Abstract
The aim of this study is to explore the construction of rat spinal cord injury model guided by Allen's model. Methods: Male rats aged 4–5 weeks and weighing about 250 g are selected as subjects in the Animal Laboratory Center of XX Hospital. Rats are divided into two groups, which are experimental group 1 and experimental group 2, respectively, so as to construct spinal cord injury model in rats. The first group is given 300 g.cm hitting force of T10 spinal cord, and the second group is given 500 g.cm hitting force of T10 spinal cord. Within 25 days after spinal cord injury in Allen's rats, the survival, neurological function, diet, motor ability, tactile ability and auditory ability of the two groups are monitored and evaluated daily. Results: In terms of survival, the survival rate of rats in group 1 is 85%, while that of rats in group 2 is 21%, and there is a concentrated death phenomenon in group 2. In terms of neurological function recovery, experimental group 1 is stable and gets 7 points and experimental group 2 is stable and gets 3 points. In terms of diet, the experimental group 1 is stable and gets 5 points and the experimental group 2 is stable and gets 2 points. In terms of motor ability, the experimental group 1 is stable and gets 5 points and the experimental group 2 is stable and gets 2 points. In tactile sense, experimental group 1 is stable and gets 17 points and experimental group 2 is stable and gets 12 points. It can be seen that the post-operative recovery ability of the experimental group 1 is better than that of the experimental group 2. Conclusion: Under the guidance of Allen's model, compared with the group 2, the experimental group 1 of the rat spinal cord injury model has better recovery in each index. It can be seen that the smaller impact strength is more beneficial to the recovery of rats after spinal cord injury surgery.
Keywords: Allen's, Model, Rat, Score, Recovery
1. Introduction
At present, there are many studies on spinal cord injury at home and abroad, including the mechanism of spinal cord injury, repair and regeneration after injury, etc. (Liu et al., 2017). In the study of the mechanism of spinal cord injury, it mainly includes primary injury and secondary injury (Lakes and Allen, 2016). The former is mostly caused by mechanical force directly injuring nerve cells and endothelial cells (Sparrey et al., 2016). The unbalanced movement of tissue after injury results in hemorrhage and necrosis in the injured area and shear damage of nerve cell membrane and connective tissue (Xie et al., 2017). The latter has more complex mechanisms, including necrosis, infarction and apoptosis in general sense, as well as molecular level participation and excitotoxicity damage caused by glutamate. Reactive oxygen species (ROS) mediators produced by reperfusion after injury (He et al., 2017) are mainly superoxide free radicals and peroxide free radicals, which are directly related to neuronal apoptosis (Corder et al., 2017). At the microscopic level, it also includes axon swelling, lamellar separation, or even demyelination after spinal cord injury, and the rupture of myelin sheath to form debris, which eventually becomes glial scar (Furman et al., 2016). It also participates in ischemia-reperfusion injury and excitotoxicity of glutamate, which eventually leads to cell death (Ma et al., 2016). Regarding spinal cord repair, in the traditional view, mammalian brain and spinal cord injury cannot regenerate. As a pessimistic conclusion (Hu et al., 2016a, Hu et al., 2016b), it has been introduced into traditional neurosurgery course. If the symptoms of hemiplegia or total paralysis cannot be improved within 24 h after injury, there will be no significant functional recovery (Theodore et al., 2016).
Although post-traumatic care and comprehensive treatment have been improved and life expectancy has been prolonged after spinal cord injury, so far, the prognosis of functional recovery after spinal cord injury is still hopeless (Cheng et al., 2017). In recent years, there have been many studies on the repair of spinal cord injury. The repair ability of nerve injury depends on various factors (Kim et al., 2016). In all vertebrates, peripheral nerve injury (such as peripheral nerve amputation) causes axonal regeneration of motor neurons in certain specific environments, including target organ reinnervation and functional recovery (Hu et al., 2016a, Hu et al., 2016b). Axonal regeneration of the central nervous system depends on phylogenesis (Chen et al., 2016).
In conclusion, in this study, the construction of rat spinal cord injury model guided by Allen's model has been studied. The results show that under the guidance of Allen's model, compared with the group 2, the recovery of experimental group 1 of spinal cord injury model is better, indicating that smaller impact strength is more beneficial to the recovery of spinal cord injury in rats. The innovation of this study is to study the construction of rat spinal cord injury model guided by Allen's model from different impact strength, and to analyze the recovery of rats in detail. The results of this study still provide some guidance for future research, so this study is a valuable research topic.
2. Method
2.1. Subjects and groups
40 healthy adult male rats weighing 250 g are provided by Animal Laboratory of XX Hospital. After purchase, it is necessary to ensure rich nutrition. In a warm, comfortable and quiet living environment, rats are fed exclusively for 24 h. Temperature is maintained at 20–23 °C and humidity is controlled at 55%–60%. After one month of feeding, the operation is performed. All experimental animals are handled and operated in accordance with the Requirements for Guiding Opinions on Treating Experimental Animals issued by the Ministry of Science and Technology of the People's Republic of China and agreed by animal management agencies, and in accordance with relevant regulations.
Grouping: Animals are randomly divided into 2 groups of 20 animals each. Under the same conditions, rats in the experimental group 1 and the experimental group 2 are dissected to expose the spinal cord of the T10 segment. The exposure time is the same, and the rats are given a hit amount of 300 g.cm and 500 g.cm, respectively. At the same time points on the 1st, 2nd, 4th, 8th, 12th, 16th, 20th, and 25th day after surgery, the rats are scored for functional recovery to observe the effects of different impacts on the recovery of body function.
2.2. Experimental method
Preoperative preparation and anesthesia: All instruments are sterilized by high pressure steam. Normal male rats weighing about 250 g are selected. At 0.5 cm above the middle point of the right groin, ketamine (50 mg/kg) and diazepam (2.5 mg/kg) are injected intraperitoneally (Shanghai Kunya Co., Ltd., China) to anesthetize rats. The anesthesia is successful when the muscles of the rats are relaxed and the hind limbs of the rats are clamped (Shanghai Kunya Co., Ltd., China) without contraction. Rats are immobilized prone. At the intersection of thoracic trapezius tendons (about 10–12 vertebrae), the skin is prepared up to 19, 10, 11 and 4 cm above and below the center. After depilation, iodine tincture and alcohol are used to carry out routine disinfection and sterile operation sheets are laid.
Skin incision and location: The middle part of the back of the rat body is cut horizontally (Shanghai Kunya Co., Ltd., China), about 5 cm. Skin and subcutaneous fascia are cut successively, and the parts to be operated on are exposed. Muscles are clearly visible and the protective film on the surface of the vertebral body can be clearly seen. The bilateral protective membranes are located approximately parallel to the 12th lumbar body at the nearest junction to the midline. Glass needles are used to pull away skin tissues and fully display transverse processes, laminae and spinous processes. Special scissors (Shanghai Kunya Co., Ltd., China) are used to cut the tendons and muscles connected to them along the spinous process. A knife edge about the distance of T8-T11 appears, and the depth of the knife reaches the position of the joint. In order to avoid excessive blood loss, the skin and muscle tissue covering the surface of vertebral body and articular process are treated and cleaned by slightly pulling and cutting, so that the joint, vertebral body and lateral wall can be completely presented in the field of vision. At the same time, T10, T11 and T12 are also exposed. Among them, the tail of T10 should be visible. It is connected with the neutral position of T11 and the head-side position of T12, which is a sign of operation. In order, the upper and middle ligaments and the lower ligaments are all cut off. Special props are used to pull the ligaments of T9-11 apart. The first step is to take the midpoint of the caudal wall of T11 vertebral body as a position point, and the junction of the subprocessus incision of the head and lateral wall of the caudal foramen and the vertebral body as another position point. Scissors are used to create a slightly deeper suture for osteotomy markers. Along the osteotomy line, the vertebral body is cut. The spinous process of T11 is raised to enlarge the suture space between the upper and lower parts, and to clean up all the remaining tissue cells in the middle of the suture. The next step is to clean up half or all of the back of the T10 vertebral body and expose the spinal cord. Combined with the fixing groove and pliers made by ourselves, the spinal cord of rats is fixed on it and placed under the spinal cord injury striking equipment made by ourselves. The height is adjusted to keep it in place. 300 g.cm and 500 g.cm are given respectively to achieve the construction of spinal cord injury model. The spinal cord is destroyed for 3 min by beating the rat's head continuously. When the spinal cord injury is completed, the color of spinal cord congestion gradually becomes dim. When the rat's legs are completely free of muscle tension, the gelatin sponge is used to control blood loss. Then, the skin, muscle and other tissues of rats are gradually sutured. Gentamicin is then injected from the abdomen of the rats for four days.
2.3. Main observed indicators
Two groups of rats are given different degrees of damage to the rat spinal cord injury model. Rats are scored for functional recovery at the same time each day on the 1st, 2nd, 4th, 8th, 12th, 16th, 20th, and 25th day after surgery to determine the effect of different strokes on recovery of body function in rats. Rats are placed in a warm, well-lit, and open environment. In combination with the characteristics of the life of the rats, the functional recovery of the rats is scored at 6 pm on each day of the test date.
Samples: Rats in groups 1 and 2 are fed for 10 days after spinal cord injury. The rats to be prepared are anesthetized with ketamine injected from the abdomen. The rat's chest is opened to expose the heart. Rat veins are intubated. The left atrium is opened with a scalpel and saline is slowly injected into the blood vessels from the vein. The formaldehyde buffer solution of 80 g/L is slowly injected into the left atrium of rats after the colored liquid comes out. When the left atrium smells bad, it is necessary to stop injection after 5 min and the spinal cord is completely exposed. Meanwhile, the T9 and T10 vertebrae are completely removed, the spinal cord is cut off, and specimens are taken away. The injured area is labeled and placed in 150 g/L formaldehyde solution, then fixed. Then, according to the sequence, dehydration, transparency, wax immersion, paraffin embedding and other operations are carried out on the spinal cord tissue cells of rats. The spinal cord injury site is taken as the origin for continuous sectioning with a thickness of 8um. Toluidine is used for dyeing. Under the optical microscope (Shanghai Kunya Co., Ltd., China), the structure of spinal cord tissue and cells are observed.
2.4. Statistical processing
The measurement data are expressed by the standard deviation of mean soil, and the variance analysis of complete random design is carried out by SPSS16.0 statistical software. The results of the three groups are different after comparison. LSD-t test is used to test the homogeneity of variance between the two groups, and P < 0.05 is the significant difference.
3. Results
3.1. Survival of rats after spinal cord injury
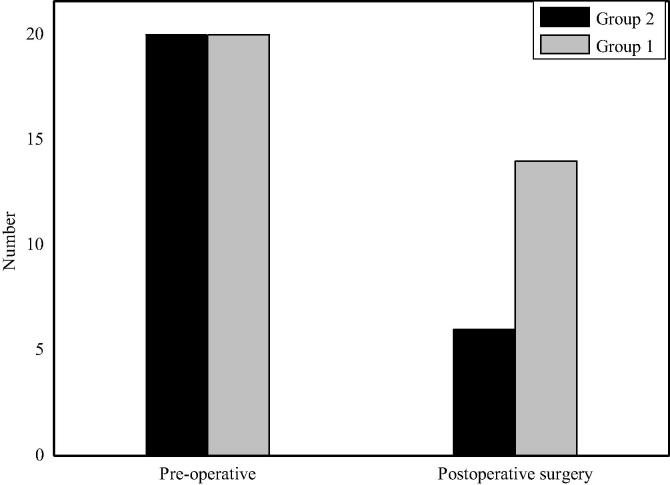
The mortality analysis of rats after Allen's spinal cord injury is shown in Fig. 1. From the picture, it can be clearly seen that compared with the death of rats in the experimental group 2, the survival rate of rats in the experimental group 1 after the same intervention and nursing care is significantly higher. In addition, most rats in group 2 die centrally, and no such centralized death occurs in group 1, which is in line with the requirements of spinal cord injury rat model. It can be seen that the size of the impact affects the survival of rats. The greater the impact is, the lower the survival rate of rats is and the slower the recovery of spinal cord function is.
Fig. 1.
Mortality analysis of rats after Allen's spinal cord injury.
3.2. Neurological function after spinal cord injury in rats
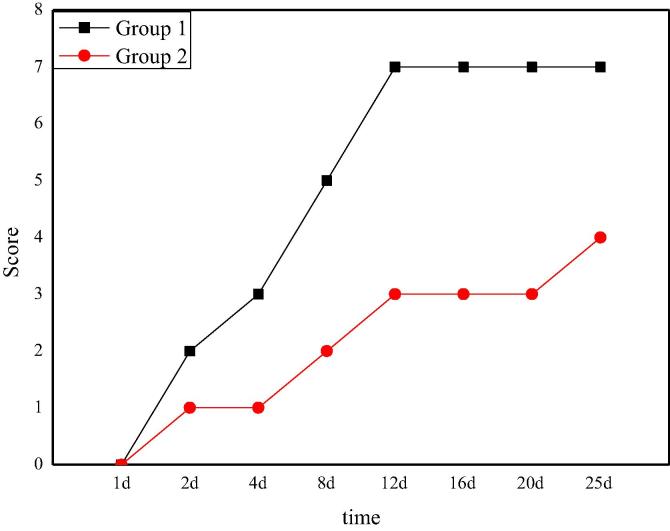
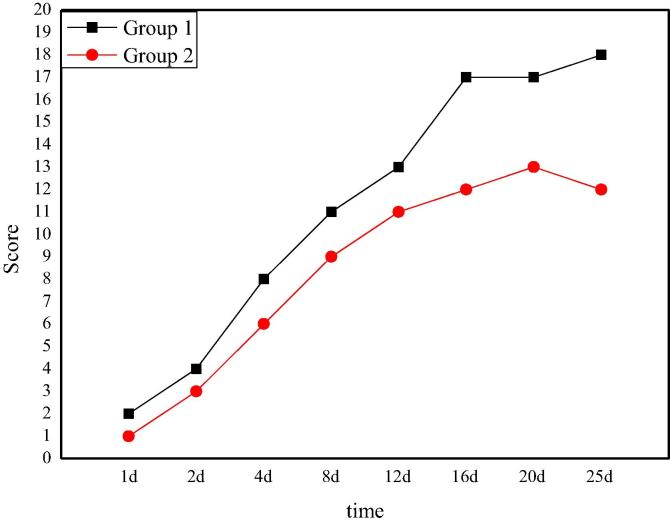
The neurological recovery score of rats after Allen's spinal cord injury is shown in Fig. 2. From the picture, it can be seen that after the spinal cord injury operation, the neurological function of the two groups of rats is temporarily 0. The neurological function of group 1 recovers gradually with the passage of time, and finally reaches 7 points on the 12th day. Compared with the recovery of nerve function in the group 1, the recovery of nerve function in the group 2 is not enough and reaches a stable level on the 12th day with a score of 3. The difference of nerve function recovery between the two groups is obvious, which also shows that the nerve function recovery of rats with spinal cord injury model based on Allen's has significant difference under different beating volume.
Fig. 2.
The score of neurological recovery after Allen's spinal cord injury in rats.
3.3. Action recovery of rats after spinal cord injury
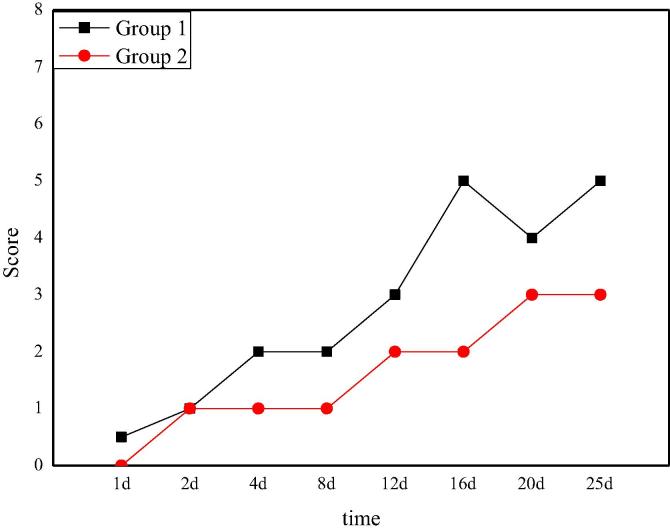
The action recovery score of rats after Allen's spinal cord injury is shown in Fig. 3. From the data and curves in the graph, it can be clearly seen that after spinal cord injury surgery, rats in the experimental group 1 still have a little action ability, while rats in the group 2 have completely lost their action ability. After several days of the same recovery nursing, the action ability of the two groups of rats is gradually restored, but the situation of the rats in the group 1 is significantly better. On the 16th day, the score of group 1 achieves stability with a score of 5. On the fifth day, the group 2 achieves stability with a score of 2. Comparing the two groups, it can be seen that the action ability of rats in the group 1 is better than that in the group 2, which indicates that the smaller the impact is, the better the action ability of rats is.
Fig. 3.
Allen's score of action recovery ability in rats after spinal cord injury operation.
3.4. Analysis of auditory ability in rats after spinal cord injury
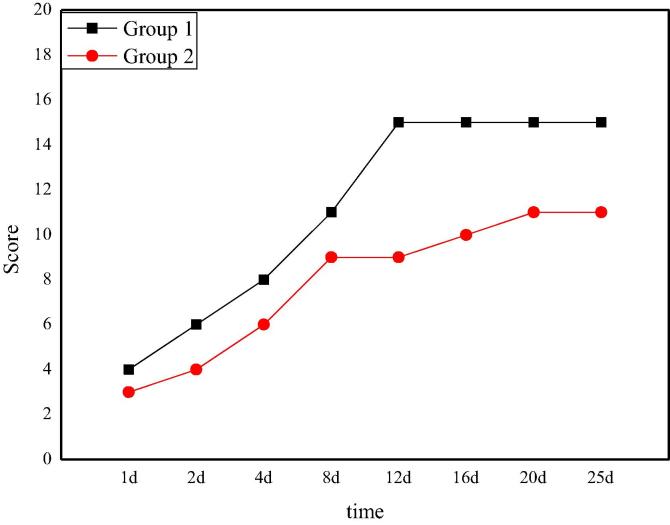
The auditory ability score of rats after Allen's spinal cord injury is shown in Fig. 4. From the data and curves in the figure, it can be clearly seen that after spinal cord injury surgery, the auditory ability of rats in the experimental group 1 and the experimental group 2 has not been completely lost. In the following days, the hearing ability of the two groups is well restored and nursed, and the hearing ability is gradually restored. However, the rats in group 1 recover better. On the 12th day, the score of group 1 achieves stability with a score of 15. On the eighth day, the experimental group 2 achieves stability with a score of 8. Comparing the two groups, it can be seen that the recovery of rat's auditory ability in group 1 is more positive than that in group 2, which indicates that the hitting power has a great influence on the recovery of rat's auditory ability.
Fig. 4.
The score of auditory recovery after Allen's spinal cord injury in rats.
3.5. The score of tactile ability in rats after Allen's spinal cord injury
The score of tactile ability in rats after Allen's spinal cord injury is as shown in Fig. 5. From Fig. 5, it can be observed that the tactile recovery ability of the experimental group 1 is better than that of the experimental group 2 when the score is used to evaluate the tactile recovery of rats after spinal cord injury. The score of group 1 is 17 when it reaches stability and the score of group 2 is 12 points. However, the time of stabilization of the two groups is basically the same. Therefore, after spinal cord injury surgery, the rats in group 1 perform better in tactile recovery, which also shows that different hitting power would lead to different recovery effects.
Fig. 5.
The score of tactile ability in rats after Allen's spinal cord injury.
3.6. The score of dietary ability in rats after Allen's spinal cord injury
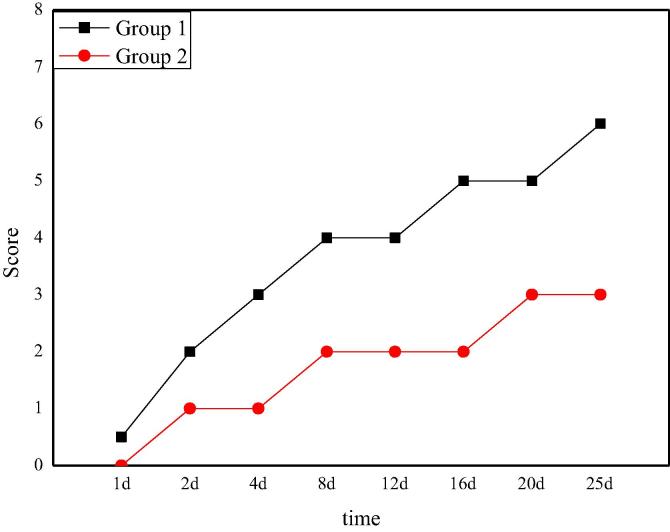
The dietary recovery score of rats after Allen's spinal cord injury is shown in Fig. 6. From the picture, it can be seen that after the spinal cord injury operation, the nerve function of the two groups of rats is close to zero temporarily. The dietary ability of rats in group 1 gradually recovers over time, and finally reaches a stable level of 5 points on the 16th day. Compared with the recovery of nerve function in the group 1, the recovery of tactile function in the group 2 is not obvious enough, and reaches a stable level on the 20th day with a score of 3. The difference of tactile function recovery between the two groups is obvious, which also shows that the recovery of nerve function of rats is more optimistic under a smaller hitting power based on Allen's spinal cord injury model.
Fig. 6.
The dietary recovery score of rats after Allen's spinal cord injury.
4. Discussion
In this study, the construction of rat spinal cord injury model guided by Allen's model has been mainly studied. Male rats aged 4–5 weeks and weighing about 250 g are selected as subjects in the Animal Laboratory Center of XX Hospital. Rats are divided into two groups, which are experimental group 1 and experimental group 2, respectively, so as to construct spinal cord injury model in rats. The first group is given 300 g.cm hitting force of T10 spinal cord, and the second group is given 500 g.cm hitting force of T10 spinal cord. Within 25 days after spinal cord injury in Allen's rats, the survival, neurological function, diet, motor ability, tactile ability and auditory ability of the two groups are monitored and evaluated daily. The results show that, no matter in survival, neurological function, diet, motor ability, tactile ability and auditory ability, the recovery of group 1 after spinal cord injury treated with 300 g.cm hitting force of T10 segment is better than that of group 2 after spinal cord injury treated with 500 g.cm hitting force. It shows that the smaller impact force is more beneficial to the construction of spinal cord injury model in rats under the guidance of Allen's model.
Therefore, the construction of spinal cord injury model in rats under the guidance of Allen's model is studied through different groups and the intervention methods of hitting force. The experimental group 1 with 300 g.cm hitting force of T10 segment spinal cord is closer to the actual experimental requirements for the construction of rat spinal cord injury model under the guidance of Allen's model. The intervention with hitting force as the core under Allen's model has important research value. The research of this study is also limited by some limitations, such as the number of samples is not large enough, the results obtained are slightly less convincing, and the conditions required by the experiment cannot be fully satisfied due to the limitations of conditions. In the follow-up study, the number of rat samples can be increased, and the experiment can be carried out under the conditions that fully meet the requirements, which will reduce the interference caused by some other factors. This study has important reference value for later researchers.
Funded by the fund
Tianjin Natural Science Foundation: Project number: 17JCY13JC27300.
Tianjin Health Bureau Foundation: Project number: 2015KR20.
Footnotes
Peer review under responsibility of King Saud University.
References
- Chen Y., Xiong M., Dong Y. Chemical control of grafted human PSC-derived neurons in a mouse model of Parkinson’s disease. Cell Stem Cell. 2016;18(6):817–826. doi: 10.1016/j.stem.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L., Duan B., Huang T. Identification of spinal circuits involved in touch-evoked dynamic mechanical pain. Nat. Neurosci. 2017;20(6):804. doi: 10.1038/nn.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder G., Tawfik V.L., Wang D. Loss of μ opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nat. Med. 2017;23(2):164. doi: 10.1038/nm.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman J.L., Sompol P., Kraner S.D. Blockade of astrocytic calcineurin/NFAT signaling helps to normalize hippocampal synaptic function and plasticity in a rat model of traumatic brain injury. J. Neurosci. 2016;36(5):1502–1515. doi: 10.1523/JNEUROSCI.1930-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Zhao J., Peng X. Molecular mechanism of MiR-136-5p targeting NF-κB/A20 in the IL-17-mediated inflammatory response after spinal cord injury. Cell. Physiol. Biochem. 2017;44(3):1224–1241. doi: 10.1159/000485452. [DOI] [PubMed] [Google Scholar]
- Hu J., Ni S., Cao Y. The angiogenic effect of microRNA-21 targeting TIMP3 through the regulation of MMP2 and MMP9. PLoS ONE. 2016;11(2) doi: 10.1371/journal.pone.0149537. 149537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Lang Y., Zhang T. Lentivirus-mediated PGC-1α overexpression protects against traumatic spinal cord injury in rats. Neuroscience. 2016;328:40–49. doi: 10.1016/j.neuroscience.2016.04.031. [DOI] [PubMed] [Google Scholar]
- Kim R.B., Irvin C.W., Tilva K.R. State of the field: an informatics-based systematic review of the SOD1-G93A amyotrophic lateral sclerosis transgenic mouse model. Amyotrophic Lateral Sclerosis Frontotemporal Degeneration. 2016;17(1–2):1–14. doi: 10.3109/21678421.2015.1047455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakes E.H., Allen K.D. Gait analysis methods for rodent models of arthritic disorders: reviews and recommendations. Osteoarthritis Cartilage. 2016;24(11):1837–1849. doi: 10.1016/j.joca.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhou L.J., Wang J. TNF-α differentially regulates synaptic plasticity in the hippocampus and spinal cord by microglia-dependent mechanisms after peripheral nerve injury. J. Neurosci. 2017;37(4):871–881. doi: 10.1523/JNEUROSCI.2235-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z., Zhang Y.P., Liu W. A controlled spinal cord contusion for the rhesus macaque monkey. Exp. Neurol. 2016;279:261–273. doi: 10.1016/j.expneurol.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Sparrey C.J., Salegio E.A., Camisa W. Mechanical design and analysis of a unilateral cervical spinal cord contusion injury model in non-human primates. J. Neurotrauma. 2016;33(12):1136–1149. doi: 10.1089/neu.2015.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore N., Hlubek R., Danielson J. First human implantation of a bioresorbable polymer scaffold for acute traumatic spinal cord injury: a clinical pilot study for safety and feasibility. Neurosurgery. 2016;79(2):305–312. doi: 10.1227/NEU.0000000000001283. [DOI] [PubMed] [Google Scholar]
- Xie X., Ma L., Xi K. MicroRNA-183 suppresses neuropathic pain and expression of AMPA receptors by targeting mTOR/VEGF signaling pathway. Cell. Physiol. Biochem. 2017;41(1):181–192. doi: 10.1159/000455987. [DOI] [PubMed] [Google Scholar]