Abstract
A number of publications have described the use of adeno-associated virus (AAV) for the delivery of anti-HIV and anti-simian immunodeficiency virus (SIV) monoclonal antibodies (mAbs) to rhesus monkeys. Anti-drug antibodies (ADAs) have been frequently observed, and long-term AAV-mediated delivery has been inconsistent. Here, we investigated different AAV vector strategies and delivery schemes to rhesus monkeys using the rhesus monkey mAb 4L6. We compared 4L6 immunoglobulin G1 (IgG1) delivery using the AAV1 versus the AAV8 serotype with a cytomegalovirus (CMV) promoter and the use of a muscle-specific versus a liver-specific promoter. Long-term expression levels of 4L6 IgG1 following AAV8-mediated gene transfer were comparable to those following AAV1-mediated gene transfer. AAV1-mediated gene transfer, using a muscle-specific promoter, showed robust ADAs and transiently low 4L6 IgG1 levels that ultimately declined to below detectable levels. Intravenous AAV8-mediated gene transfer, using a liver-specific promoter, also resulted in low levels of delivered 4L6 IgG1, but those low levels were maintained in the absence of any detectable ADAs. Booster injections using AAV1-CMV allowed for increased 4L6 IgG1 serum levels in animals that were primed with AAV8 but not with AAV1. Our results suggest that liver-directed expression may help to limit ADAs and that re-administration of AAV of a different serotype can result in successful long-term delivery of an immunogenic antibody.
Keywords: AAV vector, AAV-antibody delivery, anti-drug antibody responses, monoclonal antibody, AAV1, AAV8, CMV promoter, Desmin promoter, TBG promoter, prime-boost immunization
Introduction
The efficacy of passively administered anti-HIV broadly neutralizing antibodies (bnAbs) has been demonstrated in a number of trials in monkeys and in humans.1, 2, 3 Whereas passive transfer is a reasonable approach to prevent and treat HIV infection, it has practical limitations for large-scale and long-term use. The immense costs associated with monoclonal antibody (mAb) production, the limited bioavailability following infusion, the need for repeated infusions over a prolonged period, and adherence will likely be problematic, especially in regions of the developing world. Lifelong delivery following a single administration of the adeno-associated virus (AAV) vector encoding the desired anti-HIV bnAb is a promising alternative.4
Multiple preclinical studies in monkeys have shown that AAV-delivered bnAbs and antibody-like molecules can protect against acquisition of immunodeficiency virus infection5, 6, 7, 8, 9, 10 and are also capable of controlling virus replication once infected.11 Nonetheless, the effectiveness of the AAV-antibody approach has been hindered by the emergence of unwanted immune responses following AAV administration. These immune responses, including anti-drug antibodies (ADAs), limit the bioavailability of AAV-delivered bnAbs and consequently reduce or eliminate their antiviral effect.12, 13, 14 The degree of immunogenicity is dependent on a variety of factors. Whereas autologous administered “self” proteins may not be expected to elicit immune responses, they do in hosts who have mutations or are missing the protein entirely.15 mAbs may be viewed as foreign/nonself, especially when the variable domain regions are vastly diverged from the host germline and/or when the constant regions are from a different species.12,16
In a previous study, we attempted to deliver the anti-simian immunodeficiency virus (SIV) antibody 4L6 immunoglobulin G1 (IgG1) to six rhesus monkeys. Following AAV1-mediated gene transfer, all six monkeys developed readily detectable ADAs that severely restricted bioavailability of 4L6 IgG1 in serum in five of the six monkeys for the 14 weeks of measurement.6 With the goal of comparing head to head several vector strategies and delivery schemes, we designed 3-monkey pilot studies to investigate AAV-mediated delivery of the 4L6 IgG1 mAb. Here, we describe how liver-directed AAV-antibody gene transfer can avoid the emergence of ADAs and that subsequent intramuscular re-administration of AAV of a different serotype can result in sustained high-level delivery of an immunogenic antibody.
Results
Antibody Delivery Using AAV1-CMV as Compared to AAV8-CMV
In our previous monkey experiment, we utilized a bicistronic single-stranded AAV (ssAAV) vector construct with the capsid of the AAV1 serotype.6 Following intramuscular inoculation, the serum levels of the AAV-delivered 4L6 IgG1 mAb were at 47–150 μg/mL at peak (weeks 3–4) but then precipitously dropped to 0–7 μg/mL by week 14 in five of the six monkeys, whereas only one monkey maintained a reasonable 4L6 IgG1 concentration of 38 μg/mL at week 14. 4L6-IgG1-specific ADAs were detected in all six monkeys.6,12 Here, we describe 3-monkey pilot experiments using 4L6 IgG1 as our model antibody. We redesigned our initial bicistronic ssAAV vector encoding the 4L6 mAb by modifying the 3′ UTR: the woodchuck hepatitis virus post-transcriptional regulatory element (WPRE) was removed (due to safety concerns),17 and microRNA-binding sites (miRNAbs) were inserted into all constructs (Figure 1). The selected miRNAbs are complementary in sequence to the microRNAs of miR-142-3p18, 19, 20 and miR-126-3p,21 which are both highly expressed in antigen-presenting cells (APCs). The idea behind including these two miRNAbs is that inadvertently AAV-transduced APCs may no longer express the 4L6 IgG1 mAb, which could avoid or blunt adaptive immune responses, including ADAs.
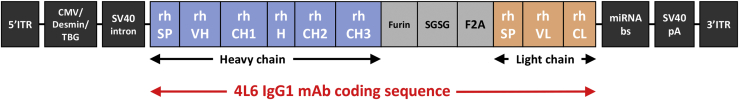
Figure 1.
AAV Vector Design
The AAV expression vector contains the following: a CMV promoter, a muscle-specific desmin promoter, or a liver-specific TBG promoter; a SV40 intron; a SV40 polyA signal; constant regions of IgG1 heavy and light chains from rhesus (rh) monkey, a rhesus signal peptide (SP), and the rh variable domains (VH and VL) of the 4L6 sequences that originate from recombinant anti-SIV Fab sequences derived from the bone marrow of SIV-infected rhesus monkeys. The expression cassette is flanked by AAV serotype 2 inverted terminal repeats (ITR). In this bicistronic single-stranded AAV (ssAAV) vector, both heavy and light chains of IgG are expressed from one open reading frame using a F2A “self-processing” peptide from foot-and-mouth disease virus. The furin cleavage sequence “RKRR” for the cellular protease furin is added for removal of amino acids that were left on the heavy chain C terminus following F2A self-processing. The peptide linker “SGSG” is added for improved furin enzyme-mediated cleavage. The 3′ UTR contains multiple binding sites for conserved endogenous microRNAs (miRNAs) that are specifically expressed in antigen-presenting cells (miRNAbs). This is designed to render the mAb transcripts sensitive to translational inhibition by the miRNAs expressed in antigen-presenting cells. Abbreviations are as follows: mAb, monoclonal antibody; CMV, cytomegalovirus; TBG, thyroxine-binding globulin; SV40, simian virus 40; VH, variable heavy domain; CH, constant heavy domain; H, hinge; VL, variable light domain; CL, constant light domain; pA, polyadenylation signal; and rh, of rhesus monkey origin.
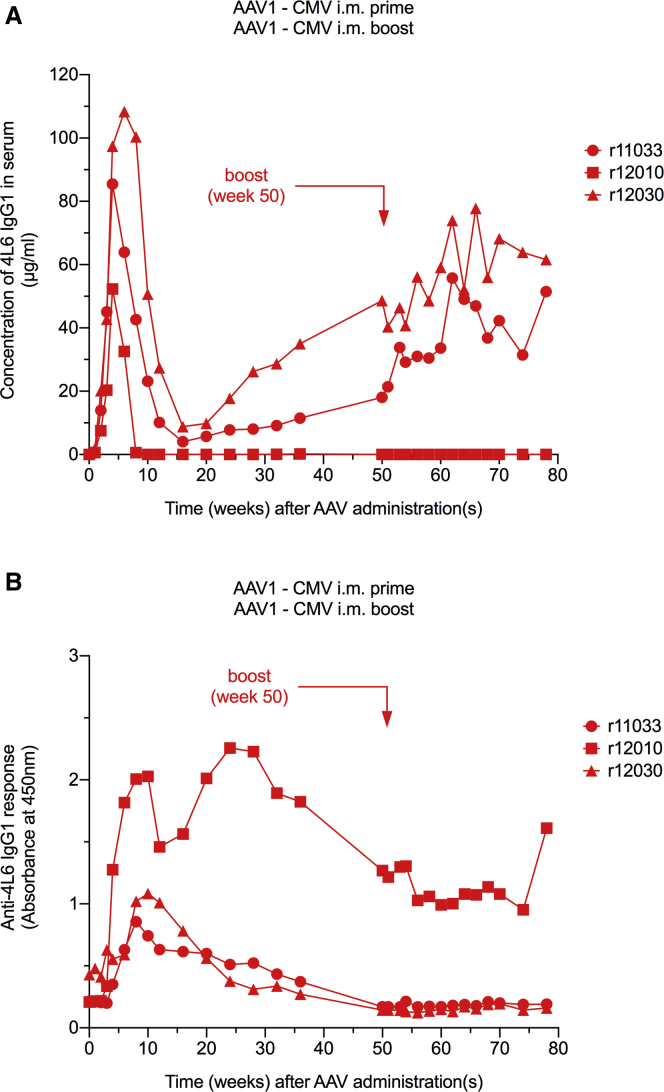
In our first group, we enrolled three AAV1-negative monkeys. Each monkey was inoculated intramuscularly with 0.25 × 1013 AAV1 particles harboring the redesigned 4L6 IgG1 expression cassette with a cytomegalovirus (CMV) promoter.22 Serum levels of the AAV-delivered mAb were measured by ELISA using SIVmac239 gp120 as capture antigen and recombinant (r)4L6 IgG1 as standard. Following AAV administration, levels of 4L6 IgG1 peaked at 52–108 μg/mL within 4–6 weeks but declined considerably thereafter. Whereas two animals had a nadir concentration of 4 and 9 μg/mL at week 16, rhesus monkey r12010 had undetectable 4L6 IgG1 at week 10 throughout week 78 (Figure 2A). The decline in mAb levels was associated with the emergence of ADAs in all three animals (Figure 2B). ADAs in r12010 were strong and persisted for the duration of the study. The other two animals exhibited moderate ADAs that gradually declined to baseline by week 50; the gradual decline in ADAs was associated with a gradual increase in 4L6 IgG1 levels between weeks 16 and 50 (Figures 2A and 2B).
Figure 2.
AAV1 Prime/AAV1 Boost-Mediated Antibody Delivery Using the CMV Promoter
(A) Levels of the monoclonal antibody (mAb) 4L6 IgG1 in serum following intramuscular (i.m.) AAV1 administrations. Intramuscular AAV1 boost had little or no effect on 4L6 IgG1 levels in AAV1-seropositive animals. (B) Anti-drug antibody (ADA) responses following i.m. AAV1 administration. Inclusion of microRNA-binding site (miRNAb) sequences into the rAAV genome did not limit ADA responses. No anamnestic humoral response was observed following AAV1 boost.
We next assessed the possibility of boosting mAb levels in these AAV1 recipients. The three animals that received AAV1-CMV into the quadriceps muscle were injected with the same AAV1-CMV construct to the deltoid and biceps muscle at week 50. At the time of the boost, mAb levels were at 0, 18, and 49 μg/mL. Minimal or no boosting effects in 4L6 IgG1 levels were observed in the weeks following AAV1-CMV re-administration. At week 78, the study endpoint, mAb levels were at 0, 51, and 62 μg/mL, respectively (Figure 2A). Interestingly, no anamnestic ADA response was observed following the AAV1 booster inoculation (Figure 2B).
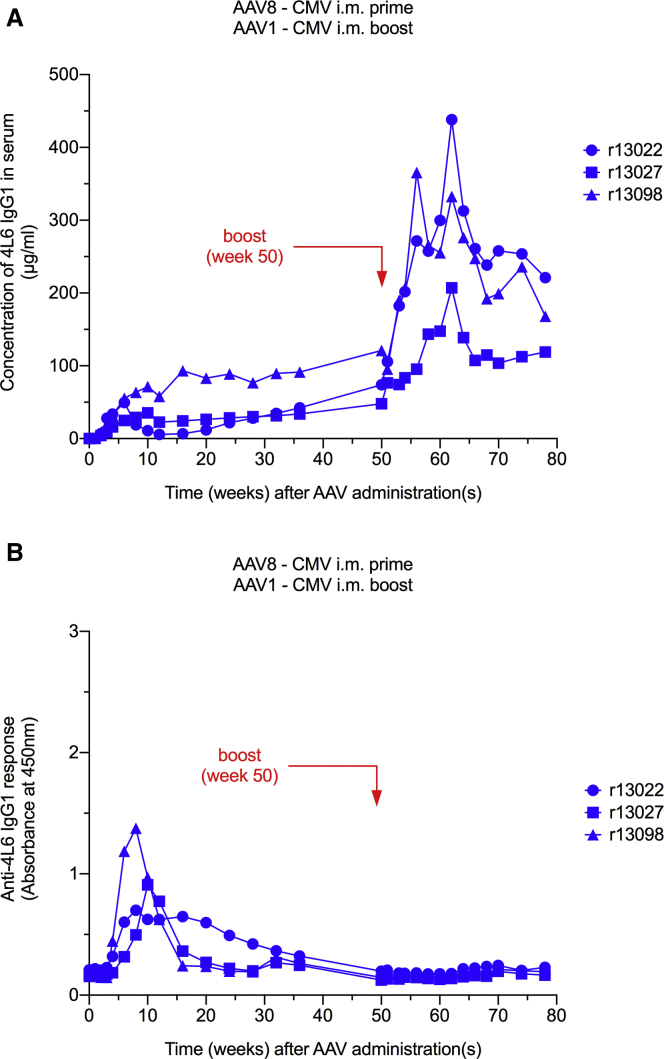
In our second group, three AAV1/AAV8 double-negative monkeys were injected intramuscularly with 0.25 × 1013 AAV8 particles harboring the same expression cassette as in group 1. Following the AAV8-CMV inoculation, serum levels of 4L6 IgG1 were in the range of 35–71 μg/mL at peak (within 6–10 weeks). Two of the three monkeys showed just a slight decrease in mAb levels following the peak; only rhesus monkey r13022 had a 10-fold drop between weeks 6 and 12. At week 12, the nadir concentrations were at 6, 23, and 58 μg/mL. By week 50, levels of 4L6 IgG1 gradually rose to 74, 48, and 121 μg/mL, respectively (Figure 3A). ADA responses were detected in all three animals. From the time of the peak at weeks 8–10, ADAs gradually decreased to baseline in all three animals by week 50 (Figure 3B). Interestingly, 4L6 IgG1 levels did not appear to be affected by the emergence of ADAs in r13098, the monkey with the strongest initial ADA response in the group of three (Figures 3A and 3B).
Figure 3.
AAV8 Prime/AAV1 Boost-Mediated Antibody Delivery Using the CMV Promoter
(A) AAV8-mediated gene transfer resulted in stable 4L6 IgG1 levels in three of three monkeys. An AAV1 boost markedly increased the levels of 4L6 IgG1 in AAV1 seropositive animals. (B) Anti-drug antibody (ADA) responses following intramuscular (i.m.) AAV8 prime and subsequent AAV1 boost administrations. AAV8-mediated gene transfer resulted in moderate ADA responses that resolved by week 50. No anamnestic humoral response was observed following AAV1 boost.
As in group 1, we tested the possibility of boosting mAb levels in serum. The three AAV8-CMV recipients were injected intramuscularly with AAV1-CMV at week 50. In contrast to group 1, group 2 displayed a boosting effect in 4L6 IgG1 levels within the first 12 weeks following the booster inoculation. At week 62, mAb levels were at 438, 207, and 333 μg/mL, respectively, i.e., an average 4.3-fold increase in 4L6 IgG1 levels. However, these boosted mAb levels subsequently normalized to 221, 119, and 168 μg/mL, respectively, by week 78 (Figure 3A). As observed in group 1, the monkeys in group 2 also did not elicit an anamnestic ADA response following the AAV1-CMV booster inoculation (Figure 3B).
Muscle-Directed AAV-Antibody Delivery
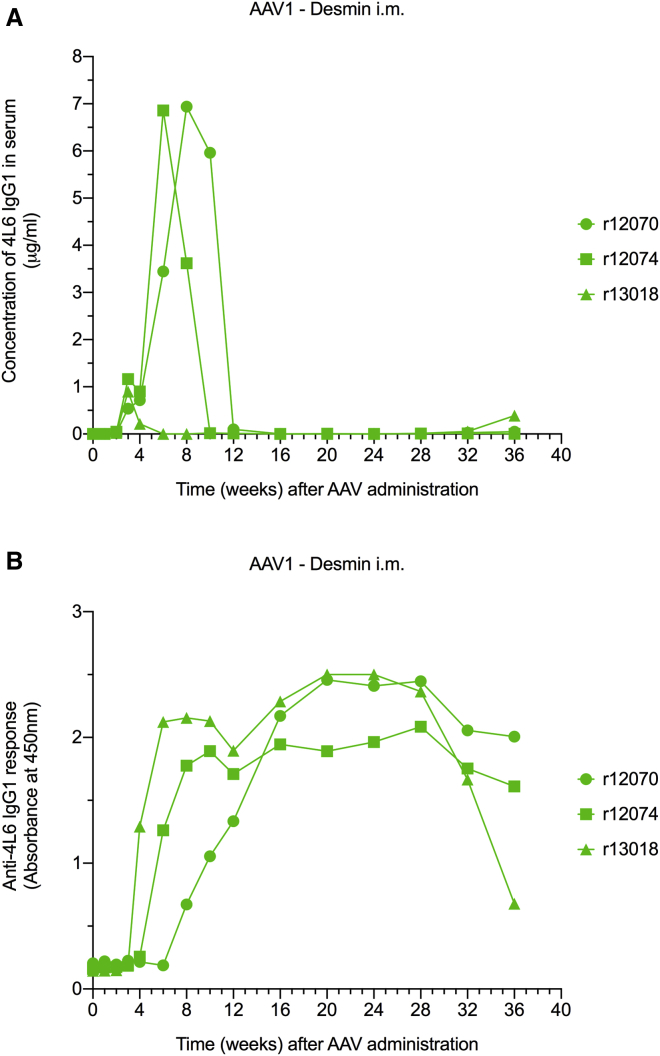
Intramuscular injection of AAV leads to local transduction of myocytes. However, a fraction of the intramuscularly injected AAV may also disseminate to other sites23,24 and/or transduce other cell types, including cells of the immune system. This could facilitate the triggering of unwanted immune responses, such as ADAs. Since the ubiquitous CMV promoter drives transgene expression in most cell types,22 we explored the possibility of containing transgene expression to the muscle by using a muscle-specific promoter. In our third group, we enrolled three AAV1-negative monkeys. Each monkey was injected intramuscularly with 0.25 × 1013 AAV1 particles harboring the 4L6 IgG1 expression cassette with a desmin promoter.25 Serum levels of the 4L6 IgG1 mAb peaked to 1–7 μg/mL between weeks 3 and 8 and subsequently dropped precipitously to undetectable levels in all three animals by week 16 (Figure 4A). The decline in mAb levels was associated with robust ADAs that persisted through week 36, the study endpoint (Figure 4B).
Figure 4.
AAV1-Mediated Antibody Delivery Using the Muscle-Specific Desmin Promoter
(A) AAV1-mediated gene transfer under a muscle-specific promoter resulted in low levels of 4L6 IgG1 that were undetectable by week 16. (B) Anti-drug antibody (ADA) responses following intramuscular (i.m.) AAV1 administration. AAV-mediated gene transfer under a muscle-specific promoter resulted in robust ADA responses.
Liver-Directed AAV-Antibody Delivery
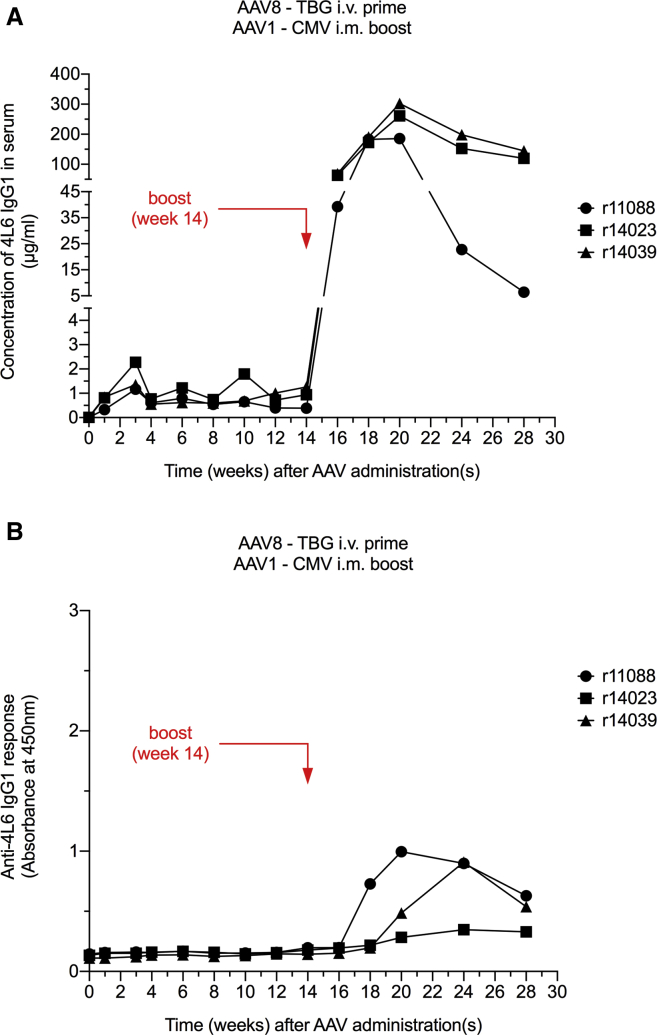
It has been suggested that liver-directed transgene expression is able to induce tolerance toward the transgene product and prevent the emergence of immune responses, including ADAs.26, 27, 28, 29, 30, 31, 32, 33, 34 We thus designed a 4L6 IgG1 expression cassette with a liver-specific promoter to investigate whether liver-directed AAV-antibody delivery could avoid unwanted ADAs. Three AAV1/AAV8 double-negative monkeys were injected intravenously with 0.25 × 1013 AAV8 particles harboring the 4L6 IgG1 expression cassette with a thyroxine-binding globulin (TBG) promoter.35, 36, 37, 38 Following AAV8-TBG inoculation, serum levels of 4L6 IgG1 were detectable but relatively low in all three animals, ranging from 0.3 to 2.3 μg/mL through week 14 (Figure 5A). Notably, none of the three monkeys developed any detectable ADA responses (Figure 5B). At week 14, we conducted an AAV booster inoculation. The three AAV8-TBG recipients were intramuscularly injected with 0.25 × 1013 AAV1 particles harboring the 4L6 IgG1 expression cassette with a CMV promoter. Within 6 weeks following the boost, serum levels of delivered 4L6 IgG1 peaked to 186–302 μg/mL at week 20. In the subsequent weeks, mAb levels declined to 6, 120, and 145 μg/mL (Figure 5A). ADA responses were detected as early as 4 weeks following the boost (week 18), but these ADAs were weak and had a downward trend by week 28 (Figure 5B).
Figure 5.
AAV8 Prime/AAV1 Boost-Mediated Antibody Delivery Using the Liver-Specific TBG Promoter (Prime) and the CMV Promoter (Boost)
(A) AAV8-mediated gene transfer under a liver-specific TBG promoter resulted in low levels of 4L6 IgG1. An AAV1-CMV boost strongly elevated the levels of 4L6 IgG1 in serum in AAV8-seropositive animals. (B) Anti-drug antibody (ADA) responses following intravenous (i.v.) AAV8 prime inoculation using the TBG promoter and subsequent intramuscular (i.m.) AAV1 boost administration using the CMV promoter. AAV8-mediated gene transfer under a liver-specific TBG promoter avoided ADA responses. An AAV1-CMV boost elicited moderate ADAs that appeared to decrease by week 28.
Group Comparison
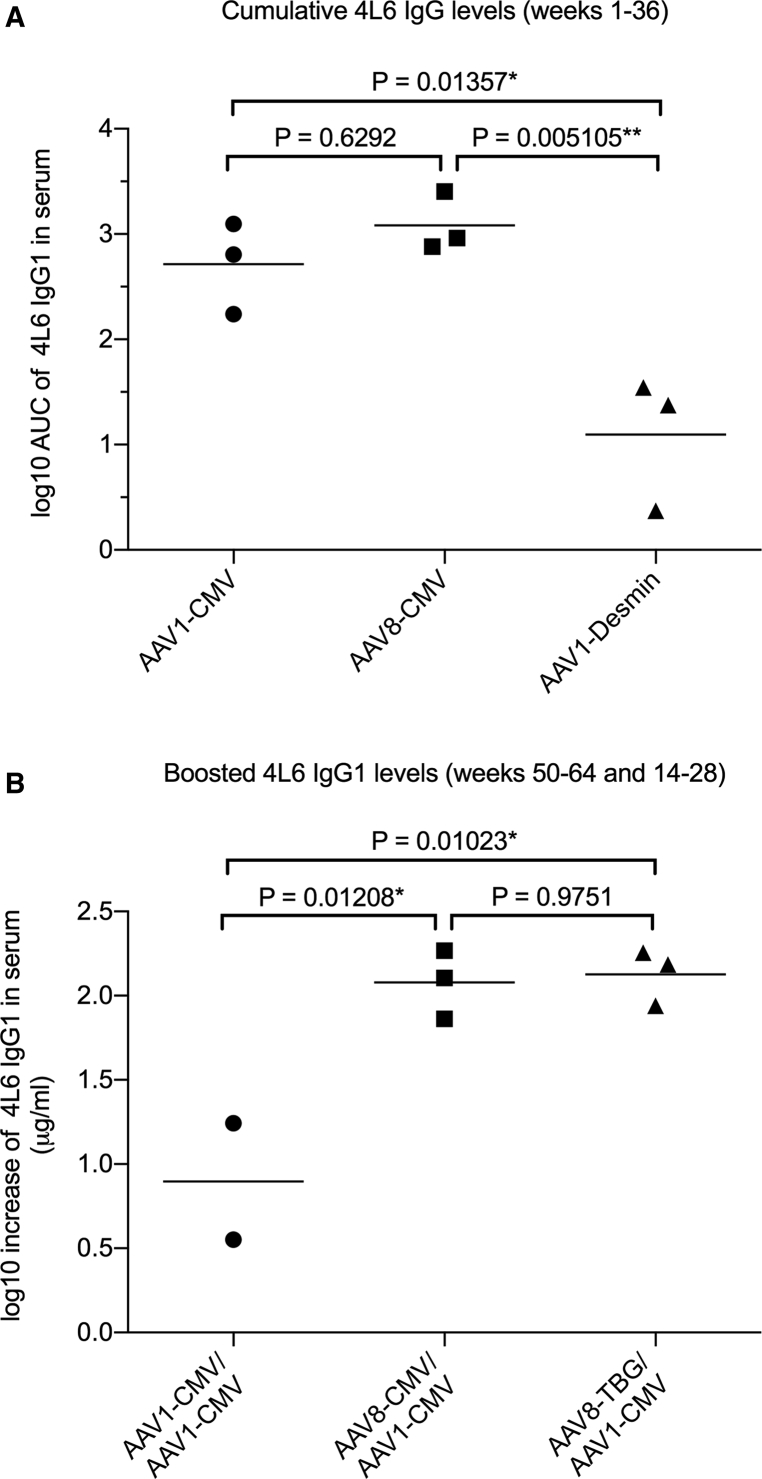
Two of the three monkeys that received AAV1-CMV maintained 4L6 IgG1 levels throughout the duration of the study (Figure 2A). In the AAV8-CMV group, the 4L6 IgG1 mAb was delivered successfully to all three monkeys (Figure 3A). Following AAV administration, the serum levels of 4L6 IgG1 rose to a peak and subsequently declined. Whereas the decline in mAb levels was followed by a rebound in five of the six animals, rhesus monkey r12010 had undetectable 4L6 IgG1 by week 10 throughout the end of the study (Figures 2A and 3A). Cumulative mAb levels in both groups, AAV1-CMV and AAV8-CMV, were comparable and not significantly different. In contrast, delivery of 4L6 IgG1 to the third group that received AAV1-desmin was poor and significantly lower than the other two groups that utilized the CMV promoter (Figure 6A).
Figure 6.
Statistical Analysis of AAV-Delivered 4L6 IgG1 Levels in Serum
(A) Levels of AAV-delivered 4L6 IgG1 in serum were compared in the three groups enrolled in the study: AAV1-CMV, AAV8-CMV, and AAV1-desmin. The area under the curve (AUC) of 4L6 IgG1 levels through week 36 was calculated, and the three groups were analyzed by one-way ANOVA Tukey’s multiple comparisons test; *p < 0.05; **p < 0.01. (B) The effect of an AAV1-CMV boost was compared in the three groups enrolled in the study: AAV1-CMV prime/AAV1-CMV boost (week 50), AAV8-CMV prime/AAV1-CMV boost (week 50), and AAV8-TBG prime/AAV1-CMV boost (week 14). Levels of 4L6 IgG1 following AAV booster inoculation (weeks 50–64 and 14–28) were averaged and compared to the levels at the time of the boost (week 50 or 14). The boosting effect is expressed as net-increase difference in 4L6 IgG1 as compared to the time of the boost. The values were analyzed by one-way ANOVA Tukey’s multiple comparisons test; *p < 0.05. Monkey r12010 in the AAV1-CMV/AAV1-CMV group was excluded from the analysis due to undetectable levels of 4L6 IgG1 at the time and following the time of the boost.
The animals that received AAV1-CMV (Figure 2), AAV8-CMV (Figure 3), and AAV8-TBG (Figure 5) were boosted with AAV1-CMV at either week 50 or week 14. Serum levels of AAV-delivered 4L6 IgG1 were followed for 14 weeks from the time of the AAV1-CMV boost and compared to the levels at the time of the boost. Animals that received AAV1-CMV showed no increased 4L6 IgG1 levels following the AAV1-CMV boost. Animals that received either AAV8-CMV or AAV8-TBG showed significantly increased 4L6 IgG1 levels following the AAV1-CMV boost (Figure 6B).
Discussion
Following administration of AAV1-CMV-4L6 or AAV8-CMV-4L6 by the intramuscular route (Figures 2 and 3), there was an early and strong ADA response to the delivered 4L6 mAb. This greatly suppressed the early 4L6 IgG1 concentrations in serum. However, the ADAs gradually declined in five of the six monkeys, eventually reaching levels below the limit of detection beyond week 36. This gradual decline in ADA levels corresponded to a gradual increase from trough levels in the 4L6 IgG1 concentration between weeks 16 and 50. This is a pattern that we have occasionally, but rarely, seen in our AAV delivery experiments using eight other mAbs, all constant domain-rhesusized (C-rhesusized) versions of human anti-HIV mAbs.11,12 Most commonly, the early appearance of strong ADAs has driven the delivered mAb levels to undetectable or marginally detectable levels. It is worth noting that the assay for ADA measures free but not bound anti-antibodies; thus, the absence of detection of ADAs may simply mean that their levels are not saturating, i.e., not in excess of the delivered antibody levels.
What are some of the factors that may influence the appearance of ADAs to therapeutic antibodies? Martinez-Navio et al.12 have shown that ADAs predominantly target the variable domains of an antibody and that the magnitude of the ADA response to AAV-delivered mAbs correlates directly with the distance of the variable domains of the antibody from germline. This is a problem because the potent anti-HIV bnAbs that one would like to deliver by AAV to the host typically have unusually long variable domains, are hypermutated, and/or harbor unusual structures.2,39,40 The host origin of the delivered mAb may also be a factor. The 4L6 mAb used here, as well as the 5L7 mAb in our previous publication,6 are of rhesus monkey origin.5,41,42 They also have very long heavy chain complementarity-determining region 3 (H-CDR3) regions in their variable domains.12 Whereas the eight anti-HIV mAbs that we have used for AAV delivery have been rhesusized in their constant regions, their variable domains still originate from the human germline. Whereas the 4L6 mAb and the 5L7 mAb have still quite consistently elicited ADAs following intramuscular AAV administration, far greater consistency of long-term delivery has been achieved with these rhesus monkey mAbs than with the eight C-rhesusized human anti-HIV mAbs. Cell types that may be expressing the mAb may also be a factor, dependent upon the route of administration, AAV dose, promoter used, and AAV serotype: muscle cells, lymphocytes, APCs, liver cells.
Will ADAs be a problem when human anti-HIV mAbs are delivered to humans via AAV? A recently published human trial of AAV to deliver the human anti-HIV mAb PG9 revealed readily detectable ADAs in ten of thirteen volunteers in four dosage groups and zero measureable delivery of the PG9 mAb.13,43 Further human phase 1 trials are certainly warranted using different vector designs and different mAbs to expand the database of knowledge regarding AAV delivery of anti-HIV mAbs to people and how consistently ADAs appear to be a problem in people. But, in the meantime, monkey models can be used to understand the ADA problem better and to examine ways by which the appearance of ADAs may be circumvented. The concentrations of mAbs needed to protect against the acquisition of infection are likely to be considerably less than the concentrations needed to control virus replication in an already-infected individual. Given the remarkable case of the functional cure of the Miami monkey,11,44,45 further testing in both monkeys and people appears warranted.
Previous studies have suggested that a low titer of pre-existing anti-AAV8 antibodies (≤1:160) does not influence the take of AAV8 following intramuscular administration.24 We investigated whether levels of 4L6 IgG1 in serum could be boosted in monkeys that had previously received AAV1-4L6 by a subsequent second intramuscular administration of AAV1-4L6. Little or no boosting of 4L6 IgG1 levels was observed. However, a second administration of AAV1-4L6 to monkeys that had previously received AAV8-4L6 resulted in significant boosting in the levels of the delivered 4L6 mAb. At least two factors may be contributing to this boosting effect: the tolerizing potential of AAV8 and the lack of major cross-reactivity between AAV8 and AAV1 serotypes.
We have identified in our pilot monkey studies two strategies that may help to overcome the problems of ADA and inconsistency of delivery: liver targeting and use of prime and boost. Liver-targeted expression has been previously shown to have a tolerogenic effect toward the AAV-delivered transgene product.26, 27, 28, 29, 30, 31, 32, 33, 34 Furthermore, AAV8 has been shown to transduce APCs poorly, as compared to AAV1,46 and to express well in liver.35, 36, 37, 38 Consistent with this, our results show no ADA to the 4L6 mAb when the AAV8 vector was administered intravenously using a liver-specific TBG promoter for expression despite the presence of readily detectable 4L6 IgG1 levels in serum for the 14 weeks of measurement. Boosting with the AAV1-CMV vector given intramuscularly showed a tremendous increase in 4L6 IgG1 levels that persisted at high levels for the 14 weeks of follow-up in at least two of the three monkeys. The boosting was also accompanied by the emergence of ADAs. Further work will be needed to examine the optimal conditions of the tolerizing liver-directed AAV8 prime, the timing of the AAV1 boost, and the dose of the AAV1 boost for their effects on the emergence and persistence of ADAs and the ability to achieve long-term persistence of the delivered mAb at reasonable levels.
Materials and Methods
rAAV Vector Constructs
The 4L6 mAb variable domain sequences (VH [variable heavy]; VL [variable light]) originate from the recombinant anti-SIV macaque antibody-binding fragment (Fab) “346-16h” that derived from the bone marrow of a SIV-infected rhesus monkey following Phage library selection.5,41,42 The coding sequence of the full-length 4L6 IgG1 was constructed by fusing the variable heavy domain to the constant heavy domains of rhesus IgG1 (based on NCBI: AAF14058 and AAQ57555) and by fusing the variable light domain to the rhesus kappa constant light domain (based on NCBI: AAD02577). A bicistronic DNA sequence was then designed in silico, codon optimized for rhesus monkeys, and gene synthesized (GenScript) as described previously.47 The synthesized DNA fragment was subsequently cloned into select ssAAV vector plasmids that contained a ubiquitous CMV promoter, a muscle-specific desmin promoter, or a liver-specific TBG promoter. All three ssAAV vector plasmids contained a simian virus 40 (SV40) intron, miRNAbs as described previously,18, 19, 20, 21 a SV40 polyA site, and the inverted terminal repeats (ITRs) of the AAV2 serotype.
rAAVs
Production of rAAV particles was accomplished as described previously.48 In short, HEK293 cells were transfected with a select rAAV vector plasmid and two helper plasmids to allow generation of infectious AAV particles. rAAV vector genomes were encapsidated with either the AAV1 or the AAV8 serotype capsid as indicated. After harvesting transfected cells and cell culture supernatant, rAAV was purified by three sequential CsCl centrifugation steps. Vector genome number was assessed by real-time PCR, and the purity of the preparation was verified by electron microscopy and silver-stained SDS-PAGE.
Rhesus Macaques and AAV Delivery
The twelve animals used in our studies were Indian-origin rhesus macaques (Macaca mulatta), housed at the Wisconsin National Primate Research Center. They were cared for in accordance with the guidelines of the Weatherall Report under a protocol approved by the University of Wisconsin Graduate School Animal Care and Use Committee. Animals were tested negative for the presence of antibodies to the AAV1 and the AAV8 capsids prior to AAV inoculations with the respective serotype. At the time of AAV prime inoculations, the average age of the animals was 2.8 years (ranging from 1.6 to 4.6), and the average weight was 4.0 kg (ranging from 2.4 to 7.4). A total of six females and six males were enrolled in the study. The twelve enrolled animals were separated into four groups of three. Nine of the twelve animals received rAAVs by intramuscular inoculation (AAV1-CMV, AAV8-CMV, and AAV1-desmin): 0.25 × 1013 vector genomes per kg; four 0.5 mL injections per animal, two injections per quadriceps muscle. Three of the 12 animals received rAAV by intravenous injection (AAV8-TBG): 0.25 × 1013 vector genomes per kg; one 2 mL injection into the saphenous vein. Where indicated (week 14 or 50), nine of the twelve animals received intramuscular AAV1-CMV booster inoculations: 0.25 × 1013 vector genomes per kg; four 0.5 mL injections per animal. Two injections per quadriceps muscle were performed. When intramuscular prime inoculations were conducted on the quadriceps muscles, then booster inoculations were conducted on the left and right deltoid and biceps muscles (four 0.5 mL injections per animal).
In Vivo mAb Quantification
HEK293T cells (ATCC) were expanded and then transfected with a rAAV vector plasmid encoding the 4L6 IgG1 mAb. Complete D10 cell culture medium was replaced with serum-free DMEM medium, 18 h after transfection (Gibco). The antibody-containing medium was harvested 90 h after transfection, precleared by centrifugation, and filtered through a 0.22-mm pore-size membrane (Nalgene). The 4L6 IgG1 was then affinity purified using protein A Sepharose 4 Fast Flow (GE Healthcare), concentrated and desalted, followed by protein quantification with a Nanodrop spectrometer (Thermo Fisher Scientific). To measure the concentration of 4L6 IgG1 in vivo, we performed a SIVmac239 gp120 (Immune Tech)/anti-rhesus IgG-horseradish peroxidase (HRP) ELISA (Southern Biotech) as previously described.6 Absorbance at 450 nm was compared to a serial dilution of purified mAb produced in HEK293T cells, and the amount of antibody in serum was determined based on the mAb standard curve.
ADA Responses
Humoral responses to the AAV-delivered 4L6 IgG1 mAb were measured by an antibody capture ELISA, as previously described.6,12 Plates were coated with purified 4L6 IgG1. After coating and blocking, the plates were incubated with antisera from the AAV-immunized monkeys. For detection, a HRP-conjugated anti-human lambda light-chain antibody was used (Southern Biotech). This secondary antibody did not cross-react with the coated mAb on the plates, since 4L6 IgG1 harbors a kappa light chain.
Statistical Analysis
All analyses were performed in Prism 6 (GraphPad). Comparisons between two groups were performed using the unpaired two-tailed t test with Welch’s correction. Comparisons among three groups were performed using the one-way ANOVA Tukey’s multiple comparison test. All values are expressed as mean. p values of <0.05 (*) and <0.01 (**) were considered significant.
Author Contributions
The study was conceived and designed by S.P.F., J.M.M.-N., and R.C.D. The experiments were performed by S.P.F., J.M.M.-N., E.G.R., and G.G. Reagents that were used in the study were generated by S.P.F., J.M.M.-N., E.G.R., and G.G. Data analysis was performed by S.P.F., J.M.M.-N., E.G.R., G.G., and R.C.D. The manuscript was composed by S.P.F., J.M.M.-N., and R.C.D.
Conflicts of Interest
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Acknowledgments
The authors thank Sònia Pedreño-López, Pratibha D. Selvakumar, William A. Lauer, and Kimberly L. Weisgrau for technical assistance; Jun Xie, Qin Su, Ran He, and Shaoyong Li of the Gene Therapy Core at University of Massachusetts Medical School for excellent AAV vector preparation and supportive advice; the Wisconsin National Primate Research Center veterinary staff for professional animal care; and Nancy Schultz-Darken and Wendy Newton for animal experiment planning and conduct. The data presented in this paper are listed in the main paper. Materials are available with an appropriate material transfer agreement. This project was supported by National Institutes of Health (NIH) grants P01 AI100263, R01 AI098446, and U19 AI095985 (to R.C.D.) and a P51 base grant RR000167 (Wisconsin National Primate Research Center), also from the NIH. We also acknowledge support from the Miami Center for AIDS Research (to J.M.M.-N.) at the University of Miami Miller School of Medicine, funded by grant P30AI073961 from the NIH.
References
- 1.Caskey M., Klein F., Nussenzweig M.C. Broadly neutralizing anti-HIV-1 monoclonal antibodies in the clinic. Nat. Med. 2019;25:547–553. doi: 10.1038/s41591-019-0412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sok D., Burton D.R. Recent progress in broadly neutralizing antibodies to HIV. Nat. Immunol. 2018;19:1179–1188. doi: 10.1038/s41590-018-0235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen Y.Z., Caskey M. Broadly neutralizing antibodies for treatment and prevention of HIV-1 infection. Curr. Opin. HIV AIDS. 2018;13:366–373. doi: 10.1097/COH.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs S.P., Desrosiers R.C. Promise and problems associated with the use of recombinant AAV for the delivery of anti-HIV antibodies. Mol. Ther. Methods Clin. Dev. 2016;3:16068. doi: 10.1038/mtm.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson P.R., Schnepp B.C., Zhang J., Connell M.J., Greene S.M., Yuste E., Desrosiers R.C., Clark K.R. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat. Med. 2009;15:901–906. doi: 10.1038/nm.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs S.P., Martinez-Navio J.M., Piatak M., Jr., Lifson J.D., Gao G., Desrosiers R.C. AAV-Delivered Antibody Mediates Significant Protective Effects against SIVmac239 Challenge in the Absence of Neutralizing Activity. PLoS Pathog. 2015;11:e1005090. doi: 10.1371/journal.ppat.1005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunders K.O., Wang L., Joyce M.G., Yang Z.Y., Balazs A.B., Cheng C., Ko S.Y., Kong W.P., Rudicell R.S., Georgiev I.S. Broadly Neutralizing Human Immunodeficiency Virus Type 1 Antibody Gene Transfer Protects Nonhuman Primates from Mucosal Simian-Human Immunodeficiency Virus Infection. J. Virol. 2015;89:8334–8345. doi: 10.1128/JVI.00908-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardner M.R., Kattenhorn L.M., Kondur H.R., von Schaewen M., Dorfman T., Chiang J.J., Haworth K.G., Decker J.M., Alpert M.D., Bailey C.C. AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature. 2015;519:87–91. doi: 10.1038/nature14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welles H.C., Jennewein M.F., Mason R.D., Narpala S., Wang L., Cheng C., Zhang Y., Todd J.P., Lifson J.D., Balazs A.B. Vectored delivery of anti-SIV envelope targeting mAb via AAV8 protects rhesus macaques from repeated limiting dose intrarectal swarm SIVsmE660 challenge. PLoS Pathog. 2018;14:e1007395. doi: 10.1371/journal.ppat.1007395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner M.R., Fellinger C.H., Kattenhorn L.M., Davis-Gardner M.E., Weber J.A., Alfant B., Zhou A.S., Prasad N.R., Kondur H.R., Newton W.A. AAV-delivered eCD4-Ig protects rhesus macaques from high-dose SIVmac239 challenges. Sci. Transl. Med. 2019;11:eaau5409. doi: 10.1126/scitranslmed.aau5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Navio J.M., Fuchs S.P., Pantry S.N., Lauer W.A., Duggan N.N., Keele B.F., Rakasz E.G., Gao G., Lifson J.D., Desrosiers R.C. Adeno-Associated Virus Delivery of Anti-HIV Monoclonal Antibodies Can Drive Long-Term Virologic Suppression. Immunity. 2019;50:567–575.e5. doi: 10.1016/j.immuni.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez-Navio J.M., Fuchs S.P., Pedreño-López S., Rakasz E.G., Gao G., Desrosiers R.C. Host Anti-antibody Responses Following Adeno-associated Virus-mediated Delivery of Antibodies Against HIV and SIV in Rhesus Monkeys. Mol. Ther. 2016;24:76–86. doi: 10.1038/mt.2015.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Priddy F.H., Lewis D.J.M., Gelderblom H.C., Hassanin H., Streatfield C., LaBranche C., Hare J., Cox J.H., Dally L., Bendel D. Adeno-associated virus vectored immunoprophylaxis to prevent HIV in healthy adults: a phase 1 randomised controlled trial. Lancet HIV. 2019;6:e230–e239. doi: 10.1016/S2352-3018(19)30003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardner M.R., Fetzer I., Kattenhorn L.M., Davis-Gardner M.E., Zhou A.S., Alfant B., Weber J.A., Kondur H.R., Martinez-Navio J.M., Fuchs S.P. Anti-drug Antibody Responses Impair Prophylaxis Mediated by AAV-Delivered HIV-1 Broadly Neutralizing Antibodies. Mol. Ther. 2019;27:650–660. doi: 10.1016/j.ymthe.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao O., Hoffman B.E., Moghimi B., Nayak S., Cooper M., Zhou S., Ertl H.C., High K.A., Herzog R.W. Impact of the underlying mutation and the route of vector administration on immune responses to factor IX in gene therapy for hemophilia B. Mol. Ther. 2009;17:1733–1742. doi: 10.1038/mt.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Meer P.J., Kooijman M., Brinks V., Gispen-de Wied C.C., Silva-Lima B., Moors E.H., Schellekens H. Immunogenicity of mAbs in non-human primates during nonclinical safety assessment. MAbs. 2013;5:810–816. doi: 10.4161/mabs.25234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schambach A., Bohne J., Baum C., Hermann F.G., Egerer L., von Laer D., Giroglou T. Woodchuck hepatitis virus post-transcriptional regulatory element deleted from X protein and promoter sequences enhances retroviral vector titer and expression. Gene Ther. 2006;13:641–645. doi: 10.1038/sj.gt.3302698. [DOI] [PubMed] [Google Scholar]
- 18.Xiao Y., Muhuri M., Li S., Qin W., Xu G., Luo L., Li J., Letizia A.J., Wang S.K., Chan Y.K. Circumventing cellular immunity by miR142-mediated regulation sufficiently supports rAAV-delivered OVA expression without activating humoral immunity. JCI Insight. 2019;4:e99052. doi: 10.1172/jci.insight.99052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Majowicz A., Maczuga P., Kwikkers K.L., van der Marel S., van Logtenstein R., Petry H., van Deventer S.J., Konstantinova P., Ferreira V. Mir-142-3p target sequences reduce transgene-directed immunogenicity following intramuscular adeno-associated virus 1 vector-mediated gene delivery. J. Gene Med. 2013;15:219–232. doi: 10.1002/jgm.2712. [DOI] [PubMed] [Google Scholar]
- 20.Boisgerault F., Gross D.A., Ferrand M., Poupiot J., Darocha S., Richard I., Galy A. Prolonged gene expression in muscle is achieved without active immune tolerance using microrRNA 142.3p-regulated rAAV gene transfer. Hum. Gene Ther. 2013;24:393–405. doi: 10.1089/hum.2012.208. [DOI] [PubMed] [Google Scholar]
- 21.Agudo J., Ruzo A., Tung N., Salmon H., Leboeuf M., Hashimoto D., Becker C., Garrett-Sinha L.A., Baccarini A., Merad M., Brown B.D. The miR-126-VEGFR2 axis controls the innate response to pathogen-associated nucleic acids. Nat. Immunol. 2014;15:54–62. doi: 10.1038/ni.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985;41:521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- 23.Greig J.A., Peng H., Ohlstein J., Medina-Jaszek C.A., Ahonkhai O., Mentzinger A., Grant R.L., Roy S., Chen S.J., Bell P. Intramuscular injection of AAV8 in mice and macaques is associated with substantial hepatic targeting and transgene expression. PLoS ONE. 2014;9:e112268. doi: 10.1371/journal.pone.0112268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greig J.A., Calcedo R., Grant R.L., Peng H., Medina-Jaszek C.A., Ahonkhai O., Qin Q., Roy S., Tretiakova A.P., Wilson J.M. Intramuscular administration of AAV overcomes pre-existing neutralizing antibodies in rhesus macaques. Vaccine. 2016;34:6323–6329. doi: 10.1016/j.vaccine.2016.10.053. [DOI] [PubMed] [Google Scholar]
- 25.Li Z.L., Paulin D. High level desmin expression depends on a muscle-specific enhancer. J. Biol. Chem. 1991;266:6562–6570. [PubMed] [Google Scholar]
- 26.Herzog R.W. Complexity of immune responses to AAV transgene products - Example of factor IX. Cell. Immunol. 2019;342:103658. doi: 10.1016/j.cellimm.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keeler G.D., Markusic D.M., Hoffman B.E. Liver induced transgene tolerance with AAV vectors. Cell. Immunol. 2019;342:103728. doi: 10.1016/j.cellimm.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherman A., Biswas M., Herzog R.W. Tolerance induction in hemophilia: innovation and accomplishments. Curr. Opin. Hematol. 2018;25:365–372. doi: 10.1097/MOH.0000000000000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vandamme C., Adjali O., Mingozzi F. Unraveling the Complex Story of Immune Responses to AAV Vectors Trial After Trial. Hum. Gene Ther. 2017;28:1061–1074. doi: 10.1089/hum.2017.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherman A., Biswas M., Herzog R.W. Innovative Approaches for Immune Tolerance to Factor VIII in the Treatment of Hemophilia A. Front. Immunol. 2017;8:1604. doi: 10.3389/fimmu.2017.01604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doerfler P.A., Nayak S., Corti M., Morel L., Herzog R.W., Byrne B.J. Targeted approaches to induce immune tolerance for Pompe disease therapy. Mol. Ther. Methods Clin. Dev. 2016;3:15053. doi: 10.1038/mtm.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kattenhorn L.M., Tipper C.H., Stoica L., Geraghty D.S., Wright T.L., Clark K.R., Wadsworth S.C. Adeno-Associated Virus Gene Therapy for Liver Disease. Hum. Gene Ther. 2016;27:947–961. doi: 10.1089/hum.2016.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colella P., Ronzitti G., Mingozzi F. Emerging Issues in AAV-Mediated In Vivo Gene Therapy. Mol. Ther. Methods Clin. Dev. 2017;8:87–104. doi: 10.1016/j.omtm.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mingozzi F., High K.A. Overcoming the Host Immune Response to Adeno-Associated Virus Gene Delivery Vectors: The Race Between Clearance, Tolerance, Neutralization, and Escape. Annu. Rev. Virol. 2017;4:511–534. doi: 10.1146/annurev-virology-101416-041936. [DOI] [PubMed] [Google Scholar]
- 35.Gao G., Lu Y., Calcedo R., Grant R.L., Bell P., Wang L., Figueredo J., Lock M., Wilson J.M. Biology of AAV serotype vectors in liver-directed gene transfer to nonhuman primates. Mol. Ther. 2006;13:77–87. doi: 10.1016/j.ymthe.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 36.Wang L., Calcedo R., Wang H., Bell P., Grant R., Vandenberghe L.H., Sanmiguel J., Morizono H., Batshaw M.L., Wilson J.M. The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques. Mol. Ther. 2010;18:126–134. doi: 10.1038/mt.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L., Bell P., Lin J., Calcedo R., Tarantal A.F., Wilson J.M. AAV8-mediated hepatic gene transfer in infant rhesus monkeys (Macaca mulatta) Mol. Ther. 2011;19:2012–2020. doi: 10.1038/mt.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greig J.A., Nordin J.M., Bote E., Makaron L., Garnett M.E., Kattenhorn L.M., Bell P., Goode T., Wilson J.M. Impact of intravenous infusion time on AAV8 vector pharmacokinetics, safety, and liver transduction in cynomolgus macaques. Mol. Ther. Methods Clin. Dev. 2016;3:16079. doi: 10.1038/mtm.2016.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burton D.R., Hangartner L. Broadly Neutralizing Antibodies to HIV and Their Role in Vaccine Design. Annu. Rev. Immunol. 2016;34:635–659. doi: 10.1146/annurev-immunol-041015-055515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burton D.R., Mascola J.R. Antibody responses to envelope glycoproteins in HIV-1 infection. Nat. Immunol. 2015;16:571–576. doi: 10.1038/ni.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reitter J.N., Means R.E., Desrosiers R.C. A role for carbohydrates in immune evasion in AIDS. Nat. Med. 1998;4:679–684. doi: 10.1038/nm0698-679. [DOI] [PubMed] [Google Scholar]
- 42.Johnson W.E., Sanford H., Schwall L., Burton D.R., Parren P.W., Robinson J.E., Desrosiers R.C. Assorted mutations in the envelope gene of simian immunodeficiency virus lead to loss of neutralization resistance against antibodies representing a broad spectrum of specificities. J. Virol. 2003;77:9993–10003. doi: 10.1128/JVI.77.18.9993-10003.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caskey M. Delivery of anti-HIV bNAbs by viral vectors. Lancet HIV. 2019;6:e207–e208. doi: 10.1016/S2352-3018(19)30041-4. [DOI] [PubMed] [Google Scholar]
- 44.Liberatore R.A., Ho D.D. The Miami Monkey: A Sunny Alternative to the Berlin Patient. Immunity. 2019;50:537–539. doi: 10.1016/j.immuni.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Haigwood N.L., Hessell A.J. Antibodies Tip the Balance Towards an HIV Cure. Trends Immunol. 2019;40:375–377. doi: 10.1016/j.it.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Lu Y., Song S. Distinct immune responses to transgene products from rAAV1 and rAAV8 vectors. Proc. Natl. Acad. Sci. USA. 2009;106:17158–17162. doi: 10.1073/pnas.0909520106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fuchs S.P., Martinez-Navio J.M., Gao G., Desrosiers R.C. Recombinant AAV Vectors for Enhanced Expression of Authentic IgG. PLoS ONE. 2016;11:e0158009. doi: 10.1371/journal.pone.0158009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mueller C., Ratner D., Zhong L., Esteves-Sena M., Gao G. Production and discovery of novel recombinant adeno-associated viral vectors. Curr. Protoc. Microbiol. 2012;26 doi: 10.1002/9780471729259.mc14d01s26. 14D.1.1–14D.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]