Abstract
Background
Poliomyelitis is a debilitating and deadly infection. Despite exponential growth in medical science, there is still no cure for the disease, which is caused by three types of wild polioviruses: types 1, 2, and 3. According to the Global Polio Eradication Initiative (GPEI), wild poliovirus is still in circulation in three countries, and fresh cases have been reported even in the year 2018.
Due to the administration of live vaccines, the risk for vaccine‐derived poliovirus (VDPV) is high in areas that are free from wild polioviruses. This is evident based on the fact that VDPV caused 20 outbreaks between 2000 and 2011.
Recent recommendations from the World Health Organization favoured the inclusion of inactivated poliovirus vaccine (IPV) in the global immunisation schedule. IPV can be delivered in two ways: intramuscularly and intradermally. IPV was previously administered intramuscularly, but shortages in vaccine supplies, coupled with the higher costs of the vaccines, led to the innovation of delivering a fractional dose (one‐fifth) of IPV intradermally. However, there is uncertainty regarding the efficacy, immunogenicity, and safety of an intradermal, fractional dose of IPV compared to an intramuscular, full dose of IPV.
Objectives
To compare the immunogenicity and efficacy of an inactivated poliovirus vaccine (IPV) in equivalent immunisation schedules using fractional‐dose IPV given via the intradermal route versus full‐dose IPV given via the intramuscular route.
Search methods
We searched CENTRAL, MEDLINE, Embase, 10 other databases, and two trial registers up to February 2019. We also searched the GPEI website and scanned the bibliographies of key studies and reviews in order to identify any additional published and unpublished trials in this area not captured by our electronic searches.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs of healthy individuals of any age who are eligible for immunisation with IPV, comparing intradermal fractional‐dose (one‐fifth) IPV to intramuscular full‐dose IPV.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We included 13 RCTs involving a total of 7292 participants, both children (n = 6402) and adults (n = 890). Nine studies were conducted in middle‐income countries, three studies in high‐income countries, and only one study in a low‐income country. Five studies did not report methods of randomisation, and one study failed to conceal the allocations. Eleven studies did not blind participants, and six studies did not blind outcome assessments. Two studies had high attrition rates, and one study selectively reported the results. Three studies were funded by pharmaceutical companies.
Paralytic poliomyelitis. No study reported data on this outcome.
Seroconversion rates. These were significantly higher for all three types of wild poliovirus for children given intramuscular full‐dose IPV after a single primary dose and two primary doses, but only significantly higher for type two wild poliovirus given intramuscularly after three primary doses: • dose one (six studies): poliovirus type 1 (odds ratio (OR) 0.30, 95% confidence interval (CI) 0.22 to 0.41; 2570 children); poliovirus type 2 (OR 0.43, 95% CI 0.31 to 0.60; 2567 children); poliovirus type 3 (OR 0.19, 95% CI 0.12 to 0.30; 2571 children); • dose two (three studies): poliovirus type 1 (OR 0.23, 95% CI 0.16 to 0.33; 981 children); poliovirus type 2 (OR 0.41, 95% CI 0.28 to 0.60; 853 children); and poliovirus type 3 (OR 0.12, 95% CI 0.07 to 0.22; 855 children); and • dose three (three studies): poliovirus type 1 (OR 0.45, 95% CI 0.07 to 3.15; 973 children); poliovirus type 2 (OR 0.34, 95% CI 0.19 to 0.63; 973 children); and poliovirus type 3 (OR 0.18, 95% CI 0.01 to 2.58; 973 children).
Using the GRADE approach, we rated the certainty of the evidence as low or very low for seroconversion rate (after a single, two, or three primary doses) for all three poliovirus types due to significant risk of bias, heterogeneity, and indirectness in applicability/generalisability.
Geometric mean titres. No study reported mean antibody titres. Median antibody titres were higher for intramuscular full‐dose IPV (7 studies with 4887 children); although these studies also reported a rise in antibody titres in the intradermal group, none reported the duration for which the titres remained high.
Any vaccine‐related adverse event. Five studies (2217 children) reported more adverse events, such as fever and redness, in the intradermal group, whilst two studies (1904 children) reported more adverse events in the intramuscular group.
Authors' conclusions
There is low‐ and very low‐certainty evidence that intramuscular full‐dose IPV may result in a slight increase in seroconversion rates for all three types of wild poliovirus, compared with intradermal fractional‐dose IPV. We are uncertain whether intradermal fractional‐dose (one‐fifth) IPV has better protective effects and causes fewer adverse events in children than intramuscular full‐dose IPV.
Plain language summary
Effectiveness of equivalent schedules of full‐dose inactivated poliovirus vaccine injected into muscle compared to a smaller dose injected into skin
Background
Polio is a disabling disease that is only preventable via vaccination. There are two types of polio vaccines: live poliovirus vaccine delivered orally (by mouth – so‐called OPV) and inactivated (killed) poliovirus vaccine (IPV). OPV is the mainstay of polio eradication but carries a risk of causing vaccine‐associated polio. This is not the case for IPV, which also has fewer side effects and can be given to people with low immunity, making it vital for the complete elimination of poliovirus. Killed vaccines can be given via injection either into the muscles (intramuscular) or into the skin (intradermal).
Review question
How effective is a small dose of IPV injected into the skin compared to a full dose of IPV injected into muscle in similar schedules?
Study characteristics
The database searches, up‐to‐date to February 2019, found 13 randomised controlled trials (a type of experiment in which participants are randomly assigned to one of two or more treatment groups). Three studies comprised 890 adult participants; a further 10 studies comprised 6402 infants and children.
Nine studies were conducted in middle‐income countries; three studies in high‐income countries; and one study in a low‐income country. The studies had a duration of 2 to 19 months. Three studies were supported financially by drug companies, and three studies received the vaccines from the pharmaceutical company. There is overall limited confidence in the quality of the included studies since, for example, in most trials the recipient or assessor (or both) were aware of the vaccine being given.
Key results
The review included 13 studies with a total of 7292 participants (6402 children and 890 adults). Where possible, we combined the results of similar studies in a meta‐analysis (a statistical method of combining the results of multiple single studies to calculate an overall effect).
There are three types of wild poliovirus: types 1, 2, and 3. We found that the number of antibody responses to the vaccine (measured using something called seroconversion rates) in children was higher in the group that received the vaccine by intramuscular injection compared to the group that had a similar number of injections given intradermally, after one single dose (6 studies, 2571 children) and two doses (3 studies, 981 children) for all three types of poliovirus, and after three doses for type 2 poliovirus (3 studies, 973 children).
The vaccines produce antibodies against all three types of poliovirus. The quantity of antibodies produced by the vaccines (measured as geometric median titres) was higher in children receiving a full dose of IPV via intramuscular route for all three types of poliovirus (7 studies, 4887 children).
Five studies (2217 children) reported more adverse events, such as fever and redness, in the intradermal group, whilst two studies (1904 children) reported more adverse events in the intramuscular group.
None of the included studies reported data on the occurrence of paralytic poliomyelitis.
Certainty of the evidence
Based on the evidence, intramuscular full‐dose IPV may result in a slight increase in seroconversion rates for all three types of wild poliovirus when compared with intradermal fractional‐dose IPV. We are uncertain if a fractional dose of IPV given intradermally is better than a full dose of IPV given intramuscularly at producing antibodies for all three types of poliovirus or reducing adverse effects.
Summary of findings
Background
Description of the condition
Poliomyelitis (polio) is a debilitating and deadly contagious disease caused by three different types of wild poliovirus, each of which has slightly different capsid proteins (CDC 2002; Grassly 2013; Minor 2014). Though wild type 2 has been eradicated, and no case due to wild type 3 has been reported since 2012, wild type 1 is still prevalent (GPEI 2010). The poliovirus is a small ribonucleic acid (RNA) that measures a mere 30 nm in diameter, with a viral genome of 7500 nucleotides. It spreads through person‐to‐person contact: the virus enters through the mouth and nose, multiplies in the intestine, and is then shed in enormous quantities through faeces into the environment (a gram of stool can contain several million virus particles) (Dowdle 2002). Lack of sanitation and poor hygiene that results in the faecal contamination of food and drink facilitate its transmission (Dowdle 2002). It can affect people of any age, but primarily occurs in children under five years old (Dowdle 2002).
The Global Polio Eradication Initiative has succeeded in reducing the incidence of polio, from 350,000 reported cases across 125 endemic countries in 1988, to 33 cases in 2018 (WHO 2018). Despite these efforts, wild poliovirus remains uninterrupted in three regions: Afghanistan, Nigeria, and Pakistan (WHO 2018); in 2016, polio cases were again detected in Nigeria after a period of two years, and although significant progress has been made in Pakistan (99% of poliovirus has been eradicated since 2014), surveillances still reveal the circulation of poliovirus (GPEI 2018).
Paralytic polio may be suspected in cases where a child under 15 years of age presents with acute flaccid paralysis (AFP) ‐ sudden onset of limp or droopy muscles in the absence of another cause ‐ or in a person of any age presenting with polio‐like symptoms (GPEI 2010). Each case presenting with AFP must be reported and tested for poliovirus within 48 hours of onset, even if polio is not suspected, due to difficulties in differentiating polio from other diseases at early stages of infection (GPEI 2010). Diagnosis is confirmed by a subsequent laboratory analysis of two stool specimens, taken 24 to 48 hours apart, within 14 days of onset of paralysis, as virus excretion decreases after two weeks. Where polio is suspected and it is not possible to obtain samples within the desired time frame, additional stool specimens are collected from up to five healthy individuals in close contact with the individual presenting with symptoms of polio, to improve the sensitivity and specificity of poliovirus detection (WHO 2009). Although the majority of people infected with wild poliovirus are asymptomatic (approximately 99.5%) (GPEI 2010), the disease has the potential to cause irreparable paralysis by damaging the nervous system. It is reported that one in every 200 infections leads to paralysis, which most commonly affects the lower limbs, but in severe cases can involve the muscles of the torso and result in quadriplegia (GPEI 2010). In individuals with bulbar polio, the brainstem is affected, which leads to reduced breathing capacity and difficulty in swallowing and speaking. At least 5% to 10% of affected patients die once their breathing muscles become paralysed (GPEI 2010).
Despite exponential growth in medical sciences, there is still no cure for poliomyelitis, as it is a permanent, lower motor neuron paralysis. Available treatments, such as antispasmodics or physical therapies, provide only symptomatic relief. However, polio can be prevented through immunisation, which can either be live attenuated or inactivated/killed. The attenuated virus in the vaccine replicates in the intestine and stimulates the immune system to produce antibodies against all three types of wild poliovirus. During the process of replication, however, the vaccine viruses mutate, and, after many replications become neuro‐virulent, that is able to cause paralysis in the recipient or his/her contacts; this is known as vaccine‐derived poliovirus (VDPV) (GPEI 2018). VDPV are rare strains of poliovirus that have mutated from the vaccine strains of oral polio vaccine (OPV), and are known to cause two to four cases of vaccine‐associated poliomyelitis per cohort of one million children. VDPV are further classified as cVDPV (i.e. circulating VDPV) and iVDPV (i.e. immunodeficiency‐related vaccine‐derived poliovirus). cVDPV becomes a threat when the population is under‐immunised and the excreted virus becomes virulent and starts circulating. There were approximately 20 outbreaks of cVDPV leading to 580 cases between 2000 and 2011 (GPEI 2018). Prolonged replication of the vaccine virus in people with immunodeficiency and with an inability to clear intestinal vaccine‐virus infection leads to prolonged excretion of iVDPV, and over 100 cases have been reported (GPEI 2018). In the event of an outbreak due to VDPV, antiviral polio drugs are now available for use in combination with OPV (McKinlay 2014). Due to the risk of vaccine‐associated poliomyelitis, an inactivated poliovirus vaccine (IPV) is required for complete elimination of polio (CDC 2002; Grassly 2013).
Description of the intervention
On 25 January 2013, the World Health Organization (WHO) approved an action plan for the eradication and containment of all wild polioviruses in order that no child would suffer paralytic poliomyelitis (CDC 2013; WHO 2013). IPV may be a prudent choice in the fight against polio. It is a killed vaccine and cannot mutate into neuro‐virulent forms. Furthermore, it has been reported to provide adequate immunogenicity against wild poliovirus (Nelson 2012). IPV can be delivered either intramuscularly or intradermally. Intramuscular IPV is the most widely used IPV.
Given that immune responses are more effective when vaccines are delivered directly into the skin (intradermal) than into muscles (intramuscular), it is possible that a lower vaccine dose (one‐fifth) of IPV injected intradermally may be equally or even more immunogenic than a vaccine given intramuscularly. With the development of different delivery methods, intradermal administration of vaccines has become easier. The fractional‐IPV innovation was developed in response to programmatic developments (Okayasu 2017), including:
global shortage of IPV;
removal of type 2‐containing component of the oral polio vaccine in April 2016, in response to declaring the eradication of type 2 wild poliovirus in 2015 and the need to provide baseline/boosting of type 2 polio immunity; and
cost‐savings for immunisation programmes in routine immunisation or supplementary activities, particularly in large countries.
Intradermal administration of vaccines also requires a different skill set in healthcare professionals, since improper administration of the vaccine can affect its immunogenicity (Okayasu 2017).
How the intervention might work
Intradermal fractionated IPV could be an immensely efficient method of antipoliovirus immunisation. Dendritic cells, including Langerhans cells, are concentrated in the dermis and serve as mediators between innate and adaptive immune responses (Lambert 2008). After recognising antigens, these cells release cytokines and activate the cell‐mediated, innate immune response. They also act as antigen‐presenting cells with a peptide/major histocompatibility complex (MHC) and generate B‐cell response (Lambert 2008; Palucka 2010). As dendritic cells induce migration of T cells, the generated immune response (i.e. immunogenicity) is more effective and may be further enhanced by the use of specific adjuvants (Palucka 2010).
Why it is important to do this review
As the global eradication of wild poliovirus progresses, vaccine viruses will become the main source of polioviruses and could conceivably prompt new outbreaks of polio across the world. Affordable IPV choices need to be available for any nation wishing to proceed with polio immunisation. As part of its strategic approach, the Global Polio Eradication Initiative is pursuing a dose‐reduction strategy of intradermal inoculation of fractional IPV (20% or 0.1 mL). Though trials have established the efficacy of full‐dose intramuscular IPV, intradermal IPV, if proved to be as efficacious and immunogenic, may be an alternative that low‐ and middle‐income countries could exploit. This review aimed to find out whether fractionated IPV administered through the intradermal route is as efficacious and immunogenic as full‐dose IPV administrated through the intramuscular route for preventing poliomyelitis in infants, children, and adults. It might help policymakers to weigh the advantages and disadvantages of this treatment.
The WHO Strategic Advisory Group of Experts (SAGE) on Immunization currently recommends the use of two doses of fractional IPV over one full dose of intramuscular IPV (WHO 2016). This recommendation was made during the development of this review and highlights the need to provide further information to inform future policies.
Objectives
To compare the immunogenicity and efficacy of an inactivated poliovirus vaccine (IPV) in equivalent immunisation schedules using fractional‐dose IPV given via the intradermal route versus full‐dose IPV given via the intramuscular route.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs, with the exception of cross‐over trials, which are extremely rare in this area.
Types of participants
Healthy individuals of any age group who qualify for immunisation with IPV, irrespective of HIV status, feeding habits, and birth weight (for infants); who may or may not have been exposed to OPV at birth; and who may also have received other vaccines for their age (e.g. diphtheria and tetanus toxoids and pertussis vaccine (DPT), bacille Calmette‐Guérin vaccine (BCG), etc.), provided there was a gap of at least two weeks prior to or after receiving IPV.
Types of interventions
Intervention
Fractional‐dose IPV given via the intradermal route.
Comparison/control
Full‐dose IPV given via the intramuscular route.
Types of outcome measures
Primary outcomes
Paralytic poliomyelitis
Seroconversion rate
Geometric mean titres of antibodies for wild poliovirus types 1, 2, and 3
Any vaccine‐related adverse event after each dose (early or late events; e.g. injection site reactions, pyrexia, haematomas, or gastroenteritis, etc.), measured by the number of participants in an arm having an adverse event/total number of participants in that arm
Secondary outcomes
Reciprocal antibody titres (as calculated by the Kärber method and expressed as 1/dilution) seven days after the first dose, second dose, third dose, and one month after the receiving the third primary dose
Serum immunoglobulin A (IgA) levels (measured by the enzyme‐linked immunosorbent assay (ELISA), expressed by the number of children with detectable IgA levels in that arm/total number of participants in that arm) seven days after the first dose, second dose, third dose, and one month after receiving the third primary dose
Poliovirus shedding in stool after seven days and after the first month of each dose (we analysed both qualitative and quantitative measures separately; we provided a narrative description of values given as interquartile ranges, medians, etc.; when mentioned, we pooled the number of participants in each arm with virus shedding as proportions in each arm)
Vaccine‐associated paralytic polio (VAPP), measured by the number of participants in each arm with VAPP/total number of participants in that arm (Ciapponi 2014)
Search methods for identification of studies
We ran the first searches for this review in October 2015 and updated them in April 2017, March 2018, and February 2019.
Electronic searches
We searched the following electronic sources up to February 2019.
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 2) in the Cochrane Library, which includes the Cochrane Psychosocial, Developmental and Learning Problems Group Specialized Register (searched 13 February 2019).
MEDLINE Ovid (1946 to January Week 5 2019).
MEDLINE In‐Process and Other Non‐indexed Citations Ovid (searched 13 February 2019).
MEDLINE Epub Ahead of Print Ovid (searched 13 February 2019).
Embase Ovid (1974 to 12 February 2019).
Science Citation Index Web of Science (SCI; 1970 to 14 February 2019).
Conference Proceedings Citation Index ‐ Science Web of Science (CPCI‐S; 1990 to 14 February 2019).
IndMED (indmed.nic.in; searched 14 February 2019).
Cochrane Database of Systematic Reviews (CDSR; 2019, Issue 2), part of the Cochrane Library (searched 13 February 2019).
Database of Abstracts of Reviews of Effects (DARE; 2015, Issue 2), part of the Cochrane Library (final issue of DARE searched 27 April 2017).
LILACS (Latin American and Caribbean Health Science Information Database; lilacs.bvsalud.org/en; searched 14 February 2019).
Trip database (www.tripdatabase.com; searched 14 February 2019).
WHOLIS (World Health Organization Library Database; kohahq.searo.who.int; searched 14 February 2019).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 14 February 2019).
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; who.int/ictrp/en; searched 14 February 2019).
The search strategies and exact search dates for each database are shown in Appendix 1. We did not limit our searches by date, language, or publication status, and where necessary we sought translation and data extraction of studies written in languages other than English (Criteria for considering studies for this review).
Searching other resources
We scanned the bibliographies of key studies and reviews to identify any additional published and unpublished trials that our electronic searches failed to capture. We also searched the websites of relevant organisations, including the Global Polio Eradication Initiative (polioeradication.org), to identify any ongoing or unpublished studies.
Data collection and analysis
Selection of studies
After removal of duplicates, two review authors (AA and NJ) screened the titles and abstracts yielded by the searches and separated them into two groups: 'excluded' and 'not excluded'. Next, they retrieved the full texts of those records deemed 'not excluded', and independently assessed them for relevance against the selection criteria (Criteria for considering studies for this review). Any discrepancies were resolved by discussion and in consultation with a third review author (MS) who acted as an arbiter. The selection process is described in a PRISMA flow diagram, per the PRISMA guidelines (Moher 2009).
Data extraction and management
Two review authors (AA and NJ) independently extracted and recorded data on each of the following criteria onto separate, pre‐piloted study report forms.
General information (study identifier, date of extraction, title, authors, and source of study if not published)
Study characteristics (study design, participants, and inclusion or exclusion criteria used in the study)
Details of the interventions (including vaccine schedule and dosage, comparison details, duration of follow‐up)
Outcomes, as described in the Types of outcome measures section
Details for the 'Risk of bias' assessment
Any disagreements were resolved through discussion with a third review author (MS).
Three review authors (AC, NJ, and SS) entered the extracted data into Review Manager 5 (RevMan 5) for analysis (Review Manager 2014).
Assessment of risk of bias in included studies
We assessed the risk of bias in each included study using Cochrane's 'Risk of bias' tool (Higgins 2011a). For each domain listed below, three review authors (KKT, AC, and SS) independently judged the risk of bias as low, high, or unclear as per our protocol (Jaiswal 2015), using the criteria set out in Table 7. One review author (MS) acted as the arbiter in case of disagreements.
2. Criteria for assigning 'Risk of bias' judgements.
| Domain | Rating | Criteria |
| Random sequence generation | Low risk of bias | Study used a random method, such as a computer‐generated system or random number table, to generate the allocation sequence and described the approach in sufficient detail. Drawing of lots, tossing of coin, shuffling of cards, or throwing dice was considered adequate if a person who was not otherwise involved in the recruitment of participants performed the procedure. |
| Unclear risk of bias | Method used to generate the allocation sequence was not described. | |
| High risk of bias | Study used a non‐random method, such as dates, names, or admittance numbers, to generate the allocation sequence of participants. | |
| Allocation concealment | Low risk of bias | Allocation of participants to study groups was concealed from participants and investigators using a central independent unit, on‐site locked computer, identical syringes or schedules (used by an independent pharmacist or investigator), or opaque, sealed envelopes. |
| Unclear risk of bias | Method for allocation concealment was not described or was not described in sufficient detail to permit a judgement of low or high risk of bias. | |
| High risk of bias | Allocations were not concealed and were known to both participants and investigators. | |
| Blinding of participants and personnel | Low risk of bias | Parents/guardians or recipients (in cases of older children/adults) were blinded; blinding was described in sufficient detail; and it was unlikely that the blinding could have been broken. |
| Unclear risk of bias | Blinding was not described or was not described in sufficient detail to permit a judgement of low or high risk of bias. | |
| High risk of bias | Parents or recipients were not blinded; blinding was broken; or it was likely that the outcome could have been affected by the lack of blinding. | |
| Blinding of outcome assessment | Low risk of bias | Outcome assessment was blinded; details of blinding were described in sufficient detail; and it was unlikely that the blinding could have been broken, or there was no blinding but the outcome assessment was unlikely to have been affected by the lack of blinding. |
| Unclear risk of bias | Blinding was not described or was not described in sufficient detail to permit a judgement of low or high risk of bias. | |
| High risk of bias | Outcome assessment was not blinded; blinding was broken; or it was likely that the outcome assessment could have been influenced by the lack of blinding. | |
| Incomplete outcome data | Low risk of bias | No missing data; the reasons for the missing data were unrelated to the true outcome; or the study used appropriate methods to impute the data |
| Unclear risk of bias | Insufficient information to permit a judgement of low or high risk of bias | |
| High risk of bias | Reasons for missing data were related to the true outcome, or the study used inappropriate methods to impute the data. | |
| Selective outcome reporting | Low risk of bias | Study protocol was available, and all prespecified outcomes were reported and in the manner specified; or study protocol was not available, but it was clear that all prespecified outcomes had been reported. |
| Unclear risk of bias | Insufficient information to permit a judgement of low or high risk of bias | |
| High risk of bias | Study protocol was available, but not all of the study’s prespecified outcomes were reported, or not all were reported in prespecified way, or one or more were reported incorrectly; or outcomes were reported that were not prespecified; or study did not have a protocol, and not all expected outcomes were reported. | |
| Other bias | Low risk of bias | No other sources of bias |
| Unclear risk of bias | Insufficient information to judge that an important risk of bias exists | |
| High risk of bias | Other important potential sources of bias (e.g. studies were privately funded) exist. |
Random sequence generation
Allocation concealment
Blinding of participants and study personnel
Blinding of outcome assessments
Reporting of incomplete outcome data
Selective reporting
Other potential sources of bias (we used data pertaining to the study's funding source(s) to populate the column in the 'Risk of bias' table)
We used these judgements to draw a 'Risk of bias' graph, expressed as percentages, and a 'Risk of bias' summary graph employing RevMan 5 (Review Manager 2014).
Measures of treatment effect
Dichotomous data
We used odds ratios (OR) and presented these with 95% confidence intervals (CIs). See Appendix 2 for additional methods we had planned to use but did not (Jaiswal 2015).
Unit of analysis issues
We analysed trials on adults (aged 18 years and above) separately from trials on children (under 18 years of age).
Cluster‐randomised controlled trials
We stated in our protocol that we would combine the adjusted measures of effects of cluster‐randomised trials (Jaiswal 2015); however, we did not encounter any such trials. Our methods for managing cluster‐randomised trials are summarised in Appendix 2.
Studies with multiple intervention arms
We combined the data from all eligible intervention arms, and compared them with the combined data from all eligible control groups, making single pair‐wise comparisons; we did not use data from arms including interventions not relevant to this review. For dichotomous outcomes we summed the number of participants with events and the total number of participants across the groups (Higgins 2011b). In the case of continuous outcomes, we combined the mean and standard deviations using the formulae described in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c).
Dealing with missing data
We investigated and reported the reasons, numbers, and characteristics of dropouts in the Characteristics of included studies tables. Where possible, we compared the protocols of the included studies to their respective published reports, and contacted the corresponding authors of included studies to seek clarification or missing data (or both) when necessary. The methods we will use if possible in future updates of this review are shown in Appendix 2.
Assessment of heterogeneity
We assessed clinical and methodological variation across studies by comparing differences in settings and important participant characteristics (such as age of study participants, exposure to OPV, age of first dose, etc.), as well as trial characteristics (study design, interventions, outcomes, and risk of bias) to identify the source of any observed heterogeneity. We evaluated statistical heterogeneity in the included studies using the Chi2 test (significance set at P value < 0.10) and the I2 statistic (Higgins 2003). We based our interpretations of I2 on the thresholds listed below (Deeks 2011).
0% to 40% as probably not important
30% to 60% as moderate heterogeneity
50% to 90% as substantial heterogeneity
75% to 100% as considerable heterogeneity
Assessment of reporting biases
We were not able to construct funnel plots (plotting trial effects against inverse standard errors of effects) to assess for reporting biases as planned (Jaiswal 2015), as there were fewer than 10 studies for all outcomes included in the meta‐analysis. The methods we will use if possible in future updates of this review are shown in Appendix 2.
Data synthesis
Where possible, we pooled data using the random‐effects model with Mantel‐Haenszel weighting, as there was heterogeneity in the included studies with regard to the schedule and number of doses of polio vaccines administered intradermally and intramuscularly. We conducted a sensitivity analysis using the fixed‐effect model to test the robustness of this decision (see Sensitivity analysis).
Where a meta‐analysis was not possible, we provided a narrative synthesis of the results.
'Summary of findings' table
Having imported the data from RevMan 5 (Review Manager 2014), we used GRADEpro GDT to create a 'Summary of findings' table for the comparison 'Equivalent schedules of intradermal fractional‐dose inactivated poliovirus vaccine compared to intramuscular inactivated poliovirus vaccine for the prevention of poliomyelitis in children' (GRADEpro GDT). The table reports the absolute and relative effects for each primary outcome, as well as a rating of the certainty of the evidence, and the number of participants and studies contributing data.
Two review authors (AC and NJ) used the GRADE approach to assess the overall certainty of the body of evidence for each primary outcome (Guyatt 2011); another review author (MS) arbitrated in the case of disagreement. The evidence was downgraded by one level from high to moderate certainty (or by two levels to low or very low certainty, depending on the extent of the violation) for the following criteria: study limitations (risk of bias); indirectness of evidence; inconsistency; imprecision of effect estimates; and publication bias.
Subgroup analysis and investigation of heterogeneity
Where possible, we performed subgroup analysis based on the low‐, middle‐, and high‐income country classification of the World Bank, to assess the efficacy and immunogenicity of intradermal IPV against intramuscular IPV (World Bank 2014). We were unable to conduct our other preplanned subgroup analyses (Jaiswal 2015), which we have archived for use in future updates of this review (Appendix 2).
Sensitivity analysis
We performed a sensitivity analysis using a fixed‐effect model to test the robustness of our decision to pool results using a random‐effects model. We were unable to conduct any of our other preplanned sensitivity analyses (Jaiswal 2015), which we have archived for use in future updates of this review (Appendix 2).
Results
Description of studies
Results of the search
Our literature search retrieved a total of 7438 records, of which 2457 were discarded as duplicates. We screened the titles and abstracts of the remaining 4981 records, excluded 4948 irrelevant records and retrieved 33 full‐text reports, which we assessed against our inclusion criteria (Criteria for considering studies for this review). We included 13 studies (from 15 reports) and excluded 14 studies (from 15 reports), as shown in Figure 1. One study is awaiting classification (NCT02347423), as it was not clear from the available report whether relevant interventions were used, and two studies are ongoing (NCT02847026; NCT03016949).
1.
Study flow (PRISMA) diagram.
We contacted the corresponding authors of all included studies for clarification regarding allocation concealment and for data regarding virus shedding in stools. We received replies from four authors (Anand 2015; Estívariz 2012; Resik 2013; Troy 2015), who provided clarification regarding allocation concealment. We were unable to obtain any unpublished data.
Included studies
We included 13 RCTs in this review (Anand 2015; Cadorna‐Carlos 2012; Clarke 2016; Estívariz 2012; Gamage 2018; Mohammed 2010; Resik 2010; Resik 2013; Resik 2015; Resik 2017; Soonawala 2013; Tejeda Fuentes 2011; Troy 2015). Of these, three studies included 890 adult participants (Resik 2017; Soonawala 2013; Troy 2015), and 10 studies included 6402 infants and children (Anand 2015; Cadorna‐Carlos 2012; Clarke 2016; Estívariz 2012; Gamage 2018; Mohammed 2010; Resik 2010; Resik 2013; Resik 2015; Tejeda Fuentes 2011). We contacted the corresponding authors of all included studies for clarification regarding allocation concealment and for missing data regarding virus shedding in stools.
See the Characteristics of included studies tables for a detailed description of the studies.
Study design
All included studies were RCTs of fractional doses (one‐fifth) of IPV delivered intradermally compared with full doses of IPV delivered intramuscularly.
Seven trials had more than two arms (Anand 2015; Clarke 2016; Estívariz 2012; Gamage 2018; Resik 2015; Soonawala 2013; Troy 2015). Soonawala 2013 had four study arms, one of which was an intramuscular, fractional‐dose IPV arm. We did not consider the data from the other arms of this study in this review as they did not involve the interventions in question. Three studies had five arms (Anand 2015; Estívariz 2012; Resik 2015). Estívariz 2012, compared bivalent with trivalent OPV and, in addition to the two arms relevant to this review (i.e. fractional‐dose IPV delivered intradermally and full‐dose IPV delivered intramuscularly), had an arm combining intradermal fractional‐dose IPV and bivalent OPV (bOPV). Anand 2015 also had five study arms, and compared OPV preparations by different manufacturers along with intradermal fractional‐dose IPV and intramuscular full‐dose IPV, whereas Resik 2015 compared the administration of intradermal fractional‐dose IPV using different devices and IPV vaccine. The Gambian study by Clarke 2016 had eight study arms, comparing intramuscular full‐dose IPV and intradermal fractional‐dose IPV alone or in combination with measles and yellow fever vaccines. Troy 2015 had four arms, of which two administered two‐fifths of IPV given intradermally and intramuscularly. These were not relevant to this review, and hence the data were not considered. We included the other two arms, involving fractional‐dose intradermal IPV and full‐dose intramuscular IPV, in the current review. The Sri Lankan study had three arms: intramuscular full‐dose IPV, intradermal fractional‐dose IPV, and no IPV vaccine (Gamage 2018); we excluded the no‐IPV arm from this review as it was not relevant.
Participants
Three studies recruited adult participants only (Resik 2017; Soonawala 2013; Troy 2015). Troy 2015 recruited only adults who were HIV positive, and Resik 2017 recruited only male participants, whereas Soonawala 2013 included both men and women irrespective of any comorbidities.
Nine studies included children younger than two years of age (Anand 2015; Cadorna‐Carlos 2012; Clarke 2016; Estívariz 2012; Mohammed 2010; Resik 2010; Resik 2013; Resik 2015; Tejeda Fuentes 2011). Only one study, Resik 2015, recruited children older than 12 months of age and up to 20 months of age, whereas three studies recruited newborns (Mohammed 2010; Resik 2010; Resik 2013), and five studies recruited infants up to nine months of age (Anand 2015; Cadorna‐Carlos 2012; Clarke 2016; Estívariz 2012; Tejeda Fuentes 2011).
The male‐to‐female ratio was equally distributed in 12 of the 13 included trials (Anand 2015; Cadorna‐Carlos 2012; Clarke 2016; Estívariz 2012; Gamage 2018; Mohammed 2010; Resik 2010; Resik 2013; Resik 2015; Soonawala 2013; Tejeda Fuentes 2011; Troy 2015). One trial included males only (Resik 2017). None of these trials included children or infants who were HIV positive.
See the Characteristics of included studies tables for further details on the participants included in each trial.
Location, setting, and duration of studies
One study was from a low‐income country (Clarke 2016), and three studies were from high‐income countries according to the World Bank classification (Mohammed 2010; Soonawala 2013; Troy 2015). The remaining nine studies were conducted in middle‐income countries (both low‐middle and high‐middle) (Anand 2015; Cadorna‐Carlos 2012; Estívariz 2012; Gamage 2018; Resik 2010; Resik 2013; Resik 2015; Resik 2017; Tejeda Fuentes 2011).
The duration of studies ranged from two months in two studies, Gamage 2018; Resik 2015, to 19 months in one study (Soonawala 2013).
Interventions
Six studies compared single doses of the intended interventions (intradermal fractional‐dose IPV and intramuscular full‐dose IPV) (Cadorna‐Carlos 2012; Clarke 2016; Estívariz 2012; Gamage 2018; Resik 2015; Resik 2017). Two studies compared booster doses of the intended interventions in adults (Soonawala 2013; Troy 2015). One study, Resik 2013, compared two doses of intradermal fractional‐dose IPV to two doses of intramuscular full‐dose IPV. The four remaining studies compared three doses of intradermal fractional‐dose IPV to intramuscular full‐dose IPV (Anand 2015; Mohammed 2010; Resik 2010; Tejeda Fuentes 2011). The different dosing schedules and different number of doses given in the studies brought an element of heterogeneity to the results of this systematic review.
Outcomes
Primary outcomes
None of the included studies measured paralytic poliomyelitis. Eight studies assessed seroconversion rates after each dose of the vaccines (intramuscular IPV or intradermal fractional‐dose IPV): six in children, Anand 2015; Clarke 2016; Estívariz 2012; Mohammed 2010; Resik 2010; Resik 2013, and two in adults, Resik 2017; Troy 2015. Seven studies assessed geometric median titres (Cadorna‐Carlos 2012; Clarke 2016; Estívariz 2012; Mohammed 2010; Resik 2010; Resik 2013; Resik 2015). Ten studies assessed adverse events: seven in children, Cadorna‐Carlos 2012; Clarke 2016; Mohammed 2010; Resik 2010; Resik 2013; Resik 2015; Tejeda Fuentes 2011, and three in adults, Resik 2017; Soonawala 2013; Troy 2015.
Secondary outcomes
Three studies measured reciprocal antibody titres (Cadorna‐Carlos 2012; Clarke 2016; Resik 2015). None of the studies assessed serum IgA levels or VAPP. Only two studies assessed poliovirus shedding after an OPV challenge dose (Gamage 2018; Mohammed 2010).
Source of funding and conflict of interests
None of the included studies mentioned any conflicts of interest. Two studies were supported by drug companies (Cadorna‐Carlos 2012; Estívariz 2012), and in one study, Estívariz 2012, the funding agency was listed as an affiliation of those authors providing statistical amongst other support to the study. Pharmaceutical companies supplied the vaccines in three studies (Estívariz 2012; Mohammed 2010; Resik 2017), and manufacturing companies supplied the needle‐free devices in two studies (Resik 2010; Troy 2015). In Troy 2015, the manufacturing company also supported the salaries of two of the authors.
Excluded studies
See the Characteristics of excluded studies tables for further details.
We excluded 13 studies that did not meet our inclusion criteria after full‐text screening (Aaby 2007; Bakker 2011; Bégué 1998; Choudhury 2011; Cuba IPV Study Group 2007; Grassly 2014; Klein 2012; Li 2016; NCT00871000; Nirmal 1998; O’Ryan 2015; Verdijk 2013; WHO Collaborative Study 1996). Of these studies, 10 used different interventions (i.e. they did not use intradermal IPV) (Aaby 2007; Bakker 2011; Bégué 1998; Choudhury 2011; Cuba IPV Study Group 2007; Klein 2012; Li 2016; NCT00871000; O’Ryan 2015; WHO Collaborative Study 1996); one was a systematic review and not an RCT (Grassly 2014); one compared two different schedules of intradermal IPV (Nirmal 1998); and one compared two different types of intramuscular IPV (Verdijk 2013). See Figure 1.
Studies awaiting classification
One RCT is awaiting classification (NCT02347423). It is a multicentric, phase 2 trial conducted in the Dominican Republic comparing three reduced doses of aluminium hydroxide‐based inactivated poliovirus vaccines from the Statens Serum Institut (IPV‐AI‐SSI) with a full dose of non‐adjuvated IPV‐AI‐SSI. This trial included 824 six‐week‐old infants of both sexes who received either intervention or control at 6, 10, and 14 weeks of age. The trial measured seroconversion rates, type‐specific geometric mean titres, type‐specific seroprotection rates, reverse cumulative titre distribution, and adverse events following each dose. It was supported by the Bill & Melinda Gates Foundation, Quintiles Inc, Larix A/S, and Statens Serum Institut. See the Characteristics of studies awaiting classification table for further details.
Ongoing studies
We found two ongoing studies: one from Bangladesh, NCT02847026, and one from Uruguay, NCT03016949.
The Bangladesh study enrolled 1144 infants of both sexes at six weeks of age (NCT02847026). This multi‐arm study compared different schedules of IPV in combination with two different rotavirus vaccines.
The Uruguay study, another multi‐arm study, intends to include 1493 infants aged between five and seven weeks old (NCT03016949). It will compare two or three doses of full‐dose IPV given intramuscularly with two or three fractional doses of IPV given intradermally in the following schedule combinations: 6 and 14 weeks; 10 and 14 weeks; 14 and 36 weeks; 6, 14, and 36 weeks; and 10, 14, and 36 weeks. This study is supported by the Bill & Melinda Gates Foundation and the Fedic Corporation.
See the Characteristics of ongoing studies tables for further details.
Risk of bias in included studies
We assessed the risk of bias in each included study for each of domains provided in the Assessment of risk of bias in included studies section. We summarised the findings of this assessment in Figure 2 and Figure 3. See the 'Risk of bias' tables beneath the Characteristics of included studies tables for further details.
2.
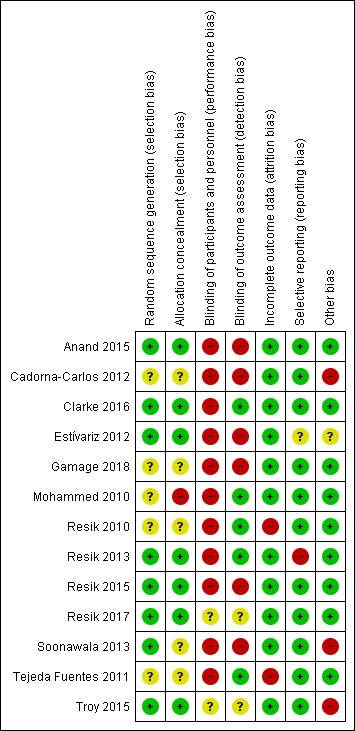
Risk of bias summary: review authors' judgements about each 'Risk of bias' item for each included study.
3.
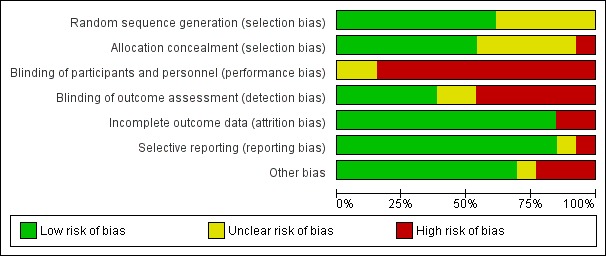
Risk of bias graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
Allocation
Random sequence generation (selection bias)
We judged five studies as at unclear risk of bias for this domain as they did not mention the method of randomisation (Cadorna‐Carlos 2012; Gamage 2018; Mohammed 2010; Resik 2010; Tejeda Fuentes 2011). We assessed the remaining eight studies as at low risk of selection bias due to random sequence generation as details describing a satisfactory method were provided (Anand 2015; Clarke 2016; Estívariz 2012; Resik 2013; Resik 2015; Resik 2017; Soonawala 2013; Troy 2015).
Allocation concealment (selection bias)
We evaluated one study, Mohammed 2010, as at high risk of bias for this domain as the parents knew which vaccine their children would be given. We rated five studies as at unclear risk of bias (Cadorna‐Carlos 2012; Gamage 2018; Resik 2010; Soonawala 2013; Tejeda Fuentes 2011). Soonawala 2013 used sealed envelopes but did not specify whether or not the envelopes were opaque. The other four studies did not report on allocation concealment. We judged the remaining seven trials to be at low risk of bias as the studies concealed the allocations satisfactorily (Anand 2015; Clarke 2016; Estívariz 2012; Resik 2013; Resik 2015; Resik 2017; Troy 2015).
Blinding
Blinding of participants and personnel (performance bias)
We assessed 11 studies as at high risk of performance bias since the participants were not blinded (Anand 2015; Cadorna‐Carlos 2012; Clarke 2016; Estívariz 2012; Gamage 2018; Mohammed 2010; Resik 2010; Resik 2013; Resik 2015; Soonawala 2013; Tejeda Fuentes 2011). We judged two studies to be at unclear risk of performance bias as blinding of participants and personnel was not mentioned (Resik 2017; Troy 2015).
Blinding of outcome assessment (detection bias)
We rated five studies as at low risk of detection bias as they used laboratories or outcome assessors blinded to the intervention (Clarke 2016; Mohammed 2010; Resik 2010; Resik 2013; Tejeda Fuentes 2011). We rated six studies as at high risk of bias as they were open‐label or unblinded studies (Anand 2015; Cadorna‐Carlos 2012; Estívariz 2012; Gamage 2018; Resik 2015; Soonawala 2013). We rated the two remaining studies as at unclear risk of detection bias as they did not mention blinding (Resik 2017; Troy 2015).
Incomplete outcome data
We judged two studies with attrition of more than 15% to be at high risk of bias for this domain (Resik 2010; Tejeda Fuentes 2011). We considered the 11 remaining studies to be at low risk of attrition bias as attrition was much lower (Anand 2015; Cadorna‐Carlos 2012; Clarke 2016; Estívariz 2012; Gamage 2018; Mohammed 2010; Resik 2013; Resik 2015; Resik 2017; Soonawala 2013; Troy 2015).
Selective reporting
We compared the protocols of 11 trials to the respective published reports to identify any unreported outcomes (Anand 2015; Cadorna‐Carlos 2012; Clarke 2016; Estívariz 2012; Gamage 2018; Mohammed 2010; Resik 2013; Resik 2010; Resik 2015; Resik 2017; Soonawala 2013). One of these studies, Resik 2013, did not report on all prespecified outcomes and was therefore rated as at high risk of bias. We rated another study, Estívariz 2012, as at unclear risk of reporting bias as we could not distinguish clearly between the prespecified and reported outcomes. We considered the other nine studies to be at low risk of bias as all they reported on the prespecified outcomes (Anand 2015; Cadorna‐Carlos 2012; Clarke 2016; Gamage 2018; Mohammed 2010; Resik 2010; Resik 2015; Resik 2017; Soonawala 2013).
For the two remaining studies for which protocols were not available, we compared the outcomes in the Methods section with those reported in the Results section. We rated both studies as at low risk of reporting bias (Tejeda Fuentes 2011; Troy 2015).
We did not construct funnel plots for publication bias as there were fewer than 10 studies in each analysis.
Other potential sources of bias
We rated three studies as at high risk of other bias as they were funded by drug companies (Cadorna‐Carlos 2012; Soonawala 2013; Troy 2015). We rated one study as at unclear risk of other bias as one author was an employee from the vaccine company (Estívariz 2012). We identified no other sources of bias in the nine remaining studies and so rated them as at low risk of other bias (Anand 2015; Clarke 2016; Gamage 2018; Mohammed 2010; Resik 2010; Resik 2013; Resik 2015; Resik 2017; Tejeda Fuentes 2011).
Effects of interventions
See: Table 1
Summary of findings for the main comparison. Equivalent schedules of intradermal fractional‐dose inactivated poliovirus vaccine compared to intramuscular inactivated poliovirus vaccine for the prevention of poliomyelitis in children.
| Equivalent schedules of intradermal fractional‐dose inactivated poliovirus vaccine compared to intramuscular inactivated poliovirus vaccine for the prevention of poliomyelitis in children | ||||||
| Patient or population: children Setting: community Intervention: intradermal fractional‐dose inactivated poliovirus vaccine Comparison: intramuscular inactivated poliovirus vaccine | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with intramuscular IPV | Risk with intradermal fractional‐dose IPV | |||||
| Paralytic poliomyelitis (not measured) | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported by any of the included studies. |
| Seroconversion rate (after a single primary dose) | Type 1 poliovirus | OR 0.30 (0.22 to 0.41) | 2570 (6 RCTs) |
⊕⊕⊝⊝ Lowa,b | ||
| 721 per 1000 | 437 per 1000 (363 to 515) | |||||
| Type 2 poliovirus | OR 0.43 (0.31 to 0.60) | 2567 (6 RCTs) |
⊕⊕⊝⊝ Lowb,d | |||
| 773 per 1000 | 595 per 1000 (514 to 672) | |||||
| Type 3 poliovirus | OR 0.19 (0.12 to 0.30) | 2571 (6 RCTs) |
⊕⊝⊝⊝ Very lowa,b,d | |||
| 753 per 1000 | 366 per 1000 (268 to 477) | |||||
| Seroconversion rate (after 2 primary doses) | Type 1 poliovirus | OR 0.23 (0.16 to 0.33) | 981 (3 RCTs) |
⊕⊕⊝⊝ Lowb,e | ||
| 826 per 1000 | 522 per 1000 (432 to 611) | |||||
| Type 2 poliovirus | OR 0.41 (0.28 to 0.60) | 853 (3 RCTs) |
⊕⊕⊝⊝ Lowb,f | |||
| 846 per 1000 | 693 per 1000 (606 to 767) | |||||
| Type 3 poliovirus | OR 0.12 (0.07 to 0.22) | 855 (3 RCTs) |
⊕⊕⊝⊝ Lowb,e | |||
| 941 per 1000 | 657 per 1000 (528 to 778) | |||||
| Seroconversion rate (after 3 primary doses) | Type 1 poliovirus | OR 0.45 (0.07 to 3.15) | 973 (3 RCTs) |
⊕⊝⊝⊝ Very lowb,d,e | ||
| 913 per 1000 | 825 per 1000 (423 to 970) | |||||
| Type 2 poliovirus | OR 0.34 (0.19 to 0.63) | 973 (3 RCTs) |
⊕⊕⊝⊝ Lowa,b | |||
| 969 per 1000 | 914 per 1000 (855 to 951) | |||||
| Type 3 poliovirus | OR 0.18 (0.01 to 2.58) | 973 (3 RCTs) |
⊕⊝⊝⊝ Very lowb,c,d,f | |||
| 979 per 1000 | 894 per 1000 (320 to 991) | |||||
| Geometric mean titres | 7 studies reported median titres rather than mean titres. In all 7 studies, antibody titres showed better protective effects with intradermal fractional‐dose (1/5) IPV, albeit in fewer numbers of participants. | ‐ | 4887 (7 RCTs) |
⊕⊝⊝⊝ Very lowb,d,e | ||
| Vaccine‐related adverse events | 5 studies reported more adverse events in the intradermal group, whilst 2 studies reported more adverse events in the intramuscular group. The most common adverse events were redness, tenderness, erythema, and fever. | ‐ | 4121 (7 RCTs) |
⊕⊝⊝⊝ Very lowb,c,d | See Table 6 for full results. | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IPV: inactivated poliovirus vaccine; OR: odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for risk of bias: one study had high attrition; one study did not report all of the prespecified outcomes; and all studies included in this analysis were open‐label. bDowngraded one level due to indirectness: there were different schedules of the intervention and comparisons. cDowngraded one level due to limitations in the design: there was the potential for high risk of bias due to high attrition in one study and unclear methods of randomisation, and all of the studies included in this analysis were open‐label. dDowngraded one level due to heterogeneity: there was significant heterogeneity in the studies included in this analysis. eDowngraded one level due to limitations in the design: there was the potential for bias due to attrition and selective reporting, and the studies included in this analysis were not blinded. fDowngraded one level due to imprecision: the effect estimate had wide CI.
We have reported exact P values where possible, or as reported by the study authors. For P values less than 0.001, we express P < 0.001.
Primary outcomes
Paralytic poliomyelitis
None of the included studies reported data on the occurrence of paralytic poliomyelitis.
One study from India, Estívariz 2012, reported one participant with wild poliovirus type 3 infection who received a full dose of IPV delivered intramuscularly.
Seroconversion rate
We conducted all analyses using a random‐effects model and performed subgroup analyses based on the World Bank classification of countries where possible. We also performed a sensitivity analysis using the fixed‐effect model.
Seroconversion rates in children
After a single primary dose
Type 1 poliovirus: seroconversion rates were significantly higher in children who received intramuscular full‐dose IPV (odd ratio (OR) 0.30, 95% confidence interval (CI) 0.22 to 0.41, P < 0.001; 6 studies, 2570 children; heterogeneity: Tau2 = 0.00; Chi2 = 3.93, df = 4 (P = 0.42); I2 = 0%). Results of a subgroup analysis found no significant difference between the groups in low‐income countries (OR 0.51, 95% CI 0.15 to 1.70, P = 0.27; 1 study, 700 children), whereas there were significantly higher seroconversion rates in children given intramuscular full‐dose IPV from middle‐income countries (OR 0.25, 95% CI 0.17 to 0.37, P < 0.001; 4 studies, 1502 children; heterogeneity: Tau2 = 0.00; Chi2 = 1.17, df = 2 (P = 0.56), I2 = 0%) and high‐income countries (OR 0.41, 95% CI 0.23 to 0.75, P = 0.003; 1 study, 368 children). See Analysis 1.1.
Type 2 poliovirus: seroconversion rates favoured children given intramuscular full‐dose IPV (OR 0.43, 95% CI 0.31 to 0.60, P < 0.001; 6 studies, 2567 children; heterogeneity: Tau2 = 0.05; Chi2 = 7.43, df = 5 (P = 0.19); I2 = 33%). A subgroup analysis showed no difference in seroconversion rates amongst children receiving intramuscular full‐dose IPV or intradermal fractional‐dose IPV in low‐income countries (OR 0.51, 95% CI 0.05 to 5.65, P = 0.58; 1 study, 700 children), but found a significant difference in favour of intramuscular full‐dose IPV in both middle‐income countries (OR 0.40, 95% CI 0.23 to 0.69, P = 0.001; 4 studies, 1502 children; heterogeneity: Tau2 = 0.16; Chi2 = 7.57, df = 3 (P = 0.06), I2 = 60%) and high‐income countries (OR 0.43, 95% CI 0.26 to 0.71, P = 0.001; 1 study, 365 children). See Analysis 1.2.
Type 3 poliovirus: seroconversion rates favoured children given intramuscular full‐dose IPV (OR 0.19, 95% CI 0.12 to 0.30, P < 0.001; 6 studies, 2571 children; heterogeneity: Tau2 = 0.23; Chi2 = 15.19, df = 5 (P = 0.010), I2 = 67%). A subgroup analysis also favoured children receiving intramuscular full‐dose IPV over those receiving intradermal fractional‐dose IPV in low‐income countries (OR 0.38, 95% CI 0.18 to 0.84, P = 0.02; 1 study, 700 children), middle‐income countries (OR 0.18, 95% CI 0.10 to 0.33, P < 0.001; 4 studies, 1502 children; heterogeneity: Tau2 = 0.27; Chi2 = 10.01, df = 3 (P = 0.02), I2 = 70%), and high‐income countries (OR 0.12, 95% CI 0.07 to 0.22, P < 0.001; 1 study, 369 children). See Analysis 1.3.
1.1. Analysis.
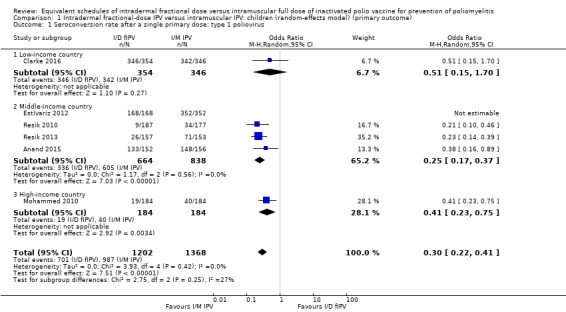
Comparison 1 Intradermal fractional‐dose IPV versus intramuscular IPV: children (random‐effects model) (primary outcome), Outcome 1 Seroconversion rate after a single primary dose: type 1 poliovirus.
1.2. Analysis.
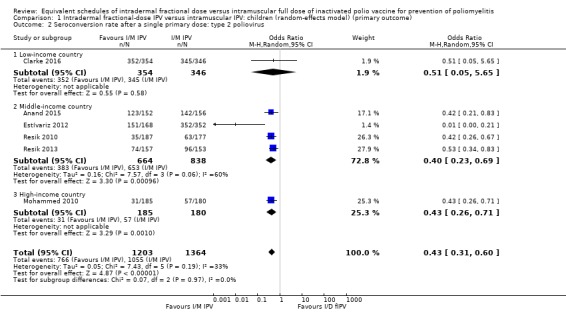
Comparison 1 Intradermal fractional‐dose IPV versus intramuscular IPV: children (random‐effects model) (primary outcome), Outcome 2 Seroconversion rate after a single primary dose: type 2 poliovirus.
1.3. Analysis.
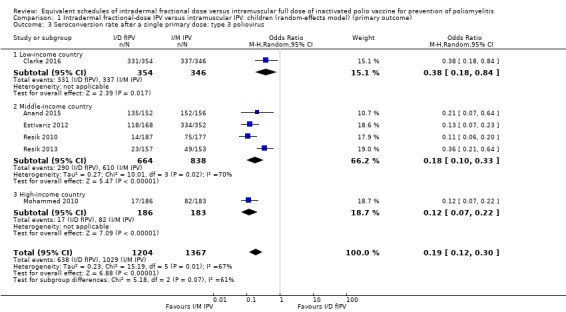
Comparison 1 Intradermal fractional‐dose IPV versus intramuscular IPV: children (random‐effects model) (primary outcome), Outcome 3 Seroconversion rate after a single primary dose: type 3 poliovirus.
After two primary doses
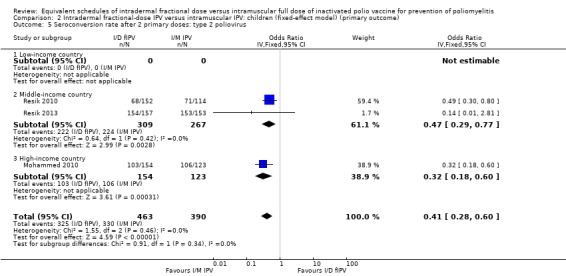
Type 1 poliovirus: seroconversion rates were significantly higher in children receiving intramuscular full‐dose IPV (OR 0.23, 95% CI 0.16 to 0.33, P < 0.001; 3 studies, 981 children; heterogeneity: Tau2 = 0.00; Chi2 = 1.63, df = 2 (P = 0.44), I2 = 0%). No studies from low‐income countries reported data on seroconversion rates after two doses of IPV. In studies conducted in middle‐income countries, the group given intramuscular full‐dose IPV had a significantly higher seroconversion rate (OR 0.19, 95% CI 0.07 to 0.47, P < 0.001; 2 studies, 674 children; heterogeneity: Tau2 = 0.20; Chi2 = 1.18, df = 1 (P = 0.28), I2 = 16%). The subgroup for high‐income countries also had seroconversion rates favouring the intramuscular full‐dose IPV group (OR 0.27, 95% CI 0.15 to 0.50, P < 0.001; 1 study, 307 children). See Analysis 1.4.
Type 2 poliovirus: seroconversion rates favoured children given intramuscular full‐dose IPV (OR 0.41, 95% CI 0.28 to 0.60, P < 0.001; 3 studies, 853 children; heterogeneity: Tau2 = 0.00; Chi2 = 1.55, df = 2 (P = 0.46), I2 = 0%). A subgroup analysis favoured intramuscular full‐dose IPV over intradermal fractional‐dose IPV in both middle‐income countries (OR 0.47, 95% CI 0.29 to 0.77, P = 0.003; 2 studies, 576 children; heterogeneity: Tau2 = 0.00; Chi2 = 0.64, df = 1 (P = 0.42), I2 = 0%) and high‐income countries (OR 0.32, 95% CI 0.18 to 0.60, P < 0.001; 1 study, 277 children). No studies from low‐income countries reported seroconversion rate after two doses of IPV. See Analysis 1.5.
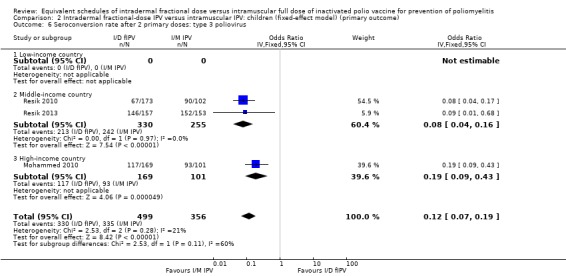
Type 3 poliovirus: seroconversion rates favoured children given intramuscular full‐dose IPV (OR 0.12, 95% CI 0.07 to 0.22, P < 0.001; 3 studies, 855 children; heterogeneity: Tau2 = 0.06; Chi2 = 2.53, df = 2 (P = 0.28), I2 = 21%). A subgroup analysis for both middle‐income countries (OR 0.08, 95% CI 0.04 to 0.16, P < 0.001; 2 studies, 585 participants; heterogeneity: Tau2 = 0.00; Chi2 = 0.00, df = 1 (P = 0.97), I2 = 0%) and high‐income countries (OR 0.19, 95% CI 0.09 to 0.43, P < 0.001; 1 study, 270 children) favoured the intramuscular full‐dose IPV group. See Analysis 1.6.
1.4. Analysis.
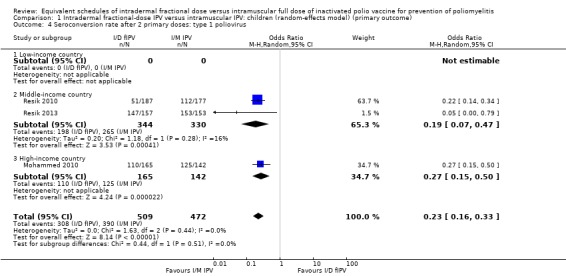
Comparison 1 Intradermal fractional‐dose IPV versus intramuscular IPV: children (random‐effects model) (primary outcome), Outcome 4 Seroconversion rate after 2 primary doses: type 1 poliovirus.
1.5. Analysis.
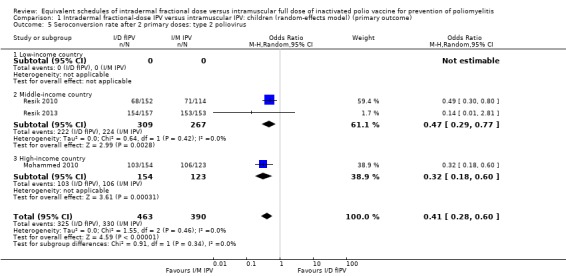
Comparison 1 Intradermal fractional‐dose IPV versus intramuscular IPV: children (random‐effects model) (primary outcome), Outcome 5 Seroconversion rate after 2 primary doses: type 2 poliovirus.
1.6. Analysis.
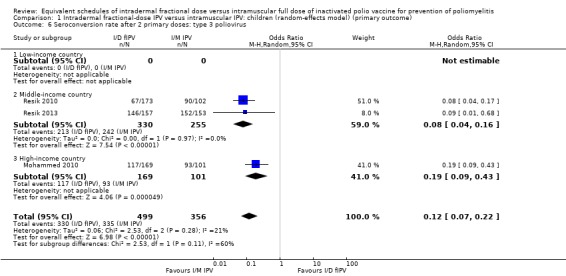
Comparison 1 Intradermal fractional‐dose IPV versus intramuscular IPV: children (random‐effects model) (primary outcome), Outcome 6 Seroconversion rate after 2 primary doses: type 3 poliovirus.
After three primary doses
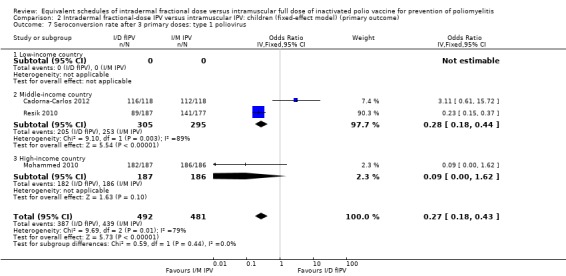
Type 1 poliovirus: seroconversion rates were not statistically significant in children receiving intramuscular full‐dose IPV (OR 0.45, 95% CI 0.07 to 3.15, P = 0.42; 3 studies, 973 children; heterogeneity: Tau2 = 2.19; Chi2 = 9.73, df = 2 (P = 0.008), I2 = 79%). A subgroup analysis found that in studies conducted in middle‐income countries seroconversion rates amongst children were not significantly higher in children receiving full‐dose IPV (OR 0.75, 95% CI 0.06 to 9.59, P = 0.83; 2 studies, 600 children; heterogeneity: Tau2 = 3.03; Chi2 = 9.18, df = 1 (P = 0.002), I2 = 89%), whereas the subgroup for high‐income countries showed no difference between the two groups (OR 0.09, 95% CI 0.00 to 1.62, P = 0.10; 1 study, 373 children). No studies from low‐income countries reported seroconversion rates after three doses of IPV. See Analysis 1.7.
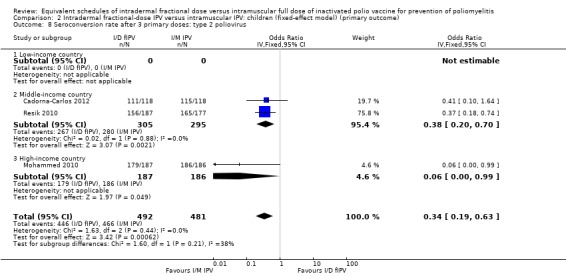
Type 2 poliovirus: seroconversion rates favoured children given intramuscular full‐dose IPV (OR 0.34, 95% CI 0.19 to 0.63, P < 0.001; 3 studies, 973 children; heterogeneity: Tau2 = 0.00; Chi2 = 1.72, df = 2 (P = 0.42), I2 = 0%). A subgroup analysis favoured intramuscular full‐dose IPV over intradermal fractional‐dose IPV in both middle‐income countries (OR 0.38, 95% CI 0.20 to 0.70, P = 0.002; 2 studies, 600 children; heterogeneity: Tau2 = 0.00; Chi2 = 0.02, df = 1 (P = 0.88), I2 = 0%) and high‐income countries (OR 0.06, 95% CI 0.00 to 0.99, P = 0.05; 1 study, 373 children). No studies from low‐income countries reported on seroconversion rates after three doses of IPV. See Analysis 1.8.
Type 3 poliovirus: seroconversion rates were not statistically significant (OR 0.18, 95% CI 0.01 to 2.58, P = 0.21; 3 studies, 973 children; heterogeneity: Tau2 = 4.75; Chi2 = 18.70, df = 2 (P < 0.001), I2 = 89%). A subgroup analysis for middle‐income (OR 0.21, 95% CI 0.01 to 7.07, P = 0.39; 2 studies, 600 children; heterogeneity: Tau2 = 6.08; Chi2 = 18.59, df = 1 (P < 0.001), I2 = 95%) and high‐income countries (OR 0.11, 95% CI 0.01 to 2.05, P = 0.14; 1 study, 373 participants) was also not statistically significant, although the effect estimate favoured full‐dose IPV. No studies from low‐income countries reported on seroconversion rates after three doses of IPV. See Analysis 1.9.
1.7. Analysis.
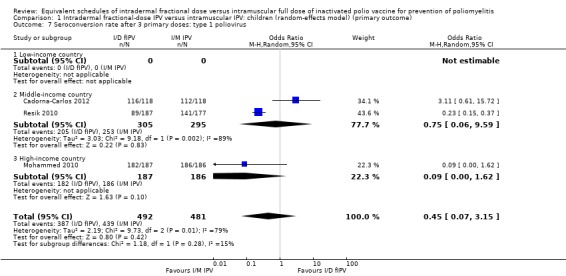
Comparison 1 Intradermal fractional‐dose IPV versus intramuscular IPV: children (random‐effects model) (primary outcome), Outcome 7 Seroconversion rate after 3 primary doses: type 1 poliovirus.
1.8. Analysis.
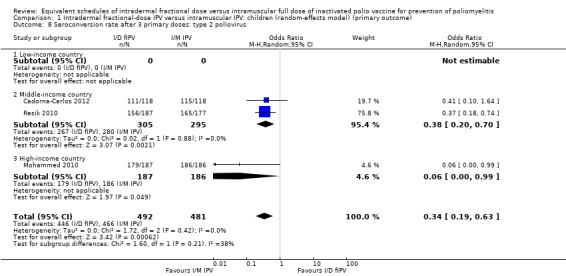
Comparison 1 Intradermal fractional‐dose IPV versus intramuscular IPV: children (random‐effects model) (primary outcome), Outcome 8 Seroconversion rate after 3 primary doses: type 2 poliovirus.
1.9. Analysis.
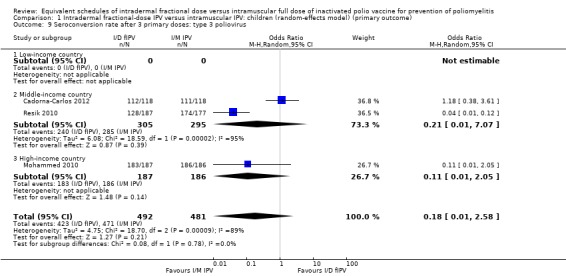
Comparison 1 Intradermal fractional‐dose IPV versus intramuscular IPV: children (random‐effects model) (primary outcome), Outcome 9 Seroconversion rate after 3 primary doses: type 3 poliovirus.
Sensitivity analysis
Sensitivity analyses showed that the fixed‐effect and random‐effects models produced similar findings for seroconversion rates for polio types 1, 2, and 3 in children after a single primary dose (see Analysis 2.1; Analysis 2.2; Analysis 2.3) and after two primary doses (see Analysis 2.4; Analysis 2.5; Analysis 2.6). After three primary doses, the sensitivity analyses showed similar seroconversion rate effects for the fixed‐effect and random‐effects models for type 1 (Analysis 2.7) and type 2 (Analysis 2.8) only. For type 3, the fixed‐effect analysis showed that seroconversion rates favoured the group given intramuscular full‐dose IPV (OR 0.22, 95% CI 0.10 to 0.48; 3 studies, 973 participants; Analysis 2.9), whereas the random‐effects model showed no clear difference between the two interventions (OR 0.18, 95% CI 0.01 to 2.58; 3 studies, 973 participants; Analysis 1.9).
2.1. Analysis.
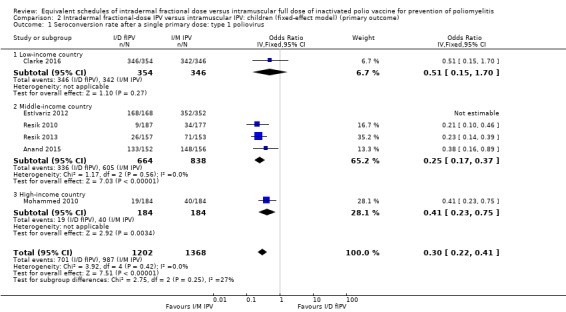
Comparison 2 Intradermal fractional‐dose IPV versus intramuscular IPV: children (fixed‐effect model) (primary outcome), Outcome 1 Seroconversion rate after a single primary dose: type 1 poliovirus.
2.2. Analysis.
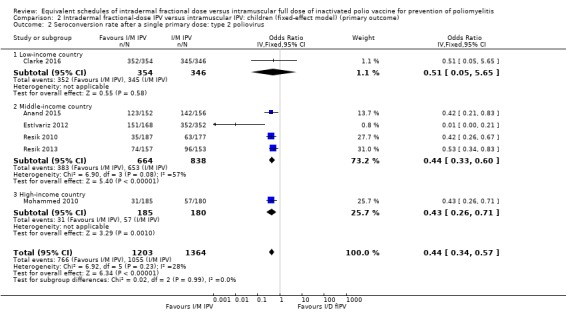
Comparison 2 Intradermal fractional‐dose IPV versus intramuscular IPV: children (fixed‐effect model) (primary outcome), Outcome 2 Seroconversion rate after a single primary dose: type 2 poliovirus.
2.3. Analysis.
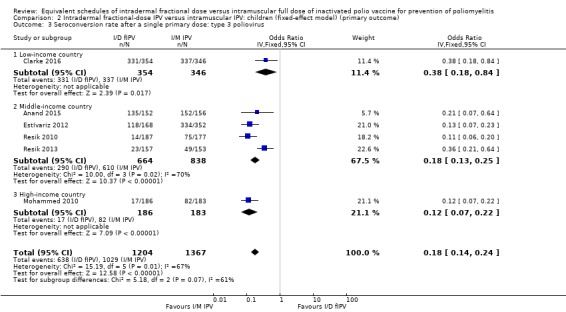
Comparison 2 Intradermal fractional‐dose IPV versus intramuscular IPV: children (fixed‐effect model) (primary outcome), Outcome 3 Seroconversion rate after a single primary dose: type 3 poliovirus.
2.4. Analysis.
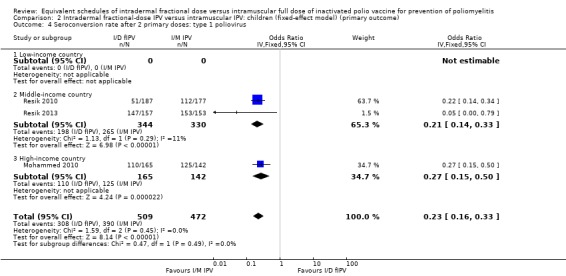
Comparison 2 Intradermal fractional‐dose IPV versus intramuscular IPV: children (fixed‐effect model) (primary outcome), Outcome 4 Seroconversion rate after 2 primary doses: type 1 poliovirus.
2.5. Analysis.
Comparison 2 Intradermal fractional‐dose IPV versus intramuscular IPV: children (fixed‐effect model) (primary outcome), Outcome 5 Seroconversion rate after 2 primary doses: type 2 poliovirus.
2.6. Analysis.
Comparison 2 Intradermal fractional‐dose IPV versus intramuscular IPV: children (fixed‐effect model) (primary outcome), Outcome 6 Seroconversion rate after 2 primary doses: type 3 poliovirus.
2.7. Analysis.
Comparison 2 Intradermal fractional‐dose IPV versus intramuscular IPV: children (fixed‐effect model) (primary outcome), Outcome 7 Seroconversion rate after 3 primary doses: type 1 poliovirus.
2.8. Analysis.
Comparison 2 Intradermal fractional‐dose IPV versus intramuscular IPV: children (fixed‐effect model) (primary outcome), Outcome 8 Seroconversion rate after 3 primary doses: type 2 poliovirus.
2.9. Analysis.
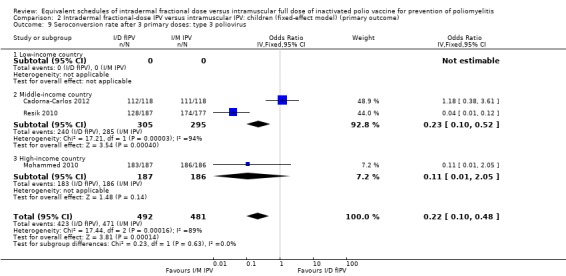
Comparison 2 Intradermal fractional‐dose IPV versus intramuscular IPV: children (fixed‐effect model) (primary outcome), Outcome 9 Seroconversion rate after 3 primary doses: type 3 poliovirus.
Seroconversion rates in adults
Only two studies (565 participants) reported data on seroconversion rates in adults (Resik 2017; Troy 2015). We were unable to pool these results in a meta‐analysis due to high levels of clinical heterogeneity. Troy 2015 recruited adults who were HIV positive, whilst Resik 2017 recruited healthy adult males only. Both studies reported data on seroconversions post‐booster doses and showed no significant difference in seroconversion rates at one‐month postintervention (results not shown).
Geometric mean titres
Seven studies (4887 participants) measured geometric titres (Cadorna‐Carlos 2012; Clarke 2016; Estívariz 2012; Mohammed 2010; Resik 2010; Resik 2013; Resik 2015). We were unable to pool these data in a meta‐analysis since all seven studies reported geometric titres as median, as opposed to mean titres. Consequently, we have presented a narrative summary of the results from each study in the section below. Detailed results are tabulated in Table 8.
3. Geometric titres: children (median titres reported in included studies that could not be used in a meta‐analysis).
| Study ID (location) | Number of doses given | Type of antibody | Median antibody titres: intradermal median (range) | Median antibody titres: intramuscular median (range) |
| Cadorna‐Carlos 2012 (the Philippines) | 3 primary doses given; end of primary series vaccination | Antipolio antibody type 1 | 221 (188 to 259) | 585 (482 to 710) |
| Antipolio antibody type 2 | 234 (186 to 294) | 795 (638 to 992) | ||
| Antipolio antibody type 3 | 194 (157 to 240) | 774 (622 to 963) | ||
| 1 booster dose given; booster series | Antipolio antibody type 1 | 2833 (2392 to 3356) | 6666 (5613 to 7916) | |
| Antipolio antibody type 2 | 3210 (2672 to 3857) | 6522 (5540 to 7678) | ||
| Antipolio antibody type 3 | 4498 (3608 to 5607) | 11,952 (10,046 to 14,220) | ||
| Clarke 2016 (The Gambia) | Single primary dose of intramuscular IPV or intradermal fractional‐dose IPV | Antipoliovirus type 1 |
|
|
| Antipoliovirus type 2 |
|
|
||
| Antipoliovirus type 3 |
|
|
||
| Estívariz 2012 (India) | Single supplementary dose; 28 days after vaccination | Antipolio antibody type 1 | > 1448 (> 1448 to > 1448) |
|
| Antipolio antibody type 2 | 724 (455 to 910) |
|
||
| Antipolio antibody type 3 | 202 (28 to 724) |
|
||
| Mohammed 2010 (Oman) | 3 primary doses given; end of primary vaccination series | Antipolio antibody type 1 | 228 (228 to 456) | 724 (575 to 912) |
| Antipolio antibody type 2 | 287 (228 to 456) | 1149 (912 to 1149) | ||
| Antipolio antibody type 3 | 362 (287 to 456) | > 1448 (> 1448 to > 1448) | ||
| Resik 2010 (Cuba) | 3 primary doses at 6, 10, and 14 weeks; median titres reported at 18 weeks of age (4 weeks after the 3rd dose) | Antipolio antibody type 1 | 19 (19 to 22) | 85 (54 to 99) |
| Antipolio antibody type 2 | 45 (45 to 54) | 214 (178 to 295) | ||
| Antipolio antibody type 3 | 32 (24 to 45) | 295 (214 to 355) | ||
| Resik 2013 (Cuba) | 2 doses of vaccine at 4 months and 8 months of age; titres after 30 days of 2nd dose | Antipolio antibody type 1 | 450 (357 to 566) | > 1448 (> 1448 to > 1448) |
| Antipolio antibody type 2 | 898 (713 to > 1448) | > 1448 (> 1448 to > 1448) | ||
| Antipolio antibody type 3 | 71 (36 to 113) | 898 (566 to > 1448) | ||
| Resik 2015 (Cuba) | Single dose; 21 days after the dose | Antipolio antibody type 1 |
|
4499 (3573 to 5664) |
| Antipolio antibody type 2 |
|
2839 (2255 to 3573) | ||
| Antipolio antibody type 3 |
|
4499 (3573 to 4499) |
BCG: bacille Calmette‐Guérin vaccine GSK: GlaxoSmithKline ID: identifier IPV: inactivated polio vaccine
Single‐study results
Cadorna‐Carlos 2012 (461 participants) compared both primary and booster doses. Three primary doses were given. The median antibody titre against intradermal poliovirus type 1 was 221 (range = 188 to 259); type 2 was 234 (range = 186 to 294); and type 3 was 194 (range = 157 to 240), whilst the median antibody titre against intramuscular poliovirus type 1 was 585 (range = 482 to 710); type 2 was 795 (range = 638 to 992); and type 3 was 774 (range = 622 to 963). Only one booster dose was given. The median antibody titre against intradermal poliovirus type 1 was 2833 (range = 2392 to 3356); type 2 was 3210 (range = 2672 to 3857); and type 3 was 4498 (range = 3608 to 5607), whilst the median antibody titre against intramuscular poliovirus type 1 was 6666 (range = 5613 to 7916); type 2 was 6522 (range = 5540 to 7678); and type 3 was 11,952 (range = 10,046 to 14,220).
Clarke 2016 (1504 participants) compared a single primary dose of intramuscular or intradermal injectable IPV and assessed antibody titres against three types of polioviruses (types 1, 2, and 3). The median antibody titre against intradermal poliovirus type 1 was 256 (range = 256 to 256) from needle/syringe and 256 (128 to 256) from jet injection; type 2 was 256 (range = 256 to 512) for needle/syringe and 256 (range = 128 to 256) for jet injection; and type 3 was 512 (range = 512 to 512) for needle/syringe and 256 (range = 256 to 512) for jet injection. The median antibody titre against intramuscular poliovirus type 1 was 512 (range = 256 to 512) from needle/syringe and 512 (range = 256 to 512) from jet injection; type 2 was 512 (range = 512 to 512) from needle/syringe and 512 (range = 256 to 512) from jet injection; and type 3 was 1024 (range = 512 to 1024) from needle/syringe and 512 (range = 512 to 1024) from jet injection.
Estívariz 2012 (1002 participants) used a single dose of either intramuscular or intradermal injectable IPV and recorded median titres after 28 days against all three types of poliovirus. The median antibody titre against intradermal poliovirus type 1 was > 1448 (range = > 1448 to > 1448); type 2 was 724 (range = 455 to 910); and type 3 was 202 (range = 28 to 724). The median antibody titre against intramuscular poliovirus type 1 was > 1448 (range = > 1448 to > 1448) for both GlaxoSmithKline (GSK) and Panacea Biotec groups; type 2 was > 1448 (range = 1176 to > 1448) for both GSK and Panacea Biotec groups; and type 3 was 455 (range = 181 to 910) for the GSK group and 362 (range = 288 to 724) for the Panacea Biotec group.
Mohammed 2010 (400 participants) used three doses of the vaccine (i.e. either intramuscular full‐dose IPV or intradermal fractional‐dose IPV) as the primary series. The median antibody titre against intradermal poliovirus type 1 was 228 (range = 228 to 456); type 2 was 287 (range = 228 to 456); and type 3 was 362 (range = 287 to 456). The median antibody titre against intramuscular poliovirus type 1 was 724 (range = 575 to 912); type 2 was 1149 (range = 912 to 1149); and type 3 was > 1448 (range = > 1448 to > 1448).
Resik 2010 (471 participants) used three primary doses at 6, 10, and 14 weeks, and reported median titres at 18 weeks of age (i.e. four weeks after the third dose). The median antibody titre against intradermal poliovirus type 1 was 19 (range = 19 to 22); type 2 was 45 (range = 45 to 54); and type 3 was 32 (range = 24 to 45). The median antibody titre against intramuscular poliovirus type 1 was 85 (range = 54 to 99); type 2 was 214 (range = 178 to 295); and type 3 was 295 (range = 214 to 355).
Resik 2013 (320 participants) used two primary doses, given at four and eight months of age, and measured titres after 30 days of the second dose. The median antibody titre against intradermal poliovirus type 1 was 450 (range = 357 to 566); type 2 was 898 (range = 713 to > 1448); and type 3 was 71 (range = 36 to 113). The median antibody titre against intramuscular poliovirus type 1 was > 1448 (range = > 1448 to > 1448); type 2 was > 1448 (range = > 1448 to > 1448); and type 3 was 898 (range = 566 to > 1448).
Resik 2015 (729 participants) used a single full dose of intramuscular or a fractional dose of intradermal injectable IPV and recorded the median antibody titre for all three types of poliovirus. The median antibody titre against intradermal fractional‐dose poliovirus type 1 was 1423 (range = 1130 to 1791) from BCG syringe, 1423 (range = 1423 to 1791) from injector X, 898 (range = 713 to 1130) from injector Y, and 1423 (range = 1130 to 1423) from injector Z; type 2 was 1130 (range = 898 to 1423) from BCG syringe, 1130 (range = 713 to 1423) from injector X, 566 (range = 450 to 713) from injector Y, and 1130 (range = 898 to 1130) from injector Z; and type 3 was 1130 (range = 713 to 1423) from BCG syringe, 1423 (range = 1130 to 1791) from injector X, 566 (range = 357 to 713) from injector Y, and 1423 (range = 898 to 1791) from injector Z. The median antibody titre against intramuscular poliovirus type 1 was 4499 (range = 3573 to 5664); type 2 was 2839 (range = 2255 to 3573); and type 3 was 4499 (range = 3573 to 4499).
Any vaccine‐related adverse event
The studies did not report on adverse events uniformly, therefore we were unable to conduct a meta‐analysis of the data. Instead, we have provided a summary of the individual results from the studies below; see results with children in Table 6 and adults in Table 9.
1. Adverse events: children.
| Study ID (location) | Doses | Adverse events | Intradermal (number of individuals with events/total number of individuals) | Intramuscular (number of individuals with events/total number of individuals) |
| Cadorna‐Carlos 2012 (the Philippines) | 3 primary doses of intramuscular IPV or fractional dose of intradermal IPV | Solicited injection site reactions | ||
| Tenderness | 71/109 | 59/114 | ||
| Erythema | 82/109 | 34/114 | ||
| Swelling | 25/109 | 11/114 | ||
| Solicited systemic reactions | ||||
| Fever | 7/109 | 12/114 | ||
| Vomiting | 18/109 | 25/114 | ||
| Crying abnormal | 40/109 | 36/114 | ||
| Drowsiness | 44/109 | 41/114 | ||
| Appetite lost | 19/109 | 23/114 | ||
| Irritability | 58/109 | 51/114 | ||
| Clarke 2016 (The Gambia) | Single primary dose of intramuscular IPV or fractional dose of intradermal IPV | Redness and swelling on tenderness | 3 (needle syringe) 2 (jet injector) |
2 (needle syringe) 5 (jet injector) |
| Mohammed 2010 (Oman) | 3 primary doses of intramuscular IPV or fractional dose of intradermal IPV | 42 serious events (39 = infectious disease, 2 = anaemia, 1 = fall) | 18 events | 24 events |
| Resik 2010 (Cuba) | Dose 1 | Temperature | 39/187 | 34/177 |
| Redness | 25/187 | 8/177 | ||
| Induration | 4/187 | 2/177 | ||
| Pain | 1/187 | 1/177 | ||
| Combination | 26/187 | 9/177 | ||
| Dose 2 | Temperature | 21/187 | 41/177 | |
| Redness | 8/187 | 3/177 | ||
| Induration | 3/187 | 0/177 | ||
| Pain | 1/187 | 1/177 | ||
| Combination | 1/187 | 0/177 | ||
| Dose 3 | Temperature | 11/187 | 28/177 | |
| Redness | 19/187 | 0/177 | ||
| Induration | 5/187 | 0/177 | ||
| Pain | 0/187 | 0/177 | ||
| Combination | 1/187 | 0/177 | ||
| Resik 2013 (Cuba) | Dose 1 | Temp ≥ 38 °C | 1/157 | 2/153 |
| Redness | 47/157 | 3/153 | ||
| Induration | 11/157 | 2/153 | ||
| Other | 1/157 | 1/153 | ||
| Dose 2 | Temp ≥ 38 °C | 2/157 | 0/153 | |
| Redness | 37/157 | 2/153 | ||
| Induration | 14/157 | 1/153 | ||
| Other | 0/157 | 0/153 | ||
| Resik 2015 (Cuba) | Single primary dose of intramuscular IPV or fractional dose of intradermal IPV | Fever | 13/583 | 8/146 |
| Redness | 9/583 | 2/146 | ||
| Induration | 15/583 | 0/146 | ||
| Infiltration | 14/583 | 0/146 | ||
| Tejeda Fuentes 2011 (Cuba) | Dose 1 | General adverse events (temperature 37 to 37.9 °C) | 38 | 32 |
| General adverse events (temperature above 37.9 °C) | 1 | 2 | ||
| General adverse events (crying < 1 hour) | 3 | 4 | ||
| Local adverse events (redness) | 23 | 8 | ||
| Local adverse events (induration) | 4 | 2 | ||
| Local adverse events (pain) | 1 | 1 | ||
| Combination of all local adverse events | 26 | 9 | ||
| Dose 2 | General adverse events (temperature 37 to 37.9 °C) | 21 | 41 | |
| General adverse events (temperature above 37.9 °C) | 0 | 0 | ||
| General adverse events (crying < 1 hour) | 1 | 2 | ||
| Local adverse events (redness) | 8 | 3 | ||
| Local adverse events (induration) | 3 | 0 | ||
| Local adverse events (pain) | 1 | 1 | ||
| Combination of all local adverse events | 1 | 0 | ||
| Dose 3 | General adverse events (temperature 37 to 37.9 °C) | 11 | 28 | |
| General adverse events (temperature above 37.9 °C) | 0 | 0 | ||
| General adverse events (crying < 1 hour) | 0 | 0 | ||
| Local adverse events (redness) | 19 | 0 | ||
| Local adverse events (induration) | 5 | 0 | ||
| Local adverse events (pain) | 0 | 0 | ||
| Combination of all local adverse events | 1 | 0 | ||
ID: identifier IPV: inactivated poliovirus vaccine
4. Adverse events: adults.
| Study ID (location) | Adverse events | Intradermal (number of individuals with events/total number of individuals) | Intramuscular (number of individuals with events/total number of individuals) |
| Resik 2017 (Cuba) | Any adverse event | 28/268 | 19/266 |
| Soonawala 2013 (the Netherlands) | Vaccine delivery (pain) | 2/32 | 19/62 |
| Erythema | 28/32 | 34/62 | |
| Swelling | 19/32 | 12/62 | |
| Induration | 11/32 | 14/62 | |
| Stiffness | 5/32 | 22/62 | |
| Soreness | 5/32 | 33/62 | |
| Systemic side effects | Intradermal | Intramuscular | |
| Fever | 0 | 0 | |
| Myalgia | 3/32 | 5/62 | |
| Fatigue | 10/32 | 14/62 | |
| Headache | 8/32 | 12/62 | |
| Troy 2015 (USA) | Any adverse event | 29/63 | 18/64 |
| Fever | 2/63 | 0/64 | |
| Rash | 1/63 | 4/64 | |
| Redness at injection site | 22/63 | 4/64 | |
| Swelling at injection site | 7/63 | 3/64 | |
| Tenderness at injection site | 8/63 | 11/64 | |
| Itching at injection site | 4/63 | 0/64 |
ID: identifier
Single‐study results
The most commonly recorded adverse events were pain, redness, fever, and induration in eight studies with 4312 participants (Cadorna‐Carlos 2012; Clarke 2016; Resik 2010; Resik 2013; Resik 2015; Soonawala 2013; Tejeda Fuentes 2011; Troy 2015).
There were equal numbers of participants with local and systemic adverse events in both the intradermal and intramuscular groups in seven studies (3217 participants) in children, Cadorna‐Carlos 2012; Resik 2010; Resik 2013; Resik 2015; Resik 2017; Tejeda Fuentes 2011; Troy 2015, and three studies (659 participants) in adults, Resik 2017; Soonawala 2013; Troy 2015.
Two studies did not report adverse events (Estívariz 2012; Gamage 2018).
Adverse event: children
Five studies (1888 participants) reported adverse events at all doses in children (Cadorna‐Carlos 2012; Mohammed 2010; Resik 2010; Resik 2013; Tejeda Fuentes 2011). Two studies (2233 participants) reported adverse events with a single primary dose (Clarke 2016; Resik 2015). The most commonly recorded adverse events were redness, tenderness, erythema, and fever.
Five studies (2217 participants) reported more adverse events in the intradermal group than the intramuscular group (Cadorna‐Carlos 2012; Resik 2010; Resik 2013; Resik 2015; Tejeda Fuentes 2011). In three of these studies, there were almost equal numbers of participants with local and systemic adverse events in both the intradermal and intramuscular groups (Cadorna‐Carlos 2012; Resik 2010; Resik 2013). One study, Mohammed 2010, reported 42 adverse events in total, 24 in the intramuscular group and 18 in the intradermal group. Another study, Clarke 2016, also reported more adverse events in the intramuscular group (n = 7) compared with the intradermal group (n = 5).
See Table 6.
Adverse event: adults
Three studies with 659 adult participants reported an approximately equal number of participants with adverse events in both the intradermal and intramuscular groups (Resik 2017; Soonawala 2013; Troy 2015). In one study, rash and tenderness at the injection site were reported more frequently in the intradermal group (Troy 2015), whilst in another study, stiffness and soreness at the injection site were reported more frequently in the intradermal group (Soonawala 2013).
See Table 9.
Secondary outcomes
Reciprocal antibody titres
Three studies in children reported data on reciprocal antibody titres as seroprevalence/seroprotection rates (Cadorna‐Carlos 2012; Clarke 2016; Resik 2015). Analysis 3.1, Analysis 3.2, and Analysis 3.3 show the seroprotection rates for antipoliovirus antibodies types 1, 2, and 3, respectively. There was no significant difference in seroprevalence/seroprotection rates amongst children given intramuscular full‐dose IPV compared to those given intradermal fractional‐dose IPV at postintervention for type 1 poliovirus (OR 1.42, 95% CI 0.59 to 3.39; 3 studies, 1661 participants; heterogeneity: I2 = 0%) or type 2 poliovirus (OR 3.10, 95% CI 0.50 to 19.44; 3 studies, 1659 participants; heterogeneity: I2 = 0%). However, for type 3 poliovirus, children receiving intramuscular full‐dose IPV had better seroprotection rates (OR 2.94, 95% CI 1.44 to 6.00; 3 studies, 1659 participants; heterogeneity: I2 = 0%).
3.1. Analysis.
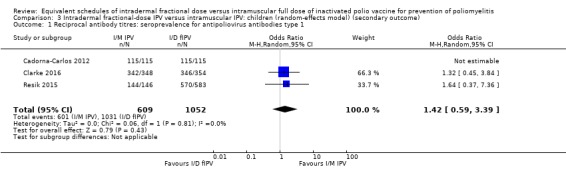
Comparison 3 Intradermal fractional‐dose IPV versus intramuscular IPV: children (random‐effects model) (secondary outcome), Outcome 1 Reciprocal antibody titres: seroprevalence for antipoliovirus antibodies type 1.
3.2. Analysis.
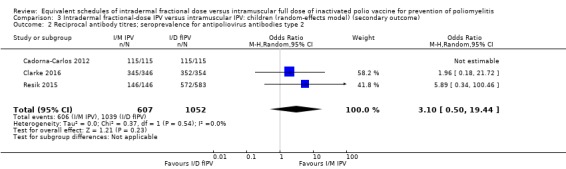
Comparison 3 Intradermal fractional‐dose IPV versus intramuscular IPV: children (random‐effects model) (secondary outcome), Outcome 2 Reciprocal antibody titres; seroprevalence for antipoliovirus antibodies type 2.
3.3. Analysis.
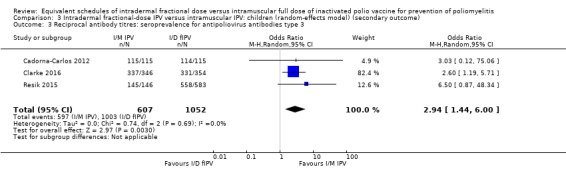
Comparison 3 Intradermal fractional‐dose IPV versus intramuscular IPV: children (random‐effects model) (secondary outcome), Outcome 3 Reciprocal antibody titres: seroprevalence for antipoliovirus antibodies type 3.
None of the studies with adult participants reported data on reciprocal antibody titres.
Sensitivity analysis
A sensitivity analysis for 'reciprocal antibody titres: seroprevalence for antipoliovirus antibodies types 1, 2, and 3' using the fixed‐effect model showed similar results (Analysis 4.1; Analysis 4.2; Analysis 4.3) to the random‐effects model (Analysis 3.1; Analysis 3.2; Analysis 3.3).
4.1. Analysis.
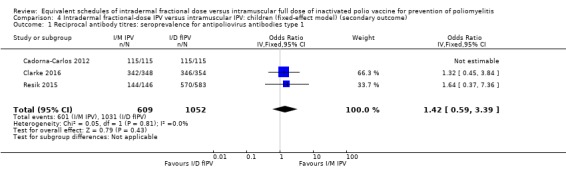
Comparison 4 Intradermal fractional‐dose IPV versus intramuscular IPV: children (fixed‐effect model) (secondary outcome), Outcome 1 Reciprocal antibody titres: seroprevalence for antipoliovirus antibodies type 1.
4.2. Analysis.
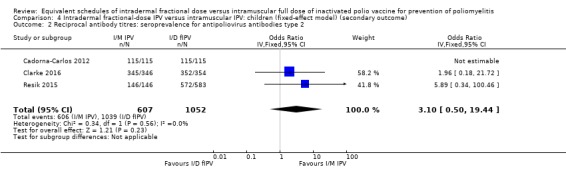
Comparison 4 Intradermal fractional‐dose IPV versus intramuscular IPV: children (fixed‐effect model) (secondary outcome), Outcome 2 Reciprocal antibody titres: seroprevalence for antipoliovirus antibodies type 2.
4.3. Analysis.
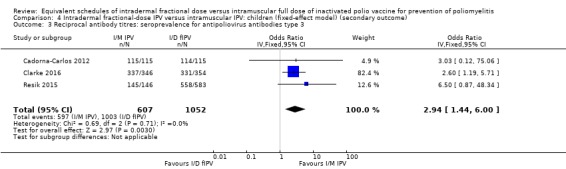
Comparison 4 Intradermal fractional‐dose IPV versus intramuscular IPV: children (fixed‐effect model) (secondary outcome), Outcome 3 Reciprocal antibody titres: seroprevalence for antipoliovirus antibodies type 3.
Serum immunoglobulin (IgA) levels
None of the included studies reported data on serum IgA levels.
Poliovirus shedding in stool
Faecal shedding after OPV is a proxy of mucosal immunity, and a more frequent excretion indicates lower mucosal immunity. Two studies reported poliovirus shedding after a challenge dose of OPV and found no difference between the two vaccines (intramuscular or intradermal) (Gamage 2018; Mohammed 2010). We were unable to pool the data from these studies due to differences in the frequency of doses of the two IPV vaccines (intramuscular and intradermal) used.
Single‐study results
Gamage 2018 found no difference in poliovirus shedding after the booster dose of either of the vaccines (intramuscular or intradermal) was given to children aged 10 to 12 years old. Mohammed 2010 also did not find a clear difference in poliovirus shedding after the primary doses of the two vaccines (intramuscular or intradermal) were given to younger children.
Vaccine‐associated paralytic poliomyelitis (VAPP)
None of the included studies reported data on VAPP.
Discussion
Summary of main results
This review included 13 RCTs with 7292 participants, from low‐, middle‐, and high‐income countries, comparing intradermal fractional‐dose (one‐fifth) IPV with intramuscular full‐dose IPV. The studies involved infants and children (n = 6402) and adults (n = 890). The studies ranged from 2 to 19 months in duration.
The studies included in this review did not report on the occurrence of paralytic poliomyelitis but did report on seroconversion rates for all three types of poliovirus. We found that seroconversion was greater in participants given intramuscular full‐dose IPV than in participants given intradermal fractional‐dose IPV; however, we considered the certainty of the evidence to be low and very low due to limitations in design (risk of bias) and indirectness, meaning that we are not confident that the estimated effect reflects the true effect. The studies also reported data on geometric median titres, which were higher in the intramuscular full‐dose IPV group than in the intradermal fractional‐dose IPV group for an equal number of primary vaccine doses.
Three studies reported data on reciprocal antibody titres as seroprotection/seroprevalence rates. We found a significant difference in seroprotection/prevalence rates between children given intramuscular full‐dose IPV and those given intradermal fractional‐dose IPV for type 3 poliovirus in favour of intramuscular IPV. Two studies reported data on poliovirus shedding after OPV challenge dose, finding no difference between groups. No studies reported data on serum IgA levels or VAPP.
Overall completeness and applicability of evidence
The studies included in this review represent the three economic divisions of the world (i.e. low‐income, middle‐income, and high‐income countries). Furthermore, study participants comprised all relevant age groups (i.e. children and adults). The body of evidence is thus representative of the population relevant to the review question. However, the interventions used in the studies differed in the number of doses and age of administration which introduced heterogeneity in the evidence base, thereby preventing generalisation.
The included studies did not report data on all of our planned outcomes (Jaiswal 2015). No studies recorded the incidence of paralytic poliomyelitis. Furthermore, none of the included studies evaluated the impact of intradermal fractional‐dose IPV on mucosal immunity, which was projected to be one of the major advantages of intradermal administration of the vaccine. We found two ongoing studies (NCT02847026; NCT03016949), of which only one is evaluating mucosal immunity (NCT02847026). The results of these studies are much anticipated.
The available evidence does not provide any information about the skill and accuracy of those involved in injecting vaccines, and no comparison of the costs involved in developing such skills is available. The lack of information about the costs involved could indirectly affect the applicability of the evidence. In the current review, the reported outcomes also differed across studies, hence a meta‐analysis was not always possible for all outcomes. A narrative synthesis of evidence also has applicability issues.
We combined the data on seroconversion rates in meta‐analyses and found low‐ and very low‐certainty evidence of better performance of intramuscular full‐dose IPV compared to intradermal fractional‐dose IPV. During the course of this review, the WHO recommended two doses of fractional intradermal IPV over one full dose of intramuscular IPV (WHO 2016). The current review was not designed for such analysis. However, this analysis can be incorporated in future updates, since more studies may follow the WHO recommendation.
Quality of the evidence
Using the GRADE approach (Schünemann 2011), we assessed the certainty of the evidence as low and very low for seroconversion rates, as shown in Table 1. We downgraded the certainty of the evidence due to serious risk of bias and indirectness, which means that we are not confident that the estimated effect of a full dose of IPV given intramuscularly compared to the same frequency of a fractional dose of IPV given intradermally on seroconversion rates is the true effect. Although we were unable to pool the data on geometric mean antibody titres in a meta‐analysis, we have provided a narrative synthesis and rated the certainty of the evidence for this outcome as very low due to limitations in study design, indirectness in the evidence, and the heterogenous nature of the studies (Table 1). We also rated the certainty of the body of evidence for vaccine‐related adverse events as very low due to indirectness, risk of bias, and heterogeneity (Table 1). Although data on adverse events were not suitable for meta‐analysis, we conducted a narrative synthesis and applied the GRADE approach, which showed the evidence to be of very low certainty. None of the included studies reported data on paralytic poliomyelitis, hence we could not assess the certainty of the evidence for this outcome.
Potential biases in the review process
We were not able to assess publication bias due to the small number of included studies. In addition, we were unable to pool the data for most outcomes in a meta‐analysis and have therefore provided a narrative, and thus somewhat subjective, synthesis.
It was challenging to extract the data from one study, Tejeda Fuentes 2011, as it was written in Spanish.
Agreements and disagreements with other studies or reviews
This review compared intradermal and intramuscular routes of administration for IPV. A previous literature review, Nelson 2012, also discussed intradermal IPV but did not compare it with the intramuscular route of administration. Another review, Anand 2017, compared two fractional doses with one full dose of IPV and found better immunogenicity with two doses of fractional IPV given intradermally. The current systematic review compared doses, in similar frequencies, of both fractional‐dose intradermal IPV and full‐dose intramuscular IPV, whereas the review by Anand and colleagues compared cumulative responses after two primary doses of fractional‐dose IPV with singular responses after the first primary dose of intramuscular IPV. Anand 2017 had based the inferences on the post hoc analysis of four studies and found results favouring fractional‐dose IPV, whereas the current review, which was based on 13 studies, found better seroconversion rates in children receiving full‐dose intramuscular IPV compared to children receiving doses of intradermal IPV in equivalent schedules. WHO SAGE currently recommends two doses of fractional‐dose IPV intradermally over one full dose of intramuscular IPV (GPEI 2017; WHO 2016). We have not compared the effects of different dosing schedules in this review as the WHO recommendations were available only after the protocol for this review was published. However, this analysis can be incorporated in future updates of this review, since more studies may follow the WHO recommendation.
The current review found no evidence comparing mucosal immunity between the two vaccines. However, another review comparing OPV and IPV found that OPV‐primed participants receiving IPV boosters had better mucosal immune responses (Parker 2015). The authors of a narrative review, Okayasu 2017, commented on the immune responses by the two routes and found that fractional‐dose IPV was not superior.
Authors' conclusions
Implications for practice.
There is low‐ and very low‐certainty evidence suggesting better seroconversion rates with full‐dose inactivated poliovirus vaccine (IPV) delivered intramuscularly compared to fractional‐dose IPV delivered intradermally. However, antibody titres show protective levels with a fractional (one‐fifth) dose of IPV delivered intradermally, albeit in fewer participants. The current evidence does not address immunocompromised or low‐birthweight babies. The available evidence for adults is scarce. The current systematic review does not describe the costs involved in training people in the technical skills required to administer vaccines intradermally.
The current systematic review found that the complete immunisation schedules with intramuscular and intradermal IPV are both effective for producing protective antibodies, but per‐dose efficacy of fractional‐dose IPV is lower. Fractional‐dose IPV can be an alternative in places where easy access to vaccination services is available but IPV supply is scarce.
Implications for research.
Based on the currently available evidence, there is a need for studies that compare both routes of IPV administration (i.e. intradermal and intramuscular) in real‐world settings, and that take into account the uncertainties surrounding the role of the vaccinator's technical skills in fractional‐dose IPV administration. Unanswered questions include the impact on mucosal immunity, number of doses, impact on low‐birthweight or immunocompromised babies, and the effect on oral polio vaccine‐primed participants. A study designed to assess the mucosal immunity generated via both routes is ongoing, but it is important that more studies of this kind are conducted. Future research should also describe the costs involved in training people in the technical skills required for intradermal versus intramuscular administration, along with the cost of the vaccine. This will better enable consumers to draw a comprehensive overview of the route and dose of the vaccines.
Acknowledgements
Cochrane Developmental, Psychosocial and Learning Problems: Geraldine Macdonald, Co‐ordinating Editor, and Joanne Duffield, Managing Editor, for providing support at all levels of the review. Margaret Anderson, Information Specialist, for providing us with the search strategy and helping search the databases and compile the searches for inclusion in the review.
We would also like to acknowledge the efforts made by Elena Ojeda Ruiz, who extracted data from the non‐English study (Tejeda Fuentes 2011). Cochrane Task Exchange helped us get in contact with Elena.
We would like to acknowledge Ms Monika Verma, Senior Research Fellow at the Department of Pediatrics, Postgraduate Institute of Medical Education and Research, Chandigarh, India, for helping us populate the Characteristics of included studies tables. We would also like to acknowledge Nikita Jaiswal, Assistant Professor of Medical Microbiology, Maharishi Markandeshwar Institute of Medical Science and Research, Ambala, Haryana, India, for providing support during various stages of the review.
Finally, we would like to acknowledge the following reviewers for their contributions to an earlier version of this review: Ali Faisal Saleem, Aga Khan University, Pakistan; Mike Famulare, Institute for Disease Modeling, Bellevue, WA, USA; and Anna Chaimani, Paris Descartes University, Paris, France.
Appendices
Appendix 1. Search strategies
CENTRAL
Searched 1 October 2015 [286 records] Searched 18 April 2017 [319 new records] Searched 20 March 2018 [421 new records] Searched 13 February 2019 [100 new records]
#1MeSH descriptor: [Poliovirus Vaccine, Inactivated] explode all trees #2(polio* NEAR/5 (inactiv* or in‐activ* or inject* or killed)):ti,ab,kw #3(Salk or IPV* or eIPV*):ti,ab,kw #4(polio* NEAR/5 (intradermal* or intra‐dermal*)):ti,ab,kw #5(polio* NEAR/5 (intramusc* or intra‐musc*)):ti,ab,kw #6(polio* NEAR/5 fractional*):ti,ab,kw #7{or #1‐#6} #8((intimate next partner or interpersonal or inter‐personal) NEAR/5 violence):ti,ab,kw #9#7 NOT #8 in Trials
MEDLINE Ovid
Searched 21 September 2015 [612 records] Searched 19 April 2017 [734 records. Records include MEDLINE In‐Process & Other Non‐Indexed Citations, MEDLINE(R) Daily and MEDLINE(R) 1946 to Present] Searched 20 March 2018 [43 new records] Searched 13 February 2019 [23 new records]
We used the Cochrane highly sensitive search strategy to identify randomized trials in MEDLINE (Lefebvre 2011).
1. Poliovirus Vaccine, Inactivated/ 2. (polio$ adj5 (inactiv$ or in‐activ$ or inject$ or killed)).tw. 3. (Salk or IPV$ or eIPV$).tw. 4. (polio$ adj5 (intradermal$ or intra‐dermal$)).tw. 5. (polio$ adj5 (intramusc$ or intra‐musc$)).tw. 6. (polio$ adj5 fractional$).tw. 7. or/1‐6 8. ((intimate partner or interpersonal or inter‐personal) adj violence).tw. 9. 7 not 8 10. randomized controlled trial.pt. 11. controlled clinical trial.pt. 12. randomi#ed.ab. 13. placebo$.ab. 14. drug therapy.fs. 15. randomly.ab. 16. trial.ab. 17. groups.ab. 18. or/10‐17 19. exp animals/ not humans.sh. 20. 18 not 19 21. 9 and 20
MEDLINE In‐Process & Other Non‐Indexed Citations Ovid
Searched 1 October 2015 [35 records] Searched 19 April 2017 [Records included with MEDLINE total] Searched 21 March 2018 [76 new records] Searched 13 February 2019 [22 new records]
1 (polio$ adj5 (inactiv$ or in‐activ$ or inject$ or killed)).tw,kf. 2 (Salk or IPV$ or eIPV$).tw,kf. 3 (polio$ adj5 (intradermal$ or intra‐dermal$)).tw,kf. 4 (polio$ adj5 (intramusc$ or intra‐musc$)).tw,kf. 5 (polio$ adj5 fractional$).tw,kf. 6 or/1‐5 7 ((intimate partner or interpersonal or inter‐personal) adj violence).tw,kf. 8 6 not 7 9 (random$ or control$ or group$ or cluster$ or placebo$ or trial$ or assign$ or prospectiv$ or meta‐analysis or systematic review or longitudinal$).tw,kf. 10 8 and 9
MEDLINE Epub Ahead of Print Ovid
Searched 21 March 2018 [23 records] Searched 13 February 2019 [25 records]
1 (polio$ adj5 (inactiv$ or in‐activ$ or inject$ or killed)).tw,kf. 2 (Salk or IPV$ or eIPV$).tw,kf. 3 (polio$ adj5 (intradermal$ or intra‐dermal$)).tw,kf. 4 (polio$ adj5 (intramusc$ or intra‐musc$)).tw,kf. 5 (polio$ adj5 fractional$).tw,kf. 6 or/1‐5 7 ((intimate partner or interpersonal or inter‐personal) adj violence).tw,kf. 8 6 not 7 9 (random$ or control$ or group$ or cluster$ or placebo$ or trial$ or assign$ or prospectiv$ or meta‐analysis or systematic review or longitudinal$).tw,kf. 10 8 and 9
Embase Ovid
Searched 11 September 2015 [973 records] searched 27 April 2017 [150 new records] Searched 21 March 2018 [68 new records] Searched 13 February 2019 [78 new records]
1 poliomyelitis vaccine/ 2 (polio$ adj5 (inactiv$ or in‐activ$ or inject$ or killed)).tw. 3 inactivated vaccine/ 4 poliomyelitis/ 5 3 and 4 6 1 or 2 or 5 7 (Salk or IPV$ or eIPV$).tw. 8 (polio$ adj5 (intradermal$ or intra‐dermal$)).tw. 9 (polio$ adj5 (intramusc$ or intra‐musc$)).tw. 10 (polio$ adj5 fractional$).tw. 11 or/6‐10 12 ((intimate partner or interpersonal or inter‐personal) adj violence).tw. 13 11 not 12 14 Randomized controlled trial/ 15 controlled clinical trial/ 16 Single blind procedure/ 17 Double blind procedure/ 18 triple blind procedure/ 19 Crossover procedure/ 20 (crossover or cross‐over).tw. 21 ((singl$ or doubl$ or tripl$ or trebl$) adj1 (blind$ or mask$)).tw. 22 Placebo/ 23 placebo.tw. 24 prospective.tw. 25 factorial$.tw. 26 random$.tw. 27 assign$.ab. 28 allocat$.tw. 29 volunteer$.ab. 30 or/14‐29 31 13 and 30
Science Citation Index Web of Science
Searched 14 September 2015 [693 records] Searched 27 April 2017 [111 new records] Searched 21 March 2018 [73 new records] Searched 14 February 2019 [56 new records]
#10 #9 AND #8 DocType=All document types; Language=All languages; #9 TS=(random* or trial* or control*or placebo* or blind*) DocType=All document types; Language=All languages; #8 #6 not #7 DocType=All document types; Language=All languages; #7 TS=(("intimate partner" or interpersonal or inter‐personal) NEAR/2 violence) DocType=All document types; Language=All languages; #6 #5 OR #4 OR #3 OR #2 OR #1 DocType=All document types; Language=All languages; #5 TS= (polio* NEAR/5 fractional*) DocType=All document types; Language=All languages; #4 TS=(polio* NEAR/5 (intramusc* or intra‐musc*)) DocType=All document types; Language=All languages; #3 TS=(polio* NEAR/5 (intradermal* or intra‐dermal*)) DocType=All document types; Language=All languages; #2 TS=(Salk or IPV* or eIPV*) DocType=All document types; Language=All languages; #1 TS=(polio* NEAR/5 (inactiv* or in‐activ* or inject* or killed)) DocType=All document types; Language=All languages;
Conference Proceedings Citation Index ‐ Science Web of Science
Searched 14 September 2015 [39 records] Searched 27 April 2017 [14 new records] Searched 21 March 2018 [5 new records] Searched 14 February 2019 [0 new records]
#10 #9 AND #8 DocType=All document types; Language=All languages; #9 TS=(random* or trial* or control*or placebo* or blind*) DocType=All document types; Language=All languages; #8 #6 not #7 DocType=All document types; Language=All languages; #7 TS=(("intimate partner" or interpersonal or inter‐personal) NEAR/2 violence) DocType=All document types; Language=All languages; #6 #5 OR #4 OR #3 OR #2 OR #1 DocType=All document types; Language=All languages; #5 TS= (polio* NEAR/5 fractional*) DocType=All document types; Language=All languages; #4 TS=(polio* NEAR/5 (intramusc* or intra‐musc*)) DocType=All document types; Language=All languages; #3 TS=(polio* NEAR/5 (intradermal* or intra‐dermal*)) DocType=All document types; Language=All languages; #2 TS=(Salk or IPV* or eIPV*) DocType=All document types; Language=All languages; #1 TS=(polio* NEAR/5 (inactiv* or in‐activ* or inject* or killed)) DocType=All document types; Language=All languages;
IndMED (http://indmed.nic.in/)
Searched 13 September 2015 [39 records] Searched 27 April 2017 [39 records] Searched 21 March 2018 [0 new records] Searched 14 February 2019 [0 new records]
We searched Indmed using the Advanced search, without applying any limits, and used the default setting 'Anywhere'.
Poliomyelitis AND vaccine AND inactivated
We also conducted a simple search using key words "polio", "vaccine", "intradermal", intramuscular".
CDSR
Searched 13 September 2015 [4 records] Searched 18 April 2017 [5 records] Searched 20 March 2018 [3 records] Searched 13 February 2019 [0 new records]
#1MeSH descriptor: [Poliovirus Vaccine, Inactivated] explode all trees #2(polio* NEAR/5 (inactiv* or in‐activ* or inject* or killed)):ti,ab,kw #3(Salk or IPV* or eIPV*):ti,ab,kw #4(polio* NEAR/5 (intradermal* or intra‐dermal*)):ti,ab,kw #5(polio* NEAR/5 (intramusc* or intra‐musc*)):ti,ab,kw #6(polio* NEAR/5 fractional*):ti,ab,kw #7{or #1‐#6} #8((intimate next partner or interpersonal or inter‐personal) NEAR/5 violence):ti,ab,kw #9#7 NOT #8 in Cochrane Reviews, Cochrane Protocols
DARE
Searched 27 April 2017 [4 records]. Database ceased adding new records in 2015.
#1[mh "Poliovirus Vaccine, Inactivated"] #2(polio* near/5 (inactiv* or in‐activ* or inject* or killed)) #3(Salk or IPV* or eIPV*) #4(polio* near/5 (intradermal* or intra‐dermal*)) #5(polio* near/5 (intramusc* or intra‐musc*)) #6(polio* near/5 fractional*) #7{or #1‐#6} #8(("intimate partner" or interpersonal or inter‐personal) next violen*) #9#7 not #8 in Other Reviews
LILACS (http://lilacs.bvsalud.org/en/)
Searched 14 September 2015 [3 records] Searched 27 April 2017 [2 records] Searched 21 March 2018 [0 new records] Searched 14 February 2019 [0 new records]
(mh:("poliovirus vaccine, inactivated")) OR (tw:((polio* AND (intramusc* OR fractional OR intradermal* OR inactiv* OR in‐activ* OR inject* OR killed)) OR (salk OR ipv OR eipv))) AND (instance:"regional") AND ( db:("LILACS") AND type_of_study:("clinical_trials") AND limit:("humans"))
TRIP database (www.tripdatabase.com)
Searched 13 September 2015 [1476 records] Searched 21 March 2018 [3 records]. Search limited to primary research Searched 14 February 2019 [12 records]. Search limited to primary research
polio AND vaccine AND (intramuscular OR intradermal)
WHOLIS
Accessed at dosei.who.int on 14 September 2015 [143 records]
subject "POLIOVIRUS VACCINES OR POLIOVIRUS VACCINE INACTIVATED" OR words or phrase "polio$ OR IPV OR eIPV OR SALK" AND words or phrase "RANDOM$ OR TRIAL$ OR PLACEBO$
Accessed at bvsalud.org on 27 April 2017 [1 record] and 21 March 2018 [0 new records]
(mh:(("poliovirus vaccine, inactivated"))) OR (tw:((polio* AND (intramusc* OR fractional OR intradermal* OR inactiv* OR in‐activ* OR inject* OR killed)) )) OR (tw:((salk OR ipv OR eipv))) AND (instance:"regional") AND ( db:("WHOLIS") AND year_cluster:("2015" OR "2016" OR "2017")) AND (instance:"regional")
Accessed at kohahq.searo.who.int on 14 February 2019 [0 new records]
Search on :POLIOVIRUS VACCINES OR POLIOVIRUS VACCINE INACTIVATED [Subject descriptor] or polio* OR IPV OR eIPV OR SALK [kEYWords] and RANDOM* OR TRIAL* OR PLACEBO* [kEYWords]
ClinicalTrials.gov (https://clinicaltrials.gov/)
Searched 13 September 2015 [248 records]. Searched all available years Searched 18 April 2017 [279 records]. Searched all available years Searched 21 March 2018 [8 records]. Searched first posted from 18 April 2017 to 21 March 2018 Searched 14 February 2019 [12 records]. Searched first posted from 21 March 2018 to 14 February 2019
All studies
Disease: poliomyelitis
Others: poliovirus vaccine OR poliomyelitis vaccine
WHO ICTRP (www.who.int/ictrp/en/)
Searched 13 September 2015 [68 records]. Searched all available years Searched 18 April 2017 [87 records]. Searched all available years Searched 21 March 2018 [16 records]. Searched first posted from 1 January 2017 to 21 March 2018 Searched 14 February 2019 [8 records]. Searched first posted from 21 March 2018 to 14 February 2019
We searched the basic search option using the search term " Polio vaccine".
We also searched the advanced search option using the following search terms: Title| Inactivated Polio vaccine Condition| Polio* Intervention|(intradermal OR intramuscular)
Appendix 2. Methods archived for use in future updates of this review
| Section | Methods |
| Measures of treatment effect |
Continous data We will calculate mean differences (MD) with 95% confidence intervals (CIs) for continuous outcome data measured on the same scale (Deeks 2011). For continuous data measured on different scales, we will compute standardised mean differences (SMD) with 95% CIs. |
| Unit of analysis issues |
Cluster‐randomised trials If possible, we will combine the adjusted measures of effects of cluster‐randomised trials with the results of non‐cluster‐randomised trials using inverse variance methods. We will contact the study authors to request individual participant data to calculate an estimate of the intracluster correlation coefficient (ICC), or use an ICC estimate from a comparable study if the ICC is not mentioned in the published report. If this is not possible, we will exclude those studies from the meta‐analysis, but include them in the narrative synthesis. |
| Dealing with missing data | We will impute missing continuous data using standard deviations (SDs) from other studies or by computing SDs using standard errors and probability values. We will calculate and impute the missing SDs for changes from baseline using a correlation coefficient, which describes how similar the baseline and final measurements were across participants (Higgins 2011d). The correlation coefficient in the group (experimental or control), Corr, can be calculated as: Corr = (SD2baseline + SD2final ‐ SD2change) / 2 × SDbaseline × SDfinal. We will also contact the authors of any future trials for which only final values were reported. |
| Assessment of reporting biases | Should we include and combine a sufficient number of studies (n = 10), we will assess reporting bias by constructing and visually inspecting funnel plots (constructed by plotting trial effects against inverse standard errors of effects). We will interpret these plots within the context of study sizes and methodological rigour, i.e. whether the asymmetrical funnel plot is due to the effect of poorly conducted small studies, reporting biases such as selective outcome reporting or location bias, or genuine heterogeneity (Sterne 2011). |
| Subgroup analysis and investigation of heterogeneity | We will undertake subgroup analysis based on age at first vaccination and HIV status. We will conduct the following additional subgroup analyses for children: age of children (under 5 years of age versus 5 years of age and over); feeding practices (exclusively breastfed versus not exclusively breastfed); and birthweight (≥ 2.5 kg versus < 2.5 kg). |
| Sensitivity analysis | We will perform a sensitivity analysis to compare the results of analyses with imputed missing data to those without imputed missing data. We will also perform a sensitivity analysis based on risk of bias, i.e. we will exclude studies at high risk and repeat the analysis (Ciapponi 2014). For the primary analysis, we will include all studies that fulfil the inclusion criteria (Criteria for considering studies for this review), but we will perform a sensitivity analysis for trials with higher attrition (> 20%) and those with differences in methodology. We will examine the percentages of dropouts overall in each trial and randomisation arm and evaluate whether an intention‐to‐treat (ITT) analysis was performed or could be performed from the published information. Where possible, we will conduct ITT analyses (treating the missing data as unsuccessful events). |
See Jaiswal 2015.
Data and analyses
Comparison 1. Intradermal fractional‐dose IPV versus intramuscular IPV: children (random‐effects model) (primary outcome).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seroconversion rate after a single primary dose: type 1 poliovirus | 6 | 2570 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.22, 0.41] |
| 1.1 Low‐income country | 1 | 700 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.15, 1.70] |
| 1.2 Middle‐income country | 4 | 1502 | Odds Ratio (M‐H, Random, 95% CI) | 0.25 [0.17, 0.37] |
| 1.3 High‐income country | 1 | 368 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.23, 0.75] |
| 2 Seroconversion rate after a single primary dose: type 2 poliovirus | 6 | 2567 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.31, 0.60] |
| 2.1 Low‐income country | 1 | 700 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.05, 5.65] |
| 2.2 Middle‐income country | 4 | 1502 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.23, 0.69] |
| 2.3 High‐income country | 1 | 365 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.26, 0.71] |
| 3 Seroconversion rate after a single primary dose: type 3 poliovirus | 6 | 2571 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.12, 0.30] |
| 3.1 Low‐income country | 1 | 700 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.18, 0.84] |
| 3.2 Middle‐income country | 4 | 1502 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.10, 0.33] |
| 3.3 High‐income country | 1 | 369 | Odds Ratio (M‐H, Random, 95% CI) | 0.12 [0.07, 0.22] |
| 4 Seroconversion rate after 2 primary doses: type 1 poliovirus | 3 | 981 | Odds Ratio (M‐H, Random, 95% CI) | 0.23 [0.16, 0.33] |
| 4.1 Low‐income country | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Middle‐income country | 2 | 674 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.07, 0.47] |
| 4.3 High‐income country | 1 | 307 | Odds Ratio (M‐H, Random, 95% CI) | 0.27 [0.15, 0.50] |
| 5 Seroconversion rate after 2 primary doses: type 2 poliovirus | 3 | 853 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.28, 0.60] |
| 5.1 Low‐income country | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Middle‐income country | 2 | 576 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.29, 0.77] |
| 5.3 High‐income country | 1 | 277 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.18, 0.60] |
| 6 Seroconversion rate after 2 primary doses: type 3 poliovirus | 3 | 855 | Odds Ratio (M‐H, Random, 95% CI) | 0.12 [0.07, 0.22] |
| 6.1 Low‐income country | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Middle‐income country | 2 | 585 | Odds Ratio (M‐H, Random, 95% CI) | 0.08 [0.04, 0.16] |
| 6.3 High‐income country | 1 | 270 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.09, 0.43] |
| 7 Seroconversion rate after 3 primary doses: type 1 poliovirus | 3 | 973 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.07, 3.15] |
| 7.1 Low‐income country | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Middle‐income country | 2 | 600 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.06, 9.59] |
| 7.3 High‐income country | 1 | 373 | Odds Ratio (M‐H, Random, 95% CI) | 0.09 [0.00, 1.62] |
| 8 Seroconversion rate after 3 primary doses: type 2 poliovirus | 3 | 973 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.19, 0.63] |
| 8.1 Low‐income country | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Middle‐income country | 2 | 600 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.20, 0.70] |
| 8.3 High‐income country | 1 | 373 | Odds Ratio (M‐H, Random, 95% CI) | 0.06 [0.00, 0.99] |
| 9 Seroconversion rate after 3 primary doses: type 3 poliovirus | 3 | 973 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 2.58] |
| 9.1 Low‐income country | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Middle‐income country | 2 | 600 | Odds Ratio (M‐H, Random, 95% CI) | 0.21 [0.01, 7.07] |
| 9.3 High‐income country | 1 | 373 | Odds Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.05] |
Comparison 2. Intradermal fractional‐dose IPV versus intramuscular IPV: children (fixed‐effect model) (primary outcome).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seroconversion rate after a single primary dose: type 1 poliovirus | 6 | 2570 | Odds Ratio (IV, Fixed, 95% CI) | 0.30 [0.22, 0.41] |
| 1.1 Low‐income country | 1 | 700 | Odds Ratio (IV, Fixed, 95% CI) | 0.51 [0.15, 1.70] |
| 1.2 Middle‐income country | 4 | 1502 | Odds Ratio (IV, Fixed, 95% CI) | 0.25 [0.17, 0.37] |
| 1.3 High‐income country | 1 | 368 | Odds Ratio (IV, Fixed, 95% CI) | 0.41 [0.23, 0.75] |
| 2 Seroconversion rate after a single primary dose: type 2 poliovirus | 6 | 2567 | Odds Ratio (IV, Fixed, 95% CI) | 0.44 [0.34, 0.57] |
| 2.1 Low‐income country | 1 | 700 | Odds Ratio (IV, Fixed, 95% CI) | 0.51 [0.05, 5.65] |
| 2.2 Middle‐income country | 4 | 1502 | Odds Ratio (IV, Fixed, 95% CI) | 0.44 [0.33, 0.60] |
| 2.3 High‐income country | 1 | 365 | Odds Ratio (IV, Fixed, 95% CI) | 0.43 [0.26, 0.71] |
| 3 Seroconversion rate after a single primary dose: type 3 poliovirus | 6 | 2571 | Odds Ratio (IV, Fixed, 95% CI) | 0.18 [0.14, 0.24] |
| 3.1 Low‐income country | 1 | 700 | Odds Ratio (IV, Fixed, 95% CI) | 0.38 [0.18, 0.84] |
| 3.2 Middle‐income country | 4 | 1502 | Odds Ratio (IV, Fixed, 95% CI) | 0.18 [0.13, 0.25] |
| 3.3 High‐income country | 1 | 369 | Odds Ratio (IV, Fixed, 95% CI) | 0.12 [0.07, 0.22] |
| 4 Seroconversion rate after 2 primary doses: type 1 poliovirus | 3 | 981 | Odds Ratio (IV, Fixed, 95% CI) | 0.23 [0.16, 0.33] |
| 4.1 Low‐income country | 0 | 0 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Middle‐income country | 2 | 674 | Odds Ratio (IV, Fixed, 95% CI) | 0.21 [0.14, 0.33] |
| 4.3 High‐income country | 1 | 307 | Odds Ratio (IV, Fixed, 95% CI) | 0.27 [0.15, 0.50] |
| 5 Seroconversion rate after 2 primary doses: type 2 poliovirus | 3 | 853 | Odds Ratio (IV, Fixed, 95% CI) | 0.41 [0.28, 0.60] |
| 5.1 Low‐income country | 0 | 0 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Middle‐income country | 2 | 576 | Odds Ratio (IV, Fixed, 95% CI) | 0.47 [0.29, 0.77] |
| 5.3 High‐income country | 1 | 277 | Odds Ratio (IV, Fixed, 95% CI) | 0.32 [0.18, 0.60] |
| 6 Seroconversion rate after 2 primary doses: type 3 poliovirus | 3 | 855 | Odds Ratio (IV, Fixed, 95% CI) | 0.12 [0.07, 0.19] |
| 6.1 Low‐income country | 0 | 0 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Middle‐income country | 2 | 585 | Odds Ratio (IV, Fixed, 95% CI) | 0.08 [0.04, 0.16] |
| 6.3 High‐income country | 1 | 270 | Odds Ratio (IV, Fixed, 95% CI) | 0.19 [0.09, 0.43] |
| 7 Seroconversion rate after 3 primary doses: type 1 poliovirus | 3 | 973 | Odds Ratio (IV, Fixed, 95% CI) | 0.27 [0.18, 0.43] |
| 7.1 Low‐income country | 0 | 0 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Middle‐income country | 2 | 600 | Odds Ratio (IV, Fixed, 95% CI) | 0.28 [0.18, 0.44] |
| 7.3 High‐income country | 1 | 373 | Odds Ratio (IV, Fixed, 95% CI) | 0.09 [0.00, 1.62] |
| 8 Seroconversion rate after 3 primary doses: type 2 poliovirus | 3 | 973 | Odds Ratio (IV, Fixed, 95% CI) | 0.34 [0.19, 0.63] |
| 8.1 Low‐income country | 0 | 0 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Middle‐income country | 2 | 600 | Odds Ratio (IV, Fixed, 95% CI) | 0.38 [0.20, 0.70] |
| 8.3 High‐income country | 1 | 373 | Odds Ratio (IV, Fixed, 95% CI) | 0.06 [0.00, 0.99] |
| 9 Seroconversion rate after 3 primary doses: type 3 poliovirus | 3 | 973 | Odds Ratio (IV, Fixed, 95% CI) | 0.22 [0.10, 0.48] |
| 9.1 Low‐income country | 0 | 0 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Middle‐income country | 2 | 600 | Odds Ratio (IV, Fixed, 95% CI) | 0.23 [0.10, 0.52] |
| 9.3 High‐income country | 1 | 373 | Odds Ratio (IV, Fixed, 95% CI) | 0.11 [0.01, 2.05] |
Comparison 3. Intradermal fractional‐dose IPV versus intramuscular IPV: children (random‐effects model) (secondary outcome).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reciprocal antibody titres: seroprevalence for antipoliovirus antibodies type 1 | 3 | 1661 | Odds Ratio (M‐H, Random, 95% CI) | 1.42 [0.59, 3.39] |
| 2 Reciprocal antibody titres; seroprevalence for antipoliovirus antibodies type 2 | 3 | 1659 | Odds Ratio (M‐H, Random, 95% CI) | 3.10 [0.50, 19.44] |
| 3 Reciprocal antibody titres: seroprevalence for antipoliovirus antibodies type 3 | 3 | 1659 | Odds Ratio (M‐H, Random, 95% CI) | 2.94 [1.44, 6.00] |
Comparison 4. Intradermal fractional‐dose IPV versus intramuscular IPV: children (fixed‐effect model) (secondary outcome).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reciprocal antibody titres: seroprevalence for antipoliovirus antibodies type 1 | 3 | 1661 | Odds Ratio (IV, Fixed, 95% CI) | 1.42 [0.59, 3.39] |
| 2 Reciprocal antibody titres: seroprevalence for antipoliovirus antibodies type 2 | 3 | 1659 | Odds Ratio (IV, Fixed, 95% CI) | 3.10 [0.50, 19.44] |
| 3 Reciprocal antibody titres: seroprevalence for antipoliovirus antibodies type 3 | 3 | 1659 | Odds Ratio (IV, Fixed, 95% CI) | 2.94 [1.44, 6.00] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anand 2015.
| Methods |
Study design: open‐label, multi‐arm, randomised controlled trial Location: Mirpur, Bangladesh Duration: 12 months
|
|
| Participants |
Inclusion criteria: "Infants were recruited at age 6–7 weeks (42–51 days), if the parents were willing to participate, comply with study procedures, and provide written informed consent" (quote) Exclusion criteria: "Exclusion criteria included (1) receipt of any polio vaccine before enrolment; (2) diagnosis or suspicion of immunodeficiency or a bleeding disorder; (3) known allergy to polio vaccines or constituents; (4) any acute illness such as vomiting, diarrhoea or infection immediately before enrolment; and (5) an infant who was part of a multiple birth." Also, "Enrolled participants were withdrawn from the study if requested by their parents or if they received polio vaccine outside of the study." (quote) Sample size: 975 total. 326 participants included in the review, as follows.
Number of withdrawals/loss to follow‐up: 53 out of 975; 18 of 326 included in this review Age:
Sex (male:female):
|
|
| Interventions |
Intervention (n = 164): fractional‐dose (0.1 mL) of IPV administered* intradermally using NanoPass Microjet 600 (MJ600), a microneedle device with 3 microneedles (0.6 mm in length) that attaches to intradermal syringe Comparator (n = 162): intramuscular IPV (0.5 mL) administered* using standard needle and syringe *IPV and fractional‐dose IPV were administered in the anterolateral aspect of thigh, opposite the site used for routine immunisations. The other 3 arms of the trial not included in this review were:
|
|
| Outcomes |
Timing of outcome assessment: immunogenicity outcomes were reported at 14 weeks, 15 weeks, and 18 weeks. |
|
| Notes |
Funding source(s): Centers for Disease Control and Prevention Conflict(s) of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: infants randomly assigned to 1 of 5 arms using a block randomisation scheme that was 65 blocks in size; each block had a size of 18 |
| Allocation concealment (selection bias) | Low risk | Comment: allocation ratio was 4:4:3:3:4; allocations were random |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: published paper states use of ITT approach for primary endpoint |
| Selective reporting (reporting bias) | Low risk | Comment: reported on all outcomes |
| Other bias | Low risk | Comment: not funded by any drug company |
Cadorna‐Carlos 2012.
| Methods |
Study design: open‐label, parallel‐design randomised controlled trial Location: Manila, the Philippines Duration: 10 months (6 months primary series and 4 months of booster series) The enrolment of participants and recording of outcomes were as follows.
|
|
| Participants |
Inclusion criteria: healthy infants Exclusion criteria: "Participants were excluded either at the time of screening (0 to 7 days after birth, at which time the study was explained) or at the first vaccination (6 weeks of age) if they had illnesses or health issues (established by clinical examination and/ or medical history), which could have interfered with the study, or a congenital or acquired immunodeficiency, or human immunodeficiency virus, hepatitis B antigen, or hepatitis C seropositivity." (quote) Sample size: 236
Number of withdrawals/loss to follow‐up: for the primary immunisation series, there were 6 withdrawals/discontinuations, 3 in the intervention group and 3 in the comparator group. For the booster series, there were again 6 withdrawals, 2 in the intervention group and 4 in the comparator group. Age: mean age was 45.5 days Sex (male:female):
|
|
| Interventions |
Intervention (n = 118): one‐fifth dose of IPV given through intradermal route at 6, 10, and 14 weeks of age. It was administered in the right upper arm with a syringe mounted with a 13‐millimetre to 30‐gauge needle. Comparator (n = 118): full dose of IPV given through intramuscular route at 6, 10, and 14 weeks of age. It was given in the anterolateral aspect of the right thigh with a syringe fitted with a 16‐millimetre to 25‐gauge needle. |
|
| Outcomes |
Timing of outcome assessment: safety was assessed 7 days following the vaccination, and immunogenicity was recorded within 1 month of vaccination |
|
| Notes |
Funding source(s): Sanofi Pasteur Conflict(s) of interest: the principal investigator received funds for attending congress and also previously received funds for several clinical studies from Sanofi Pasteur. 1 author is an employee of Sanofi Pasteur. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: methods of randomisation not mentioned or explained |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition rate less than 5%, and reasons for attrition provided |
| Selective reporting (reporting bias) | Low risk | Comment: reported on all outcomes in Results section |
| Other bias | High risk | Comment: funded by drug company |
Clarke 2016.
| Methods |
Study design: randomised, phase 4, non‐inferiority trial Location: peri‐urban government clinics, west Gambia Duration: 11 months
|
|
| Participants |
Inclusion criteria: "To be eligible, infants had to be aged 9–10 months (inclusive); to have received at least three doses of trivalent OPV up to 28 days before recruitment; to have not received any measles, rubella, yellow fever, or inactivated poliovirus vaccines; and to be clinically healthy with no indications of clinically significant chronic health problems" (quote) Exclusion criteria: any deviation from the inclusion criteria resulted in exclusion Sample size: 1504; 754 included in this review Number of withdrawals/loss to follow‐up: 82; 54 of 754 Age 9.6 (range = 9.0 to 11.0) months Sex (males:females): 49:51% |
|
| Interventions | The trial has 8 arms, as follows.
Intervention (n = 378): we used arms 6 and 8 as the intervention in this review. Comparator (n = 376): we used arms 2 and 3 as the comparator in this review. We used the arms where IPV (ID or IM) was administered alone. |
|
| Outcomes |
Timing of outcome assessment: 4 to 6 weeks postvaccination |
|
| Notes |
Funding source(s): Bill & Melinda Gates Foundation Conflict(s) of interest: 3 authors have received grants from the vaccine companies (GSK, Pfizer, and Sanofi Pasteur). 1 author was an employee of the Bill & Melinda Gates Foundation, and 1 author has received grants from the same in past. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: stratified block randomisation, with a block size of 32; stratification based on sex; random sequences electronically generated |
| Allocation concealment (selection bias) | Low risk | Comment: sealed envelopes with sequential numbers used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: parents were unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: laboratory assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 7% attrition rate (< 15%) and also performed ITT analysis |
| Selective reporting (reporting bias) | Low risk | Comment: study reported on all outcomes described in protocol |
| Other bias | Low risk | Comment: funded by the Bill & Melinda Gates Foundation, which is not a vaccine manufacturer or drug company |
Estívariz 2012.
| Methods |
Study design: unblinded, multi‐arm randomised controlled trial Location: Moradabad district, Uttar Pradesh, India; sites were in a high‐risk area of Moradabad for polio, based on low coverage of routine poliovirus vaccines and recent reports of poliovirus circulation Duration: 11 months
|
|
| Participants |
Inclusion criteria: "For inclusion, participants had to be 6–9 months old at enrolment; live within 30 km of a study site; and have no immunodeficiency, chronic disease, contraindication for venepuncture, severe malnutrition, or acute infection." (quote) Exclusion criteria: not reported Sample size: 1002, data for which 602 were included in the review Number of withdrawals/loss to follow‐up: 133; 82 (from 602) were loss to follow‐up/withdrawals Age: 6 to 9 months Sex (male:female): 430:572 |
|
| Interventions | The study had 5 arms, of which data from 3 were considered in this review. Intervention (n = 202): 1 fractional dose (0.1 mL or 1/5 of a dose) of IPV by GSK (group 1) Control (n = 800):
*We combined the data from groups 2 and 3 into a single group. **We did not consider the following groups in this review: group 4 (1 dose of mOPV type 1 by Panacea (potency 10^6.15 TCID50 in 0.1 mL)) and group 5 (1 dose of mOPV type 1 (potency 10^6.8 TCID50 in 0.1 mL) by Sanofi Pasteur). |
|
| Outcomes |
Timing of outcome assessment: 0, 7, and 28th day after vaccination |
|
| Notes |
Funding source(s):
Conflict(s) of interest: 2 authors were employees of Panacea Biotec. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐generated block randomisation, in blocks of 4 |
| Allocation concealment (selection bias) | Low risk | Quote: "The infant was assigned a study number, the parent picked a opaque‐sealed envelope from a basket for allocation to a study group" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: unblinded study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: unblinded study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 869 out of 1002 children completed the study. Data for 602 participants were included in this review, 520 of which completed the study. Attrition rate was less than 15% and reasons provided. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Although the protocol describes the measurements of IgA and IgM through ELISA, there is no mention of them in the published paper. |
| Other bias | Unclear risk | Comment: study author team includes companies providing the vaccine |
Gamage 2018.
| Methods |
Study design: randomised controlled trial Location: Kalutara District, Sri Lanka Duration: 2 months
|
|
| Participants |
Inclusion criteria: "Children between 10 and 12 years of age residing in Kalutara District of Sri Lanka were eligible for enrolment." (quote) Exclusion criteria: "The exclusion criteria were contraindication for venipuncture, sick child requiring hospitalization for acute or chronic condition, and diagnosis or suspicion of congenital immunodeficiency disorder in the subject or an immediate family member." (quote) Sample size: 304; 203 included in this review Number of withdrawals/loss to follow‐up: none Age: median age = 11 (interquartile range = 10 to 11.5) years Sex (male:female): 148:156 |
|
| Interventions |
Intervention (n = 101): "Fractional one dose (0.1 ml) of inactivated poliovirus vaccine administered intradermally" (quote) Comparator (n = 102): "Full one dose (0.5 ml) of inactivated poliovirus vaccine administered intramuscularly" (quote) We did not include the third arm with no IPV (n = 101) in the review. |
|
| Outcomes |
Primary outcome: "Difference in proportion of children excreting vaccine polioviruses (by serotype) between arms among those who serologically respond to IPV measured by isolation of poliovirus in stool" (quote) Secondary outcome: "Difference in duration of poliovirus excretion between arms measured by isolation of poliovirus in stool" (quote) Timing of outcome assessment: after vaccination |
|
| Notes |
Funding source(s):
Conflict(s) of interest: no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: details of randomisation not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Comment: details not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition in the study was 2% |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes mentioned in the protocol reported in the publication |
| Other bias | Low risk | Comment: funded by the World Health Organization and Rotary International |
Mohammed 2010.
| Methods |
Study design: multicentric randomised controlled trial Location: Oman Duration: 10 months
|
|
| Participants |
Inclusion criteria: "Newborns were eligible for participation if oral or written informed consent was obtained from the parents, if the infant’s Apgar score at 5 minutes was 9 or 10, if the infant’s birth weight was at least 2.5 kg, if the infant was healthy (not requiring hospitalization), and if the family was not planning to move out of the study area during the study period." (quote) Exclusion criteria: "Infants were excluded if a diagnosis or suspicion of immunodeficiency disorder in the infant or a family member was revealed." (quote) Sample size: 400; 373 completed the study
Number of withdrawals/loss to follow‐up: 27 Age: newborns at birth Sex (male:female):
|
|
| Interventions |
Intervention (n = 200): intradermal IPV fractional dose (one‐fifth or 0.1 mL) given at 2, 4, and 6 months of age using a needle‐free device (Biojector) Comparator (n = 200): intramuscular full‐dose IPV given at 2, 4, and 6 months using an auto‐disable syringe |
|
| Outcomes |
Timing of outcome assessment: immunogenicity outcomes were assessed at the age of 2, 4, 6, and 7 months. Timing for adverse events assessment was not stated. |
|
| Notes |
Funding source(s):
Conflict(s) of interest: no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no details on randomisation methods |
| Allocation concealment (selection bias) | High risk | Comment: parents aware of children's vaccine assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: laboratory unaware of vaccination group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 7% of participants excluded from final assessment of immunogenicity |
| Selective reporting (reporting bias) | Low risk | Comment: reported on all outcomes |
| Other bias | Low risk | Comment: none of the study authors are from the company that provided the vaccine |
Resik 2010.
| Methods |
Study design: randomised controlled trial involving 3 maternity hospitals and 15 vaccination sites in 5 districts Location: Camagüey Province, Cuba Duration: 8 months
|
|
| Participants |
Inclusion criteria: "Newborns were eligible for participation and subjects were eligible to continue if (1) informed consent was obtained, (2) the infant’s Apgar score was 9 at 5 min, (3) the infant’s birth weight was 12.5 kg, (4) the infant was healthy at 6 weeks on the basis of a medical examination and was being breast fed, and (5) the infant’s weight to height ratio was above 10th percentile on the growth chart" (quote) Exclusion criteria: "(1) suffering from an acute or chronic disease, (2) temperature 137.0C at vaccination visit or in the last 24 hr before visit, (3) given a diagnosis of seizure illness, (4) on immunosuppressive therapy or immunostimulant therapy (during the previous month), and (5) given a diagnosis of or suspected to have severe allergic or immunodeficiency disorder. In addition, if a subject fell from above 10th percentile to below 10th percentile weight‐to‐height ratio on the growth curve during the study period, then the subject was also excluded." (quote) Sample size: 471
Number of withdrawals/loss to follow‐up: 131
Age: newborn Sex (male:female):
|
|
| Interventions |
Intervention (n = 235): primary doses of fractional intradermal IPV (0.1 mL or one‐fifth) with full‐dose intramuscular IPV (0.5 mL) given at 6, 10, and 14 weeks of age Comparator (n = 236): intradermal IPV administered by a needle‐free device (Biojector 2000) and intramuscular full‐dose IPV administered using needle and syringe |
|
| Outcomes |
Timing of outcome assessment: immunogenicity outcomes were assessed at 6, 10, 14, and 18 weeks of age; adverse events were recorded at 1 hour, 24, 48, and 72 hours, and until 7 days after vaccination |
|
| Notes |
Funding source(s):
Conflict(s) of interest: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: methods of randomisation not mentioned clearly in published paper |
| Allocation concealment (selection bias) | Unclear risk | Comment: methods not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: laboratory investigators blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: attrition rates higher than 15% (intradermal IPV = 21%, intramuscular IPV = 25%; overall attrition = 22.7%), and ITT analysis not performed |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes described in protocol were reported in published paper |
| Other bias | Low risk | Comment: none |
Resik 2013.
| Methods |
Study design: multicentric (4 districts and 13 polyclinics), unblinded intervention, randomised controlled trial Location: Cuba Duration: not mentioned
|
|
| Participants |
Inclusion criteria: "Infants born during either March or April 2009 in the participating health center catchment areas were eligible for participation." "Participation was contingent on provision of informed consent by the parent or guardian, an Apgar score of 9 or more at 5 minutes (according to a review of records), a birth weight of 2.5 kg or more (according to records), a medical examination suggesting that the infant was healthy and breast‐fed, and a weight for height above the 10th percentile on a growth chart at the age of 4 months" (quotes) Exclusion criteria: "If an infant’s weight for height fell below the 10th percentile on the growth curve during the study period, the infant was withdrawn from the study." (quote) Sample size: 310
Number of withdrawals/loss to follow‐up: none Age: 4 to 5 months at enrolment Sex (male:female):
|
|
| Interventions |
Intervention (n = 157): 2 doses of fractional (0.1 mL or one‐fifth) IPV delivered intradermally at 4 and 8 months of age Comparator (n = 153): 2 doses of full‐dose (0.5 mL) IPV delivered intramuscularly at 4 and 8 months of age |
|
| Outcomes |
Timing of outcome assessment: 7 days and 30 days after the second vaccination |
|
| Notes |
Funding source(s):
Conflict(s) of interest: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: used random number generators |
| Allocation concealment (selection bias) | Low risk | Comment: used opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: laboratory investigators blinded, but trial investigators unblinded, hence high risk of bias for adverse events |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition rate less than 15% |
| Selective reporting (reporting bias) | High risk | Comment: outcome for IgA, IgG, and IgM prespecified in protocol but not evaluated and therefore not reported |
| Other bias | Low risk | Comment: none |
Resik 2015.
| Methods |
Study design: single‐centre, unblinded randomised controlled trial Location: Cuba Duration: 2 months
|
|
| Participants |
Inclusion criteria: "Children who were born between May 2011 and January 2012 were selected through health center registers. Only children who received two doses of OPV in February and April 2012, as per Cuban immunization policy, were eligible to participate in the study" (quote) Exclusion criteria: not reported Sample size: 729
Number of withdrawals/loss to follow‐up: 1 Age: 12 months to 20 months Sex: not reported |
|
| Interventions |
Intervention (n = 583): fractional dose of IPV given intradermally by the following methods.
We combined the data from all 4 groups for use in this review. Comparator (n = 146): intramuscular full dose (0.5 mL) of IPV given via needle and syringe |
|
| Outcomes |
Timing of outcome assessment: adverse events were reported after 1 hour and at 1st, 2nd, 3rd, and 7th day postvaccination. Seroconversions and median titres were recorded at days 0, 3, 7, and 21. |
|
| Notes |
Funding source(s):
Conflict(s) of interest: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: children selected from health centre population lists using simple random sampling; randomisation lists generated through computers. In Cuba, the population lists at the health centre level are considered to be complete and up‐to‐date. |
| Allocation concealment (selection bias) | Low risk | Comment: used opaque (non‐transparent), sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: only 1 participant lost to follow‐up at end of trial |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes described in protocol and Methods section were reported on in published paper |
| Other bias | Low risk | Comment: none |
Resik 2017.
| Methods |
Study design: 2‐arm, non‐inferiority randomised controlled clinical trial Location: 14 health centres in Camaguey Province, Cuba Duration: 9 months
|
|
| Participants |
Inclusion criteria: "Healthy adult males aged 15–30 years were eligible if they had a history of receiving 6 doses of poliovirus vaccine during childhood as part of the Cuban National Immunization Program and gave voluntary informed consent." (quote) Exclusion criteria: "poliovirus vaccination after 12 years of age, known or suspected exposure to wild poliovirus, receipt of any vaccination in the preceding 3 months, or known contraindications to vaccination" (quote) Sample size: 534
Number of withdrawals/loss to follow‐up: 31 Age:
Sex: all 534 enrolled participants were male |
|
| Interventions |
Intervention (n = 268): 2 doses of fractional (0.1 mL) IPV delivered intradermally, 28 days apart Comparator (n = 266): 2 doses of full‐dose (0.5 mL) IPV delivered intramuscularly, 28 days apart |
|
| Outcomes |
Timing of outcome assessment: assessment for safety outcomes was reported at 7, 28, and 56 days |
|
| Notes |
Funding source(s):
Conflict(s) of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization list was containing participant numbers and their corresponding study arms was prepared by study biostatistician independently of the study investigators and distributed to participating health centers" |
| Allocation concealment (selection bias) | Low risk | Quote: "Enrolled participants chose a sealed opaque envelope from available envelopes that contained the assigned participant number, which corresponded to one of 2 study arms, as indicated on randomisation list" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: blinding not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: blinding not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition rate of 5.8% |
| Selective reporting (reporting bias) | Low risk | Comment: published paper reported on all outcomes prespecified in protocol |
| Other bias | Low risk | Comment: study vaccines donated by Bilthoven Biologicals. Financial support provided by the Ministry of Health, Labour and Welfare, Japan, and the Norwegian Agency for Development Cooperation |
Soonawala 2013.
| Methods |
Study design: single‐centre, non‐inferiority randomised controlled trial Location: Leiden University Medical Center, the Netherlands Duration: 19 months
|
|
| Participants |
Inclusion criteria: "Healthy dutch adult volunteers who had received exactly 6 combined DTP‐IPV vaccinations according to the National Immunization Programme were included." (quote) Exclusion criteria: "Those who had received any IPV booster after 10 years of age were excluded. Receipt of OPV was also an exclusion criteria." (quote) Sample size: 125; 94 included in the review Number of withdrawals/loss to follow‐up: 1 Age:
Sex (male:female):
|
|
| Interventions |
Intervention (n = 32): 0.1 mL IPV given intradermally by PharmaJet jet injector Control (n = 62):
*We combined the data from arms 1 and 2 into a single group (n = 62). **We did not consider the data from third arm (i.e. 0.1 mL intramuscular dose of IPV, n = 31) in this review. |
|
| Outcomes |
Timing of outcome assessment: 28 days after vaccination of a fractional booster dose |
|
| Notes |
Funding source(s): Netherlands Vaccine Institute, the Netherlands Conflict(s) of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: used random number generator to generate random sequences |
| Allocation concealment (selection bias) | Unclear risk | Comment: sealed envelopes randomly numbered, but unclear if opaque (non‐transparent) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants accounted for in safety assessment and less than 5% loss to follow‐up for immunogenicity assessment |
| Selective reporting (reporting bias) | Low risk | Comment: reported on all outcomes |
| Other bias | High risk | Comment: funding agency part of the research team |
Tejeda Fuentes 2011.
| Methods |
Study design: randomised controlled trial Location: Cuba Duration: 8 months
|
|
| Participants |
Inclusion criteria: healthy newborns Exclusion criteria: not reported Sample size: 471
Number of withdrawals/loss to follow‐up: 107 loss to follow‐up Age: newborn Sex: not reported |
|
| Interventions |
Intervention (n = 187): fractional dose of IPV given intradermally in 3 primary doses at 6, 10, and 14 weeks of age Control (n = 177): full dose of IPV given intramuscularly in 3 primary doses at 6, 10, and 14 weeks of age |
|
| Outcomes |
Timing of outcome assessment: 1 week after administration of the vaccine |
|
| Notes |
Funding source(s): World Health Organization Conflict(s) of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of randomisation not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 22.7% attrition |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported |
| Other bias | Low risk | Comment: funded by the World Health Organization |
Troy 2015.
| Methods |
Study design: multi‐arm, parallel‐group randomised controlled trial Location: Norfolk, Virginia, USA Duration: 10 months
|
|
| Participants |
Inclusion criteria: adults over age 18 with documented HIV infection and an HIV load < 400 copies/mL at the most recent measurements Exclusion criteria: current acute illness, pregnancy, or history of allergic reaction to any component of IPV Sample size: 231; data from 132 included in the review:
Number of withdrawals/loss to follow‐up: 99 Age: > 18 years
Sex (male:female):
|
|
| Interventions |
Intervention (n = 66): IPV booster given 20% (0.1 mL) standard dose of IPV intradermally Control (n = 66): IPV booster given full dose (0.5 mL) of IPV intramuscularly 2 arms receiving 40% of IPV intramuscularly or intradermally were not considered, as follows.
|
|
| Outcomes |
Timing of outcome assessment: 1 month after administration of booster dose |
|
| Notes |
Funding source(s):
Conflict(s) of interest: 2 authors were employees of NanoPass Technologies; other authors reported no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: used computer‐generated random sequence, done in 3 blocks of 77 |
| Allocation concealment (selection bias) | Low risk | Comment: used computer‐generated random sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition rate less than 5% |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes described in protocol were reported on |
| Other bias | High risk | Comment: salaries of 2 study authors supported by NanoPass Technologies, Israel |
BCG: bacille Calmette‐Guérin vaccine CO2: carbon dioxide ELISA: enzyme‐linked immunosorbent assay ID: intradermal IgA: immunoglobulin A IgG: immunoglobulin G IgM: immunoglobulin M IM: intramuscular IPV: inactivated poliovirus vaccine ITT: intention‐to‐treat mOPV: monovalent oral poliovirus vaccine OPV: oral poliovirus vaccine TCID50: 50% tissue culture infective dose
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aaby 2007 | Different interventions. Did not use intradermal IPV |
| Bakker 2011 | Did not compare intradermal IPV versus intramuscular IPV |
| Bégué 1998 | Different interventions. Did not use intradermal IPV |
| Choudhury 2011 | Study aimed to determine the schedules for IPV. Did not compare intramuscular IPV and intradermal IPV |
| Cuba IPV Study Group 2007 | Study aimed to determine the schedules for IPV. Did not use intramuscular IPV. Compared 3 doses of intramuscular IPV with 2 doses of intramuscular IPV and a control group with no intramuscular IPV |
| Gamage 2019 | Not an RCT |
| Grassly 2014 | Not an RCT |
| Klein 2012 | Different interventions and study aims |
| Li 2016 | Did not compare fractional‐dose intradermal IPV versus full‐dose intramuscular IPV |
| NCT00871000 | Did not compare fractional‐dose intradermal IPV versus full‐dose intramuscular IPV |
| Nirmal 1998 | RCT comparing 2 doses of intradermal IPV and 3 doses of intradermal IPV |
| O’Ryan 2015 | Different interventions. Compared IPV‐IPV‐IPV with IPV‐OPV‐IPV and IPV‐IPV‐bOPV |
| Verdijk 2013 | Phase I trial comparing 2 different intramuscular IPVs |
| WHO Collaborative Study 1996 | Compared OPV and IPV |
bOPV: bivalent oral polio vaccine IPV: inactivated polio vaccine OPV: oral polio vaccine RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
NCT02347423.
| Methods |
Public title: 3 adjuvated reduced dose IPV‐Al SSI and non‐adjuvated full dose IPV SSI given as primary vaccinations to infants Scientific title: Immunogenicity and safety of 3 adjuvated reduced dose inactivated polio vaccines (IPV‐Al SSI) and non‐adjuvated full dose IPV SSI, given as primary vaccinations to infants at 6, 10 and 14 weeks of age Design: phase 2, multicentre, multi‐arm, single‐blinded randomised controlled trial Location: Dominican Republic |
| Participants |
Target sample size: 824 Inclusion criteria:
Exclusion criteria:
|
| Interventions | 3 investigational, reduced‐dose adjuvated IPV‐Al SSI vaccines and full‐dose IPV SSI vaccine will be investigated in 4 parallel groups. Intervention:
Comparator: IPV SSI; 3 vaccinations of IPV SSI (containing 40 DU of type 1, 8 DU of type 2, and 32 DU of type 3) given at 6, 10, and 14 weeks of age |
| Outcomes |
Primary outcome
Secondary outcome
Timing of outcome assessment:
|
| Notes |
Start date: February 2015 Contact person: Ingrid Kromann, Statens Serum Institut (e‐mail address not provided) Funding source(s):
Conflict of interest(s): none stated |
DU: Dobson unit GMT: geometric mean titre IPV: inactivated polio vaccine IPV‐AI SSI: reduced‐dose IPV manufactured by Statens Serum Institut IPV SSI: full‐dose IPV manufactured by Statens Serum Institut OPV: oral polio vaccine
Characteristics of ongoing studies [ordered by study ID]
NCT02847026.
| Trial name or title |
Public title: Fractional inactivated poliovirus vaccine booster and rotavirus study Scientific title: Immunogenicity of a booster dose of fractional inactivated poliovirus vaccine (fIPV) delivered intradermally concomitantly with rotavirus vaccines |
| Methods |
Desing: open‐label, randomised, parallel‐group trial Location: Dhaka, Bangladesh |
| Participants | Children aged 6 weeks Target sample size: 1144 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Multi‐arm study that will compare the booster dose of fractional IPV and full‐dose IPV given after various schedules of primary doses. These arms will be compared to each other.
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | September 2016 |
| Contact information |
Name: CJ Snider (e‐mail: csnider@cdc.gov) Address: ICDDR, Bangladesh |
| Notes |
Funding source(s): Centers for Disease Control and Prevention, ICDDR, Bangladesh Conflict(s) of interest: not reported |
NCT03016949.
| Trial name or title |
Public title: A study to evaluate immunogenicity of various schedules of inactivated polio vaccine Scientific title: A phase 3, open‐label, randomised trial to evaluate humoral immunogenicity of various schedules of intramuscular full dose and intradermal fractional dose of inactivated polio vaccine in infants |
| Methods |
Design: open‐label, phase III randomised controlled trial Location: Montevideo, Uruguay |
| Participants | Children (male and female) aged 5 to 7 weeks old Target sample size: 1493 infants, randomised into 6 groups Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Comparator: IPV given intramuscularly (IM) in various schedules Intervention: fractional‐dose IPV (fIPV) given intradermally (ID) in various schedules Groups:
|
| Outcomes |
Primary outcome: seroconversion Secondary outcomes:
Timing of outcome assessment: at 6, 18, 36, and 40 weeks of age |
| Starting date | July 2017 |
| Contact information |
Name: Stella Gutierrez, MD (Principal Investigator; email not reported) Address: CASMU (Centro Asistencial del Sindicato Médico del Uruguay) Polyclinic, Avendia 8 de Octubre 3310, Montevideo, Uruguay |
| Notes |
Funding source(s):
Conflict(s) of interest: not reported |
ICDDR: International Centre for Diarrhoeal Disease Research IgA: immunoglobulin IPV: inactivated poliovirus vaccine OPV: oral polio vaccine
Differences between protocol and review
Title. We changed the title of the review to clarify that the comparisons performed were for equivalent schedules of fractional dose IPV given intradermally versus full dose of IPV given intramuscularly.
Authors. Two review authors, Anil Chauhan and Shreya Singh, joined the author team at the review stage (i.e. post‐publication of the protocol).
-
We planned to search ProQuest Dissertations & Theses database but did not have access as the subscription was no longer available at the time of searching.
We searched two additional databases not listed in our protocol (Jaiswal 2015): Ovid MEDLINE Epub Ahead of Print and Trip.
Data collection and analysis. We were unable to use all of our preplanned methods (Jaiswal 2015). See Appendix 2.
Measures of treatment effect. We had planned to use the risk ratio (RR) (Jaiswal 2015). Instead, we used the odds ratio (OR) as the measure of effect because the OR is a better measure than the RR of rare events such as poliomyelitis (Deeks 2002).
'Summary of findings' table (beneath Data synthesis). We changed 'quality of the evidence' to 'certainty of the evidence' to align with current practice.
Subgroup analysis and investigation of heterogeneity. We had planned to conduct subgroup analyses according to the type of polio virus (i.e. types 1, 2, and 3), but analysed them separately instead, as findings from multiple subgroups may be misleading.
Contributions of authors
| Roles and responsibilities | ||
| Review stage | Task | Who undertook the task? |
| Protocol | Draft the protocol | NJ, MS, KKT, AA, HK |
| Review | Run the search (provided by the Information Specialist) | HK |
| Select which trials to include (2 review authors + 1 arbiter) | NJ and AA. Arbiter = MS | |
| Retrieval of full‐text reports | NJ and AA | |
| Extract data from trials (2 review authors + 1 arbiter) | NJ, KKT, AA. Arbiter = MS | |
| Enter data into RevMan 5 (Review Manager 2014) | NJ, AC, and SS | |
| Conduct the 'Risk of bias' assessment | KKT, AC, SS. Arbiter = MS | |
| Carry out the analysis | NJ, MS, SS | |
| Interpret the analysis | NJ, MS, SS | |
| Apply GRADE (2 review authors + 1 arbiter) | NJ, AC. Arbiter = MS | |
| Draft the final review | NJ, MS, SS, AC, AA | |
| Update | Update the review | NJ, SS, AA, AC, MS |
NJ and MS share overall responsibility for the review.
Sources of support
Internal sources
-
India Council of Medical Research (ICMR), Centre for Advanced Research in Evidence‐Based Child Health, Postgraduate Institute of Medical Education & Research (PGIMER), Chandigarh, India.
Salaries (Nishant Jaiswal, Amit Agarwal, Anil Chauhan, Kiran Kumar Thumburu) and technical assistance
External sources
None, Other.
Declarations of interest
Nishant Jaiswal ‐ none known. Shreya Singh ‐ none known Amit Agarwal ‐ none known. Anil Chauhan ‐ none known Kiran K Thumburu ‐ none known. Harpreet Kaur ‐ none known. Meenu Singh ‐ none known.
None of the review authors has received any benefit or favour from vaccine‐manufacturing companies.
New
References
References to studies included in this review
Anand 2015 {published data only}
- Anand A. Regarding your published trial on IPV in the Vaccine journal [personal communication]. Email to: N Jaiswal 04 May 2016.
- Anand A, Zaman K, Estívariz CF, Yunus M, Gary HE, Weldon WC, et al. Early priming with inactivated poliovirus vaccine (IPV) and intradermal fractional dose IPV administered by a microneedle device: a randomized controlled trial. Vaccine 2015;33(48):6816‐22. [DOI: 10.1016/j.vaccine.2015.09.039; PUBMED: 26476367] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cadorna‐Carlos 2012 {published data only}
- Cadorna‐Carlos J, Vidor E, Bonnet MC. Randomized controlled study of fractional doses of inactivated poliovirus vaccine administered intradermally with a needle in the Philippines. International Journal of Infectious Diseases 2012;16(2):e110‐6. [DOI: 10.1016/j.ijid.2011.10.002; PUBMED: 22153001] [DOI] [PubMed] [Google Scholar]
Clarke 2016 {published data only}
- Clarke E, Saidu Y, Adetifa JU, Adigweme I, Hydara MB, Bashorun AO, et al. Safety and immunogenicity of inactivated poliovirus vaccine when given with measles–rubella combined vaccine and yellow fever vaccine and when given via different administration routes: a phase 4, randomised, non‐inferiority trial in The Gambia. Lancet. Global Health 2016;4(8):e534‐47. [DOI: 10.1016/S2214-109X(16)30075-4; PUBMED: 27364568] [DOI] [PubMed] [Google Scholar]
- NCT01847872. IPV clinical trial ‐ The Gambia (IPV) [A phase 4, randomized trial to assess the safety and immunogenicity of inactivated poliovirus vaccine when given concomitantly with measles and rubella combined vaccine and yellow fever vaccine at nine months and when administered via different vaccination routes]. clinicaltrials.gov/ct2/show/NCT01847872 (first received 19 April 2013).
Estívariz 2012 {published data only}
- Estívarez CF. Details regarding your published paper about "Immunogenicity of supplemental doses of poliovirus vaccine for children aged 6‐9 months in Moradabad, India: a community‐based, randomised controlled trial. Lancet 2012 [personal communication]. Email to: KK Thumburu 11 March 2016. [DOI] [PubMed]
- Estívariz CF, Jafari H, Sutter RW, John TJ, Jain V, Agarwal A, et al. Immunogenicity of supplemental doses of poliovirus vaccine for children aged 6‐9 months in Moradabad, India: a community‐based, randomised controlled trial. Lancet. Infectious Diseases 2012;12(2):128‐35. [DOI: 10.1016/S1473-3099(11)70190-6; PUBMED: 22071249] [DOI] [PubMed] [Google Scholar]
Gamage 2018 {published data only}
- ACTRN12616000124437p. Comparison of boosting of mucosal immunity after full and fractional dose of inactivated poliovirus vaccine, clinical trial in children in Sri Lanka, 2016 [Comparison of boosting of mucosal immunity after full and fractional dose of inactivated poliovirus vaccine, a randomized clinical trial in 300 children aged 10‐12 years of age living in Kalutara district of Sri Lanka, 2016]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=369725 (first received 3 December 2015).
- Gamage D, Mach O, Palihawadana P, Zhang Y, Weldon WC, Oberste MS, et al. Boosting of mucosal immunity after fractional‐dose inactivated poliovirus vaccine. Journal of Infectious Diseases 2018;218(12):1876‐82. [DOI: 10.1093/infdis/jiy389; PUBMED: 29982532] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohammed 2010 {published data only}
- Mohammed AJ, AlAwaidy S, Bawikar S, Kurup PJ, Elamir E, Shaban MMA, et al. Fractional doses of inactivated poliovirus vaccine in Oman. New England Journal of Medicine 2010;362(25):2351‐9. [DOI: 10.1056/NEJMoa0909383; PUBMED: 20573923] [DOI] [PubMed] [Google Scholar]
Resik 2010 {published data only}
- Resik S, Tejeda A, Lago PM, Diaz M, Carmenates A, Sarmiento L, et al. Randomized controlled clinical trial of fractional doses of inactivated poliovirus vaccine administered intradermally by needle‐free device in Cuba. Journal of Infectious Diseases 2010;201(9):1344‐52. [DOI: 10.1086/651611; PUBMED: 20350164] [DOI] [PubMed] [Google Scholar]
Resik 2013 {published data only}
- Resik S, Tejeda A, Sutter RW, Diaz M, Sarmiento L, Alemañi N, et al. Priming after a fractional dose of inactivated poliovirus vaccine. New England Journal of Medicine 2013;368(5):416‐24. [DOI: 10.1056/NEJMoa1202541; PUBMED: 23363495] [DOI] [PubMed] [Google Scholar]
- Sutter RW. Regarding your published paper about "Priming after a Fractional Dose of Inactivated Poliovirus Vaccine" published in NEJM 2013 [personal communication]. Email to: KK Thumburu 04 February 2016. [DOI] [PubMed]
Resik 2015 {published data only}
- Resik S, Tejeda A, Mach O, Fonseca M, Diaz M, Alemany N, et al. Immune responses after fractional doses of inactivated poliovirus vaccine using newly developed intradermal jet injectors: a randomized controlled trial in Cuba. Vaccine 2015;33(2):307‐13. [DOI: 10.1016/j.vaccine.2014.11.025; PUBMED: 25448109] [DOI] [PubMed] [Google Scholar]
Resik 2017 {published data only}
- Resik S, Tejeda A, Diaz M, Okayasu H, Sein C, Molodecky NA, et al. Boosting immune responses following fractional‐dose inactivated poliovirus vaccine: a randomized, controlled trial. Journal of Infectious Diseases 2017;215(2):175‐82. [DOI: 10.1093/infdis/jiw492; PUBMED: 28073858] [DOI] [PubMed] [Google Scholar]
Soonawala 2013 {published data only}
- Soonawala D, Verdijk P, Wijmenga‐Monsuur AJ, Boog CJ, Koedam P, Visser LG, et al. Intradermal fractional booster dose of inactivated poliomyelitis vaccine with a jet injector in healthy adults. Vaccine 2013;31(36):3688‐94. [DOI: 10.1016/j.vaccine.2013.05.104; PUBMED: 23770332] [DOI] [PubMed] [Google Scholar]
Tejeda Fuentes 2011 {published data only}
- Tejeda Fuentes A, Armas López J, Silva Sosa M, Alemañy Bueno N, Carmenate García A, García González G, et al. Reactogenicity associated to the intradermally administered inactivated poliovirus vaccine with a needle‐free injector [Reactogenicidad asociada a la vacuna de poliovirus inactivada administrada por vía intradérmica con un inyector sin aguja]. Revista Cubana de Medicina Tropical 2011;63(1):38‐43. [PUBMED: 23437535] [PubMed] [Google Scholar]
Troy 2015 {published data only}
- Troy S. Regarding your paper "Comparison of the Immunogenicity of Various Booster Doses of Inactivated Polio Vaccine Delivered Intradermally Versus Intramuscularly to HIV‐Infected Adults" [personal communication]. Email to: A Agarwal 06 May 2016. [DOI] [PMC free article] [PubMed]
- Troy SB, Kouiavskaia D, Siik J, Kochba E, Beydoun H, Mirochnitchenko O, et al. Comparison of the immunogenicity of various booster doses of inactivated polio vaccine delivered intradermally versus intramuscularly to HIV‐infected adults. Journal of Infectious Diseases 2015;211(12):1969‐76. [DOI: 10.1093/infdis/jiu841; PMC4539908; PUBMED: 25567841] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aaby 2007 {published data only}
- Aaby P, Garly ML, Nielsen J, Ravn H, Martins C, Balé C, et al. Increased female‐male mortality ratio associated with inactivated polio and diphtheria‐tetanus‐pertussis vaccines: observations from vaccination trials in Guinea‐Bissau. Pediatric Infectious Disease Journal 2007;26(3):247‐52. [DOI: 10.1097/01.inf.0000256735.05098.01; PUBMED: 17484223] [DOI] [PubMed] [Google Scholar]
Bakker 2011 {published data only}
- Bakker WA, Thomassen YE, Van't Oever AG, Westdijk J, Oijen MG, Sundermann LC, et al. Inactivated polio vaccine development for technology transfer using attenuated Sabin poliovirus strains to shift from Salk‐IPV to Sabin‐IPV. Vaccine 2011;29(41):7188‐96. [DOI: 10.1016/j.vaccine.2011.05.079; PUBMED: 21651934] [DOI] [PubMed] [Google Scholar]
Bégué 1998 {published data only}
- Bégué PC, Grimprel EM, Giovannangeli MD, Abitbol VI. Comparative reactogenicity and immunogenicity of booster doses of diphtheria‐tetanus‐acellular pertussis‐inactivated poliovirus vaccine and diphtheria‐tetanus‐inactivated poliovirus vaccine in preadolescents. Pediatric Infectious Disease Journal 1998;17(9):804‐9. [DOI: 10.1097/00006454-199809000-00011; PUBMED: 9779766] [DOI] [PubMed] [Google Scholar]
Choudhury 2011 {published data only}
- Choudhury P, Thacker N, Gargano LM, Weiss PS, Vashishtha VM, Amladi T, et al. Attitudes and perceptions of private paediatricians regarding polio immunization in India. Vaccine 2011;29(46):8317‐22. [DOI: 10.1016/j.vaccine.2011.08.099; PUBMED: 21893150] [DOI] [PubMed] [Google Scholar]
Cuba IPV Study Group 2007 {published data only}
- The Cuba IPV Study Collaborative Group. Randomized, placebo‐controlled trial of inactivated poliovirus vaccine in Cuba. New England Journal of Medicine 2007;356(15):1536‐44. [DOI: 10.1056/NEJMoa054960; PUBMED: 17429085] [DOI] [PubMed] [Google Scholar]
Gamage 2019 {published data only}
- Gamage D, Mach O, Ginige S, Weldon WC, Oberste MS, Jeyaseelan V, et al. Poliovirus type 2 seroprevalence following full‐ or fractional‐dose inactivated poliovirus vaccine in the period after Sabin type 2 withdrawal in Sri Lanka. Journal of Infectious Diseases 2019;219(12):1887‐92. [DOI: 10.1093/infdis/jiz026; PUBMED: 30649505] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grassly 2014 {published data only}
- Grassly NC. Immunogenicity and effectiveness of routine immunization with 1 or 2 doses of inactivated poliovirus vaccine: systematic review and meta‐analysis. Journal of Infectious Diseases 2014;210(Suppl 1):S439‐46. [DOI: 10.1093/infdis/jit601; PMC4197908; PUBMED: 24634499] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klein 2012 {published data only}
- Klein NP, Weston WM, Kuriyakose S, Kolhe D, Howe B, Friedland LR, et al. An open‐label, randomized, multi‐center study of the immunogenicity and safety of DTaP‐IPV (KinrixTM) co‐administered with MMR vaccine with or without varicella vaccine in healthy pre‐school age children. Vaccine 2012;30(3):668‐74. [DOI: 10.1016/j.vaccine.2011.10.065; PUBMED: 22064267] [DOI] [PubMed] [Google Scholar]
Li 2016 {published data only}
- Li R, Li CG, Li Y, Liu Y, Zhao H, Chen X, et al. Primary and booster vaccination with an inactivated poliovirus vaccine (IPV) is immunogenic and well‐tolerated in infants and toddlers in China. Vaccine 2016;34(12):1436‐43. [DOI: 10.1016/j.vaccine.2016.02.010; PUBMED: 26873055] [DOI] [PubMed] [Google Scholar]
NCT00871000 {published data only}
- NCT00871000. Immunogenicity and safety of Boostrix polio vaccine as a booster dose in 5 to 6‐year‐old children [Immunogenicity and safety of GSK Biologicals' dTpa‐IPV vaccine (Boostrix polio) as a booster dose in 5 to 6‐year‐old children]. clinicaltrials.gov/ct2/show/NCT00871000 (first received 26 March 2009).
Nirmal 1998 {published data only}
- Nirmal S, Cherian T, Samuel BU, Rajasingh J, Raghupathy P, John TJ. Immune response of infants to fractional doses of intradermally administered inactivated poliovirus vaccine. Vaccine 1998;16(9‐10):928‐31. [DOI: 10.1016/s0264-410x(97)00293-4; PUBMED: 9682339] [DOI] [PubMed] [Google Scholar]
O’Ryan 2015 {published data only}
- O'Ryan M, Bandyopadhyay AS, Villena R, Espinoza M, Novoa J, Weldon WC, et al. Inactivated poliovirus vaccine given alone or in a sequential schedule with bivalent oral poliovirus vaccine in Chilean infants: a randomised, controlled, open‐label, phase 4, non‐inferiority study. Lancet. Infectious Diseases 2015;15(11):1273‐82. [DOI: 10.1016/S1473-3099(15)00219-4] [DOI] [PubMed] [Google Scholar]
Verdijk 2013 {published data only}
- Verdijk P, Rots NY, Oijen MG, Oberste MS, Boog CJ, Okayasu H, et al. Safety and immunogenicity of inactivated poliovirus vaccine based on Sabin strains with and without aluminium hydroxide: a phase I trial in healthy adults. Vaccine 2013;31(47):5531‐6. [DOI: 10.1016/j.vaccine.2013.09.021; PUBMED: 24063976] [DOI] [PubMed] [Google Scholar]
WHO Collaborative Study 1996 {published data only}
- WHO Collaborative Study Group on Oral and Inactivated Poliovirus Vaccines. Combined immunization of infants with oral and inactivated poliovirus vaccines: results of a randomized trial in The Gambia, Oman, and Thailand. Bulleten of the World Health Organization 1996;74(3):253‐68. [PMC2486925] [PMC free article] [PubMed] [Google Scholar]
- WHO Collaborative Study Group on Oral and Inactivated Poliovirus Vaccines. Combined immunization of infants with oral and inactivated poliovirus vaccines: results of a randomized trial in The Gambia, Oman, and Thailand. Journal of Infectious Diseases 1997;175(Suppl 1):S215‐27. [DOI: 10.1093/infdis/175.supplement_1.s215; PUBMED: 9203720] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT02347423 {published data only}
- NCT02347423. 3 adjuvated reduced dose IPV‐Al SSI and non‐adjuvated full dose IPV SSI given as primary vaccinations to infants [Immunogenicity and safety of 3 adjuvated reduced dose inactivated polio vaccines (IPV‐Al SSI) and non‐adjuvated full dose IPV SSI, given as primary vaccinations to infants at 6, 10 and 14 weeks of age]. clinicaltrials.gov/ct2/show/record/NCT02347423 (first received 15 January 2015).
References to ongoing studies
NCT02847026 {unpublished data only}
- NCT02847026. Fractional inactivated poliovirus vaccine booster and rotavirus study (fIPV) [Immunogenicity of a booster dose of fractional inactivated poliovirus vaccine (fIPV) delivered intradermally concomitantly with rotavirus vaccines]. clinicaltrials.gov/ct2/show/NCT02847026 (first received 25 July 2016).
NCT03016949 {unpublished data only}
- NCT03016949. A study to evaluate immunogenicity of various schedules of inactivated polio vaccine [A phase 3, open‐label, randomized trial to evaluate humoral immunogenicity of various schedules of intramuscular full dose and intradermal fractional dose of inactivated polio vaccine in infants]. clinicaltrials.gov/ct2/show/NCT03016949 (first received 5 January 2017).
Additional references
Anand 2017
- Anand A, Molodecky NA, Pallansch MA, Sutter RW. Immunogenicity to poliovirus type 2 following two doses of fractional intradermal inactivated poliovirus vaccine: a novel dose sparing immunization schedule. Vaccine 2017;35(22):2993‐8. [DOI: 10.1016/j.vaccine.2017.03.008; PUBMED: 28434691] [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2002
- Centers for Disease Control and Prevention. Progress toward global eradication of poliomyelitis, 2001. Morbidity and Mortality Weekly Report 2002;51(12):253‐6. [PUBMED: 11939704] [PubMed] [Google Scholar]
CDC 2013
- Centers for Disease Control and Prevention. Assessing the risks for poliovirus outbreaks in polio‐free countries ‐ Africa, 2012‐2013. Morbidity and Mortality Weekly Report 2013;62(37):768‐72. [PMC4585360; PUBMED: 24048153] [PMC free article] [PubMed] [Google Scholar]
Ciapponi 2014
- Ciapponi A, Bardach A, Rey Ares L, Glujovsky D, Cafferata ML, Romano M, et al. Sequential inactivated (IPV) and live oral (OPV) poliovirus vaccines for preventing poliomyelitis. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD011260] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2002
- Deeks JJ. Issues in the selection of a summary statistic for meta‐analysis of clinical trials with binary outcomes. Statistics in Medicine 2002;21(11):1575‐600. [DOI: 10.1002/sim.1188; PUBMED: 12111921] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG, editor(s), on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analysis. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dowdle 2002
- Dowdle WR, Gary HE, Sanders R, Loon AM. Can post‐eradication laboratory containment of wild polioviruses be achieved?. Bulletin of the World Health Organization 2002;80(4):311‐6. [PMC2567771; PUBMED: 12075368] [PMC free article] [PubMed] [Google Scholar]
GPEI 2010
- Global Polio Eradication Initiative. Polio + prevention. bit.ly/19VK8ok (accessed 2 May 2014).
GPEI 2017
- Global Polio Eradication Initiative. Use of fractional dose IPV in routine immunization programmes: considerations for decision‐making. www.who.int/immunization/diseases/poliomyelitis/endgame_objective2/inactivated_polio_vaccine/fIPV_considerations_for_decision‐making_April2017.pdf (assessed 10 October 2017).
GPEI 2018
- Global Polio Eradication Initiative. Vaccine‐derived polioviruses. www.polioeradication.org/polio‐today/polio‐prevention/the‐virus/vaccine‐derived‐polio‐viruses/ (assessed 15 June 2018).
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 20 June 2017. Hamilton (ON): McMaster University (developed by Evidence Prime).
Grassly 2013
- Grassly NC. The final stages of the global eradication of poliomyelitis. Philosophical Transitions of the Royal Society of London. Series B, Biological Sciences 2013;368(1623):20120140. [DOI: 10.1098/rstb.2012.0140; PMC3720038; PUBMED: 23798688] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI: 10.1016/j.jclinepi.2010.04.026; PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI: 10.1136/bmj.327.7414.557; PMC192859; PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC, editor(s), on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG, editor(s), on behalf of the Cochrane Statistical Methods Group. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JPT, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lambert 2008
- Lambert PH, Laurent PE. Intradermal vaccine delivery: will new delivery systems transform vaccine administration?. Vaccine 2008;26(26):3197‐208. [DOI: 10.1016/j.vaccine.2008.03.095; PUBMED: 18486285] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
McKinlay 2014
- McKinlay MA, Collett MS, Hincks JR, Oberste MS, Pallansch MA, Okayasu H, et al. Progress in the development of poliovirus antiviral agents and their essential role in reducing risks that threaten eradication. Journal of Infectious Diseases 2014;210 Suppl 1:S447‐53. [DOI: 10.1093/infdis/jiu043; PUBMED: 25316866] [DOI] [PubMed] [Google Scholar]
Minor 2014
- Minor P. The polio endgame. Human Vaccines & Immunotherapeutics 2014; Vol. 10, issue 7:2106‐8. [DOI: 10.4161/21645515.2014.981115; PMC4370356; PUBMED: 25608050] [DOI] [PMC free article] [PubMed]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097; PMC2707599; PUBMED: 19621072] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nelson 2012
- Nelson KS, Janssen JM, Troy SB, Maldonado Y. Intradermal fractional dose inactivated polio vaccine: a review of the literature. Vaccine 2012;30(2):121‐5. [DOI: 10.1016/j.vaccine.2011.11.018; PUBMED: 22100886] [DOI] [PubMed] [Google Scholar]
Okayasu 2017
- Okayasu H, Sein C, Chang Blanc D, Gonzalez AR, Zehrung D, Jarrahian C, et al. Intradermal administration of fractional doses of inactivated poliovirus vaccine: a dose‐sparing option for polio immunization. Journal of Infectious Diseases 2017;216(Suppl 1):S161‐7. [DOI: 10.1093/infdis/jix038; PMC5853966; PUBMED: 28838185] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palucka 2010
- Palucka K, Banchereau J, Mellman I. Designing vaccines based on biology of human dendritic cell subsets. Immunity 2010;33(4):464‐78. [DOI: 10.1016/j.immuni.2010.10.007; PMC2975953; PUBMED: 21029958] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parker 2015
- Parker EP, Molodecky NA, Pons‐Salort M, O'Reilly KM, Grassly NC. Impact of inactivated poliovirus vaccine on mucosal immunity: implications for the polio eradication endgame. Expert Review of Vaccines 2015;14(8):1113‐23. [DOI: 10.1586/14760584.2015.1052800; PMC4673562; PUBMED: 26159938] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sterne 2011
- Sterne JAC, Egger M, Moher D, editor(s), on behalf of the Cochrane Bias Methods Group. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
WHO 2009
- World Health Organization. Case definitions for the four diseases requiring notification to WHO in all circumstances under the International Health Regulations (2005). Weekly Epidemiological Record 2009;84(7):52‐6. [www.who.int/wer/2009/wer8407/en/] [Google Scholar]
WHO 2013
- World Health Organization. Polio Eradication & Endgame Strategic Plan 2013‐2018. Geneva: WHO, 2013. [polioeradication.org/wp‐content/uploads/2016/07/PEESP_EN_A4.pdf] [Google Scholar]
WHO 2016
- World Health Organization. Polio vaccines: WHO position paper ‐ March, 2016. Weekly Epidemiological Record 2016;91(12):145‐68. [www.who.int/wer/2016/wer9112/en/] [Google Scholar]
WHO 2018
- World Health Organization. Poliomyelitis. www.who.int/mediacentre/factsheets/fs114/en/ (accessed June 2018).
World Bank 2014
- World Bank. Data: World Bank country and lending groups. bit.ly/1pUHKTk (assessed 17 September 2014).
References to other published versions of this review
Jaiswal 2015
- Jaiswal N, Singh M, Thumburu KK, Agarwal A, Kaur H. Intradermal fractional dose vs intramuscular full dose of inactivated polio vaccine for prevention of poliomyelitis. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD011780] [DOI] [PMC free article] [PubMed] [Google Scholar]


