Abstract
Bone tumor is a rare heterogeneous malignancy. Osteosarcoma is the most common bone tumor with no apparent underlying pathogenesis, and its peak incidence often occurs during puberty. The intensive application of chemotherapy rarely alters the poor prognosis of the patients in advanced stage. Despite intensive chemotherapy in clinical practice, patients still suffer from the poor prognosis, or even progression of bone tumor. We identified integrin-associated protein (IAP) Cluster of Differentiation 47 (CD47) as a target for monoclonal antibody, and use anti-CD47 antibody to block its expression in bone tumors. CD47 was highly expressed in the bone tumor rats when comparing to the healthy rats. Likewise, Western blotting assay revealed a higher protein expression of CD47 in the bone tumor cells when compared to the normal osteoblasts. Further studies have shown the association between the mRNA expression of CD47 and the disordered bone tumors development and decreased rate of overall survival of diseased rats. In addition, blocking the CD47 monoclonal antibody has been shown to drive macrophages to engulf bone tumor cells in vitro and thus inhibiting tumor metastasis in rats. Taken together, the results of this study suggested that CD47 is a key regulator of bone tumor cell metastasis and that targeting inhibition of anti-CD47 may be a new immunotherapy for bone tumors.
Keywords: Bone tumor, CD47, Monoclonal antibody, Immunotherapy
1. Introduction
Bone tumor is rare in prevalence. In the UK, approximately 400 bone tumor patients (all ages) are diagnosed annually. In the United States, approximately 650–700 children and young adults under 20 years of age are diagnosed with bone tumors each year (Cho et al., 2017). This disease occurs mostly in children and adolescents, with the highest incidence between 14 and 18 years of age (Vlychou et al., 2016). The clinical cure rate of bone tumors is very poor. Patients often need to be given limb salvage surgery to prevent tumor cells from migrating and spreading. The prognosis of the disease is extremely poor as well, with 30–40% recurrence rate and lung metastasis(Charest-Morin, Dea and Fisher, 2016).
Integrin associated protein (CD47) is a glycoprotein with a membrane receptor of about 50 kDa. It belongs to the immunoglobulin superfamily and is widely expressed on the plasma membrane of all hematopoietic cells and many other somatic cell types (Liu et al., 2017). Oldenborg et al. found that CD47 is a self-marker of rat red blood cells (RBCs) and that CD47-negative RBCs are rapidly cleared from the circulation after being engulfed by macrophages. In addition, tumor cells escape phagocytosis of macrophages by expressing CD47. The clinical significance of CD47 expression in leukemia has been extensively studied (Oldenborg, 2013). In solid tumors, including bladder cancer, CD47-expressing cells are identified as the tumor initiation population. Also, breast cancer patients with high CD47 expression had a significantly worse prognosis than those with low CD47 expression (Kaur et al., 2016). In 2015, researchers found that blocking CD47 expression was associated with enhanced T-cell-mediated clearance of immunogenic tumors, in addition to the accelerated macrophage phagocytosis (Zhang et al., 2016). Therefore, CD47 has become a hotspot in the field of immunotherapy.
Anti-tumor effects achieved by immunomodulating methods have been applied clinically. It has been shown to be an effective treatment for cancer by targeting specific antibodies to antigens. The magnificent success of PD-1 and CTLA-4 in clinical trials evidenced that targeting the immune cells is an efficient strategy (Tao et al., 2017). In addition, the anti-tumor activity of activated macrophages has been a research hotspot in recent years, especially in the study of the anchor of CD47 protein molecules and the interaction of macrophage signal-regulated protein α (SIRPα). As a transmembrane protein, CD47 binds to the SIRPα, which blocks the phagocytosis of macrophages (Oldenborg, Gresham and Lindberg, 2001). The high expression of CD47 has been reported in a variety of malignancies, and this mechanism is a self-protective response of cancer cells - they avoid phagocytic activity of macrophages. In addition, high CD47 expression in patients with bone tumors is associated with poor prognosis (Chao et al., 2010a, Chao et al., 2010b, Tseng et al., 2013, Zhang et al., 2013).
This study was aimed to investigate the effect of anti-CD47 antibody on bone tumors in rats, so as to make a full preliminary study on the further clinical application of anti-CD47 antibody.
2. Material and method
2.1. Cell line and cell culture
LM8 cells were cultured in DMEM medium added with 10% fetal bovine serum (American Life Technology, Inc.); culture of KRIB cells (Cell bank of Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences), a human osteosarcoma cell line, were carried out in RPMI-1640 medium with FBS at 37℃ in 5% CO2.
2.2. Establishment of rat bone tumor model
ACI male rats were from Shanghai Laboratory Animal Center, Chinese Academy of Sciences. LM8, a cell line extracted from murine bone tumor cells, has a very high rate of organ metastasis in ACI male rats. The bone tumor model of rats was established by subcutaneous injection of LM8 cells into the back (via the migration characteristics of LM8). B6H12 and Ab400, the monoclonal antibodies (mAbs) of Anti-CD47, were generated by the hybridoma cell lines. ACI male rats were randomly grouped to three, with 20 rats in each. B6H12 and Ab400 were used in two groups, while the rest group, as the control group, was applied with lgG immune globulin. After transplantation of the LM8 cell line, all three groups were injected with 0.5 mg of B6H12, Ab400, and IgG immunoglobulin the following day. Thereafter, the same drugs were injected intraperitoneally once a week in each group until osteosarcoma was completely cured or the rats died.
2.3. Physiological status assessment
Eight weeks after LM8 cell line transplantation, 5 rats were randomly selected from each group for physiological status assessment, including tumor development status, spleen size, etc.
2.4. Real-time quantitative PCR
With the Tiangen RNA isolation kit, we extracted the total RNA from the tumor tissues. The quality and concentration of RNA were determined using Evolution 201 ultramicrospectrophotometer. 1 μL of RNA was used to remove genomic DNA using 4 × gDNA wiper Mix. 4 μL 5 × qRT SuperMix II was added to generate cDNA at 50℃ for 15 min. SYBR Green Master Mix PCR kit was used for real-time quantification. Melt curve analysis was performed to evaluate the product. Each sample was repeated three times, and the mean C(t) value was calculated. The ΔC(t) was calculated as C(t)-C(t) GAPDH. The method for n increases or n decreases, ΔΔCt, was calculated by the calculation formula, and used the C(t) GAPDH value as the reference point.
2.5. Western blotting
Sample of tumor tissue (25 μg/lane) was injected into the SDS-PAGE gel, followed by the transferring onto a polyethylene difluoride membrane, and incubation in 5% milk TBST. Proteins on the membrane were probed at 4 °C with CD47 antibody Ab (Abcam), and following a membrane wash in TBST for 15 min, the immunoblots were incubated in suitable second antibody (1:5000; Jackson ImmunoResearch). Membrane was then incubated by the ECL reagent, and imaged (American sham Biosciences).
2.6. Antibody preparation and MTT assay
LM8 cells were inoculated in vitro and counted in 96-well plates. IgG immunoglobulin, B6H12, or Ab400 antibody groups were added to the wells at 10, 3 or 1 μg/mL per well. After incubation at 37 °C for 24, 48 and 72 h, 20 μg/mL of MTT (Promega) was added to each well for incubation to test cell viability. Four hours later, the culture medium in the wells was aspirated, MTT solubilization solution was added, and the incubation was continued for 15 min. The absorbance of each well was calculated by spectrophotometer at 590 nm and referenced to 620 nm.
2.7. In-vitro assay of macrophage phagocytosis
Peritoneal macrophages were isolated from the abdominal cavity of 6 weeks old ACI rats, and 5 × 104 macrophages were inoculated into the 24-well plate and randomized into the B6H12 group, Ab400 group, and IgG immunoglobulin group. After incubation of macrophage in serum-free medium for 2 h, 2 × 105 CFSE was added to each well to label LM8 cells. Phagocytosis was evaluated by the quantity of CFSE-positive cells every 100 macrophages, as the phagocytosis index. B6H12, Ab400 and immunoglobulin were added to the wells at 10 μg/ml, followed by 2 h of incubation at 37 °C and imaging under the confocal microscope without macrophage.
3. Results
3.1. CD47 expression decreased in B6H12 group and Ab400 group
To understand the role of CD47 in osteosarcoma, and to detect the anti-tumor effects of B6H12 and Ab400 antibodies in bone tumor tissues of ACI rats, we randomly selected five rats from three groups to extract bone tumor tissues for quantitative detection of CD47. To further validate the effect of B6H12 and Ab400 antibodies on other rat cells, we extracted venous blood from tails of the 60 rats in three groups and detected the expression of CD47 in the blood. It was found that CD47 in osteosarcoma was significantly up-regulated, compared with B6H12 and Ab400 groups. Since direct sequencing and Sanger-PCR sequencing did not reveal any other DNA mutations in the CD47 gene promoter nor methylation abnormalities, we believe that CD47 might be modulated after transcription, which may be associated with the development and metastasis of bone tumors. To further verify if CD47 is closely related to the genesis and development of bone tumors, we tested the percentage of CD47 in CD44 positive cells. CD44 is a proven marker of bone cancer stem cells (CSC). The percentage of CD47 in the three groups of CD44-positive cells is indicated in Fig. 1B. CD47 was expressed in the majority of CD44+ cells at the same time, although the proportion was different (ranging from 80% to 99%), the data suggest that CD44+ cells in bone tumors mostly expressed CD47. These data further established that targeting CD47 can reduce the activity of osteosarcoma tumor stem cells.
Fig. 1.
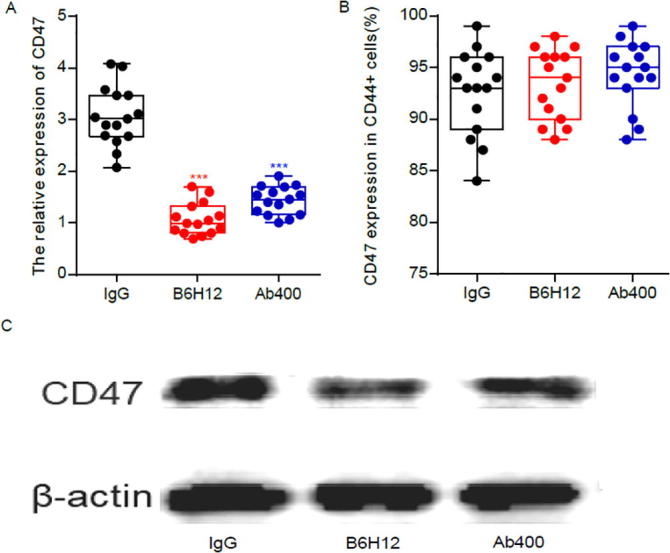
Anti-CD47 antibody potently inhibits CD47 expression (A) mRNA expression of CD47 in the three groups; (B) CD44+ cells with CD47 antibody; (C) protein expression of CD47 in the three groups.
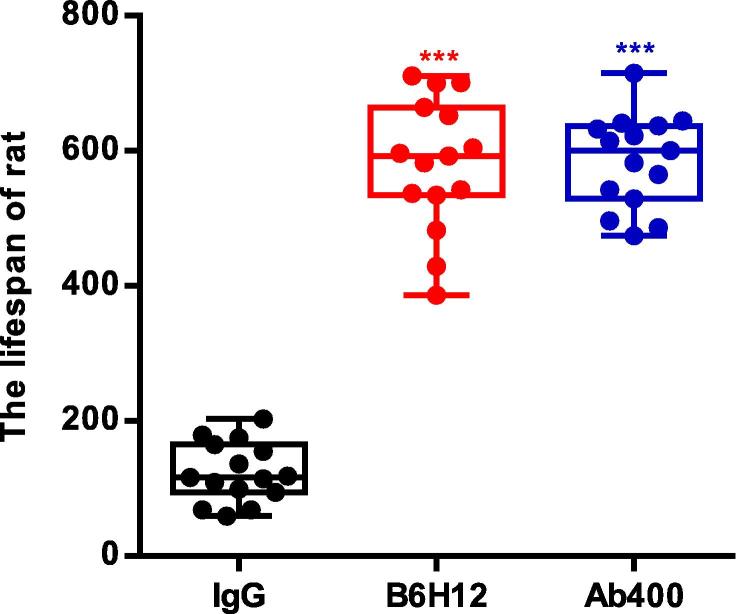
3.2. Anti-CD47 antibody prolongs life span of rats
Long-term observation and life-span test of the rats in the three groups showed that the IgG immunoglobulin group could not effectively inhibit the metastasis of bone tumors and their spreading to multiple organs, while in the two groups treated with B6H12 and Ab400 anti-CD47 antibodies, the bone tumors were effectively inhibited. Other physiological indexes of rats were also gradually go back to normal with continuous intraperitoneal injection of B6H12 and Ab400 anti-CD47 antibodies. In the final life span statistics, we found that the life span of B6H12 and Ab400 groups was significantly different from the IgG immunoglobulin group (Fig. 2).
Fig. 2.
Anti-CD47 antibodies B6H12 and Ab400 can prolong the life of ACI rats and improve the damage of bone tumors.
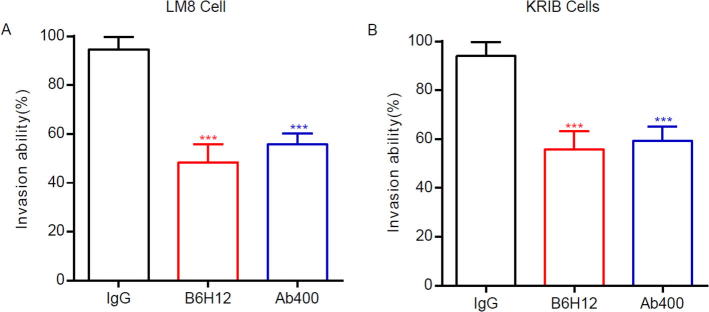
3.3. Anti-CD47 antibody reduces invasion of bone tumor cells
To further analyze the functional potency of anti-CD47 antibodies, we designed and conducted in vitro experiments to figure out whether anti-CD47 antibody can inhibit osteosarcoma cell invasion by passing through artificial membrane filter. LM8 cells and KRIB cells were divided into three groups, each using anti-CD47 antibodies Abs (B6H12 and Ab400, 500 μg/mL) and IgG immunoglobulins in five replicates. The above samples were placed in cell invasion tester for detection. The cells were counted after 22 h. As a result, anti-CD47-treated bone tumor cells were less invasive than IgG-treated cells (LM8: P <0.001; KRIB: P <0.001). These results suggested that blocking CD47 expression by antibodies can inhibit the invasion capability of bone tumor cells. We further carried out MTT cell proliferation assay by incubating normal cells and bone tumor cells with IgG, B6H12 antibody and Ab400 antibody respectively, and measured cell viability after incubation. The groups with CD47 antibodies – B6H12 and Ab400, compared with the IgG group, at the same antibody concentration and incubation time, showed no significant difference in survival of normal osteoblasts and bone tumor cells. This result indicated that the anti-CD47 antibodies are not cytotoxic and the therapeutic effect on tumor cells is not directly achieved by toxicity (see Fig. 3).
Fig. 3.
Anti-CD47 antibodies B6H12 and Ab400 inhibit the invasiveness of bone tumor cells.
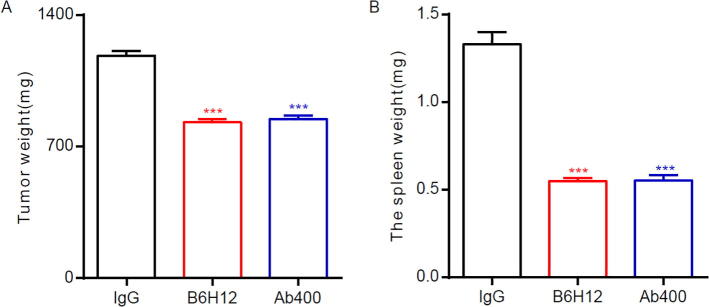
3.4. Anti-CD47 antibodies inhibit bone tumor cell metastasis in ACI rats
Bone tumor animal models were established using LAM cell lines. Most ACI rats have lung metastases after transplantation of LAM cells. To investigate how anti-CD47 antibodies alter bone tumor growth in ACI rats, we decided to establish the bone tumor models on ACI rats through injection of LAM cells. 15 days later, the success rate of building the rat bone tumor model was determined by imaging, and the successful models were selected. The rats were injected with anti-CD47 antibodies B6H12 and Ab400, 500 μg once a week; and the control group was injected with IgG antibody 500 μg once a week. After 120 days of treatment, imaging test was performed and various physiological test indicators were detected. 16 rats (80%) in the IgG -treated group were found to have tumors, while the anti-CD47 antibodies - B6H12 and Ab400 - treated groups were found to have a substantially lower probability of having tumors. 5 rats were randomly selected from each group (in total 15 rats) (Gao et al., 2017). Their tibia and spleen weights were determined after execution. The test revealed that the average tibia weight of rats treated with anti-CD47 antibodies was lower, and there was a significant difference (P < 0.001) from the control group (as shown in Fig. 4A); while the spleen weight of the control group was significantly heavier, with obvious splenomegaly in the IgG treatment group (as shown in Fig. 4B). Incidence rate of spontaneous metastasis was lower in the anti-CD47 groups than that in the IgG group (P < 0.0001). By imaging, we also found that 2 out of 20 rats (10%) in the anti-CD47 treatment groups had lung metastases. Comparatively, however, 80% of rats in the IgG treatment group had lung metastases. These results suggested that anti-CD47 can inhibit spontaneous lung metastases from xenogeneic osteosarcoma within mouse tibia phospholipids.
Fig. 4.
Anti-CD47 antibodies inhibit bone tumor cell development and splenomegaly in ACI rats.
4. Discussion
This study suggests that CD47 is critical to the tumorigenesis and development. Taking into account its indispensable expression, and its ability to modulate tumor cell growth and metastasis, its limitation of angiogenesis, as well as its response to tumor immune responses, CD47 may be an effective target for cancer treatments.
A number of recent studies have been looking at using functional blocking anti-CD47 monoclonal antibodies to inhibit, even cure, leukemia, and have achieved promising results (Gao et al., 2017a, Gao et al., 2017b, Gao et al., 2017c). For example, Sagawa et al. successfully utilized mAbs that directly against CD47 protein single strands to rapidly induce apoptosis of malignant lymphoid B cells isolated from leukemia patients, thus significantly increasing survival in mice implanted with a human lymphoblastic leukemia cell line (Jaiswal et al., 2009). In addition to direct induction of apoptosis, anti-CD47 monoclonal antibodies have other mechanisms that act on tumor cells, including stimulation of complement or antibody-dependent cytotoxicity, and induction of leukemic stem cell phagocytosis by disrupting CD47/SIRPα (signal regulatory protein α) interaction(Matozaki et al., 2009). It is worthy to note that the use of rat anti-CD47 antibodies can effectively promote phagocytosis of mouse acute myeloid leukemia cells without depleting normal hematopoietic stem cells, which demonstrates the feasibility of CD47-targeted therapies (Chao et al., 2011). Anti-CD47 monoclonal antibodies in combination with rituximab (an anti-CD20 monoclonal antibody) promotes phagocytosis of macrophages, and has synergistic effect on uprooting Hodgkin′s lymphoma (Chao et al., 2010a, Chao et al., 2010b).
Activation of CD47 induces apoptosis and also promotes cell proliferation and survival. On the other hand, tumor cell metastasis appears to be generally regulated by CD47. Therefore, when CD47 is antagonized, it can significantly reduce tumor cell metastasis, thus providing a feasible way to inhibit the spread of cancer cells (Gao et al., 2017a, Gao et al., 2017b, Gao et al., 2017c). Blocking the SIRPa/CD47 interaction to thereby promote the immune system's response to tumors, especially in the context of CD47 overexpression, seems to be a promising approach.
5. Conclusion
By using anti-CD47 antibodies in the ACI rat bone tumor models, we found that CD47 is overexpressed, whereas the use of anti-CD47 antibodies significantly reduces CD47 mRNA levels. This study also demonstrated that blocking CD47 is a potentially effective treatment for bone tumors. Therefore, we hypothesized that CD47 might be an effective target in the treatment of bone tumors.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Chao M.P., Alizadeh A.A., Tang C. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-hodgkin lymphoma. Cell. 2010;142(5):699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao M.P., Alizadeh A.A., Tang C. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res. 2011;71(4):1374–1384. doi: 10.1158/0008-5472.CAN-10-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao M.P., Jaiswal S., Weissmantsukamoto R. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci. Transl. Med. 2010;2(63):63ra94. doi: 10.1126/scitranslmed.3001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest-Morin R., Dea N., Fisher C.G. Health-related quality of life after spine surgery for primary bone tumour. Curr. Treat. Options Oncol. 2016;17(2):1–12. doi: 10.1007/s11864-015-0383-z. [DOI] [PubMed] [Google Scholar]
- Cho H.S., Park Y.K., Gupta S. Augmented reality in bone tumour resection: an experimental study. Bone Joint Res. 2017;6(3):137–143. doi: 10.1302/2046-3758.63.BJR-2016-0289.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Baig A.Q., Ali H., Sajjad W., Farahani M.R. Margin based ontology sparse vector learning algorithm and applied in biology science. Saudi J. Biolog. Sci. 2017;24:132–138. doi: 10.1016/j.sjbs.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Wang Y., Basavanagoud B., Jamil M.K. Characteristics studies of molecular structures in drugs. Saudi Pharmaceut. J. 2017;25:580–586. doi: 10.1016/j.jsps.2017.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Wang Y., Wang W., Shi L. The first multiplication atom-bond connectivity index of molecular structures in drugs. Saudi Pharmaceut. J. 2017;25:548–555. doi: 10.1016/j.jsps.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S., Jamieson C.H.M., Pang W.W. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138(2):271. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S., Elkahloun A.G., Singh S.P. A function-blocking CD47 antibody suppresses stem cell and EGF signaling in triple-negative breast cancer. Oncotarget. 2016;7(9):10133–10152. doi: 10.18632/oncotarget.7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Kwon H., Li Z. Is CD47 an innate immune checkpoint for tumor evasion? J. Hematol. Oncol. 2017;10(1):12. doi: 10.1186/s13045-016-0381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matozaki T., Murata Y., Okazawa H. Functions and molecular mechanisms of the CD47–sirpα signalling pathway. Trends Cell Biol. 2009;19(2):72–80. doi: 10.1016/j.tcb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Oldenborg P.A. CD47: a cell surface glycoprotein which regulates multiple functions of hematopoietic cells in health and disease. Isrn Hematol. 2013;5:614619. doi: 10.1155/2013/614619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenborg P.A., Gresham H.D., Lindberg F.P. CD47-signal regulatory protein α (sirpα) regulates fcγ and complement receptor–mediated phagocytosis. J. Exp. Med. 2001;193(7):855–862. doi: 10.1084/jem.193.7.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao H., Qian P., Wang F. Targeting CD47 enhances the efficacy of anti-pd-1 and ctla-4 in esophageal squamous cell cancer preclinical model. Oncol. Res. 2017;25(9) doi: 10.3727/096504017X14900505020895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng D., Volkmer J.P., Willingham S.B. Anti-CD47 antibody–mediated phagocytosis of cancer by macrophages primes an effective antitumor t-cell response. PNAS. 2013;110(27):11103–11108. doi: 10.1073/pnas.1305569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlychou M., Inagaki Y., Stacey R. Primary intraosseous meningioma: an osteosclerotic bone tumour mimicking malignancy. Clin. Sarcom. Res. 2016;6(1):14. doi: 10.1186/s13569-016-0054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Wang H., Tan S. Donor CD47 controls t cell alloresponses and is required for tolerance induction following hepatocyte allotransplantation. Sci. Rep. 2016;6:26839. doi: 10.1038/srep26839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Sime W., Juhas M. Crosstalk between colon cancer cells and macrophages via inflammatory mediators and CD47 promotes tumour cell migration. Eur. J. Cancer. 2013;49(15):3320–3334. doi: 10.1016/j.ejca.2013.06.005. [DOI] [PubMed] [Google Scholar]