Abstract
Object
To explore the role of microRNA-21 in human epithelial ovarian cancer (EOC).
Methods
We used RT-PCR to test the expressions of miRNA-21 in EOC cells and normal ovarian epithelial cells, as well as the tumor samples and the tumor-adjacent normal tissues. The vector of LV3 pGLV-H1-GFP-miR-21 was used to decrease the level expression of endogenous miR-21 in cells. Further, we investigated how miR-21 affected the biological events of EOC through determining the changes in proliferation, cycle and invasion of EOC cells, and measured the tumorigenesis in xenograft models. The association between phosphatase and tensin homolog deleted on chromosome ten (PTEN) and miR-21 were tested by RT-PCR. Next, siRNA was used to knockdown PTEN gene which help us to assess the functional association between miR-21 and PTEN in vivo and in vitro.
Results
In EOC cell lines and human epithelial ovarian tumor cells, we found that miR-21 altered the biological features of EOC cells, including suppression of proliferation and invasion and arrest of cell cycle, and also resulted in a decrease in tumorigenesis in the in vitro xenograft models. The association between PTEN and miR-21 was confirmed in previous research. From our results, the down-regulation of PTEN gave rise to the miR-21 decrease, regardless of the cells or tissues.
Conclusion
The suppression of microRNA-21 inhibits the progression of EOC profoundly. In EOC, miR-21 is negatively correlated with the expression of PTEN gene.
Keywords: Micro RNAs, Epithelial ovarian cancer, MicroRNA-21, Plasmid, Advanced glycation end-product
1. Introduction
Epithelial ovarian cancer (EOC) is a kind of cancel with high fatality rate, which is common among women (Webb and Jordan, 2017). Compared with other terminal cancer, the annual survival rate of EOC patients is lower (Van Berckelaer et al., 2016, Ramalingam, 2016). Therefore, it is of great significance to evaluate and develop new marker and testing technology so that EOC patients can be diagnozed at an early stage and the disease prognosis can be improved.
MicroRNAs (miRNA) are non-coding, endogenous, small RNAs, regulating the genetic expression negatively and horizontally after genetic transcription (Zhang et al., 2016a, Zhang et al., 2016b). miRNAs are transcribed into primary miRNAs by RNA polymerase Ⅱ in the cell nucleus. They are further processed into pre-miRNAs that are microprocessor compounds made of RNAase III and double-chain binding protein DGCR8. In EOC, miRNAs have been proved to be a major biological marker and cancer regulatory factor of EOC (Samuel and Carter, 2017). The first evidence that miRNAs involve in human cancer comes from molecule research featuring 13q14 deficiency in chronic lymphocytic leukemia. The research reveals that two kinds of miRNAs: miR-15a and miR-16-1 are closely related to leukemia (Calin et al., 2008). As researchers further their studies of miRNAs, down-regulated expression of miRNAs is found in other malignant tumors, including primary glioblstoma, papillary thyroid cancer, colon cancer, pancreatic tumor and so forth (Calin and Croce, 2006, Svoronos et al., 2016, Ji et al., 2017). Moreover, up-regulated expression of miRNAs is found in some Burkitt lymphoma and other kinds of lymphomas (Metzler et al., 2004).
MiR-21 is one of earliest miRNAs to be tested in human genome and its overexpression is noted in various tumors (Xu et al., 2013). Many researches show that miR-21 has been involved in all kinds of known cancer, including the formation, growth and transfer of the tumor (Huo et al., 2017, Lou et al., 2010, Zhang et al., 2016a, Zhang et al., 2016b). Besides, miR-21 expression is significantly correlated to the prognosis of tumor patients, suggesting it is likely to mark malignant tumors and prognosis.
In the study, we explored the EOC cell line and the normal ovarian epithelial cells, comparing the genetic expression of miR-21 and PTEN in the two groups. Moreover, we tested the expressions of miR-21 and PTEN in tumor samples of EOC or the tumor-adjacent normal tissues. Then, we knocked down miR-21 in SKOV3 and OVCAR3 cells and studied the relation between miR-21 and EOC through a series of in vitro and in vivo experiments. Besides, to further evaluate the probable molecular mechanism of miR-21 in EOC, we specifically excluded the PTNE genes in SKOV3 and OVCAR 3 cells so that we could further study the functional relation between PTEN and miR-21 in regulation of EOC.
2. Materials and methodology
2.1. Cell culture
HS832 (immortalized ovarian epithelial cell line), SKOV3 and OVCAR3 (the ovarian cancer cells) were bought from ATCC, and cultivated in DMEM with 10% of fetal calf serum, 100 U/ml penicillin and 100 μg/ml streptomycin. Culture conditions: 37 °C, 5% CO2.
2.2. Plasmid and siRNA-PTEN construction
Plasmid LV3Pglv-H1-GFP-miR-21 and siRNA are constructed by Shanghai Sangon Biotech Corporation. The constructed miR-21 plasmid included XhoI/BamHI restriction enzyme sequences: 5′-GGATCCTCAACATCAGTCT GATAAGCTACGATTCAA CATCAGTCTGATAAGCTA-3′, 5′-ACCGGTTCAACAATCAGTCTGATAAGCTATCAGCTA TCACTCAACATCATCAGTCTGATAAGCTACCCGGTGG-3′. Restriction enzyme reconstruction of the plasmid was carried out with BamHI enzyme and the plasmid was transformed into competent cells for amplification, extraction and virus packaging. PTEN siRNA sequence: 5′- AACCCACCACAGCUAGAACTT-3′, 5′-AAGUUCUAGCUGUGGUGGGTT-3′.
2.3. RNA extraction and quantitative RT-PCR
RNAs were extracted with RNA extraction kit (Bio-Rad) and they were transcribed inversely with inverse transcription kit (Invitrogen, Carlsbad, CA). To test miR-21 expression, miRNA TagMan was tested. To know relative transcript level, PCR was quantified in real-time on MX 3000P PCR machine (Stratagene, San Diego, CA). SYBR Green was used as the fluorophores. The primer sequence was: miR-21, 5′- GGGGATTTCTTGGTTTGTGAA-3′, 5′-ATACAGCTAGAAAAGTCCCTGAAAA-3′; PTEN, 5′- CGGCAGCATCAAATGTTTCAG-3′, 5′-AACTGGCAGGTAGAAGGCAACTC-3′. PCR parameters were: 95 °C 10 min, 40 circulations in 30 s, 1 min 60 °C. Each sample was tested for 3 times. Threshold values of each sample/primer were determined, average error and standard error were calculated. The PCR product was analyzed with the melting curve and tested with 1.8% AGE. mRNA expression was normalized against β-actin.
2.4. Cell proliferation analysis
SKOV3 and OVCAR3 cells were placed in the 96-hole cell culture plate (500cells/hole) respectively for 5 days. 96-water solution, single cell proliferation experiment (Promega, USA) was used to test cell proliferation. To put it simply, 15 mL MTT solution was added through each hole every 24 h to culture the cells for 2 h in 37 °C. Photometry test was followed. The wave length was 570 nm.
2.5. Cancer cell invasion experiment
Mitomycin (20 mg/mL, 30 min, Sigma Aldrich, USA) was used to prevent SKOV3 and OVCAR3 cells from proliferating. SKOV3 and OVCAR3 cells were then transferred into the top layer holes (105cell/well, Kangning Corporation, USA) in the manmade basement membrane plate and were placed in the medium without serum. The lower cavity was filled with 10% fetal calf serum medium to give play to chemical attraction. The medium was sucked after 5-hour incubation and the implant in the upper cavity was discarded. Cells invading the lower cavity were fixed with 4% PFA and stained with crystal violent to form an image. The cells in the lower cavity were counted and normalized to control conditions to measure relative invasion capacity.
2.6. In vivo EOC transplant experiment
Plasmid-transduced OVCAR3 cells were transplanted under the skin of 5-week-old, female, athymia nude mouse. The growth of EOC tumor in the mouse was measured weekly and the volume was also calculated.
2.7. Statistical analysis
Statistical processing: SPSS was applied in data analysis. Measurement data were presented in X ± s, and pairwise t-test was used for intergroup comparison. The difference was statistically significant at P < 0.05. GraphPad Prism 7.0 was used for preparation of picture.
3. Results
3.1. Expression of MiR-21 and PTEN gene in EOC cell line and EOC tumor
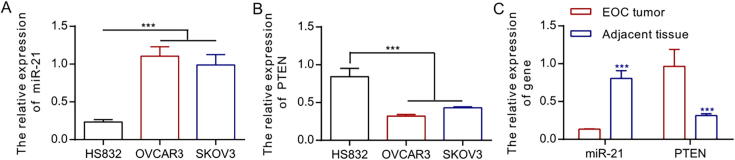
To study how MiR-21 affected EOC, we began by testing the miR-21 expression in SKOV3 and OVCAR3 cell lines. We adopted RT-PCR quantitative method to compare the genetic expression of miR-21 in OVCAR3, SKOV3 cell lines and non-tumorous, human ovarian epithelial cell HS832. Results showed the miR-21 over-expression in EOC cell line, which is higher than normal ovarian epithelial cell (Fig. 1A, *P < 0.05), while genetic expression of PTEN in OCVAR 3 and SKOVE 3 cells decreased (Fig. 1B, *P < 0.05). At the same time, to further prove our results, we tested the expressions of miR-21 in 11 EOC patients. RT-PCR showed an up-regulation of miR-21 expression in EOC in comparison with the non-tumorous ovarian epithelial tissue, with a down-regulation of PTEN (Fig. 1C, *P < 0.05).
Fig. 1.
MiR-21 is over-expressed in EOC cell line and tumor tissues, while PTEN is down-regulated.
3.2. LV3 pGLV-H1-GFP-miR-21 transduction and siRNA-PTEN’s effect on EOC cell proliferation
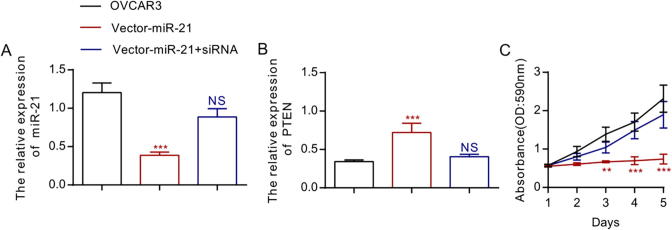
To further study miR-21’s biological function and its functional relation with PTEN, we transfected plasmid LV3 pGLV-H1-GFP-miR-21 into OVCAR3 to stably construct the EOC cell line with down-regulated miR-21. After RT-PCR analysis, obvious inhibition of miR-21 expression was proved in LV3 pGLV-H1-GFP-miR-21-transduced cell line, while the expression of PTEN genes picked up obviously (Fig. 2A and B, *P < 0.05). However, siRNA-PTEN and LV3 pGLV-H1-GFP-miR-21-transduced OVCAR3 cells, we found miR-21 expression and PTEN genetic expression did not differ from those of the OVCAR3 cell line (Fig. 2A and B). Transduced EOC cells were placed in the 96-hole plate for 5 days so that they could proliferate. The proliferation experiment showed that inhibition of miR-21 evidently inhibited OVCAR3 cells from proliferating (Fig. 2C, *P < 0.05). To further study miR-21’s functional relation with PTEN, we transduced LV3 pGLV-H1-GFP-miR-21 and siRNA-PTEN into OVCAR3 cells simultaneously. After the experiment, we found that OVCAR3 cells’ proliferation capacity was restored (Fig. 2C, *P < 0.05).
Fig. 2.
The down-regulation of MiR-21 restricts the proliferation of OVCAR3 cells, while down-regulation of PTEN promotes the proliferation of OVCAR3 cells.
3.3. LV3 pGLV-H1-GFP-miR-21 transduction and siRNA-PTEN’s invasive effect on EOC cells
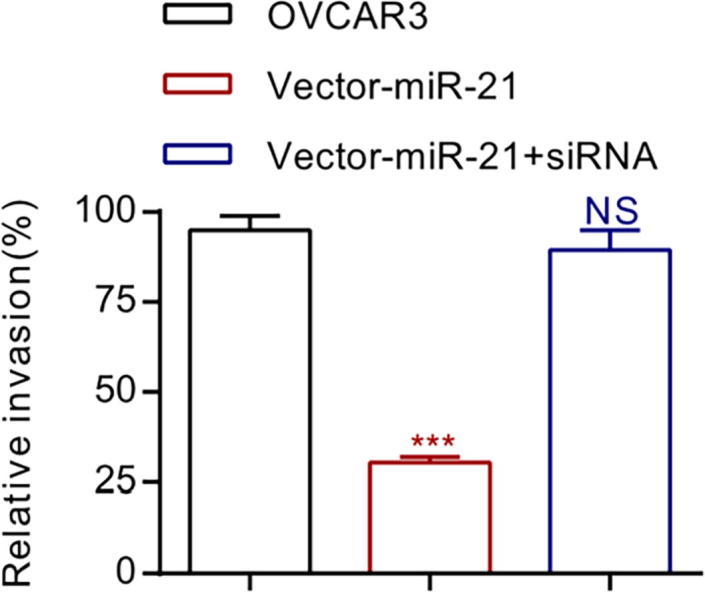
The invasion experiment was used to test LV3 pGLV-H1-GFP-miR-21-transduced OVCAR3 cells’ invasion capacity. After a 5 h test, crystal violet immune-staining method was used to stain EOC cells evading the lower cavity. Compared with the un-transduced OVCAR3 cells, we found that LV3 pGLV-H1-GFP-miR-21 plasmid transduction evidently inhibited the invasion of EOC cells (Fig. 3, *P < 0.05). Meanwhile, we found EOC cells’ invasion capacity was restored after PTEN’s synthesis was interrupted by injecting LV3 pGLV-H1-GFP-miR-21-transduced OVCAR3 cells into siRNA-PTEN (Fig. 3, *P < 0.05).
Fig. 3.
The down-regulation of MiR-21 restricts the invasion ability of OVCAR3 cells, while the down-regulation of PTEN promotes the invasion ability of OVCAR3 cells.
3.4. LV3 pGLV-H1-GFP-miR-21 transduction and siRNA-PTE’s effects on the growth in EOC in vivo
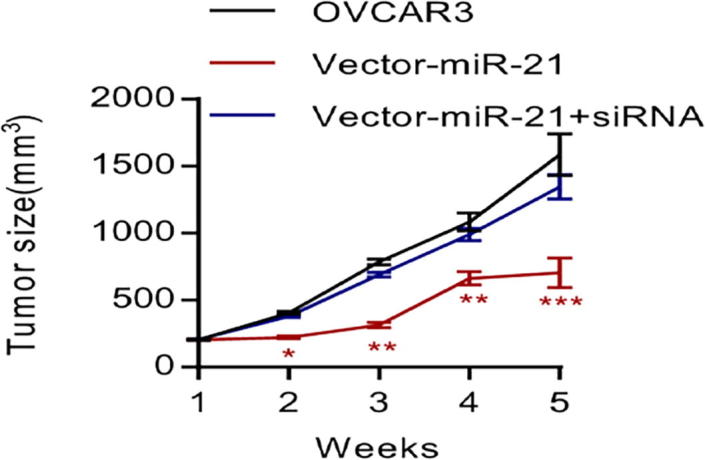
LV3 pGLV-H1-GFP-miR-21 was transduced into OVCAR3 cells and the cells were injected into nude mouse. There were no plasmid-transduced OVCAR3 cells as the control group. After 5 weeks of growth inside the mouse body, the growth of EOC explant was tested. Comparison showed that the inhibition of miR-21 also evidently inhibited the growth of EOC tumor inside the body (Fig. 4, *P < 0.05). However, no tumor inhibition was found in the group where LV3 pGLV-H1-GFP-miR-21 and siRNA-PTEN were transduced simultaneously (Fig. 4, *P < 0.05).
Fig. 4.
MiR-21 down-regulation inhibits the growth of OVCAR3 cells in vivo, while PTEN down-regulation results in the opposite.
4. Discussion
miR-21 is critical to many kinds of cancer (Shi, 2016, Khan et al., 2016). But there are few researches on the functional correlation of miR-21 with PTEN, especially in EOC. Our work aims to uncover the correlation of miR-21 with PTEN. To start with, we tested the genetic expression mode of miR-21 and PTEN in EOC to find miR-21 overexpression in EOC cell lines and clinical EOC tumor, while the expression of PTEN genes decreased obviously. This showed that EOC is driven by the overexpression of miR-21 and downregulation of PTEN, while a possible link might exist between miR-21 expression and PTEN expression in EOC.
To further study the relation, we used the plasmid transfection and siRNA technology, constructing miR-21 down-regulated EOC cell line and PTEN and miR-21 combined down-regulated cell line. Then we applied some kinds of cell function analysis to evaluate the roles played by miR-21 and PTEN in EOC. After the study, we found that miR-21 down-regulation could inhibit tumor proliferation and invasion, induce cell cycle arrest and inhibit EOC growth in vivo. Thus, miR-21 inhibited tumor both in vitro and in vivo in EOC. While in the siRNA-PTEN and LV3 pGLV-H1-GFP-miR-21cotransfection group, there was no inhibition against EOC cell line, illustrating the down-regulated PTEN expression might enhance miR-21 expression. The plasmid’s inhibiting effects was limited, so miR-21 expression was not inhibited completely, while the transduced OVCAR3 cells showed the same phenotype as OVCAR3 cells. The above results showed that miR-21 and PTEN played a crucial role in regulating EOC, and miR-21 and PTEN might be in an upstream and downstream relation.
As a kind of universal tumor inhibiting gene, dysfunction in regulating PTEN expression is mostly related to many kinds of solid tumors (Egawa et al., 2016). PTEN is regarded as the key factor affecting EOC attack and growth, which is probably due to the frequent reduction or deficiency of PTEN protein expression in cancerous tissues (Lee and Park, 2009). Besides, PTEN reduction is closely related to tumor development and poor prognosis in EOC (Lee and Park, 2009).
In all, our research reveals the regulator roles of miR-21 and PTEN in EOC. We also prove that miR-21 down-regulated tumor inhibition is inversely correlated with PTEN in EOC. These findings will undoubtedly facilitate the discovery of new biological marker or genetic target so that optimal diagnosis and treatment will be provided to EOC patients as early as possible.
Footnotes
Peer review under responsibility of King Saud University.
References
- Calin G.A., Cimmino A., Fabbri M., Ferracin M., Wojcik S.E., Shimizu M. MiR-15a and miR-16-1 cluster functions in human leukemia. P. Natl. Acad. Sci. USA. 2008;105(13):5166–5171. doi: 10.1073/pnas.0800121105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin G.A., Croce C.M. MicroRNA-cancer connection: the beginning of a new tale. Cancer Res. 2006;66(15):7390–7394. doi: 10.1158/0008-5472.CAN-06-0800. [DOI] [PubMed] [Google Scholar]
- Egawa H., Jingushi K., Hirono T., Ueda Y., Kitae K., Nakata W. The miR-130 family promotes cell migration and invasion in bladder cancer through FAK and Akt phosphorylation by regulating. PTEN Sci. Rep. – UK. 2016;6(3):20574. doi: 10.1038/srep20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo W., Zhao G., Yin J., Ouyang X., Wang Y., Yang C. Lentiviral CRISPR/Cas9 vector mediated miR-21 gene editing inhibits the epithelial to mesenchymal transition in ovarian cancer cells. J. Cancer. 2017;8(1):57. doi: 10.7150/jca.16723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W., Sun B., Su C. Targeting microRNAs in cancer gene therapy. Genes-Basel. 2017;8(1):21. doi: 10.3390/genes8010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K., Cunningham D., Peckitt C., Barton S., Tait D., Hawkins M. miR-21 expression and clinical outcome in locally advanced pancreatic cancer: exploratory analysis of the pancreatic cancer Erbitux, radiotherapy and UFT (PERU) trial. Oncotarget. 2016;7(11):12672–12681. doi: 10.18632/oncotarget.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.K., Park N.H. Prognostic value and clinicopathological significance of p53 and PTEN in epithelial ovarian cancers. Gynecol. Oncol. 2009;112(3):475–480. doi: 10.1016/j.ygyno.2008.11.031. [DOI] [PubMed] [Google Scholar]
- Lou Y., Yang X., Wang F., Cui Z., Huang Y. MicroRNA-21 promotes the cell proliferation, invasion and migration abilities in ovarian epithelial carcinomas through inhibiting the expression of PTEN protein. Int. J. Mol. Med. 2010;26(6):819–827. doi: 10.3892/ijmm_00000530. [DOI] [PubMed] [Google Scholar]
- Metzler M., Wilda M., Busch K., Viehmann S., Borkhardt A. High expression of precursor microRNA-155/BIC RNA in children with Burkitt lymphoma. Gene. Chromosome. Canc. 2004;39(2):167–169. doi: 10.1002/gcc.10316. [DOI] [PubMed] [Google Scholar]
- Ramalingam P. Morphologic, immunophenotypic, and molecular features of epithelial ovarian cancer. Oncol.-Basel. 2016;30(2):166. [PubMed] [Google Scholar]
- Samuel P., Carter D.R.F. The diagnostic and prognostic potential of microRNAs in epithelial ovarian carcinoma. Mol. Diagn. Ther. 2017;21(1):59–73. doi: 10.1007/s40291-016-0242-z. [DOI] [PubMed] [Google Scholar]
- Shi J. Considering exosomal miR-21 as a biomarker for cancer. J. Clin. Med. 2016;5(4):42. doi: 10.3390/jcm5040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoronos A.A., Engelman D.M., Slack F.J. OncomiR or tumor suppressor? The duplicity of microRNAs in cancer. Cancer Res. 2016;76(13):3666–3670. doi: 10.1158/0008-5472.CAN-16-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Berckelaer C., Brouwers A.J., Peeters D.J.E., Tjalma W., Trinh X.B., Van Dam P.A. Current and future role of circulating tumor cells in patients with epithelial ovarian cancer. Europ. J. Surg. Oncol. (EJSO) 2016;42(12):1772–1779. doi: 10.1016/j.ejso.2016.05.010. [DOI] [PubMed] [Google Scholar]
- Webb P.M., Jordan S.J. Epidemiology of epithelial ovarian cancer. Best Pract. Res. Cl. Ob. 2017;41:3–14. doi: 10.1016/j.bpobgyn.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Xu Y.Z., Xi Q.H., Ge W.L., Zhang X.Q. Identification of serum microRNA-21 as a biomarker for early detection and prognosis in human epithelial ovarian cancer. Asian Pac. J. Cancer P. 2013;14(2):1057–1060. doi: 10.7314/apjcp.2013.14.2.1057. [DOI] [PubMed] [Google Scholar]
- Zhang J., Yao T., Wang Y., Yu J., Liu Y., Lin Z. Long noncoding RNA MEG3 is downregulated in cervical cancer and affects cell proliferation and apoptosis by regulating miR-21. Cancer Biol. Ther. 2016;17(1):104–113. doi: 10.1080/15384047.2015.1108496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Nadeem L., Connor K., Xu G. Mechanisms and therapeutic targets of microRNA-associated chemoresistance in epithelial ovarian cancer. Curr. Cancer Drug Tar. 2016;16(5):429–441. doi: 10.2174/1568009616666160404121105. [DOI] [PubMed] [Google Scholar]