Abstract
In this study, ultrasound-assisted extraction of flavonoid from Crinum asiaticum was studied through response surface methodology (RSM) to gain the best extraction process of flavonoid and enhance the extraction rate of flavonoid. In the following RSM experiment, we selected the corresponding data of every factor as the center point through the single-factor experiments, then the experimental data was subjected to multiple regression analysis. According to the statistical analysis results, the results were consistent with the polynomial regression model, the determination coefficient (R2) was 0.9769. The best conditions for maximum flavonoid yield were 60% ethanol concentration, 64 °C for extraction temperature, 1:28 (v/w) solid-to-liquid ratio with extraction time for 47 min. The best response of flavonoid yield was 1.63972%. The predicted results for best reaction conditions were in good agreement with experiment values. Ultrasound-assisted extraction method can enhance the extraction rate of flavonoid significantly. It is a powerful tool to extract of important phytochemicals from nature plant.
Keywords: Ultrasound-assisted extraction, Flavonoid, Crinum asiaticum, Response surface methodology
1. Introduction
Crinum asiaticum is a plant belonging to the genus Crinum. Its leaves and bulbs have been frequently used as a medicine for its abilities of purging fire, removing toxic substances and eliminating stasis to subdue swelling in people (Kogure et al., 2011). However, few studies about the composition and pharmacological properties of the Crinum asiaticum in current research.
Flavonoids are widely found in plants in nature and are secondary metabolites of plants (Sak, 2014), and has antioxidant activity, anticancer, anti-inflammatory (Jin et al., 2010, Rokayya et al., 2014, Jantrawut et al., 2017), hepatoprotective potential and antibacterial activity. Recently, the vegetables, fruits and plants that contain a substantial number of flavonoid compounds have received more and more attention for their medical utility. However, it is a challenge to extract flavonoid compounds efficiently, which is affected by genus, extraction methods and conditions, plant growth environment and so on.
Extraction is an important means of isolating, identifying and applying valuable chemical compounds from various natural plants (Stévigny et al., 2007, Yuan et al., 2017). There are many extraction methods, including water-extracted, maceration extraction and solid-phase microextraction. Usually, the process of these traditional extraction methods is very slow, costly and with low efficiency. Several new extraction methods have been discovered during recent years. In particular, ultrasound-assisted extraction is considered to better extract natural products and has been used to extract different compounds from various sources. Its main advantages are lower cost and higher efficiency, which are mainly due to ultrasonic’s mechanical effect and acoustic cavitation effect passing through the solvent, the solvent better into the interior of the tissue, meanwhile, it provides better contact of the active ingredient between the solid phase and the liquid phase. Therefore, the solute diffuses rapidly into the solvent through the solid phase (Rostagno et al., 2003). However, it remains a challenge as how to optimize extraction conditions to maximize compound yields.
In this study, we investigated the best extraction conditions for the ultrasound-assisted extraction of flavonoid from Crinum asiaticum through RSM. RSM is a good scientific statistical method that optimizes experimental processes by studying the influence of experimental variables and their interactions on the experimental outcome, while reducing the number of experiments (Gao et al., 2017a). Most studies have proved that RSM is an effective method to optimize the extraction of flavonoids from various nature plants. In the experimental results section, we listed the results of single-factor and RSM experiments.
2. Materials and methods
2.1. Experimental materials
The Crinum asiaticum was brought from Hainan Province, China. Crinum asiaticum cut into pieces were dried to a constant weight, then grind into a uniform powder with a diameter of 0.1–0.5 mm using a grinder.
2.2. Chemical and reagents
Standard of rutin (purity: ≥98%) was purchased from Beijing Hengyuan Qitian Chemical Research Institute and the batch number is MUST-11040302. All other chemicals were analytical grade supplied by Tianjin Fuyu Fine Chemical Co., Ltd. (Tianjin, China). The visible spectrum was measured by uv-725 spectrophotometer (Shanghai, China).
2.3. Ultrasound-assisted extraction
This experiment used maximum power of 180 W and 40 kHz ultrasonic cleaning tank (JP-030ST, Jiemeng, China). Take 2.0 g sample in ultrasonic bath and sonicate at different extraction temperatures, different solid-to-liquid ratio, and different concentration ethanol for different the time of extraction. The filtrate was collected and repeatedly extracted with the same volume of solvent (2 cycles). Pre-experimental results indicate that some experimental conditions, i.e., extract 2 cycles, material size of 80 mesh, extraction working frequency of 40 kHz, are suitable for this study. Prepare three samples for each sample for analysis.
2.4. Conventional solvent extraction (CSE)
Take 5 g dried Crinum asiaticum powder in a reflux device. 100 ml 80% aqueous ethanol was added and extracted at 85 °C for 180 min. Filtrate is obtained by filtering the crude extract and concentrate solution. Do this step three times.
2.5. Determination of total flavonoid
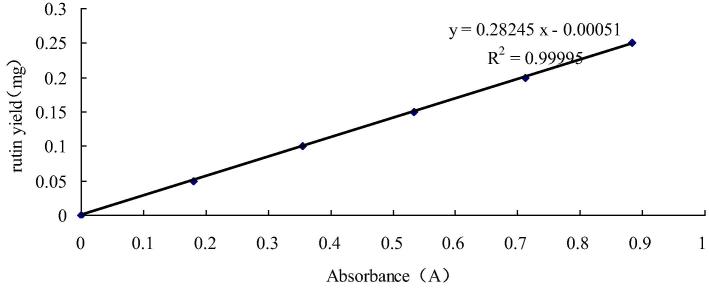
In this study, flavonoids content was measured by AlCl3 method. In short, Mix 1 ml of the sample with 1 ml of 1% (w/w) AlCl3 and let it stand for 15 min. The absorbance was determined by ultraviolet spectrophotometry at 274 nm (Gao et al., 2017b). Therutin equivalents express flavonoid content by the rutin calibration curve. The calibration curve as Fig. 1 (y = 0.28245–0.00051, where y is sample weight, x is sample absorbance) ranged 0–250 μg (R2 = 0.9999).
Fig. 1.
The calibration curve.
2.6. Experimental design
RSM was applied to establish the optimum for flavonoid yield from Crinum asiaticum. Four independent variables considered in this investigation were solid-to-liquid ratio (X1), the time of extraction (X2), the temperature of extraction (X3) and ethanol concentration (X4). Single-factor experiments were used to determine the suitable range of the four variables (Abdallah et al., 2016). The whole experimental design was composed of 29 experimental points as shown in Table 1. Five replications (25–29) were performed in the design center to estimate a pure error sun of squares. Table 1 demonstrates factors and levels of experimental.
Table 1.
Response surface analysis factors and levels.
| Variables | Level |
||
|---|---|---|---|
| −1 | 0 | 1 | |
| X1 solid-to-liquid ratio (v/w) | 10 | 20 | 30 |
| X2 extraction time (min) | 40 | 50 | 60 |
| X3 extraction temperature (°C) | 50 | 60 | 70 |
| X4 ethanol concentration (%) | 50 | 60 | 70 |
2.7. Statistical analysis
Design-Expert software 7.0 was used for experimental design. Check the accuracy of the polynomial model equation by F-test and p-value. Statistically significant: p < 0.05. All measurements were taken three times.
3. Results
3.1. Influence of Solid-to-liquid ratio to the flavonoid yield
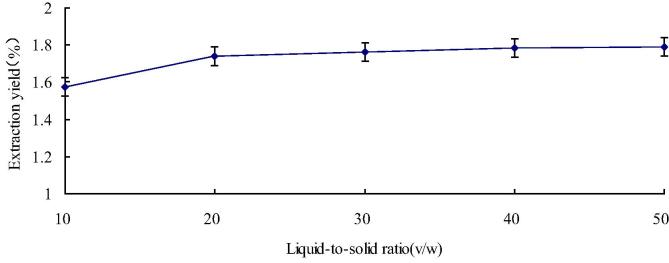
We can see the influence of solid-to-liquid ratio to flavonoids yield from Fig. 2, The ratio was set at 1:10, 1:20, 1:30, 1:40 and 1:50 (v/w) to analyze the influence of various solid-to-liquid ratios to flavonoid yield. Meanwhile, we can see other extraction parameter fitting: the concentration of ethanol was 70%, the time of extraction was 30 min and the temperature of extraction was 50 °C. It was shown that the flavonoid yield was increased from 1.57% to 1.79% as the solid-to-liquid ratio increased from 1:10 to 1:50 (v/w). This was due to the more effective constituents’ dissolvent by a large solvent volume, the extraction yield was increased as a result (Volpi, 2004). A particularly sharp rise of the flavonoid yield was shown as the solid-to-liquid ratio rose from 1:10 to 1:20, but the increase slowed down when the ratio increased further. For the best use of solvent, the 1:20 solid-to-liquid ratio was used as the conditions for subsequent studies.
Fig. 2.
Influence of solid-to-liquid ratio to the flavonoid yield.
3.2. Influence of extraction temperature to the flavonoid yield
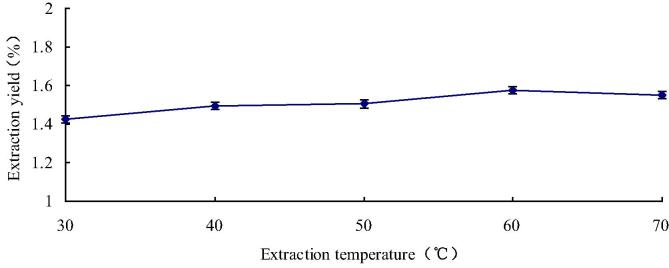
In order to investigate the influence of difference temperatures to the flavonoid’s extraction yield, experiment with extraction temperature selection from 30 °C to 70 °C. Meanwhile, we can see other extraction parameter fitting: solid-to-liquid ratio was 1:20, ethanol concentration was 70% and the time of extraction was 30 min (Khurshid et al., 2016). As shown in Fig. 3, the flavonoid yields remarkably increased from 1.42% to 1.57% as the extraction temperature was raised from 30 °C to 60 °C, but flavonoid yield gradually decreases when the extraction temperature is raised from 60 °C to 70 °C. The reason was that the appropriate temperature promoted flavonoid diffusion out of plant cells, but the higher temperature led to the flavonoid decomposition. Thus, the choice of 60 °C as the best extraction temperature conditions the BBD experiment.
Fig. 3.
Influence of different temperatures to the flavonoid extraction yield.
3.3. Influence of the time of extraction to the flavonoid yield
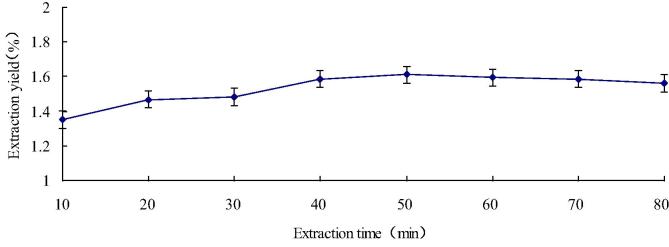
Influence of extraction time to the flavonoid yield is illustrated in Fig. 4. In this study, different time points were tested from 10 to 80 min, we can see other extraction parameter fitting: solid-to-liquid ratio was 1:20, ethanol concentration was 70% and the temperature of extraction was 60 °C. The extraction rate increased rapidly with time prolongation and reached 1.61% at 50 min, then the yield gradually declined from 50 to 80 min. The reason is that the extraction time prolongation keeps the balance of flavonoids in and out of cells (Cai et al., 2008). Therefore, the extraction time is selected for 50 min in the subsequent RSM test.
Fig. 4.
Influence of extraction time to the flavonoid yield.
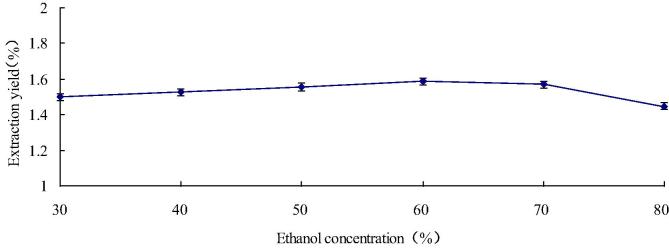
3.4. Influence of extraction concentration to the flavonoid yield
According to reports, Polar flavonoids can be extracted with lower concentrations of ethanol, and non-polar flavonoids can be extracted with higher concentrations of ethanol. In this study, different ethanol concentration points were tested from 30% to 80%, and we can see other extraction parameter fitting: solid-to-liquid ratio was 1:20, extraction time was 50 min and the temperature of extracted was 60 °C. Fig. 5 shows that the yield remarkably raised from 1.50% to 1.59% as ethanol concentration increased from 30% to 60% (Yormaz et al., 2017). But the yield declined to 1.45% the ethanol concentration gradually increases to 80%. It is because the higher concentration of ethanol blocks flavonoid diffusion from plant cells.
Fig. 5.
Influence of ethanol concentration to the flavonoid yield.
3.4.1. Building models and analyzing statistics
Based on the results of experiments on single factor, four parameters: solid-to-liquid ratio (X1), the time of extraction (X2), the temperature of extraction (X3) and the concentration of ethanol (X4) were selected as the variables for optimizing the process of flavonoid extraction. The experimental steps and their corresponding experimental results are shown in Table 2.
Table 2.
Box- Box-behnken experiments were designed with independent variables.
| No. | Solid-to-liquid ratio (v/w) X1 | Extraction time (min) X2 | Temperature (°C) X3 | Ethanol concentration (%) X4 | Extraction yield (%)a |
|---|---|---|---|---|---|
| 1 | −1(10) | −1(40) | 0(60) | 0(60) | 1.35014 |
| 2 | 1(30) | −1(40) | 0(60) | 0(60) | 1.56252 |
| 3 | −1(10) | 1(60) | 0(60) | 0(60) | 1.41429 |
| 4 | 1(30) | 1(60) | 0(60) | 0(60) | 1.62605 |
| 5 | 0(20) | 0(50) | −1(50) | −1(50) | 1.55390 |
| 6 | 0(20) | 0(50) | 1(70) | −1(50) | 1.57209 |
| 7 | 0(20) | 0(50) | −1(50) | 1(70) | 1.54066 |
| 8 | 0(20) | 0(50) | 1(70) | 1(70) | 1.57705 |
| 9 | −1(10) | 0(50) | 0(60) | −1(50) | 1.38091 |
| 10 | 1(30) | 0(50) | 0(60) | −1(50) | 1.64954 |
| 11 | −1(10) | 0(50) | 0(60) | 1(70) | 1.43199 |
| 12 | 1(30) | 0(50) | 0(60) | 1(70) | 1.61183 |
| 13 | 0(20) | −1(40) | −1(50) | 0(60) | 1.42712 |
| 14 | 0(20) | 1(60) | −1(50) | 0(60) | 1.53001 |
| 15 | 0(20) | −1(40) | 1(70) | 0(60) | 1.49953 |
| 16 | 0(20) | 1(60) | 1(70) | 0(60) | 1.53985 |
| 17 | −1(10) | 0(50) | −1(50) | 0(60) | 1.39380 |
| 18 | 1(30) | 0(50) | −1(50) | 0(60) | 1.60091 |
| 19 | −1(10) | 0(50) | 1(70) | 0(60) | 1.40136 |
| 20 | 1(30) | 0(50) | 1(70) | 0(60) | 1.59756 |
| 21 | 0(20) | −1(40) | 0(60) | −1(50) | 1.50368 |
| 22 | 0(20) | 1(60) | 0(60) | −1(50) | 1.52268 |
| 23 | 0(20) | −1(40) | 0(60) | 1(70) | 1.51179 |
| 24 | 0(20) | 1(60) | 0(60) | 1(70) | 1.53170 |
| 25 | 0(20) | 0(50) | 0(60) | 0(60) | 1.63036 |
| 26 | 0(20) | 0(50) | 0(60) | 0(60) | 1.62095 |
| 27 | 0(20) | 0(50) | 0(60) | 0(60) | 1.61560 |
| 28 | 0(20) | 0(50) | 0(60) | 0(60) | 1.62397 |
| 29 | 0(20) | 0(50) | 0(60) | 0(60) | 1.59501 |
Flavonoid yield is the percentage of the extracted flavonoid relative to the dry weight of Crinum asiaticum.
The RSM was used to obtain the relationships between the flavonoid yield and the test variables. Multiple regression analysis was used to analyze the experimental data, and it was found that the relationship between the response variable (Y) and the measured variable was a quadratic polynomial equation:
| (1) |
Table 3 demonstrates the anova of quadratic polynomial model. The Model F-value of 42.24 indicates its significance. Since “Prob > F” is less than 0.05, the model term is statistically significant. Under these circumstances, X1, X2, X3, X12, X1X4, X22, X32, X42 (P < 0.05) both significantly. The model’s determination coefficient (R2) is 0.9769, part of the F-value is meaningless (P > 0.05), reflecting model’s effectiveness. Adjusted R2 (0.9537) indicated that the total change of 95.37% for the independent variables and the model could not explain only 4.63% of the total variation. Meanwhile, coefficient of variation (C.V.% = 1.21) explained that the experimental data is reliable.
Table 3.
Response surface quadratic model anova and regression coefficient estimation.
| Parameter | Sum of Squares | df | Mean Squares | F-value | P-value |
|---|---|---|---|---|---|
| Model | 0.20 | 14 | 0.015 | 42.24 | <0.0001 |
| X1 | 0.14 | 1 | 0.14 | 391.94 | <0.0001 |
| X2 | 7.998 × 10−3 | 1 | 7.998 × 10−3 | 23.11 | 0.0003 |
| X3 | 1.658 × 10−3 | 1 | 1.658 × 10−3 | 4.79 | 0.0461 |
| X4 | 4.113 × 10−5 | 1 | 4.113 × 10−5 | 0.12 | 0.7354 |
| X1X2 | 9.619 × 10−8 | 1 | 9.619 × 10−8 | 2.776 × 10−4 | 0.9869 |
| X1X3 | 2.976 × 10−5 | 1 | 2.976 × 10−5 | 0.086 | 0.7737 |
| X1X4 | 1.971 × 10−3 | 1 | 1.971 × 10−3 | 5.69 | 0.0317 |
| X2X3 | 9.788 × 10−4 | 1 | 9.788 × 10−4 | 2.83 | 0.1148 |
| X2X4 | 2.070 × 10−7 | 1 | 2.070 × 10−7 | 5.981 × 10−4 | 0.9808 |
| X3X4 | 8.281 × 10−5 | 1 | 8.281 × 10−5 | 0.24 | 0.6323 |
| X12 | 0.032 | 1 | 0.032 | 91.21 | <0.0001 |
| X22 | 0.032 | 1 | 0.032 | 91.72 | <0.0001 |
| X32 | 0.012 | 1 | 0.012 | 34.89 | <0.0001 |
| X42 | 3.706 × 10−3 | 1 | 3.706 × 10−3 | 10.71 | 0.0056 |
| Residual | 4.846 × 10−3 | 14 | 3.461 × 10−4 | ||
| Lack of Fit | 4.118 × 10−3 | 10 | 4.118 × 10−4 | 2.26 | 0.2241 |
| Pure Error | 7.280 × 10−4 | 4 | 1.820 × 10−4 | ||
| Cor Total | 0.21 | 28 |
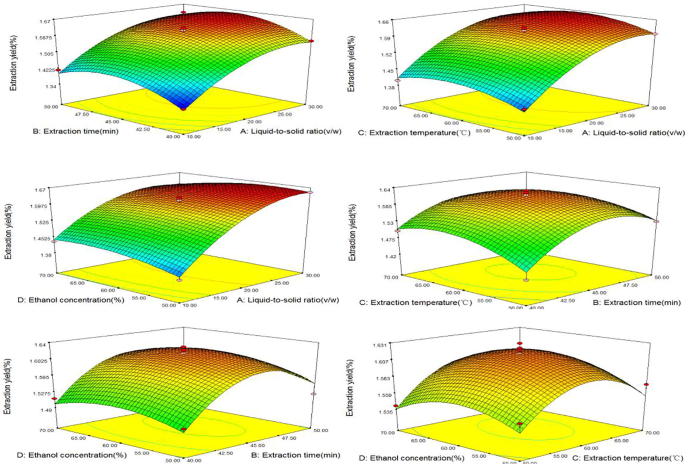
The three-dimensional response surface diagram was shown in Fig. 6. The production of flavonoids began to increase, and then declined with the extension of extraction time, extraction temperature and ethanol concentration gradually rise. As the solid-to-liquid ratio continues to increase (X1), flavonoids yield reached the maximum at a certain level.
Fig. 6.
Three-dimensional figures of interactive effects of 4 factors.
By solving the regression equation, the optimal value of the selected variable is obtained. Design-Expert software 7.0 calculated that the optioned conditions of flavonoid were 60.10% ethanol concentration, solid-to-liquid ratio was 27.56 and extraction for 46.6 min at 63.66 °C, with corresponding Y = 1.65454%. For conforming this result, trial in the next three trials under improved conditions 60% ethanol concentration, solid-to-liquid ratio was 1:28 for 47 min at 64 °C, in which the average flavonoid yield was 1.63972%. Thus, the predicted value was consistent with the actual results, indicating that the optimization parameters were available.
3.4.2. Ultrasound-assisted extraction (UAE) ompare with conventional solvent extraction (CSE)
Different methods are used to extract different natural substances, their extraction rate and efficiency are different. Table 4 explained the comparison of the extraction rates of flavonoid extraction yield rates between UAE and CSE under the best extraction conditions. As shown in Table 4, the UAE extraction rate was 1.63972%. The extraction effect is better than CSE (1.35943%). Moreover, the UAE takes only 47 min to extract and extraction temperature was 64 °C, far below the CSE (180 min and 85 °C). The result proved that UAE is better than CSE. Thus, UAE could be used as an effective method for the separation of flavonoids from Crinum asiaticum.
Table 4.
Comparison of UAE and CSE of flavonoid extraction yield from Crinum asiaticum.
| Extraction method | Solid-to-liquid ratio | Ethanol concentration (%) | Temperature (°C) | Extraction time (min) | Extraction yield (%)a |
|---|---|---|---|---|---|
| UAE | 1:28 | 60% | 64 | 47 | 1.63972 |
| CSE | 1:30 | 60% | 80 | 180 | 1.35943 |
Flavonoid yield is the percentage of the extracted flavonoid relative to the dry weight of Crinum asiaticum.
4. Discussion
At present, there are many methods for extracting flavonoids: Hot water extraction, alkali extraction and acid precipitation, organic solvent extraction, microwave-assisted extraction, ultrasound-assisted extraction, two-phase extraction, enzymatic hydrolysis, supercritical fluidextraction, etc, each method has its advantages and disadvantages. By optimizing the properties of the extracted flavonoids, extraction costs, process equipment and other influencing factors to increase the extraction rate of flavonoids and the consumption of raw material, etc. Ultrasound-assisted extraction been used to extract active ingredients from plants in recent years, its cavitation can physically destroy the cell wall and accelerate the dissolution of the active ingredient, but the ultrasonic time should’t be too long, the thermal effect of ultrasound destroys the heat sensitive substance of the extract. Therefore, this experiment selected ultrasound-assisted extraction to extract flavonoids from Crinum asiaticum.
In the experiment of extraction process of flavonoids from Crinum asiaticum, the result ofa single factor using ultrasound-assisted extraction: the solid-to-liquid ratio was 1:28, the extraction temperature was 60 °C, the extraction time was 50 min and the ethanol concentration was 60%, the measured flavonoid extraction rate was 1.61%. In this experiment,aqueous solution of ethanol was used as extractant to extract flavonoids from Crinum asiaticum. The flavonoids extracted by water extraction method have more impurities, and the high boiling point of water is inconvenient for the later concentration and purification under reduced pressure. Methanol and chloroform are toxic, and the polarity of ethyl acetate is small, which is not conducive to the extraction of flavonoid glycosides. Considering the safety and cost of the experimental process, ethanol was chosen as the extraction solvent for the flavonoids of Crinum asiaticum. RSM is a hotspot software for data analysis and experimental design in recent years. Compared with orthogonal design and uniform design, under the same variable factor, its experimental combination is small, and the interaction of each two factors will be reflected by the 3D map and the contour map. The experimental results and the effects of each other can be more intuitive and more comprehensive. The experimental results show that ultrasound-assisted extraction of flavonoids from Crinum asiaticum and the optimization of extraction parameters by RSM are scientific and economically feasible.
5. Conclusions
In this experiment, ultrasound-assisted extraction was used to extract flavonoids from Crinum asiaticum. Ultrasound-assisted effectively improves the extraction performance of flavonoids RSM is often used to optimize, extract, and evaluate experimental parameters. The results proved that the liquid-solid ratio, extraction time, extraction temperature and ethanol concentration had influence on flavonoid’s extraction rate significantly. Optimal condition for response functions 1:28 solid-to-liquid ratio, the time of extraction for 47 min, the temperature of extraction for 64 °C and 60% ethanol concentration. Under the best technological conditions, flavonoid yield achieve powder 1.63972%. The experimental results are of great significance for the application of Crinum asiaticum. The study confirmed again that the ultrasonic-assisted extraction can effectively extract important chemicals from the plant.
Acknowledgments
We would like to express sincere thanks to National Natural Science Foundation of China (41702368); Project funded by China Postdoctoral Science Foundation (2015M581467); Postdoctoral foundation of Heilongjiang Province (LBH-Z15107); Heilongjiang Postdoctoral special Foundation (LBH-TZ1613); Harbin Special Foundation for Young Technological Innovative Talented Person (2013RFQXJ150); The Scientific Research Program of Harbin University of Commerce (17XN061); Heilongjiang Natural Science Foundation (LH2019D007); Research Projects of Harbin Commercial University (18NX066), The Graduate Students Innovative Scientific Research Project of Harbin University of Commerce (YJSCX2018- 484HSCD); Heilongjiang Natural Science Foundation (LH2019H066); Project funded by China Postdoctoral Science Foundation (2019M651296).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdallah H.M., AlGhamdi D.O., Al-Salem M.S., Alattas M., El-Bassossy H.M., Alahdal A.M., Shehata I.A., Abdel-Sattar E. Effect of Viscum schimperi on advanced glycation endproducts formation. Pak. J. Pharm. Sci. 2016;29S(6):2307–2316. [PubMed] [Google Scholar]
- Cai W.R., Gu X.H., Tang J. Extraction, purification, and characterization of the polysaccharides from Opuntia milpa Alta. Carbohydr. Polym. 2008;71(3):403–410. [Google Scholar]
- Stévigny C., Rolle L., Valentini N., Zeppa G. Optimization of extraction of phenolic content from hazelnut shell using response surface methodology. J. Sci. Food Agric. 2007;87(15):2817–2822. [Google Scholar]
- Gao W., Wang Y.Q., Basavanagoud B., Jamil M.K. Characteristics studies of molecular structures in drugs. Saudi Pharm. J. 2017;25(4):580–586. doi: 10.1016/j.jsps.2017.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Wang Y.Q., Wang W.F., Shi L. The first multiplication atom-bond connectivity index of molecular structures in drugs. Saudi Pharm. J. 2017;25(4):548–555. doi: 10.1016/j.jsps.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantrawut P., Phongpradist R., Muller M., Viernstein H. Enhancement of anti-inflammatory activity of polyphenolic flavonoid rutin by encapsulation. Pak. J. Pharm. Sci. 2017;30(5):1521–1527. [PubMed] [Google Scholar]
- Jin J.H., Lim H., Kwon S.Y., Son K.H., Kim H.P. Anti-inflammatory activity of the total flavonoid fraction from Broussonetia papyrifera in combination with Lonicera japonica. Biomol. Ther. 2010;18(2):197–204. [Google Scholar]
- Khurshid Z., Naseem M., Sheikh Z., Najeeb S., Shahab S., Zafar M.S. Oral antimicrobial peptides: types and role in the oral cavity. Saudi Pharm. J. 2016;24(5):515–524. doi: 10.1016/j.jsps.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure N., Katsuta N., Kitajima M., Takayama H. Two new alkaloids from Crinum asiaticum var. sinicum. Chem. Pharm. Bull. (Tokyo) 2011;59(12):1545–1548. doi: 10.1248/cpb.59.1545. [DOI] [PubMed] [Google Scholar]
- Rokayya S., Li C.J., Zhao Y., Li Y., Sun C.H. Cabbage (Brassica oleracea L. var. capitata) phytochemicals with antioxidant and anti-inflammatory potential. Asian Pac. J. Cancer Prev. 2014;14(11):6657–6662. doi: 10.7314/apjcp.2013.14.11.6657. [DOI] [PubMed] [Google Scholar]
- Rostagno M.A., Palma M., Barroso C.G. Ultrasound-assisted extraction of soy isoflavones. J. Chromatogr. 2003;1012(2):119–128. doi: 10.1016/s0021-9673(03)01184-1. [DOI] [PubMed] [Google Scholar]
- Sak K. Characteristic features of cytotoxic activity of flavonoids on human cervical cancer cells. Asian Pac. J. Cancer Prev. 2014;15(19):8007–8019. doi: 10.7314/apjcp.2014.15.19.8007. [DOI] [PubMed] [Google Scholar]
- Volpi N. Application of high-performance capillary electrophoresis to the purification process of Escherichia coli K4 polysaccharide. J. Chroma. 2004;811(2):253–256. doi: 10.1016/j.jchromb.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Yormaz B., Kahraman H., Koksal N. Evaluation of bone demineralization in copd phenotypes. ACTA Medica Mediterr. 2017;33(4):627–636. [Google Scholar]
- Yuan Y., Liu Y., Liu M., Chen Q., Jiao Y., Liu Y., Meng Z. Optimization extraction and bioactivities of polysaccharide from wild Russula griseocarnosa. Saudi Pharm. J. 2017;25(4):523–530. doi: 10.1016/j.jsps.2017.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]