Abstract
Objective
Acupuncture is a commonly used method to provide motor-symptomatic relief for patients with Parkinson s disease (PD). Our objective was to evaluate protective effects of acupuncture treatment and potential underlying mechanisms according to the “gut-brain axis” theory.
Methods
We employed a 6-OHDA-induced PD rat model. The effects of acupuncture on disease development were assessed by behavioural tests and immunohistochistry (IHC). ELISA, qPCR and western blot (WB) were employed to measure inflammatory parameters and Fe metabolism in the substantia nigra (SN), striatum, duodenum and blood, respectively.
Results
Our data show that acupuncture can significantly increase the expression of tyrosine hydroxylase (TH), compared with untreated and madopa treated rats (P < 0.01 and P < 0.05, respectively). Furthermore we could observe significantly decreased levels of pro-inflammatory markers in the duodenum and serum (P < 0.05), reduced deposition of Fe in the substantia nigra (P < 0.05) and but no change in transferrin expression after acupuncture treatment. The mRNA ratio of DMT1/Fpn1 in the SN of acupuncture treated rats (1.1) was comparable to that of the sham group (1.0) which differed both significantly from the untreated and madopa treated groups (P < 0.05). Furthermore, after acupuncture expression of α-synuclein was decreased in the duodenum.
Conclusions
Acupuncture can reduce iron accumulation in the SN and protect the loss of dopamine neurons by promoting balanced expression of the iron importer DMT1 and the iron exporter Fpn1. Furthermore CNS iron homeostasis may be affected by reduced systemic and intestinal inflammation.
Keywords: Parkinson’s disease (PD), Acupuncture, Iron accumulation, Gut-brain
Abbreviations: SNc, substantia nigra pars compacta; GI, Gastrointestinal; SN, substantia nigral; DA, dopamine; TH, tyrosine hydroxylase; IHC, immunohistochemical; DMT1, divalent metalloion transporter 1; Fpn1, ferritin 1
1. Introduction
Parkinson’s disease (PD) is the most common motor related neurodegenerative disease up to date (Poewe et al., 2017). The prevalence of Parkinson’s disease increases with age; it affects 2990 per 100,000 individuals over the age of 70, globally (Pringsheim et al., 2014). As the aetiology and the pathogenesis of the disease are not clear, the clinical diagnosis is based on the symptoms, signs and drug sensitivity as reference, and due to the lack of objective indicators, treatment is provided mainly to improve the motor symptoms of PD (Goswami et al., 2017). In addition, there is treatment of “honeymoon” and side effects, whereas regenerated dopaminergic neurons cannot be repaired, nor further degeneration of neurons be prevented (Harrison and Dexter, 2013). Therefore, there is an urgent need for new strategies for the treatment of PD (Choong et al., 2016). In addition to standard treatments, acupuncture is the most commonly used complementary and alternative therapy for PD patients, according to a recent study (Chen et al., 2015, Zeng and Zhao, 2016).
The “gut-brain” axis consists of a bidirectional endocrine communication system between the central nervous system (CNS) and the enteric nervous system (ENS) as well as a connection of immune regulation between gut and brain (Mulak and Bonaz, 2015). ENS is a comprehensive network of gastrointestinal parietal neurons and a major participant in the “gut-brain “axis (Cryan and Dinan, 2012). In recent years, PD research has been focused on the gastrointestinal tract and the related ENS (Clairembault et al., 2015). The ENS is closely connected to the gastrointestinal myenteric macrophages (MM φ) by internal and external neural mechanisms to ensure an appropriate response to pathogens and inflammatory stimuli (Yoo and Mazmanian, 2017). MM φ is bipolar or stellate in structure, similar to the stellate structure of microglia in the brain (Gabanyi et al., 2016), and the function of MM φ can phagocytosis of neuronal fragments in order to maintain the stability of the ENS structure (Kulkarni et al., 2017), MMφ are similar to the microglia of the brain in respect of structure and function. They can be stellate in structure and phagocyte neural fragments in order to maintain the structure of the ENS, analogous to the phagocytosis of apoptotic neurons by microglia in the CNS. (Fourgeaud et al., 2016).
Gastrointestinal (GI) dysfunction is one of the most common non-motor symptoms of PD, and constipation is one of the most prominent, which precedes the movement symptoms for more than 10 years, assuming that GI dysfunction is a pre-motion sign (Fasano et al., 2015, Pagano et al., 2017). PD patients exhibit ENS accumulation of α-synuclein, which may be the basis for gastrointestinal dysfunction. There is evidence that the PD originates in the gut, then spreads to the olfactory bulb and the tail of the brain stem and from there into the brain stem and the midbrain until finally reaching the cerebral cortex (Sánchez-Ferro et al., 2015, Jankovic, 2016).
This study is based on the theory of “gut-brain-axis”, the early warning of gastrointestinal symptoms in Parkinson’s disease, and the circulation of the “stomach meridian”, and was designed to determine if acupuncture can, in fact, regulate the immune function of the gut and iron homeostasis of the body, and thereby alleviate the microenvironment of inflammation in the brain, thus to protect the loss of TH+ DA neurons in 6-OHDA-induced rats.
2. Material and methods
2.1. Rats
Sprague-Dawley male rats were provided by the Zhejiang Medical Laboratory Animal Center, and subjected to experiments after 1 week of adaption. All experimental procedures strictly complied with the regulations of Zhejiang University of traditional Chinese Medicine on the management and protection of experimental animals.
2.2. 6-OHDA lesion
6-OHDA lesions were applied as described elsewhere [Include citation of Fabrizius et al]. Briefly, 8–10 week old rats with a weight of 220–140 g were starved overnight and anesthetized by injection of 0.3% pentobarbital sodium (Shanghai Rongbai Biotechnology Co., Ltd. Cargo number P3761) (i.p.) at 150 mg/kg. During surgery rats were placed on a 36–37 °C heating pad in a stereoscopic locator (below the horizontal coordinate sinus calibrator) then 8 μg of 6-OHDA (Sigma-Aldrich-DK, added with 0.02% ascorbic acid solution) was injected at a rate of 1 μL/min into the left SNc according to Paxinos and Watson’s stereotaxic map of rat brain: TB, −2.3 mm; AP, −5.2 mm; ML, 2.1 mm; DV, −7.8 mm. All animals were closely monitored during surgery. The sham group was injected with 0.9%NaCl and otherwise treated the same.
2.3. Treatment protocols
Lesioned animals were either left untreated (sham/model group) or treated with acupuncture or madopa. Rats were randomly distributed in these groups (n = 10–14) except for weight, so that the average weight of each group was similar.
Acupuncture was performed for 15 min daily starting at day 15 post-surgery with an acupuncture needle model of Φ0.18 × 25 mm (Maanshan Bond Medical Instruments Co., Ltd. China) using the following points: Zhongwan (CV12), Tianshu (ST25), Guanyuan (CV4). During acupuncture rats were fixed by Self-made black cloth bag.
6.25 mg/kg Madopa (Shanghai Roche) was intragastricly administered daily starting at day 15 post-surgery.
Control groups were left untreated but were given the same binding force, 15 min daily starting at day 15 post-surgery.
Weight was measured in all groups once a week starting on day 15 post-surgery.
2.4. Isolation of blood and organs
Rats were sacrificed on day 46 post-surgery. Blood was taken from the heart for serum samples, and together with duodenum and SN tissue samples rapidly frozen in liquid nitrogen and transferred to −80 °C. For isolation of the SN, the intact brain tissue was stripped off, and the substantia nigra and striatum were rapidly separated according to the anatomical position. Whole brain samples were fixed with 4% paraformaldehyde.
2.5. Rotation test
On day 15 and day 45 post-surgery apomorphine hydrochloride (Sigma-Aldrich, DK) was injected (0.05 mg/kg, i.p.) and after 3 min rats were placed in a plastic basin (50 cm in diameter) and the number of rotations per minute was counted for each rat.
2.6. Suspension test
Stainless steel wire of 60 cm length and 0.3 cm diameter was hung on a foam pad 80 cm above the ground. The rats’ forepaws were placed on the wire, and time was recorded during which the rats were able to suspend the rope. Rats dropping the rope or catching the rope with only one claw for less than 3 s were considered failure. Scores were assigned according to the citation:1 = 0–4 s; 1 = 5–9 s; 2 = 10–14 s; 3 = 15–19 s; 4 = 20–24 s; 5 = 25–29 s; 6 = 25–29 s, each rat was tested three times and the average value was obtained to evaluate the limb function of each rat. Suspension tests were performed on day 15 and day 45 post-surgery.
2.7. Immunohistochemistry
Brains were fixed with 4% paraformaldehyde and embedded in paraffin. Paraffin sections (20 µm) of the SN were sliced, starting 4.8 mm from the front fontanel. Immunostaining was performed with a TH antibody (Sigma-Aldrich) and was utilized, according to manufacturer‘s instructions. Three slices of the same site were selected for each animal. The nigral area of the contralateral substantia nigra was counted using the image analysis software IPP6.0 for the injured side under 40 x magnification. The TH positive area was counted, and the average number of positive cells was calculated from three slides of each rat.
2.8. Elisa
Elisa kits for TNF-α, IL-1β, IFN-γ, Fe and transferrin were used from Nanjing Jiancheng Bioengineering Institute, China and serum samples and tissue samples from the SN and the duodenum were prepared and measurements performed according to the manufacturer‘s protocol. Sample values were calculated according to the OD value of absorbance.
2.9. Quantitative
2.9.1. RNA isolation and quantitative real-time PCR
Total RNA was extracted according to the manufacturer‘s instructions with Total RNA BioTeke (TaKaRa) then cDNA was synthesized with PrimeScriptTM RT reagent Kit (TaKaRa). cDNA was amplified by qPCR with SYBR Premix Ex TaqTM Ⅱ (TaKaRa) on a Bio-Rad iQ5 PCR thermocycler. The CT values were measured and calculated by computer software (Bio-Rad IQ5 Date Analysis), and the transcriptional level was calculated by formula the 2-△△CT formula. The following primers were used:
| Primer | Sequence (5′to3′) |
| Fpn1F | GCCTTGTTCGGACTGGTCTGTTC |
| Fpn1R | CCAGGCATGAACACGGAGATCAC |
| DMT1F | CCTGTGGCTAATGGTGGAGTTGG |
| DMT1R | GGAGATTGATGGCGATGGCTGAC |
| β-actin-F | GGAGATTACTGCCCTGGCTCCTA |
| β-actin-R | GACTCATCGTACTCCTGCTTGCTG |
2.9.2. Protein extraction and western blot
1–2 ml frozen tissue (three tissue is tested here, SN, striatum and duodenum) was placed in homogenizer, and was shredded with clean scissors. Then 400 μL lysis buffer (Keygen whole cell Lysis Assay, include: 1 ml Lysis Buffer, 5 μL phosphatase inhibitor, 1 μL protease inhibitor and 5 μL PMSF) was added in homogenizer to homogenize and then put on the ice. Tissue was grinded 15 times and after 30 min of pyrolysis, the lytic fluid was centrifuged at 4 °C for 12000 rpm for 5 min. The supernatant was separated and packed in 0.5 ml centrifugal tube, that is, whole protein extract, protein quantification (BCA method). The supernatant was stored at −70 °C.
3. Statistical analysis
The statistical analysis was carried out by SPSS13.0. The data were expressed as mean ± standard deviation (±S). One-way ANOVA analysis was used for multi-group comparison, and Dunnett was used for post-test correction. P < 0. 05 was considered statistically significant.
4. Results
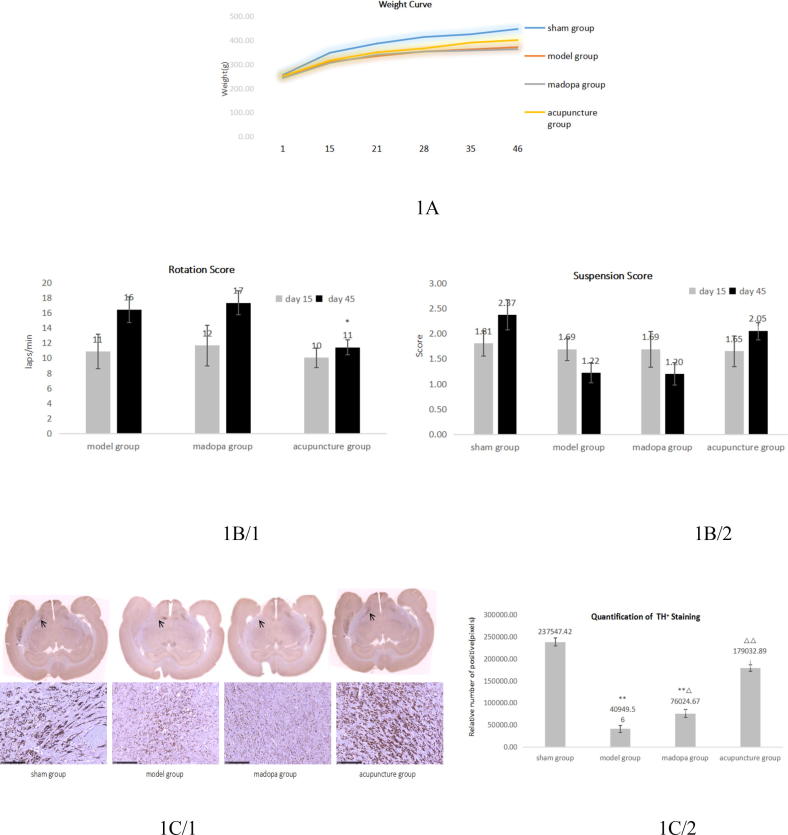
To analyse the effect of acupuncture on neural damage we applied the 6-OHDA lesion PD model to rats, which were either left untreated (model group), daily treated with madopa (madopa group) or daily treated with acupuncture (acupuncture group) from day 15 to day 45 post-surgery. Weight levels were similar for all rat groups throughout treatment (Fig. 1A). IHC staining on day 45 revealed reduced TH expression in the CSN of the model, madopa and acupuncture group, verifying the onset of neural damage. Of note, TH expression in the acupuncture group was significantly less diminished compared to sham, showing 24,6% less TH + staining than sham, as compared to the model (82,8%) madopa group (68%) (Fig. 1C). In behavioural tests we measured elevated rotation scores in all 6-OHDA lesioned rats on day 45 compared to day 15, but significantly lower rotation scores in acupunctured rats on day 45 compared to untreated and madopa treated rats, indicating a less severe damage of SN neurons (Fig. 1B left). In accordance with rotation test results, acupunctured rats showed no significant drop of suspension scores on day 45, other than rats of the madopa and model group rats, who displayed significantly lower capability of performing the suspension test on day 45 (Fig. 1B right). These data suggest that acupuncture may delay the onset of neuron damage and ameliorate motor symptoms.
Fig. 1.
Acupuncture ameliorates motor symptoms and delays onset of neuron damage in 6-OHDA lesioned rats. (A) Weight curve; sham n = 12/model n = 12/madopa n = 11/acupuncture n = 13. (B) Left: Rotation score. Rotation test was performed for each rat on day 15 and day 45. Right: Suspension score. Suspension test was performed for each rat on day 15 and day 45. Sham n = 5/model n = 5/madopa n = 11/acupuncture n = 13; SPSS13.0. One-way ANOVA analysis was used for multi-group comparison, and Dunnett and Bonferroni were used for post-test correction. *Compared with model on day 45 P < 0,05. (C) IHC: TH staining of the SN. Left: whole brain sections and representative images of the SN (40x magnification). Arrows indicate lesioned brain areas. Right: Quantification of TH + areas. 3 sections of 4 rats of each group were quantified and means ± SEM were calculated out of 12 sections in total for each group. One-way ANOVA analysis was used for multi-group comparison, and Dunnett and Bonferroni were used for post-test correction. **Compared with sham: P < 0,01; Δ compared with model: P < 0,05; ΔΔ compared with model: P < 0,01.
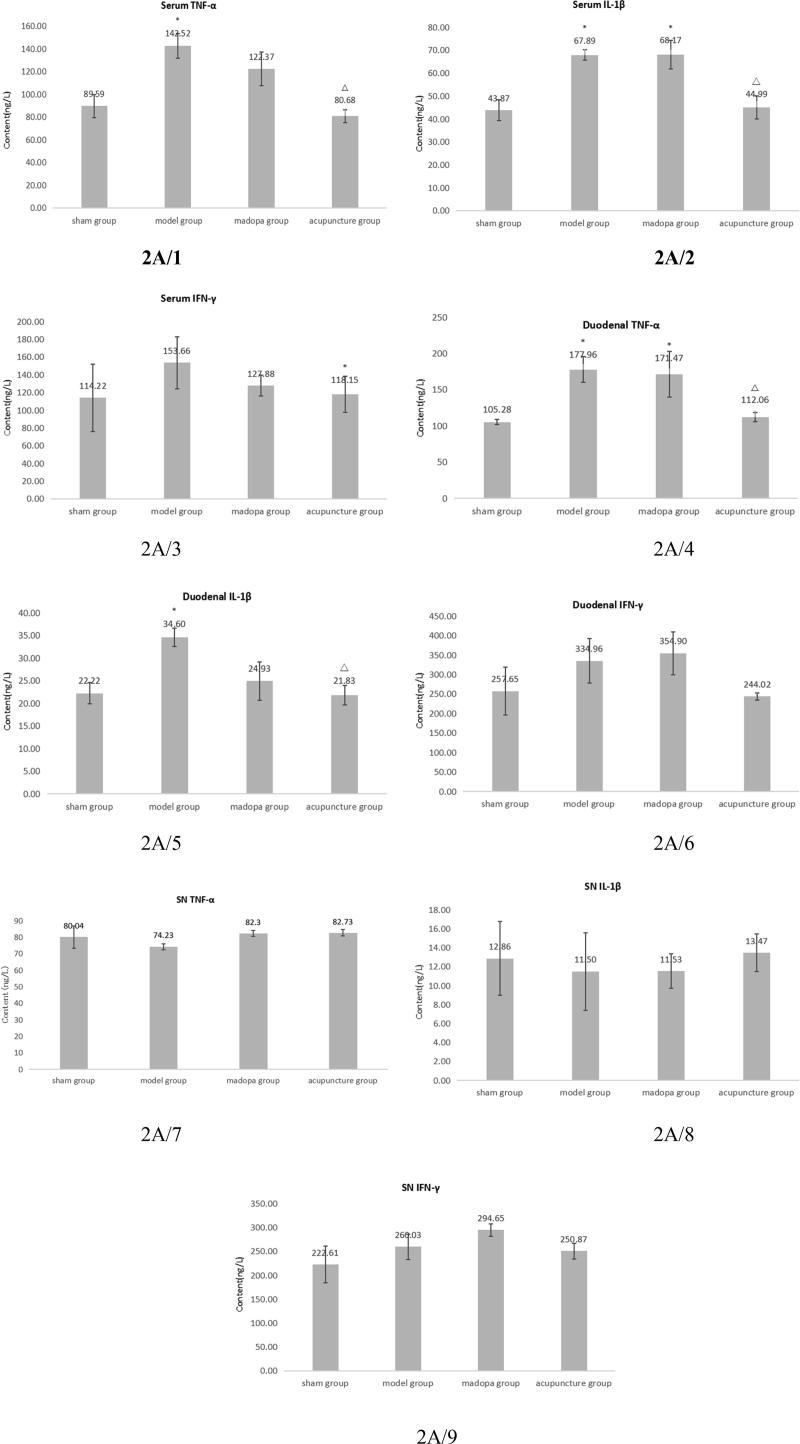
To evaluate the impact of acupuncture on the inflammatory response along the “gut-brain” axis which may be involved in mediating neuronal damage, we measured the levels of the pro-inflammatory cytokines, TNFα, IL-1β and INFγ, in serum, duodenum and the SN (Fig. 2A-C). While there were no elevated levels of proinflammatory cytokines after application of 6-OHDA lesion in the SN (Fig. 2C), we observed an increase for serum (Fig. 2A) and duodenal (Fig. 2B) TNFα and IL1β in the model group, there is statistical significance (P < 0.05). But, there were no changes in INFγ levels in any tissue, whereas cytokine levels of acupunctured rats were comparable to the sham group. These findings suggest acupuncture may have an impact on a 6-OHDA-induced inflammatory response in the duodenum.
Fig. 2.
Acupuncture has an influence on proinflammatory cytokine levels in serum and duodenum of 6-OHDA lesioned rats. ELISA for TNF-α, IL-1β and INF-γ, measured in serum (A), duodenum (B) and SN (C) of sham and 6-OHDA lesioned rats on day 46. Means ± SEM were calculated of: Sham n = 7/model n = 7/madopa n = 6/acupuncture n = 8, ELISA for each individual rat were done in triplicates. *compared with sham P < 0,05; Δcompared with model P < 0,05. SPSS13.0. One-way ANOVA analysis was used for multi-group comparison, and Dunnett and Bonferroni were used for post-test correction.
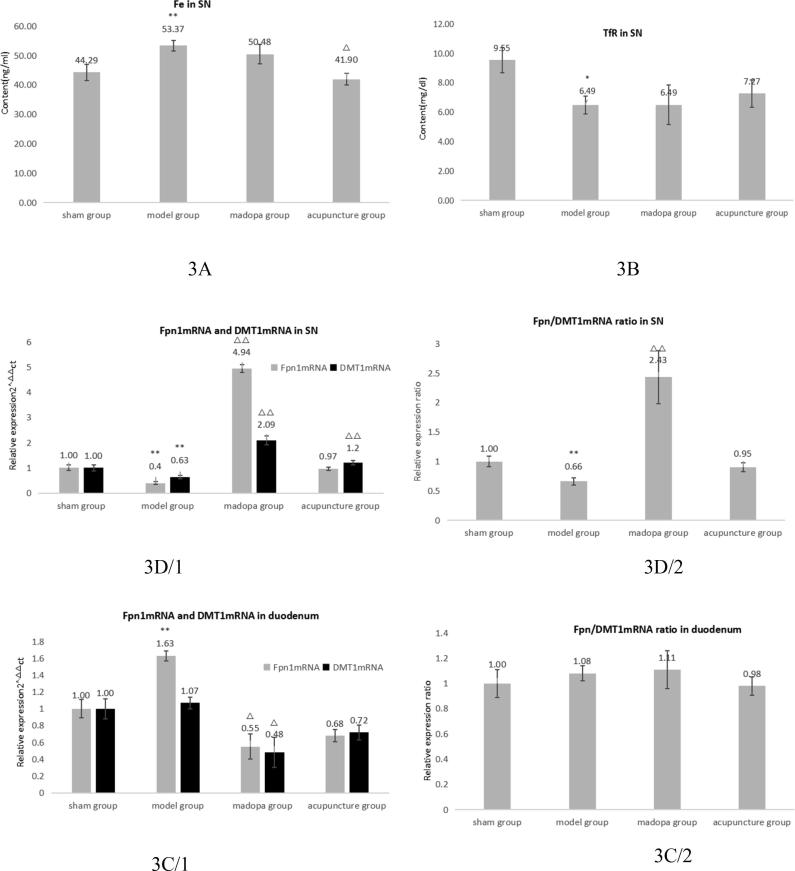
To determine if acupuncture had any influence on iron homeostasis, we measured the levels of iron and Transferrin Receptor (TfR) in tissue homogenates of the SN 46 days after 6-OHDA lesions by ELISA. Fe levels were increased (P < 0.05) in the model group compared to sham, which could be prevented by acupuncture treatment (Fig. 3A). TfR levels were (P < 0.05) decreased in all 6-OHDA lesioned rats (Fig. 3B). Next, we analysed Fpn1 and DMT1 expression in SN and duodenum. The ratio of Fpn1/DMT1 mRNA was comparable for all four groups (Fig. 3C). In contrast, Fpn1/DMT1 ratios in SN were differing significantly in the untreated rats (1, 53) and madopa treated rats (0, 45) from the sham group (1, 0), whereas acupuncture treated rats displayed a similar ratio (1, 1). This was also reflected in qPCR results for Fpn1 and DMT1 in the SN (Fig. 3D). Taken together, we conclude that acupuncture may influence on nigral iron homeostasis by promoting balanced expression of Fpn1 and DMT1.
Fig. 3.
Acupuncture has an impact on nigral iron homeostasis in 6-OHDA lesioned rats. ELISA for Fe (A) and transferrin (B) in SN. Means ± SEM were calculated of: Sham n = 7/model n = 7/madopa n = 6/acupuncture n = 8, ELISAs for each individual rat were done in triplicates. Expression of Fpn1 and DMT1 and the ratio thereof in duodenum (C) and SN (D) was measured by qRT-PCR. Means ± SEM were calculated out of: Sham n = 7 /model n = 7/madopa n = 6/acupuncture n = 8. *Compared with sham P < 0,05; **compared with sham: P < 0,01; Δcompared with model P < 0,05; ΔΔcompared with model P < 0,01. SPSS13.0. One-way ANOVA analysis was used for multi-group comparison, and Dunnett and Bonferroni were used for post-test correction.
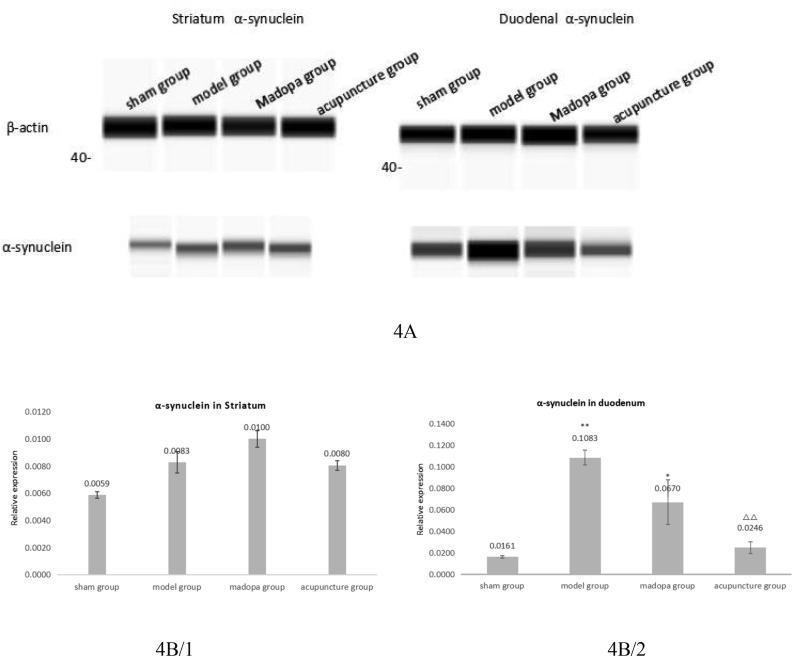
To analyse the expression of α-synuclein we performed western blots of the striatum and the duodenum. Expression levels were similar in all four groups in the stratium (Fig. 4A and 4B). In the duodenum we observed significantly decreased expression after acupuncture treatment by almost 80% compared to the model group, which indicates that acupuncture may influence on TH + DA neuron by duodenum.
Fig. 4.
Acupuncture mediates down-regulation of α-synuclein expression in the duodenum of 6-OHDA lesioned rats. Western blot analysis of α-synuclein (18 kDa) expression in the stratium and duodenum (A). Intensities of western blot signals were quantified by β-actin (43 kDa) and normalized to β-actin values. Means ± SEM were calculated from independent western blot analyses for startium (B) and duodenum (C). Sham n = 7/model n = 7/madopa n = 6/acupuncture n = 8, *Compared with sham P < 0,05; **compared with sham: P < 0,01; ΔΔcompared with model P < 0,01. SPSS13.0. One-way ANOVA analysis was used for multi-group comparison, and Dunnett and Bonferroni were used for post-test correction.
5. Discussion
Parkinson’s disease is a rapidly growing health problem in an aging society. Genetic risk has been identified, and furthermore environmental factors and epigenetic events may be the cause for the onset of the disease in most PD patients (Ritz et al., 2016). Environmental factors may have an effect on the occurrence and progression of PD through the gastrointestinal tract (GI) (Kieburtz and Wunderle, 2013). The physiological function and movement of the GI are affected by the neurotransmitter and immunological signalling produced by the central nervous system as well as by the intestine. hormones and neuropeptides, which may in turn affect the brain (Selkrig et al., 2014), as is the case for dopamine of which almost half of the total amount is produced in the gastrointestinal tract (Wall et al., 2014). Therefore, regulating gastrointestinal function may affect neuroinflammation, iron homeostasis and dopamine loss in PD.
The accumulation of iron in the SN is an early characteristic of PD (Belaidi and Bush, 2016), and the amount of nigral iron is related to the severity of motor symptoms in PD patients (Guan et al, 2017). The destruction of iron homeostasis may lead to disorder of the iron metabolism and the degeneration of DA neurons in PD patients. Glial cells play a key role in the regulation of iron homeostasis (Rathore et al., 2012). On the one hand, activated microglia release proinflammatory cytokines such as IL-1β and TNF-α, but are also involved in, regulated the import of DMT1 from microglial cells and down-regulated ferritin exportation via Fpn1, which results in the accumulation of iron in microglial cells (Wang et al., 2015). Activated microglial cells can synthesize and release lactoferrin (LF) to further enhance iron overload. These findings suggest that the synergistic effects of neuroinflammation and iron accumulation promote degeneration of Parkinson’s disease neurons. Therefore, to inhibit neuroinflammation and to balance the ferritin import transporter DMT1 and ferritin export transporter Fpn1 is particularly important in improving the degeneration of PD dopamine neurons.
TH is a key enzyme in dopamine synthesis and a marker for dopaminergic neurons (Shahnawaz Khan et al., 2012). In the MFB model, the number of TH+ DA neurons in SN is close to complete loss (10% compared to untreated) in 6-OHDA-induced rats with complete unilateral injury (Fabricius et al., 2017). Within 3 weeks after unilateral MFB injury, the striatum completely loses its dopamine content, while the number of dopaminergic neurons is gradually decreased. About 5 weeks later, the neurons are nearly completely lost, indicating that neurodegenerative activity continued when the total dopamine denervated in the striatum was stable (Sarre et al., 2004). This is perfectly in line with our observations. We measured the number TH+ DA neurons on the 46th day after initiation, and the acupuncture group showed that it can protect the loss of TH+ DA neurons, as there was significant difference (P < 0.01) to the untreated model group. It has been reported that the frequency of rotational behaviour induced by apomorphine can be used as a good indicator of the severity of dopaminergic neuron damage in substantia nigra (Hoban et al., 2013). In our experiment, we found that the rotation score increased on day 15 and on day 45, indicating that the loss of neurons was progressive, but the score of the acupuncture group was significantly lower than that of the untreated group and the madopa group, which is consistent with the TH immunohistochemical results. Through acupuncture treatment, we found that the levels of IL1-lβ, TNF-α, IFN-γ in serum and duodenum decreased compared with the untreated group (p < 0.05), balanced the ferritin import via DMT1 and ferritin export via Fpn1 in SN and reduced the accumulation of Fe in the SN. However, the expression of α-synuclein in acupuncture group was obviously decreased in the duodenum. Therefore, acupuncture may influence the inflammatory state and iron homeostasis to protect the loss of TH+ DA neurons in 6-OHDA-induced PD rats.
Therefore, acupunctural regulation of the GI may be a new tool for Parkinson’s disease treatment, as thereby amelioration of the neuroinflammatory state and iron homeostasis, and in consequence apoptosis of neurons may be mediated, which may slow DA neurodegenerative degeneration and delaying disease progression.
Acknowledgments
Acknowledgements
Thanks to Dr. Xiaoqing Jin for his support and guidance. I would like to thank President Dongsheng Huang, for giving me the opportunity to continue my study after working.
The work was financially supported byZhejiang Youth Funding of TCM (2018ZQ006), Zhejiang Medical and Health Project (NO. 2019309786) and Zhejiang Provincial Natural Science Fund of China (NO. S19H090006). In addition, thanks to Dr. Xiaoqing Jin for his support and guidance. And I am grateful to President Dongsheng Huang of Zhejiang provincial people’s hospital for giving me the chance to continue my study after working.
Declaration of Competing Interest
The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions
Lihong Li obtained funding for, participated in, and oversaw the study concept and design, data analysis and interpretation, and preparation of the manuscript. Ju Lu and Yingying Sun were doing experiments, Xiaoqing Jin was in charge of the data retrieval and preparation for analysis.
Fundings
The research was supported by Zhejiang Youth Funding of Traditional Chinese Medicine (2018ZQ006), Zhejiang Medical and Health Project (NO. 2019309786) and Zhejiang Provincial Natural science founding of china (NO. S19H090006).
Data Availability
Data sharing allows researchers to verify the results of an article, replicate the analysis, and conduct secondary analyses.
Footnotes
Peer review under responsibility of King Saud University.
References
- Belaidi A.A., Bush A.I. Iron neurochemistry in Alzheimer’s disease and Parkinson’s disease: Targets for therapeutics. J. Neurochem. 2016;139:179–197. doi: 10.1111/jnc.13425. [DOI] [PubMed] [Google Scholar]
- Chen F.P., Chang C.M., Shiu J.H., Chiu J.H., Wu T.P., Yang J.L., Kung Y.Y., Chen F.J., Chern C.M., Hwang S.J. A clinical study of integrating acupuncture and western medicine in treating patients with Parkinson’s disease. Am. J. Chin. Med. 2015;43:407–423. doi: 10.1142/S0192415X15500263. [DOI] [PubMed] [Google Scholar]
- Choong C.J., Baba K., Mochizuki H. Gene therapy for neurological disorders. Expert Opin. Biol. Ther. 2016;16:143–159. doi: 10.1517/14712598.2016.1114096. [DOI] [PubMed] [Google Scholar]
- Clairembault T., Leclair-Visonneau L., Neunlist M., Derkinderen P. Enteric glial cells: New players in Parkinson’s disease? Movement Disord. 2015;30:494–498. doi: 10.1002/mds.25979. [DOI] [PubMed] [Google Scholar]
- Cryan J.F., Dinan T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Fabricius K., Barkholt P., Jelsing J., Hansen H.H. Application of the physical disector principle for quantification of dopaminergic neuronal loss in a rat 6-Hydroxydopamine nigral lesion model of Parkinson’s disease. Front. Neuroanatomy. 2017:11. doi: 10.3389/fnana.2017.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A., Visanji N.P., Liu L.W.C., Lang A.E., Pfeiffer R.F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015;14:625–639. doi: 10.1016/S1474-4422(15)00007-1. [DOI] [PubMed] [Google Scholar]
- Fourgeaud L., Traves P.G., Tufail Y., Leal-Bailey H., Lew E.D., Burrola P.G., Lemke G., Callaway G., Zagórska A., Rothlin C.V., Nimmerjahn A., Lemke G. TAM receptors regulate multiple features of microglial physiology. Nature. 2016;532:240–244. doi: 10.1038/nature17630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabanyi I., Muller P.A., Feighery L., Oliveira T.Y., Costa-Pinto, Mucida D. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell. 2016;164:378–391. doi: 10.1016/j.cell.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami P., Joshi N., Singh S. Neurodegenerative signaling factors and mechanisms in Parkinson’s pathology. Toxicol. Vitro. 2017;43:104–112. doi: 10.1016/j.tiv.2017.06.008. [DOI] [PubMed] [Google Scholar]
- Guan X., Xuan M., Gu Q., Huang P., Liu C., Wang N., Xu X.J., Luo W., Zhang M. Regionally progressive accumulation of iron in Parkinson’s disease as measured by quantitative susceptibility mapping. NMR Biomed. 2017;30 doi: 10.1002/nbm.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison I.F., Dexter D.T. Epigenetic targeting of histone deacetylase: Therapeutic potential in Parkinson’s disease? Pharmacol. Ther. 2013;140:34–52. doi: 10.1016/j.pharmthera.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Hoban D.B., Connaughton E., Connaughton C., Hogan G., Thornton C., Mulcahy P., Moloney C.C., Dowd E. Further characterisation of the LPS model of Parkinson’s disease: a comparison of intra-nigral and intra-striatal lipopolysaccharide administration on motor function, microgliosis and nigrostriatal neurodegeneration in the rat. Brain Behav. Immun. 2013;27(1):91–100. doi: 10.1016/j.bbi.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Movement disorders in 2016: Progress in Parkinson disease and other movement disorders. Nat. Rev. Neurol. 2016;13(2):76–78. doi: 10.1038/nrneurol.2016.204. [DOI] [PubMed] [Google Scholar]
- Kieburtz K., Wunderle K.B. Parkinson’s disease: Evidence for environmental risk factors. Movement Disord. 2013;28:8–13. doi: 10.1002/mds.25150. [DOI] [PubMed] [Google Scholar]
- Kulkarni S., Micci M.A., Leser J., Shin C., Tang S.C., Fu Y.Y., Liu L.S., Li Q., Saha M., Li C.P., Enikolopov G., Becker L., Rakhilin N., Anderson M., Shen X.L., Dong X.Z., Butte M.J., Song H.J., Southard-Smith E.M., Kapur R.P., Bogunovic M., Pasricha P.J. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc. Natl. Acad. Sci. USA. 2017;114:E3709–E3718. doi: 10.1073/pnas.1619406114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulak A., Bonaz B. Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroenterol. 2015;21(37):10609–10620. doi: 10.3748/wjg.v21.i37.10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano G., Yousaf T., Wilson H., Niccolini F., Polychronis S., Chaudhuri K.R., Politis M. Constipation is not associated with dopamine transporter pathology in early drug-naïve patients with Parkinson’s disease. Eur. J. Neurol. 2017;25(2):307–312. doi: 10.1111/ene.13503. [DOI] [PubMed] [Google Scholar]
- Poewe W., Seppi K., Tanner C.M., Halliday G.M., Brundin P., Volkmann J., Schrag A.E., Lang A.E. Parkinson disease. Nat. Rev. Dis. Primers. 2017;3:17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- Pringsheim T., Jette N., Frolkis A., Steeves T.D. The prevalence of Parkinson’s primers: A systematic review and meta-analysis. Mov. Disord. 2014;29(13):1583–1590. doi: 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- Rathore K.I., Redensek A., David S. Iron homeostasis in astrocytes and microglia is differentially regulated by TNF-alpha and TGF-beta1. Glia. 2012;60:738–750. doi: 10.1002/glia.22303. [DOI] [PubMed] [Google Scholar]
- Ritz B.R., Paul K.C., Bronstein J.M. Of pesticides and men: a California story of genes and environment in Parkinson’s disease. Curr. Environ. Health Rep. 2016;3:40–52. doi: 10.1007/s40572-016-0083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Ferro Á., Rábano A., Catalán M.J., Rodríguez-Valcárcel F.C., Díez S.F., Herreros-Rodríguez J., Desojo L.V. In vivo gastric detection of α-synuclein inclusions in Parkinson’s disease. Movement Disord. 2015;30:517–524. doi: 10.1002/mds.25988. [DOI] [PubMed] [Google Scholar]
- Sarre S., Yuan H., Jonkers N., Van Hemelrijck A., Ebinger G., Michotte Y. In vivo characterization of somatodendritic dopamine release in the substantia nigra of 6-hydroxydopamine-lesioned rats. J. Neurochem. 2004;90:29–39. doi: 10.1111/j.1471-4159.2004.02471.x. [DOI] [PubMed] [Google Scholar]
- Selkrig J., Wong P., Zhang X., Pettersson S. Metabolic tinkering by the gut microbiome: Implications for brain development and function. Gut Microbes. 2014;5:369–380. doi: 10.4161/gmic.28681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahnawaz Khan, M., Tabrez, S., Priyadarshini, M., Priyamvada, S., Khan, M., 2012. Targeting Parkinson’s-tyrosine hydroxylase and oxidative stress as points of interventions. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders) 11, 369–380. [DOI] [PubMed]
- Wall R., Cryan J.F., Ross R.P., Fitzgerald G.F., Dinan T.G., Stanton C. Springer; New York: 2014. Bacterial neuroactive compounds produced by psychobiotics; pp. 221–239. [DOI] [PubMed] [Google Scholar]
- Wang J., Bi M., Liu H., Song N., Xie J. The protective effect of lactoferrin on ventral mesencephalon neurons against MPP C is notconnected with its iron binding ability. Sci. Rep. 2015;5:10729. doi: 10.1038/srep10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo B.B., Mazmanian S.K. The enteric network: Interactions between the immune and nervous systems of the gut. Immunity. 2017;46:910–926. doi: 10.1016/j.immuni.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng B.Y., Zhao K.C. Effect of acupuncture on the motor and nonmotor symptoms in Parkinson’s disease-a review of clinical studies. CNS Neurosci. Ther. 2016;22:333–341. doi: 10.1111/cns.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing allows researchers to verify the results of an article, replicate the analysis, and conduct secondary analyses.