Abstract
Background
Acetoacetate is used as an alternative energy source in the heart, and has the potential to improve cardiac function. However, the prognostic impact of acetoacetate has not been investigated in heart failure.
Methods
This study enrolled consecutive 615 hospitalized patients with heart failure. We investigated the associations between circulating acetoacetate and clinical characteristics or prognosis in HF patients.
Results
We divided the patients into two groups based on circulating acetoacetate levels (high group: acetoacetate ≥35 µmoL/L, n = 313; and low group: acetoacetate <35 µmoL/L, n = 302). The high group had an older age (68 vs. 65 years, P = 0.003) and higher log brain natriuretic peptide levels (2.43 vs. 2.23, P < 0.001) compared with the low group. In contrast, there were no significant differences in the prevalence of co-morbidities, including diabetes mellitus, chronic kidney disease, and anemia, between the two groups. During the median follow-up period of 328 days, 66 all-cause deaths occurred. The high group had a worse prognosis compared with the low group (Log rank, P = 0.041). In the Cox proportional hazard analysis, circulating acetoacetate levels (per 10 µmoL/L increase) were associated with all-cause mortality (hazard ratio 1.020, 95% confidence interval 1.010–1.030, P < 0.001).
Conclusions
Circulating acetoacetate is associated with all-cause mortality in patients with heart failure. These results provide new insights into the role of alternative cardiac metabolism in heart failure patients, and raise the possibility of acetoacetate as a novel biomarker to predict the prognosis of heart failure patients.
Keywords: Acetoacetate, Ketone body, Metabolism, Heart failure, Prognosis
1. Background
A progressive disorder that is associated with frequent mortality, heart failure is a major health problem worldwide. Community-based studies have revealed that 12–13% of their study populations had stage C heart failure, with the number of heart failure cases increasing correspondingly according to increasing ages [1], [2], [3].
The myocardium mainly uses carbohydrates and fatty acids as an energy source, with fatty acids accounting for 70% of ATP [4]. Heart failure is known to be a multi-organ syndrome with systemic and myocardial metabolic failure. Metabolic impairment of the failing heart is not a problem of only the heart, but also the skeletal muscles and other organs [5]. Ketone bodies produce small amounts of ATP in the normal heart, and become a significant alternative fuel in the failing heart [6], [7], [8]. A previous study has reported circulating ketone bodies to be increased in heart failure patients, which suggests increased production of ketone bodies in the liver [9]. The components of ketone bodies are acetoacetate, 3-hydroxybutyrate, and acetone. Acetoacetate is one of the main ketone bodies, produced by the liver, and is used particularly in the skeletal muscle, brain, and heart.
Acetoacetate is an energy source that has biological effects in various organs, and produces reactive oxygen species, stimulates insulin secretion, and increases lipid peroxidation, while 3-hydroxybutyrate does not [10], [11], [12]. Muscle regeneration is accelerated by acetoacetate through activation of the MEK1-ERK1/2 pathway [13]. Acetoacetate protects neuronal cells from oxidative stress [14]. In the myocardium, acetoacetate has beneficial effects that increase myocardial contractile function by antioxidant mechanisms [15], [16].
It has recently been reported that sodium-glucose cotransporter-2 inhibitors (SGLT2 inhibitors) increase circulating ketone bodies and decrease cardiovascular events in patients with diabetes mellitus [17], [18]. One of the possible mechanisms for this favorable outcome is that ketone bodies could be an important fuel for the failing heart [7], [8], [19]. However, the prognostic impact of circulating ketone bodies has not yet been investigated in heart failure patients. We investigated the significance of acetoacetate, a multi-functional ketone body component, and its relationship to prognosis in heart failure patients.
2. Methods
2.1. Study population
This study prospectively enrolled 638 heart failure patients who had been admitted to Fukushima Medical University Hospital between May 2016 and December 2018. Twenty-three patients who took SGLT-2 inhibitors were excluded because the said inhibitors possibly affect circulating acetoacetate levels. No patient had diabetic ketoacidosis. Finally, 615 patients were enrolled in this study. Heart failure was diagnosed by well-trained cardiologists using the American College of Cardiology Foundation and American Heart Association Guidelines [20]. We investigated the patients’ characteristics, including age, sex, New York Heart Association (NYHA) classification, etiology of heart failure, commodities, history of cancer, medications, echocardiographic parameters, and laboratory data at admission. The patients were followed up until the date of last-follow-up or for all-cause death. We also analyzed the causes of death, and cardiac death was classified by independent experienced cardiologists as death from worsened heart failure, ventricular fibrillation or ventricular tachycardia documented by electrocardiogram or implantable devices, acute coronary syndrome, or sudden death. Survival time was calculated from the sampling date of acetoacetate until the date of last-follow-up or death. We were able to follow-up on all patients. Written informed consent was obtained from all study subjects. The study protocol was approved by the Ethics Committee of Fukushima medical University (Ethical Board Approval number, 823), and was carried out in accordance with the principles outlined in the Declaration of Helsinki.
2.2. Measurement of circulating acetoacetate levels
Venous blood samples were collected for the measurement of acetoacetate levels at the time of admission, regardless of timing of feeding. The venous blood samples were immediately centrifuged at 2400 g for 10 min at 4 °C to obtain serum samples and kept frozen at −20 °C until assayed. Circulating levels of serum acetoacetate were measured by an enzymatic cycling method. The measurements were performed by BML Inc. (Tokyo, Japan), who were blind to the patients’ information.
2.3. Echocardiography
Echocardiography was performed by experienced echocardiographers. Two-dimensional echocardiographic images were obtained from the parasternal long and short axes, apical long axis, and apical 4-chamber views. The following echocardiographic parameters were investigated: left ventricular end-diastolic diameter; left ventricular end-systolic diameter; and left ventricular ejection fraction. The left ventricular ejection fraction was calculated using Simpson’s method in a four-chamber view.
2.4. Statistical analysis
Data were analyzed using the Statistical Package for Social Sciences version 26 software (SPSS Inc., Chicago, IL, USA). Continuous data are expressed as mean ± SD, and skewed data are presented as median and interquartile range. Categorical variables are expressed as numbers and percentages. The statistical significance of differences was analyzed using Student’s t-test for parametric continuous variables, and the Mann-Whitney U test for nonparametric continuous variables. Categorical variables were compared using the Chi-square test or Fisher’s exact test. The Kaplan-Meier analysis was used for the all-cause mortality, and the log rank test was used for comparisons. To assess the potential heterogeneity of associations between circulating acetoacetate levels, and all-cause mortality, we conducted subgroup analysis. Interactions between circulating acetoacetate levels and clinically important variables for heart failure patients (age, sex, NYHA classification, ischemic etiology, hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation, chronic kidney disease, anemia, and B-type natriuretic peptide) were estimated using a univariate Cox proportional hazard model. Multivariable Cox proportional analysis was performed for all-cause death. To prepare for potential confounding, we considered the following clinical factors, which are generally known in heart failure patients: age, sex, NYHA classification, ischemic etiology, hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation, chronic kidney disease, and anemia. Parameters with a P value of <0.05 by univariable analysis were entered in the multivariable analysis. A p value of <0.05 was considered statistically significant for all comparisons.
3. Results
The mean age of the study subjects was 66.5 years, and 60.6% were male. Circulating acetoacetate levels were ranged from 1 to 1690 µmoL/L (median, 35 µmoL/L). We divided all study subjects into two groups on the basis of the median circulating acetoacetate level: the low acetoacetate group (n = 302; <35 µmoL/L), and the high acetoacetate group (n = 313; ≥35 µmoL/L). The comparisons of baseline clinical characteristics are shown in Table 1. The high acetoacetate group was older (P = 0.003), and had a higher NYHA classification (P < 0.001), prevalence of hypertension (P = 0.007), and levels of log B-type natriuretic peptide (P < 0.001), compared to the low acetoacetate group. There were no significant differences regarding prevalence of diabetes mellitus, cancer, and receiving anti-diabetic drugs, and levels of hemoglobin A1c between the two groups.
Table 1.
Baseline characteristics.
| Low acetoacetate group (Acetoacetate <35 µmoL/L n = 302) | High acetoacetate group (Acetoacetate ≥35 µmoL/L n = 313) | P value | |
|---|---|---|---|
| Acetoacetate, µmoL/L* | 19 (14–25) | 84 (52–171) | <0.001 |
| Age, years | 65 ± 14 | 68 ± 15 | 0.003 |
| Male, n (%) | 185 (61) | 189 (60) | 0.824 |
| BMI, kg/m2 | 23 ± 4 | 24 ± 5 | 0.615 |
| NYHA classification I/II/III/IV | 67/144/48/43 | 41/110/81/81 | <0.001 |
| Etiology of heart failure | |||
| Ischemic, n (%) | 66 (22) | 81 (26) | 0.242 |
| Cardiomyopathy, n (%) | 79 (26) | 67 (21) | 0.166 |
| Valvular, n (%) | 57 (19) | 61 (20) | 0.847 |
| Others, n (%) | 100 (33) | 104 (33) | 0.976 |
| Co-morbidities | |||
| Hypertension, n (%) | 178 (59) | 217 (69) | 0.007 |
| Diabetes Mellitus, n (%) | 116 (38) | 140 (45) | 0.112 |
| Dyslipidemia, n (%) | 193 (64) | 199 (64) | 0.932 |
| Atrial fibrillation, n (%) | 126 (42) | 123 (39) | 0.540 |
| Chronic kidney disease | 152 (50) | 172 (55) | 0.251 |
| Anemia, n (%) | 15 (5) | 18 (6) | 0.666 |
| Cancer, n (%) | 56 (19) | 66 (21) | 0.429 |
| Medications | |||
| RAS-I, n (%) | 205 (68) | 216 (69) | 0.763 |
| Beta-blocker, n (%) | 212 (70) | 228 (73) | 0.524 |
| Diuretics, n (%) | 208 (69) | 236 (75) | 0.071 |
| Inotropic agent, n (%) | 34 (11) | 33 (11) | 0.776 |
| Anti-diabetic drugs, n (%) | 68 (23) | 78 (25) | 0.484 |
| Insulin, n (%) | 18 (6) | 13 (4) | 0.306 |
| DPP-4, n (%) | 53 (18) | 62 (20) | 0.473 |
| BG, n (%) | 8 (3) | 18 (6) | 0.056 |
| SGLT2-inhibitor, n (%) | 0 | 0 | – |
| Echocardiography | |||
| LVDd, mm | 49 ± 11 | 49 ± 11 | 0.635 |
| LVDs, mm | 36 ± 13 | 36 ± 13 | 0.797 |
| LVEF, % | 52 ± 16 | 50 ± 16 | 0.176 |
| Laboratory data | |||
| Log BNP | 2.23 ± 0.6 | 2.43 ± 0.6 | <0.001 |
| Hb, g/dL | 12.9 ± 2.0 | 12.6 ± 2.4 | 0.172 |
| eGFR, mL/min/1.73 cm2 | 58 ± 25 | 55 ± 22 | 0.250 |
| AST, IU/L* | 24 (19–34) | 26 (19–41) | 0.114 |
| ALT, IU/L* | 20 (14–30) | 21 (13–37) | 0.183 |
| BS, mg/dL | 124 ± 49 | 129 ± 57 | 0.171 |
| HbA1c, % | 6.0 ± 0.8 | 6.1 ± 0.9 | 0.898 |
Data are shown as number (%), mean ± SD, or *median (IQR).
NYHA, New York Heart association; BMI, body mass index; RAS, renin-angiotensin system; DPP, dipeptidyl peptidase; BG, biguanide; LVDd, left ventricular end-diastolic diameter; LVDs, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; BNP, B-type natriuretic peptide; Hb, hemoglobin; eGFR, estimated glomerular filtration rate; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BS, Blood Sugar; HbA1c, hemoglobin A1c; IQR, interquartile range.
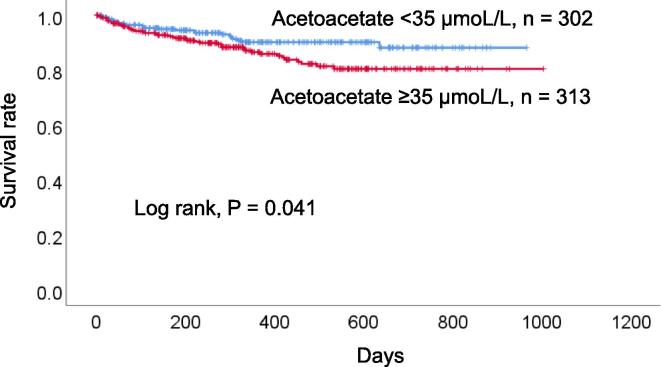
During the median follow-up period of 328 days (ranges 1 to 1004 days), 66 all-cause deaths (40 cardiac deaths, 25 non-cardiac deaths, and one unknown cause) occurred. Of those cardiac deaths, 22 were from heart failure, five each were from myocardial infarction and lethal arrhythmia, four were from sudden death, two were from multiple organ failure related to the heart, and one each was from cardiac sarcoma, and cardiac death (the detail was unknown). Of those non-cardiac deaths, seven were from infection, six were from cancer, three were from bleeding, two each were from respiratory failure, kidney failure, and natural death, and one was from multiple organ failure unrelated to the heart and mesenteric artery thrombosis. One death was due to an unknown cause. The high acetoacetate group had a worse prognosis compared with the low acetoacetate group (log rank, P = 0.041, Fig. 1).
Fig. 1.
Survival curve for all-cause death. The high acetoacetate group had a worse prognosis compared with the low acetoacetate group.
In the univariate Cox proportional hazard analysis, circulating acetoacetate level (per 10 µmoL/L increase) was associated with all-cause mortality (hazard ratio 1.020, 95% confidence interval 1.010–1.030, P < 0.001) as shown in Table 2. To assess the potential heterogeneity of circulating acetoacetate’s impact on all-cause mortality, we conducted subgroup analyses and examined interaction terms (Table 2). There were interactions between the prognostic impact of circulating acetoacetate levels and sex, NYHA classification, diabetes mellitus, and B-type natriuretic peptide. In contrast, there were no interactions between the prognostic impacts of circulating acetoacetate levels and age, ischemic etiology, hypertension, dyslipidemia, atrial fibrillation, chronic kidney disease, or anemia. In the multivariable Cox proportional hazard analysis, circulating acetoacetate level (per 10 µmoL/L increase) was associated with all-cause mortality (hazard ratio 1.010, 95% confidence interval 1.000–1.020, P = 0.019) as shown in Table 3.
Table 2.
Subgroup analysis of acetoacetate (per 10 µmoL/L increase) for all-cause death.
| Factor | Subgroup | N | HR | 95%CI | P value | InteractionP value |
|---|---|---|---|---|---|---|
| Total | 615 | 1.020 | 1.010–1.030 | <0.001 | ||
| Age | Age 67 years < | 245 | 0.932 | 0.784–1.104 | 0.402 | 0.284 |
| Age 67 years ≥ | 370 | 1.020 | 1.010–1.020 | <0.001 | ||
| Sex | Male | 374 | 1.010 | 1.001–1.020 | 0.004 | 0.005 |
| Female | 241 | 1.051 | 1.030–1.083 | <0.001 | ||
| NYHA classification | I, II | 362 | 1.041 | 1.041–1.051 | <0.001 | 0.001 |
| III, IV | 253 | 1.010 | 0.990–1.020 | 0.335 | ||
| Ischemic etiology | ± | 147 | 1.010 | 1.001–1.020 | 0.047 | 0.110 |
| – | 468 | 1.030 | 1.010–1.040 | <0.001 | ||
| Hypertension | ± | 395 | 1.010 | 1.001–1.030 | 0.011 | 0.094 |
| – | 220 | 1.030 | 1.010–1.041 | <0.001 | ||
| Diabetes mellitus | ± | 256 | 1.010 | 1.000–1.020 | 0.057 | 0.013 |
| – | 359 | 1.041 | 1.020–1.051 | <0.001 | ||
| Dyslipidemia | ± | 392 | 1.020 | 1.010–1.030 | <0.001 | 0.668 |
| – | 223 | 1.010 | 0.990–1.041 | 0.305 | ||
| Atrial fibrillation | ± | 249 | 1.030 | 1.010–1.051 | 0.015 | 0.479 |
| – | 336 | 1.020 | 1.010–1.030 | <0.001 | ||
| Chronic kidney disease | ± | 324 | 1.030 | 1.020–1.051 | <0.001 | 0.060 |
| – | 291 | 1.010 | 1.000–1.030 | 0.026 | ||
| Anemia | ± | 293 | 1.010 | 1.010–1.020 | <0.001 | 0.590 |
| – | 322 | 1.020 | 1.000–1.051 | 0.094 | ||
| Log BNP | Mean 2.3 ≥ | 340 | 1.010 | 1.001–1.020 | 0.042 | 0.007 |
| Mean 2.3 < | 275 | 1.030 | 1.020–1.041 | <0.001 |
HR, hazard ratio; CI, confidence interval; NYHA, New York Heart Association; BNP, B-type natriuretic peptide.
Table 3.
Univariable and multivariable analysis for all-cause death.
| Univariable |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| Factor | HR | 95%CI | P value | HR | 95%CI | P value |
| Acetoacetate (per 10 µmoL/L increase) | 1.020 | 1.010–1.030 | <0.001 | 1.010 | 1.000–1.020 | 0.019 |
| Age | 1.069 | 1.045–1.094 | <0.001 | 1.069 | 1.041–1.097 | <0.001 |
| Male | 1.874 | 1.079–3.523 | 0.026 | 2.576 | 1.449–4.579 | 0.001 |
| NYHA classification | 1.315 | 1.036–1.669 | 0.025 | 1.064 | 0.824–1.374 | 0.635 |
| Ischemic etiology | 1.520 | 0.905–2.552 | 0.113 | – | – | – |
| Hypertension | 0.815 | 0.497–1.335 | 0.417 | – | – | – |
| Diabetes mellitus | 1.534 | 0.946–2.489 | 0.083 | – | – | – |
| Dyslipidemia | 1.031 | 0.624–1.703 | 0.904 | – | – | – |
| Atrial fibrillation | 1.628 | 1.004–2.641 | 0.048 | 1.121 | 0.679–1.849 | 0.655 |
| Chronic kidney disease | 1.986 | 1.183–3.334 | 0.009 | 1.190 | 0.683–2.073 | 0.539 |
| Anemia | 3.641 | 2.073–6.393 | <0.001 | 2.415 | 1.353–4.309 | 0.003 |
HR, hazard ratio; CI, confidence interval; NYHA, New York Heart Association.
In addition, we analyzed the effect of diabetes mellitus on acetoacetate levels. In patients with diabetes mellitus (n = 256) and without diabetes mellitus (n = 359), acetoacetate levels were ranged from 1 to 1575 µmoL/L (median, 39 µmoL/L) and 4 to 1690 µmoL/L (median, 33 µmoL/L), respectively. There was no significant differences between heart failure patients with and without diabetes mellitus (P = 0.140).
4. Discussion
The present study is the first to describe the prognostic impact of circulating acetoacetate in heart failure patients. High levels of circulating acetoacetate are associated with older age, higher NYHA classification, hypertension, high B-type natriuretic peptide levels, and worse clinical outcome. Measurement of circulating acetoacetate might enable the evaluation of both systemic and myocardial metabolic impairment in heart failure.
Few reports described the prognostic impact of ketone bodies. Arterial ketone body ratio, which reflects hepatic mitochondrial oxidation-reduction potential, is independently associated with cardiac death in patients with acute heart failure [21]. Breath acetone level, which reflects blood ketone bodies, has been reported to be a prognostic factor in heart failure patients with reduced ejection fraction [22], [23]. In patients with hemodialysis, circulating ketone bodies predict cardiovascular events [24]. As far as we know, the prognostic impact of circulating acetoacetate has not yet been revealed. High circulating acetoacetate level was related to all-cause mortality in heart failure patients in the present study.
We were unable to fully explain the reason why acetoacetate level did not have prognostic impact in patients with NYHA III or IV, but did have a prognostic impact in patients with NYHA I or II. In high NYHA patients, acetoacetate might work as a fuel; on the other hand, in low NYHA patients, acetoacetate might work as a signaling molecule associated with reactive oxygen species [7], [25]. In addition, we also found the presence of diabetes mellitus to be associated with the prognostic impact of acetoacetate in the current study. Diabetes mellitus is closely associated with ketone body production [26], and elevated acetoacetate levels in patients with diabetes mellitus might be caused by elevated insulin resistance and decreased utilization of glucose [27]. However, in patients without diabetes mellitus, elevated acetoacetate levels could be caused by heart failure [9]. These different functions of acetoacetate might be associated with difference in prognostic impact of acetoacetate in heart failure patients with or without diabetes mellitus in this study.
Change in systemic and myocardial metabolism is a significant pathophysiology of heart failure. In heart failure patients, metabolism of ketone body in skeletal muscle changed [28]. Increased insulin resistance is closely associated with the occurrence of cardiac dysfunction [29]. In the myocardial metabolism, the failing heart has energy starvation [30]. Myocardial energy substrates change and utilization of ketone bodies is increased [7], [8]. In the current study, heart failure patients with high circulating acetoacetate might have required more acetoacetate as an alternative myocardial energy, compared with those with low circulating acetoacetate. The high acetoacetate group had worse heart failure according to higher NYHA classification, elevated B-type natriuretic peptide levels, and poor prognosis, compared with the low group. These results are compatible with those of previous studies, which reported levels of ketone bodies to be correlated with heart failure severity [9], [31], [32]. In regard to SGLT2 inhibitor, SGLT2 inhibitor increased blood ketone bodies, but on the other hand, SGLT2 inhibitor has been reported to have favorable effect to reduce cardiac events. The discrepancy of SGLT2 inhibitor with our results, which elevated circulating acetoacetate was associated with worse prognosis, might be due to adaptive response to progression of heart failure as a myocardial fuel.
Various biological functions are known about acetoacetate; it induces hepatic apoptosis through increased reactive oxygen species content [25], and it works as a signaling molecule in mediating muscle cell function [13]. Increased contractile performance and beta-adrenergic inotropism in stunned myocardium are demonstrated by circulating levels of acetoacetate [15]. Inotropes exacerbate prognosis in heart failure patients [33]. In the current study, we found that high acetoacetate was independently correlated with worse prognosis in heart failure, and that beta-adrenergic inotropism of acetoacetate, as a signaling molecule, might affect worse prognosis in heart failure patients.
An increase of blood ketone bodies is mainly due to enhancement of ketone body production in the liver. There were no significant differences regarding hepatic function in this study population based on the circulating acetoacetate levels, although high acetoacetate group had higher NYHA classification and B-type natriuretic peptide. This result suggested that high levels of circulating acetoacetate was not associated with hepatic dysfunction due to worsening heart failure. Precise mechanisms remain unknown about ketone body production in the liver among heart failure patients.
The median acetoacetate of this study (35 µmol/L) is within the reference range of under 55 µmol/L. According to this results, in hospitalized heart failure patients, 35 µmol/L would be preferable as a standard value of venous acetoacetate level. Report of circulating acetoacetate levels in heart failure patients are few. One previous study has reported that mean arterial circulating acetoacetate level after overnight fast was about 220 µmol/L in heart failure patients with reduced ejection fraction (venous circulating acetoacetate was not shown) [19]. Reasons of the difference in levels of circulating acetoacetate between our study and this previous study were as follows; first, there was difference of the vessels (vein or artery) of measuring acetoacetate. Second, study population was different because our study included various heart failure etiology such as ischemic heart failure, cardiomyopathy, and valvular heart disease.
There are several limitations to the present study. First, we measured acetoacetate levels regardless of timing of feeding. The acetoacetate levels in this study might have been changed by meals, which meant that measurements of acetoacetate at any time was correlated with prognosis in heart failure patients. Second, the present study included only variables during hospitalization for heart failure, and we did not take into consideration changes in treatment or medical parameters that include acetoacetate levels. Third, since this was a prospective observational study, the causal relationships between circulating acetoacetate levels and worse prognosis could not be fully explained. Fourth, the follow-up period of this study was a short period (median follow-up period of 328 days) to establish the role of circulating acetoacetate levels. Finally, the current study was a single-center study. Thus, our results should be considered preliminary, and a large multi-center study is necessary to establish the evidence of circulating acetoacetate in heart failure patients.
5. Conclusion
High acetoacetate level was associated with high all-cause mortality in patients with heart failure. These results provide new insights into the role of alternative cardiac metabolism in heart failure patients, and raise the possibility of acetoacetate as a novel biomarker to predict the prognosis of heart failure patients.
Acknowledgments
Acknowledgement of grant support
None.
Declaration of Competing Interest
Tetsuro Yokokawa belongs to a department supported by Actelion Pharmaceuticals Japan Ltd. Akiomi Yoshihisa and Tomofumi Misaka belong to a department supported by Fukuda Denshi Co, Ltd. These companies are not associated with the contents of this study.
Footnotes
These author takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2019.100432.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Shah A.M., Claggett B., Loehr L.R. Heart failure stages among older adults in the community: the atherosclerosis risk in communities study. Circulation. 2017;135:224–240. doi: 10.1161/CIRCULATIONAHA.116.023361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xanthakis V., Enserro D.M., Larson M.G. Prevalence, neurohormonal correlates, and prognosis of heart failure stages in the community. JACC Heart Fail. 2016;4:808–815. doi: 10.1016/j.jchf.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ammar K.A., Jacobsen S.J., Mahoney D.W. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation. 2007;115:1563–1570. doi: 10.1161/CIRCULATIONAHA.106.666818. [DOI] [PubMed] [Google Scholar]
- 4.Taegtmeyer H., Young M.E., Lopaschuk G.D. Assessing cardiac metabolism: a scientific statement from the American heart association. Circ Res. 2016;118:1659–1701. doi: 10.1161/RES.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosano G.M., Vitale C. Metabolic modulation of cardiac metabolism in heart failure. Card Fail Rev. 2018;4:99–103. doi: 10.15420/cfr.2018.18.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uchihashi M., Hoshino A., Okawa Y. Cardiac-specific Bdh1 overexpression ameliorates oxidative stress and cardiac remodeling in pressure overload-induced heart failure. Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.117.004417. [DOI] [PubMed] [Google Scholar]
- 7.Aubert G., Martin O.J., Horton J.L. The failing heart relies on ketone bodies as a fuel. Circulation. 2016;133:698–705. doi: 10.1161/CIRCULATIONAHA.115.017355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bedi K.C., Jr., Snyder N.W., Brandimarto J. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation. 2016;133:706–716. doi: 10.1161/CIRCULATIONAHA.115.017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lommi J., Kupari M., Koskinen P. Blood ketone bodies in congestive heart failure. J. Am. Coll. Cardiol. 1996;28:665–672. doi: 10.1016/0735-1097(96)00214-8. [DOI] [PubMed] [Google Scholar]
- 10.Jain S.K., McVie R., Bocchini J.A., Jr. Hyperketonemia (ketosis), oxidative stress and type 1 diabetes. Pathophysiology. 2006;13:163–170. doi: 10.1016/j.pathophys.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Macdonald M.J., Hasan N.M., Longacre M.J. Studies with leucine, beta-hydroxybutyrate and ATP citrate lyase-deficient beta cells support the acetoacetate pathway of insulin secretion. Biochim. Biophys. Acta. 2008;1780:966–972. doi: 10.1016/j.bbagen.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain S.K., McVie R. Hyperketonemia can increase lipid peroxidation and lower glutathione levels in human erythrocytes in vitro and in type 1 diabetic patients. Diabetes. 1999;48:1850–1855. doi: 10.2337/diabetes.48.9.1850. [DOI] [PubMed] [Google Scholar]
- 13.Zou X., Meng J., Li L. Acetoacetate accelerates muscle regeneration and ameliorates muscular dystrophy in mice. J. Biol. Chem. 2016;291:2181–2195. doi: 10.1074/jbc.M115.676510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noh H.S., Hah Y.S., Nilufar R. Acetoacetate protects neuronal cells from oxidative glutamate toxicity. J. Neurosci. Res. 2006;83:702–709. doi: 10.1002/jnr.20736. [DOI] [PubMed] [Google Scholar]
- 15.Squires J.E., Sun J., Caffrey J.L., Yoshishige D., Mallet R.T. Acetoacetate augments beta-adrenergic inotropism of stunned myocardium by an antioxidant mechanism. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H1340–H1347. doi: 10.1152/ajpheart.00473.2002. [DOI] [PubMed] [Google Scholar]
- 16.Russell R.R., 3rd, Mommessin J.I., Taegtmeyer H. Propionyl-L-carnitine-mediated improvement in contractile function of rat hearts oxidizing acetoacetate. Am. J. Physiol. 1995;268:H441–H447. doi: 10.1152/ajpheart.1995.268.1.H441. [DOI] [PubMed] [Google Scholar]
- 17.Zinman B., Wanner C., Lachin J.M. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 18.Ferrannini E., Mark M., Mayoux E. CV protection in the EMPA-REG OUTCOME trial: A “Thrifty Substrate” hypothesis. Diabetes Care. 2016;39:1108–1114. doi: 10.2337/dc16-0330. [DOI] [PubMed] [Google Scholar]
- 19.Voros G., Ector J., Garweg C. Increased cardiac uptake of ketone bodies and free fatty acids in human heart failure and hypertrophic left ventricular remodeling. Circ. Heart Fail. 2018;11 doi: 10.1161/CIRCHEARTFAILURE.118.004953. [DOI] [PubMed] [Google Scholar]
- 20.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi M., Ueda K., Tabata R. Arterial ketone body ratio as a prognostic indicator in acute heart failure. J. Lab. Clin. Med. 1997;129:72–80. doi: 10.1016/s0022-2143(97)90163-3. [DOI] [PubMed] [Google Scholar]
- 22.Marcondes-Braga F.G., Batista G.L., Gutz I.G. Impact of exhaled breath acetone in the prognosis of patients with Heart Failure with Reduced Ejection Fraction (HFrEF). One year of clinical follow-up. PLoS One. 2016;11 doi: 10.1371/journal.pone.0168790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcondes-Braga F.G., Batista G.L., Bacal F., Gutz I. Exhaled breath analysis in heart failure. Curr. Heart Fail Rep. 2016;13:166–171. doi: 10.1007/s11897-016-0294-8. [DOI] [PubMed] [Google Scholar]
- 24.Obokata M., Negishi K., Sunaga H. Association between circulating ketone bodies and worse outcomes in hemodialysis patients. J. Am. Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du X., Shi Z., Peng Z. Acetoacetate induces hepatocytes apoptosis by the ROS-mediated MAPKs pathway in ketotic cows. J. Cell Physiol. 2017;232:3296–3308. doi: 10.1002/jcp.25773. [DOI] [PubMed] [Google Scholar]
- 26.Sherwin R.S., Hendler R.G., Felig P. Effect of diabetes mellitus and insulin on the turnover and metabolic response to ketones in man. Diabetes. 1976;25:776–784. doi: 10.2337/diab.25.9.776. [DOI] [PubMed] [Google Scholar]
- 27.Kanikarla-Marie P., Jain S.K. Hyperketonemia and ketosis increase the risk of complications in type 1 diabetes. Free Radic. Biol. Med. 2016;95:268–277. doi: 10.1016/j.freeradbiomed.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janardhan A., Chen J., Crawford P.A. Altered systemic ketone body metabolism in advanced heart failure. Tex. Heart Inst. J. 2011;38:533–538. [PMC free article] [PubMed] [Google Scholar]
- 29.Jia G., DeMarco V.G., Sowers J.R. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat. Rev. Endocrinol. 2016;12:144–153. doi: 10.1038/nrendo.2015.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuunanen H., Knuuti J. Metabolic remodelling in human heart failure. Cardiovasc. Res. 2011;90:251–257. doi: 10.1093/cvr/cvr052. [DOI] [PubMed] [Google Scholar]
- 31.Yokokawa T., Sugano Y., Shimouchi A. Exhaled acetone concentration is related to hemodynamic severity in patients with non-ischemic chronic heart failure. Circ. J. 2016;80:1178–1186. doi: 10.1253/circj.CJ-16-0011. [DOI] [PubMed] [Google Scholar]
- 32.Yokokawa T., Sugano Y., Shimouchi A. A case of acute decompensated heart failure evaluated by series of exhaled acetone concentrations as noninvasive biomarker of heart failure severity. Int. J. Cardiol. 2016;204:112–113. doi: 10.1016/j.ijcard.2015.11.173. [DOI] [PubMed] [Google Scholar]
- 33.Mebazaa A., Motiejunaite J., Gayat E. Long-term safety of intravenous cardiovascular agents in acute heart failure: results from the European Society of Cardiology Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2018;20:332–341. doi: 10.1002/ejhf.991. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.