [2]. Sparganosis caused by the migrating procercoid larvae of Diphyllobothrium mansoni is an extremely rare disease. The most common route of infection is ingestion of contaminated water containing procercoids, which can penetrate into the intestine and migrate to the muscle or subcutaneous tissue. The second most common route of infection is ingestion of raw or partially cooked fish, frogs, snakes, or chickens. Infection in this case also occurs from migration of the procercoid through the intestine. The third most common route of infection occurs from poultice applications infested with procercoid-infected Cyclops placed on open wounds or the eyes [2]. In the past, the typical route of infection was direct oral consumption of plerocercoid-infected reptiles or amphibians, uncooked mammals such as pigs, or, most frequently, an intermediary host such as snakes or frogs [3]. Perhaps the recent economic development and advances in sanitation have influenced the routes, sites of infection, and latency period of this infection. She denied eating uncooked frogs or reptiles or drinking contaminated water. She had a good consciousness and nutritive condition, and routine hematological, urinary, and chemical investigations were within normal limit. Unfortunately, the route of infection is not known. Sparganosis is endemic in East Asian countries, it may have been exposed without the patient's consciousness.
Spargana larvae can be found in any part of the human body but have a preference for subcutaneous involvement and migration. Sparganosis most frequently manifests as a migrating subcutaneous nodule, and it presents clinically with vague or indeterminate symptoms [4]. Previous reports describe the clinical features of axillary sparganosis as an indolent palpable mass similar to an enlarged lymph node without inflammatory reactions, such as a warm sensation or painful swelling.
In the present case, diagnosis of sparganosis was confirmed by identification of the worm during surgery, but ultrasonographic findings can also be helpful for preoperative diagnosis. Characteristic ultrasonographic features of spargana include several elongated, tubular, hypoechoic structures with surrounding increased echogenicity, which correlate with pathology findings [5]. The hypoechoic tubular structure is regarded as a remnant formed by an active moving sparganum. The tubular structure appeared to have just undergone active healing after the worms passed through [6]. Pathology findings usually show a granulomatous reaction to a foreign body along a tract-like elongated cavity through which the worms have passed [7]. In addition to these findings, sparganosis can be confirmed if movement of the worm is observed during US examination [8]. US is known to be helpful in diagnosing the sparganosis [8], but in our case, the imaging findings from initial ultrasound examination were not typical for sparganosis. The initial ultrasound demonstrated a relatively circumscribed high echoic mass without internal hypoechoic tubular structures. However, the second ultrasonographic findings of elongated tubular hypoechoic structures and surrounding increased echogenicity were characteristic of spargana.
Surgical extraction is the confirmative diagnosis for sparganosis. For treatment of sparganosis, medication therapy, including praziquantel, is reported to have only limited effects [9]. Our patient underwent surgery, during which 2 dead larvae were extracted. Symptoms of the patient improved after the removal of the mass.
Human sparganosis is a rare disease, and the infection route, latency period, and symptoms of the disease are changing with economic development. Therefore, surgeons should carefully collect patient history to formulate a differential diagnosis.
To summarize, we report sparganosis with pathognomonic ultrasound imaging findings different from those obtained 6 months previously.
Although not a common condition nowadays, sparganosis should be included in the differential diagnosis of palpable subcutaneous masses with the characteristic ultrasonographic findings of elongated, tubular, hypoechoic lesions with surrounding increased echogenicity. And, if the imaging findings are not typical to sparganosis, such as a high echogenicity without internal hypoechoic tubular or elongated structures, the radiologist should consider the possibility of spargnosis.
References
- 1.Jae-Hwan P., Jee-Won C., Nariya C., Nam-Sun P., Sang-Mee G., Eun-Hee S. A surgically confirmed case of breast sparganosis showing characteristic mammography and ultrasonography findings. Korean J Parasitol. 2006;44(2):151–156. doi: 10.3347/kjp.2006.44.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seobo S., Jai-Kyung Y., In-Yong L., Kyung-Il I.M., Tai-Soon Y. A case of breast sparganosis. Korean J Parasitol. 2002;40(4):187–189. doi: 10.3347/kjp.2002.40.4.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koo M., Kim J., Kim J., Lee J., Nam S., Yang J. Cases and Literature Review of Breast Sparganosis. World J Surg. 2011;35(3):573–579. doi: 10.1007/s00268-010-0942-1. [DOI] [PubMed] [Google Scholar]
- 4.Lee Eun Kyoung, Yoo Young Bum. Axillary sparganosis which was misunderstood lymph node metastasis during neoadjuvant chemotheraphy in a breast cancer patient. Ann Surg Treat Res. 2014;87(6):336–339. doi: 10.4174/astr.2014.87.6.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi S.J., Park S.H., Kim M.J., Jung M., Ko B.H. Sparganosis of the breast and lower extremities: sonographic appearance. J Clin Ultrasound. 2014;42(7):436–438. doi: 10.1002/jcu.22146. [DOI] [PubMed] [Google Scholar]
- 6.Chung S.Y., Park K.S., Lee Y., Park C.K. Breast sparganosis: mammographic and ultrasound features. J Clin Ultrasound. 1995;23(7):447–451. doi: 10.1002/jcu.1870230711. [DOI] [PubMed] [Google Scholar]
- 7.Kim H.Y., Kang C.H., Kim J.H., Lee S., Park S.Y., Cho S.W. Intramuscular and subcutaneous sparganosis: sonographic findings. J Clini Ultrasound. 2008;36(9):570–572. doi: 10.1002/jcu.20480. [DOI] [PubMed] [Google Scholar]
- 8.Hong S.J., Kim Y.M., Seo M., Kim K.S. Breast and scrotal sparganosis: sonographic findings and pathologic correlation. J Ultrasound Med. 2010;29(11):1627–1633. doi: 10.7863/jum.2010.29.11.1627. [DOI] [PubMed] [Google Scholar]
- 9.Song E., Sohn Y., Ryu K., Min S., Shin S., Park Y. Breast sparganosis and incidentally detected subcutaneous and intramuscular sparganosis at several sites: case report and literature review. Jpn J Radiol. 2015;33(4):225–228. doi: 10.1007/s11604-015-0405-6. [DOI] [PubMed] [Google Scholar]