Editorial
Atrial fibrillation (AF) is the most common sustained arrhythmia and is associated with significant morbidity, increased risk of stroke, reduced quality of life, and increased mortality [1], [2], [3], [4]. Several studies suggest, that there are significant gender differences in clinical presentation of AF, utilization of AF therapy and clinical management of AF and outcomes [5]. On top of a higher AF-associated stroke risk, women have higher rates of dementia over time than men [6]. Additionally, in women, AF is associated with more severe or non-traditional symptoms and worse prognosis which negatively affects health-related quality of life measures [7]. Despite these gender-differences in AF symptoms and stroke risk, the AF progression rate, its mechanisms and impact on outcomes in AF in women versus men is unclear. Progression from paroxysmal to persistent or permanent AF may occur in 1–15% of the general AF population annually depending on definition of progression. Additionally, the rate of AF progression is dependent on the presence of specific risk factors [8], the number of concomitant risk factors [9] or certain combinations of risk factors, where individual risk factors may act as disease modifiers. Young-onset AF may represent a scenario, where AF is mainly maintained by concomitant or underlying risk factors rather than by increased ageing. Women are underrepresented in major AF trials, and therefore it remains unclear whether there are risk factors that specifically affect AF progression in women with young-onset AF.
In this Issue of the IJC Heart & Vasculature, Marcos et al. [10] investigated gender-differences in clinical AF risk factor profiles, AF progression rates and cardiovascular outcomes between sexes in a prospective cohort study including 497 patients with young-onset AF <60 years of age, a quarter of whom were women. Most conventional risk factors, including age, hypertension, heart failure and diabetes mellitus, were not different between sexes at baseline. However, there were a few gender-specific differences in risk factor profile: men were taller, more often presented with coronary artery disease, had a longer PR interval and higher left ventricular mass index, while women reported more often familial AF and were more likely obese. During a median follow-up of 7 years, AF progression was seen in 12% and there was no significant difference in AF progression in men compared to women. Despite these gender-specific differences in the AF risk factor profile, the absolute number of comorbidities and the mean age was comparable in men and women. This suggests, that not one individual risk factor, but more likely the absolute number of concomitant risk factors is more relevant for AF progression in young-onset AF patients and may explain the similar AF progression in this cohort of young-onset AF patients. It is also of interest that in this cohort women presented more often with familial AF, suggesting that genetics may make a larger contribution to AF-risk in women than in men (see Fig. 1).
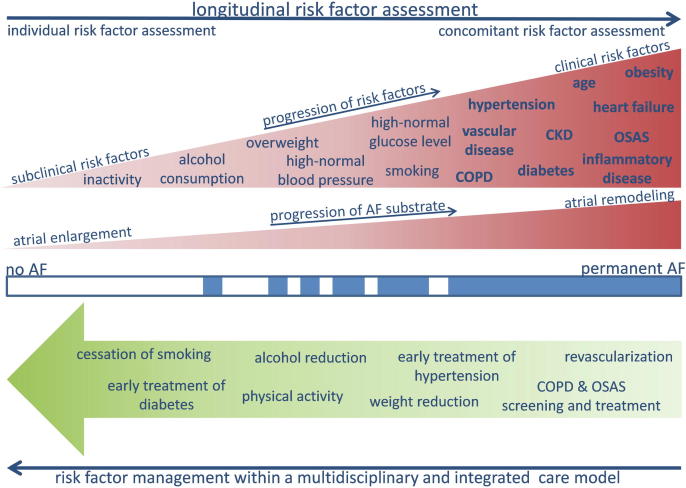
Fig. 1.
Longitudinal risk factor assessment to capture the dynamic nature of atrial fibrillation (AF) risk factors and prevent progression. Risk factors may be in a subclinical state and should be considered as precursors which often progress to clinical risk factors. The management of risk factors within a multidisciplinary and integrated care model may reverse progression of AF. Future studies are required to answer the questions whether there are gender differences in risk factors profiles and in response to risk factor modification programs.
Interestingly, the Kaplan-Meier curve suggests a trend towards a higher AF progression rate in women over time. Although the number of women was low at this time point, factors such as gender-differences in the development of new AF risk factors over time, in the treatment strategy of risk factors or in the responses to antiarrhythmic treatment may contribute to this late divergence at 3 years follow-up. An alternative explanation is that risk factors determine AF progression in young-onset AF, while intrinsic gender-specific risk factor independent mechanisms become more relevant when AF patients get older. A study of over 8.3 million participants reported higher prevalence and incidence of AF with increasing age, with disproportionate rates between men and women, peaking beyond 80 years of age [11]. Possibly, a follow-up study reporting on gender-differences in AF progression beyond the 7 years reported in the study by Marcos et al. may still unmask gender-specific AF progression differences in this or larger populations with increasing age.
There is no question that risk factor management represents an important pillar of AF management. Different groups, including the authors themselves, have demonstrated that comprehensive management of concomitant risk factors helps to maintain sinus rhythm [12], [13], [14], [15], [16]. Although a lot of effort has been invested to develop comprehensive programs for aggressive and combined risk factor management, it remains questionable, whether our current approaches for the assessment of modifiable risk factors are sufficient to detect all potential targets for risk factor management in our patients.
According to current clinical practice, Marcos et al. assessed established risk factors in a structured way at the timepoint, when AF patients present the first time in the AF Clinic [10]. However, in addition to the established risk factors, emerging risk factors such as sleep apnea [17], chronic obstructive pulmonary disease or gastrointestinal reflux [18] were not assessed in this study. Sleep apnea with a treatment indication is present in 30–60% of all patients with AF [19]. Importantly, patients with AF and sleep apnea do not report typical sleep apnea symptoms such as daytime sleepiness [19]. Therefore, sleep apnea remains to be undiagnosed and untreated in the majority of cases, if questionnaires to assess daytime sleepiness are used as prescreening tools. Additionally, risk factors may be in a subclinical state, which will be missed by standard non-longitudinal approaches. Subclinical risk factors including prehypertension [20] or exercise-induced hypertension should be considered as precursors which often progress to a clinical manifestation. As several AF risk factors may show a high day-to-day variability (e.g. sleep apnea [21], [22]) or may just occur during specific conditions (e.g. exercise induced hypertension) clinically relevant risk factors will be missed, if just one assessment is performed or the evaluation is just undertaken under resting conditions. This day-to-day variability in risk factors may be more pronounced in younger women than in older women after menopause or in men, because of the hormonal changes during the menstrual cycles. Even in AF-patients without overt risk factors, the inter-visit variability of metabolic parameters showed a close association with the risk of AF [23]. All of these factors justify a more structured and importantly longitudinal risk factor assessment to capture the dynamic nature of AF risk factors [24]. Repeated longitudinal assessment of established and emerging risk factors may be important in young-onset AF patients, particularly if no risk factor can be identified to explain the presence of AF. AF is a rare disease in the young and should be always considered as a manifestation of clinical or sub-clinical risk factors or as the presentation of an underlying (atrial) cardiomyopathy [25]. Excluding conventional AF risk factors at one timepoint should not result in the diagnosis of ‘lone AF’, which remains a diagnosis of repeated exclusions of risk factors.
It is important to note that, as with many AF studies, the current study also had an under-representation of women with only 25% of the cohort being female. A further limitation of the study by Marcos et al. [10] is that a time-to-event analysis was performed for AF progression to permanent AF only. Without structured rhythm monitoring during follow-up, the authors were unable to report on progression from paroxysmal to persistent AF. Further, the progression to permanent AF may not be an exact time-point given that the decision to not pursue rhythm control, and therefore gaining the ‘permanent AF’ label, is one that can be dynamic and differs from one patient to another, and from one healthcare provider to another. Additionally, the authors did not report on any reverse progression, i.e. reversal from permanent to less persistent or paroxysmal AF. Recent studies showed that with weight-loss and risk factor management, it is possible to halt or even reverse the progression of AF [26]. Focusing on AF progression and ignoring the possibility of AF regression in a dynamic arrhythmia such as AF may have introduced some bias.
Marcos et al. are to be congratulated on their effort to perform a unique analysis of gender-specific differences in AF-progression. They illustrate that the number of concomitant risk factors, rather than one individual risk factor, determines AF progression in patients with young-onset AF and that a few gender-specific differences in risk factor profile exist. New emerging (often non-cardiovascular) AF risk factors increase the number of potential targets for combined AF risk factor management within a multidisciplinary and integrated care model. In addition to documentation of AF progression by continuous rhythm monitoring, also longitudinal assessment of concomitant conditions might be useful to monitor risk factor progression or daily risk factor variations in AF patients. Finally, further studies are required to identify how to assess risk factors longitudinally and whether gender differences in risk factor profiles require consideration in clinical practice when it comes to personalized AF risk factor management.
Declaration of Competing Interest
None.
References
- 1.Chang T.Y., Liao J.N., Chao T.F., Vicera J.J., Lin C.Y., Tuan T.C., Lin Y.J., Chang S.L., Lo L.W., Hu Y.F., Chung F.P., Chen S.A. Oral anticoagulant use for stroke prevention in atrial fibrillation patients with difficult scenarios. Int. J. Cardiol. Heart Vasc. 2018;20:56–62. doi: 10.1016/j.ijcha.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayinde H., Schweizer M.L., Crabb V., Ayinde A., Abugroun A., Hopson J. Age modifies the risk of atrial fibrillation among athletes: a systematic literature review and meta-analysis. Int. J. Cardiol. Heart Vasc. 2018;18:25–29. doi: 10.1016/j.ijcha.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dan G.A., Dobrev D. Antiarrhythmic drugs for atrial fibrillation: Imminent impulses are emerging. Int. J. Cardiol. Heart Vasc. 2018;21:11–15. doi: 10.1016/j.ijcha.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prochnau D., von Knorre K., Figulla H.R., Schulze P.C., Surber R. Efficacy of temperature-guided cryoballoon ablation without using real-time recordings - 12-Month follow-up. Int. J. Cardiol. Heart Vasc. 2018;21:50–55. doi: 10.1016/j.ijcha.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zylla M.M., Brachmann J., Lewalter T., Hoffmann E., Kuck K.H., Andresen D., Willems S., Eckardt L., Tebbenjohanns J., Spitzer S.G., Schumacher B., Hochadel M., Senges J., Katus H.A., Thomas D. Sex-related outcome of atrial fibrillation ablation: Insights from the German Ablation Registry. Heart Rhythm. 2016;13:1837–1844. doi: 10.1016/j.hrthm.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Golive A., May H.T., Bair T.L., Jacobs V., Crandall B.G., Cutler M.J., Day J.D., Mallender C., Osborn J.S., Weiss J.P., Bunch T.J. The impact of gender on atrial fibrillation incidence and progression to Dementia. Am. J. Cardiol. 2018 Nov;1(122):1489–1495. doi: 10.1016/j.amjcard.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 7.Magnussen C., Ojeda F.M., Wild P.S., Sörensen N., Rostock T., Hoffmann B.A., Prochaska J., Lackner K.J., Beutel M.E., Blettner M., Pfeiffer N., Rzayeva N., Sinning C.R., Blankenberg S., Münzel T., Zeller T., Schnabel R.B. Atrial fibrillation manifestations risk factors and sex differences in a population-based cohort (from the gutenberg health study) Am. J. Cardiol. 2018;122:76–82. doi: 10.1016/j.amjcard.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 8.de Vos C.B., Pisters R., Nieuwlaat R., Prins M.H., Tieleman R.G., Coelen R.J., van den Heijkant A.C., Allessie M.A., Crijns H.J. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J. Am. Coll. Cardiol. 2010;55:725–731. doi: 10.1016/j.jacc.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 9.Schotten U., Verheule S., Kirchhof P., Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol. Rev. 2011;91:265–325. doi: 10.1152/physrev.00031.2009. [DOI] [PubMed] [Google Scholar]
- 10.Marcos E.G., De With R.R., Mulder B.A., Van Gelder I.C., Rienstra M. Young-onset atrial fibrillation: sex differences in clinical profile, progression rate and cardiovascular outcome. Int. J. Cardiol. Heart Vasc. 2019;25 doi: 10.1016/j.ijcha.2019.100429. (The editorial is about this manuscript) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilke T., Groth A., Mueller S., Pfannkuche M., Verheyen F., Linder R., Maywald U., Bauersachs R., Breithardt G. Incidence and prevalence of atrial fibrillation: an analysis based on 8.3 million patients. Europace. 2013;15:486–493. doi: 10.1093/europace/eus333. [DOI] [PubMed] [Google Scholar]
- 12.Rienstra M., Hobbelt A.H., Alings M., Tijssen J.G.P., Smit M.D.1, Brügemann J., Geelhoed B., Tieleman R.G., Hillege H.L., Tukkie R., Van Veldhuisen D.J., Crijns H.J.G.M., Van Gelder I.C. RACE 3 Investigators. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: results of the RACE 3 trial. Eur. Heart J. 2018;39:2987–2996. doi: 10.1093/eurheartj/ehx739. [DOI] [PubMed] [Google Scholar]
- 13.Abed H.S., Wittert G.A., Leong D.P., Shirazi M.G., Bahrami B., Middeldorp M.E., Lorimer M.F., Lau D.H., Antic N.A., Brooks A.G., Abhayaratna W.P., Kalman J.M., Sanders P. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310:2050–2060. doi: 10.1001/jama.2013.280521. [DOI] [PubMed] [Google Scholar]
- 14.Lau D.H., Nattel S., Kalman J.M., Sanders P. Modifiable risk factors and atrial fibrillation. Circulation. 2017;136:583–596. doi: 10.1161/CIRCULATIONAHA.116.023163. [DOI] [PubMed] [Google Scholar]
- 15.Pathak R.K., Middeldorp M.E., Meredith M., Mehta A.B., Mahajan R., Wong C.X., Twomey D., Elliott A.D., Kalman J.M., Abhayaratna W.P., Lau D.H., Sanders P.M. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY) J. Am. Coll. Cardiol. 2015;65:2159–2169. doi: 10.1016/j.jacc.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Pathak R.K., Middeldorp M.E., Lau D.H., Mehta A.B., Mahajan R., Twomey D., Alasady M., Hanley L., Antic N.A., McEvoy R.D., Kalman J.M., Abhayaratna W.P., Sanders P. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J. Am. Coll. Cardiol. 2014;64:2222–2231. doi: 10.1016/j.jacc.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 17.Linz D., McEvoy R.D., Cowie M.R., Somers V.K., Nattel S., Lévy P., Kalman J.M., Sanders P. Associations of obstructive sleep Apnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. 2018;3:532–540. doi: 10.1001/jamacardio.2018.0095. [DOI] [PubMed] [Google Scholar]
- 18.Linz D., Hohl M., Vollmar J., Ukena C., Mahfoud F., Böhm M. Atrial fibrillation and gastroesophageal reflux disease: the cardiogastric interaction. Europace. 2017;19:16–20. doi: 10.1093/europace/euw092. [DOI] [PubMed] [Google Scholar]
- 19.Kadhim K., Middeldorp M.E., Elliott A.D., Jones D., Hendriks J.M.L., Gallagher C., Arzt M., McEvoy R.D., Antic N.A., Mahajan R., Lau D.H., Nalliah C., Kalman J.M., Sanders P., Linz D. Self-reported daytime sleepiness and sleep-disordered breathing in patients with atrial fibrillation: SNOozE-AF. Can. J. Cardiol. 2019 Aug 1 doi: 10.1016/j.cjca.2019.07.627. [DOI] [PubMed] [Google Scholar]
- 20.Conen D., Tedrow U.B., Koplan B.A., Glynn R.J., Buring J.E., Albert C.M. Influence of systolic and diastolic blood pressure on the risk of incident atrial fibrillation in women. Circulation. 2009;119:2146–2152. doi: 10.1161/CIRCULATIONAHA.108.830042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linz D., Brooks A.G., Elliott A.D., Kalman J.M., McEvoy R.D., Lau D.H., Sanders P. Nightly variation in sleep Apnea severity as atrial fibrillation risk. J. Am. Coll. Cardiol. 2018;72:2406–2407. doi: 10.1016/j.jacc.2018.08.2159. [DOI] [PubMed] [Google Scholar]
- 22.Linz D., Brooks A.G., Elliott A.D., Nalliah C.J., Hendriks J.M.L., Middeldorp M.E., Gallagher C., Mahajan R., Kalman J.M., McEvoy R.D., Lau D.H., Sanders P. Variability of sleep Apnea severity and risk of atrial fibrillation. The VARIOSA-AF study. J. Am. Coll. Cardiol.: Clin. Electrophysiol. 2019;5:692–701. doi: 10.1016/j.jacep.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Lee S.R., Choi E.K., Han K.D., Lee S.H., Oh S. Effect of the variability of blood pressure, glucose level, total cholesterol level, and body mass index on the risk of atrial fibrillation in a healthy population. Heart Rhythm. 2019;pii: S1547–5271(19):30638–30641. doi: 10.1016/j.hrthm.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Linz D., Baumert M., Desteghe L., Kadhima K., Vernooy K., Kalman J.M., Dobrev D., Arzt M., Sastry M., Crijns H.J.G.M., Schotten U., Cowie M.R., McEvoy R.D., Heidbuchel H., Hendriks J., Sanders P., Lau D.H. Nightly sleep apnea severity in patients with atrial fibrillation: potential applications of long-term sleep apnea monitoring. Int. J. Cardiol. Heart Vasc. 2019;24 doi: 10.1016/j.ijcha.2019.100424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goette A., Kalman J.M., Aguinaga L., Akar J., Cabrera J.A., Chen S.A., Chugh S.S., Corradi D., D'Avila A., Dobrev D., Fenelon G., Gonzalez M., Hatem S.N., Helm R., Hindricks G., Ho S.Y., Hoit B., Jalife J., Kim Y.H., Lip G.Y., Ma C.S., Marcus G.M., Murray K., Nogami A., Sanders P., Uribe W., Van Wagoner D.R., Nattel S. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Heart Rhythm. 2017;14:e3–e40. doi: 10.1016/j.hrthm.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Middeldorp M.E., Pathak R.K., Meredith M., Mehta A.B., Elliott A.D., Mahajan R., Twomey D., Gallagher C., Hendriks J.M.L., Linz D., McEvoy R.D., Abhayaratna W.P., Kalman J.M., Lau D.H., Sanders P. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: the REVERSE-AF study. Europace. 2018;20:1929–1935. doi: 10.1093/europace/euy117. [DOI] [PubMed] [Google Scholar]