Short abstract
Objective
Left ventricular free wall rupture (LVFWR) is a rare but severe complication of acute myocardial infarction (AMI). During the era of pre-thrombolysis, autopsies revealed an incidence of approximately 8%.
Method
The objective of this retrospective study was to analyze the current incidence of LVFWR and to identify predictors by comparing the AMI-cohort with LVFWR to those without. The control group involved a random selection of one in every ten patients who presented with acute myocardial infarction between 2005 and 2014.
Result
A total of 5143 patients with AMI were treated at the Central Hospital, Bad Berka (71% men, median age 68 years). Out of these, seven patients with LVFWR were identified with an overall incidence of 0.14%. Clinically, LVFWR patients presented late to admission since symptom onset (median 24 h vs. 6.1 h; p < 0.0001), were more likely in cardiogenic shock (28.6% vs. 3.2%; p = 0.02) and were usually accompanied by emergency physicians (71.4% vs. 20.7%; p = 0.006). Higher troponin T (median 8.6 vs. 0.5 ng/ml; p < 0.0002), higher CRP (median 50 vs. 0.5 mg/l; p = 0.05) as well as a lower hematocrit-values (0.33 vs. 0.42; p = 0.04) were observed. All LVFWR patients were operated (100% vs. 1.6%; p < 0.001). The patients had lower rates of beta-blocker treatment (57.1% vs. 95.8%; p = 0.003). The 30-day mortality was significantly higher (42.9% vs. 6.8%; p = 0.01).
Conclusion
Compared to the thrombolytic era, the current incidence of LVFWR with AMI, who reach the hospital alive, is significantly lower. However, 30-day mortality continues to be high.
Keywords: Left ventricular aneurysm, acute coronary syndrome, myocardial infarction, complications, free wall perforation, cardiogenic shock
Introduction
Following cardiogenic shock and fatal ventricular arrhythmias, left ventricular free wall rupture (LVFWR) is ranked third as the leading cause of all infarct-related deaths.1
Post infarction LVFWR was first described by William Harvey in 1647 as a finding at autopsy of a knight who suffered severe chest pain.2 Fitzgibbon reported in 1972 the first successful surgical repair of left ventricular rupture associated with ischemic heart disease.3
The advent of primary percutaneous interventions (PCI), when compared to the pre-thrombolytic or the thrombolytic eras, has considerably reduced the rates of LVFWR;4 however the mortality continues to remain high with its incidence currently estimated to range between 0.7% and 8%, which is 8 to 10 times more frequent than other types of myocardial rupture such as papillary muscle or rupture of the interventricular septum.5
Due to the variable clinical presentations associated with high mortality, LVFWR remains a substantial diagnostic and therapeutic challenge for clinicians.
The objective of our study was to identify the incidence and possible predictors of LVFWR in patients with acute myocardial infarction.
Materials and methods
Data collection
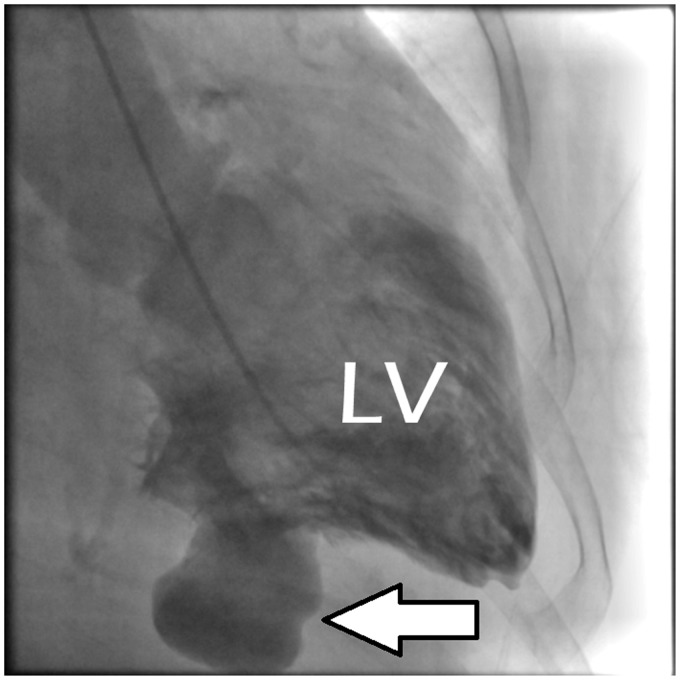
Retrospective identification of all consecutive patients presenting with LVFWR (Figure 1) from a patient cohort of acute myocardial infarction (AMI) was performed from our institutional database between January 2005 and December 2014.
Figure 1.
Example of a left ventricular (LV) free wall rupture (white arrow).
The control group was established by collecting data from 502 patients selected as a representative random sample by picking every 10th patient of the entire study population. Exclusion criteria were patients with ventricular septal defects or papillary muscle ruptures, both due to infarction. The study was approved by the institutional ethics committee.
Risk factors
To determine the potential predictors of LVFWR, the following risk factors were assessed:
Patient-related factors
Age, gender, blood pressure on admission, presence of cardiogenic shock, time of symptom onset to admission.
Procedure-related factors
The extent of coronary artery disease (one vessel disease or more), acute stent thrombosis, location of the culprit lesion on coronary angiography, and valvular pathologies.
Laboratory on admission
Creatinine, creatine kinase, troponin-T, C-reactive protein (CRP), hematocrit, white cell count, hemoglobin, and platelets were determined.
Current medications
The current medications upon diagnosis, e.g., aspirin, clopidogrel, glycoprotein IIb/IIIa receptor blocker (GPI), beta-blockers, angiotensin-converting enzyme inhibitors (ACE-I) or angiotensin receptor blockers (ARB), statins, diuretics, aldosterone antagonists, amiodarone, and digoxin.
Statistical analysis
The available data were extracted from the case files of the patients and entered into an Excel Spreadsheet, Microsoft. Continuous variables were reported as mean value ± standard deviation or median or interquartile ranges (25th–75th percentiles) as appropriate. Categorical variables were presented as absolute (n) and relative (%) frequencies. The normal distribution of variables was assessed using the D'Agostino-Pearson omnibus normality test. The T-test, Mann–Whitney test, and Fisher's exact test were used, as appropriate. All tests were two-tailed, and a probability value of p ≤ 0.05 was considered statistically significant. Statistical analysis was performed using the GraphPad Prism version 6.02 for Windows (GraphPad Software, La Jolla, CA, USA).
Results
From a total of 5143 patients presenting with acute myocardial infarction (71% of them were men, the median age was 67 years) between 2005 and 2014, seven patients with LVFWR were identified, resulting in an incidence of 0.14%. The results of the extracted data are as follows:
In univariate analysis, significant findings of the LVFWR group included delayed presentation to the hospital after the onset of symptoms (median 24 h vs. 6.1 h; p < 0.0001) with higher rates of cardiogenic shock upon presentation (28.6% vs. 3.2%; p = 0.02), frequent direct admissions through the paramedical team (71.4% vs. 20.7%; p = 0.0006). Out of the seven patients, four of them (57.1%) were females; LVFWR-patients tend to be older. The median age was 73 years, compared to the control group (68 years). Laboratory values showed higher troponin levels in LVFWR patients (median 8.6 vs. 0.5 ng/ml, p < 0.0002) and CRP levels (median 50 vs. 0.5 mg/l; p = 0.05) as well as lower hematocrit levels (0.33 vs. 0.42; p = 0.04) (Table 1).
Table 1.
Clinical characteristics.
| Variables | LVFWR, n = 7 | Controls, n = 502 | p-value |
|---|---|---|---|
| Baseline characteristics | |||
| Age (years) | 73 (61–78) | 67 (55–75) | 0.41 |
| Sex (males) | 3 (42.9%) | 356 (70.9%) | 0.20 |
| Left ventricular ejection fraction (%) | 50 (35–53) | 47(40–60) | 0.47 |
| Heart rate per minute | 68 (60–106) | 75 (65–90) | 0.86 |
| Systolic BP (mmHg) | 124 (115–149) | 130 (116–140) | 0.82 |
| Diastolic BP (mmHg) | 75 (65–84) | 80 (70–85) | 0.49 |
| Cardiogenic shock at presentation | 2 (28.6%) | 16 (3.2%) | 0.02 |
| Symptom onset to CAG time (h) | 24 (7.5–94) | 6.1 (3.3–11) | <0.0001 |
| Direct admission | 5 (71.4%) | 104 (20.7%) | 0.006 |
| Medical history | |||
| Arterial hypertension | 6 (85.7%) | 344 (68.5%) | 0.44 |
| Hypercholesterolemia | 3 (42.9%) | 152 (30.3%) | 0.44 |
| Diabetes mellitus | 1 (14.3%) | 144 (28.7%) | 0.68 |
| Past history of AMI | 1 (14.3%) | 68 (13.6%) | 1.00 |
| Past history of CABG | 1 (14.3%) | 20 (4.0%) | 0.26 |
| Valvular pathologies (> trivial) | 5 (71.4%) | 12 (2.4%) | <0.0001 |
| Laboratory values | |||
| Serum creatinine (µmol/l) | 88 (79–84) | 84 (71–103) | 0.77 |
| C-reactive protein (mg/l) | 50 (4.3–127) | 5.3 (2.6–17) | 0.05 |
| Creatine kinase (µmol/s/l) | 10 (2.2–16) | 7.1 (2.6–17) | 0.89 |
| Troponin T (ng/ml) | 8.6 (2.9–11) | 0.5 (0.07–2.2) | 0.0002 |
| Leucocytes (Gpt/l) | 9.4 (8.3–18) | 12 (9.4–14) | 0.44 |
| Hemoglobin (mmol/l) | 7.5 (6–9) | 8.7 (8.0–9.2) | 0.06 |
| Hematocrit (l/L) | 0.33 (0.31–0.43) | 0.42 (0.39–0.45) | 0.04 |
| Platelets (Gpt/l) | 249 (124–369) | 231 (189–277) | 0.51 |
| Medication on admission | |||
| Aspirin | 2 (28.6%) | 496 (98.8%) | <0.0001 |
| P2Y12-Inhibitors | 3 (42.9%) | 492 (98.4%) | <0.0001 |
| Beta blocker | 4 (57.1%) | 481 (95.8%) | 0.003 |
| ACE-I/ARB | 4 (57.1%) | 468 (93.2%) | 0.010 |
| Aldosterone antagonists | 0 (0.0%) | 64 (12.8%) | 1.00 |
| Diuretics | 4 (57.1%) | 214 (42.6%) | 0.47 |
| Statins | 4 (57.1%) | 464 (92.4%) | 0.014 |
| Digitalis | 0 (0.0%) | 52 (10.4%) | 1.00 |
| Amiodarone | 0 (0.0%) | 22 (4.4%) | 1.00 |
Bold markings denote significant values. Percentages might not sum to 100% as a result of rounding.
LVFWR: left ventricular free wall rupture, BP: blood pressure; AMI: acute myocardial infarction, CABG: coronary artery bypass grafting; ACE-I: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker.
Interestingly, the majority of the patients presented with a single-vessel disease in both the groups; the most frequent location of the culprit lesion was LAD in both groups; however, the finding was not significant. The frequency of management of patients in LVFWR group with percutaneous coronary interventions (PCI) was low (57.1% vs. 98.8%; p < 0.001), and resulted more often sub-optimal results (TIMI 3 flow after PCI: 75% vs. 92.7%; p = 0.03). Prior treatment with aspirin and beta-blockers was found to be lower in the LVFWR group (28.6% vs. 98.8% for aspirin; p < 0.0001, 57.1% vs. 95.8%; p = 0.003 for beta-blockers), possibly due to planned surgical treatment. The 30-day mortality rate was significantly higher in this group (42.9% vs. 6.8%; p = 0.01). For details, please refer to Table 2.
Table 2.
Angiographic parameters, treatment, and outcome.
| Variables | LVFWR, n = 7 | Controls, n = 502 | p-value |
|---|---|---|---|
| Coronary angiography | |||
| Coronary single vessel disease | 4 (57.1%) | 202 (40.2%) | 0.45 |
| Coronary two vessel disease | 2 (28.6%) | 188 (37.5%) | 1.00 |
| Coronary three vessel disease | 1 (14.3%) | 112 (22.3%) | 1.00 |
| Acute stent thrombosis | 1 (14.3%) | 28 (5.6%) | 0.34 |
| Culprit lesion | |||
| Right coronary artery | 3 (42.9%) | 219 (43.6%) | 1.00 |
| Left anterior descending artery | 4 (57.1%) | 228 (45.4%) | 0.71 |
| Other coronary arteries | 0 (0.0%) | 55 (11.0%) | 1.00 |
| Treatment and outcome | |||
| Percutaneous coronary intervention | 4 (57.1%) | 496 (98.8%) | <0.0001 |
| TIMI flow 3 post-PCI | 3 (75.0%) | 460 (92.7%) | 0.03 |
| Administration of glycoprotein IIb/IIIa Inhibitors | 2 (28.6%) | 214 (42.6%) | 0.13 |
| Surgical treatment | 7 (100%) | 8 (1.6%) | <0.0001 |
| Conservative management | 0 (0.0%) | 6 (1.2%) | 1.00 |
| Mortality (≤30 days) | 3 (42.9%) | 34 (6.8%) | 0.0101 |
Bold markings denote significant values. Percentages might not sum to 100% as a result of rounding.
LVFWR: left ventricular free wall rupture; PCI: percutaneous coronary intervention; TIMI: thrombolysis in myocardial infarction.
Operative treatment
All LVFWR-patients had a “blow out” type and underwent emergency/urgent surgical repair, six of them (86%) using cardio-pulmonary bypass, one patient was already on veno-arterial ECMO (extracorporeal membrane oxidation). In five patients (71.4%), the defect was repaired with a Dacron patch; three patients (43%) received CABG along with the LVFWR repair. Concomitant valve replacement was performed in two patients (29%) (Table 3).
Table 3.
Characteristics of the seven patients with left ventricular free wall rupture.
| Patient no. | Age (years) | Sex | Presentation | CAD | Location of LVFWR and timing of AMI | Medical history | Therapy | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 75 | F | Cardiogenic shock | 1 VD | Posterior wall (sub-acute) | AHt | EVCPP, MVR | Discharged (LFU 850 days) |
| 2 | 70 | M | Angina | 2 VD | Posterior wall (sub-acute) | AHt | PCI, EVCPP, MVR | Discharged (LFU 4685 days) |
| 3 | 83 | F | Cardiogenic shock | 1 VD | Anterior wall (acute) | PAD | EVCPP, CABG | Death (5 POD) |
| 4 | 78 | M | Angina | 1 VD | Anterior wall (acute) | AHt, HLP | PCI, EVCPP | Death (4 POD) |
| 5 | 68 | F | Angina | 3 VD | Posterior/lateral wall (sub-acute) | AHt, Diabetes | PCI, EVCPP, CABG | Discharged (LFU 2920 days) |
| 6 | 55 | M | Angina | 2 VD | Posterior/Lateral wall (acute) | AHt, CAD | PCI, EVCPP, CABG | Discharged (no FU) |
| 7 | 45 | F | Angina, Cardiogenic shock | 1 VD | Anterior wall (acute) | AHt | PCI, ECMO, EVCPP | Death (2 POD) |
Timing of AMI: acute: within days to a maximum of two weeks, sub-acute: more than two weeks.
AHt: arterial hypertension; AMI: acute myocardial infarction; CAD: coronary artery disease; CABG: coronary artery bypass grafting; ECMO: extracorporeal membrane oxygenation; EVCPP: endoventricular circular patch plasty; F: female; LFU: last follow-up; HLP: hyperlipidemia; M: male; MVR: mitral valve replacement; PAD: peripheral arterial disease; PCI: percutaneous coronary intervention; POD: postoperative day; VD: vessel disease.
Discussion
Current literature reports the rupture of the ventricular free wall and cardiogenic shock as the major causes of death following acute myocardial infarction (AMI), contributing to 66% of deaths due to the first AMI.6
The incidence of LVFWR during the fibrinolytic period in ST-elevation myocardial infarction (STEMI) has been lower than during pre-fibrinolytic years and is estimated to be 0.85%.7 Paradoxically, despite conferring an overall mortality-rate benefit, fibrinolytic agents have been implicated in the accelerated occurrence of LVFWR within the first 24 to 48 h after AMI.8 This may be explained due to the activation of plasmin by thrombolytic drugs, which in turn breaks down collagen. This means that plasmin prevents the repair of infarcted tissue, leaving it fragile and susceptible to rupture.9 The thrombolytic drugs also raise the potential for intramyocardial hemorrhage, which could increase the volume and pressure on the poorly healing heart; this overload can cause the heart to rupture.9
LVFWR following AMI usually occurs in elderly patients between 65 and 70 years of age; however, this may vary.10–12 The majority of our patient collective was of 70 years and above. LVFWR occurs more often in women; other risk factors include arterial hypertension without left ventricular hypertrophy, insufficient collateral network, and delayed thrombolysis. Diabetes mellitus and peripheral vascular disease are less likely in these patients, as these conditions are associated with the development of collateral circulation, which may reduce the possibility of a rupture.13
LVFWR appears in the first week (usually on the fourth or fifth day) post-AMI, although this may vary from few minutes to sometimes more than a month after AMI.1,12,14–16 It is more often reported to occur after the first (transmural) myocardial infarction.1,11,12 Four of our patients who presented with LVFWR had a history of acute myocardial infarction varying between days to a maximum of two weeks. The average time from symptom onset to admission in our patients was 24 h (range up to 94 h). Although the delay from AMI to LVFWR diagnosis is usually several days, there is no clear correlation in terms of mortality and the length of this delay.16 Formica et al. were able to demonstrate that mortality in LVFWR-patients was not affected by early (5–10 h) versus late (≥10 h) presentation; they concluded that hemodynamic status at presentation (cardiac arrest, cardiogenic shock) is the most important predictor for in-hospital mortality.16
Clinically, the patients report prolonged angina lasting for several hours. A delay in hospital admission has also been noticed, which may be mostly due to misdiagnosis. Additional triggering factors may be persistent arterial hypertension (>150 mmHg) during the first 10–24 h of the acute infarction while in hospital and physical exertion such as persistent coughing, vomiting, or agitation.1,11,12
The literature presents conflicting views on the most frequent localization of LVFWR. While some studies reported that the anterior wall was more susceptible, other research shows that the rupture is more common on the lateral or posterior wall.13,16–19 Some authors believe that the helical anatomic structure of the heart ending at the apex might reduce the rate of rupture after AMI in the anterior wall.14 Our findings of 43% anterior LVFWR were consistent with this. In a recent study of patients with LVFWR, the LAD was the culprit lesion in 46% of the cases.14 However, in the latter study non-anterior LVFWR was associated with a significantly higher in-hospital death and late mortality14 and Formica et al. demonstrated in their series that 83% of LVFWR was in the non-anterior location.16 The pathophysiological process of LVFWR involves thinning of the myocardial wall with the intensity of necrosis occurring at the distal end of the vessel (watershed area) where there is often poor collateral flow. The shearing effect of myocardial contraction against a stiffened necrotic area causes a rupture. O’Rourke first classified LVFWR as acute, subacute and chronic with the formation of pseudoaneurysm.1,20
The acute rupture is characterized by prolonged angina pectoris of sudden onset, electro-mechanical dissociation, cardiac tamponade resulting in cardiogenic shock and death within a few minutes. The rapid sequence of events does not permit any possible treatment. Electromechanical dissociation and bradycardia are typical of acute rupture.21
The sub-acute rupture is caused by a small rift on the wall that may be temporarily sealed by a clot. This type of rupture presents with cardiac tamponade, cardiogenic shock and may mimic reinfarction or right ventricular infarction. Electrocardiographically, sub-acute rupture may manifest with an increase of ST-elevation by at least one mV in affected leads, ST elevation in lead aVL as well as non-inversion of the T-wave.1,21
The formation of a pseudoaneurysm in the chronic course occurs when the bleeding is little and limited by peripheral pressure. It is usually detected at surgery or autopsy.
The rupture of the free ventricular wall can also be classified depending on clinical presentation.
“Blow-out” ruptures are manifested with sudden rupture of the infarcted area causing cardiac tamponade and cardiogenic shock,
“Stuttering” ruptures present with varying severity of symptoms without hemodynamic instability. They are characterized by a small rupture with intermittent spontaneous tamponade.1,7
The interpretation of elevated creatine kinase (CK) and creatine kinase-MB (CK-MB) and troponin levels may be quite challenging to distinguish between rupture and reinfarction. However, CRP could pose as an indicator as the levels could quickly increase for the second day and remain high (>20 mg/dl) in AMI and rupture, compared to patients with AMI only, where levels increase much slower and remain low (<10 mg/dl).1,20
CRP levels and maximum troponin values in our series also were significantly higher in LVFWR-patients compared to controls. Several authors discourage cardiac catheterization after diagnosing LVFWR since it unnecessarily delays the operation in these critically ill patients.22 However, in two contemporary case series of LVFWR-patients 91% and 100%,7,14 respectively underwent CAG prior surgical repair, which is comparable to our series where also all patients underwent CAG ahead of the operation.
Non-surgical options of postinfarction LVFWR with pericardial effusion include the instillation of fibrin glue, which induced in a case report focal peri-epicardial adhesions and closure of the rupture.23 Another group reported a percutaneous closure of an inferolateral LVFWR with an Amplatzer septal occluder.24 In acute ruptures, the rapid sequence of events rarely allows for surgical treatment.1 However, surgical repair remains the treatment of choice in subacute ventricular rupture.1,18,25 The optimal surgical treatment is the application of a Teflon or pericardial patch to the epicardial surface of the ruptured site with cyanoacrylate glue.26 In cases of unsuccessful use of a patch, resection of the rupture zone followed by a Teflon buttressed suture could be an option, this may, however, cause an excessive reduction in the size of the ventricular cavity and should, therefore, be critically considered.27 More recently sutureless repair was introduced for the treatment of postinfarction LVFWR.14 However, the series of Okamura et al. included only 6% of blow-out type ruptures, which was the case in 100% of our LVFWR-patients.27
The mortality rate for surgery in acute ruptures is high, but the mortality rate without surgery is virtually 100%.13,28 Recent larger retrospective series of LVFWR-patients reported favorable long-term outcomes at five years of 69% and 53%, respectively.14,16 Overall survival at 10 years in these publications was 63% and 50%.14,16 Some authors recommend when there is a strong suspicion of cardiac rupture, the intrapericardial administration of biological glue following pericardiocentesis.23 This ensures valuable time until the patient is led to the operating room.1 Figueras et al. recommend a conservative approach, particularly in high surgical risk patients such as those with severe chronic lung disease, renal failure, extensive myocardial infarction, or peripheral severe vascular disease. However, the authors stress upon the validation of this approach by a cohort of patients from different institutions.12
Limitations
Our research has several limitations. The retrospective data from our study has been obtained from a single tertiary center in Germany. Also, we believe that the number of patients with LVFWR may be too low to obtain definitive conclusions. However, the low number may be partly attributed to the possibility of patients being transferred to other tertiary centers within the region or may also indirectly point to the impressive advances in timely management in acute myocardial infarction. Lastly, our analysis may be seen as an attempt to shed some light on the condition, initiate discussions, and encourage further research rather than postulating a hypothesis.
Conclusions
Though the incidence of this fatal complication has seen a considerable decline due to primary percutaneous coronary angiography, our observations point to a more prolonged symptom to angiography time as one of the important predictors among the other factors mentioned above as one of the leading causes of this complication. A high level of clinical suspicion is required for the timely diagnosis and successful management of the condition. Thirty-day mortality continues to remain high. Further multicenter research with a higher number of patients may be required to provide further insight into this condition.
Contributorship
SV and MAO contributed in the data collection, data analysis, manuscript drafting and revision, and approval of the final version of the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
Ethical approval requirement was waived. There was no actual patient contact made to collect any personal and clinical information.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Guarantor
SV is the guarantor for this study.
ORCID iD
Marc-Alexander Ohlow https://orcid.org/0000-0001-6412-5935
References
- 1.Exadaktylos NI, Kranidis AI, Argyriou MOet al. Left ventricular free wall rupture during acute myocardial infarction. Early diagnosis and treatment. Hellenic J Cardiol 2002; 43: 246–252. [Google Scholar]
- 2.Willens FA, Dry TJ. A history of the heart and circulation. Philadelphia: WB Saunders, 1948, p. 73. [Google Scholar]
- 3.FitzGibbon GM, Hooper GD, Heggtveit HA. Successful surgical treatment of post-infarction external cardiac rupture. J Thoracic Cardiovasc Surg 1972; 63: 622–630. [PubMed] [Google Scholar]
- 4.Mishra PK, Pathi V, Murday A. Post myocardial infarction free wall rupture. Interact Cardiovasc Thorac Surg 2007; 6: 39–42. [DOI] [PubMed] [Google Scholar]
- 5.Yip HK, Wu CJ, Chang HWet al. Cardiac rupture complicating acute myocardial infarction in the direct percutaneous coronary intervention reperfusion era. Chest 2003; 124: 565–571. [DOI] [PubMed] [Google Scholar]
- 6.Stevenson WG, Linssen GCM, Havenith MGet al. The spectrum of death after myocardial infarction: a necropsy study. Am Heart J 1989; 118: 1182–1188. [DOI] [PubMed] [Google Scholar]
- 7.Sahibzada P, Ali N, Zahidullah M. Ventricular free wall rupture. J Ayub Med Coll Abbottabad 2009; 21: 22–26. [PubMed] [Google Scholar]
- 8.Patel MR, Meine TJ, Lindblad Let al. Cardiac tamponade in the fibrinolytic era: analysis of > 100,000 patients with ST-segment elevation myocardial infarction. Am Heart J 2006; 151: 316–322. [DOI] [PubMed] [Google Scholar]
- 9.Becker RC, Gore JM, Lambrew Cet al. A composite view of cardiac rupture in the United States National Registry of Myocardial Infarction. J Am Coll Cardiol 1996; 27: 1321–1326. [DOI] [PubMed] [Google Scholar]
- 10.London RE, London SB. Rupture of the heart. A critical analysis of 47 consecutive autopsy cases. Circulation 1965; 31: 202–208. [DOI] [PubMed] [Google Scholar]
- 11.Naiem F, De la Maza LM, Robbins SL. Cardiac Rupture during myocardial infarction. A review of 44 cases. Circulation 1972; 45: 1231–1239. [DOI] [PubMed] [Google Scholar]
- 12.Figueras J, Cortadellas J, Soler-Soler J. Left ventricular free wall rupture: clinical presentation and management. Heart 2000; 83: 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman HS, Kuhn LA, Katz AM. Clinical and electrocardiographic features of cardiac rupture following acute myocardial infarction. Am J Med 1971; 50: 709–720. [DOI] [PubMed] [Google Scholar]
- 14.Okamura H, Kimura N, Mieno Met al. Sutureless repair for postinfarction left ventricular free wall rupture. J Thorac Cardiovasc Surg 2019; 158: 771–777. [DOI] [PubMed] [Google Scholar]
- 15.Ohlow MA, Gey EM, Lauer B. Subacute covered perforation. Turk Kardiyol Dern Ars 2013; 41: 268. [DOI] [PubMed] [Google Scholar]
- 16.Formica F, Mariani S, Singh Get al. Postinfarction left ventricular free wall rupture: a 17-year single-center experience. Eur J Cardiothorac Surg 2018; 53: 150–156. [DOI] [PubMed] [Google Scholar]
- 17.O’Rourke MF. Subacute heart rupture following myocardial infarction. Clinical features of a correctable condition. Lancet 1973; 2: 124–126. [DOI] [PubMed] [Google Scholar]
- 18.Figueras J, Alcalde O, Barrabes JAet al. Changes in-hospital mortality rates in 425 patients with acute ST-elevation myocardial infarction and cardiac rupture over a 30-year period. Circulation 2008; 118: 2783–2789. [DOI] [PubMed] [Google Scholar]
- 19.Lateef F, Nimbkar N. Ventricular free wall rupture after myocardial infarction. Hong Kong J Emerg Med 2003; 10: 238–246. [Google Scholar]
- 20.Krumbhaar EB, Crowell C. Spontaneous rupture of the heart. A clinicopathological study based on 22 unpublished cases and 632 from the literature. Am J Med Sci 1925; 170: 828–856. [Google Scholar]
- 21.Pollak H, Diez W, Spiel Ret al. Early diagnosis of subacute free wall rupture complicating acute myocardial infarction. Eur Heart J 1993; 14: 640–648. [DOI] [PubMed] [Google Scholar]
- 22.Raid MH, Kraft CD, Gardner CJet al. Subacute ventricular free wall rupture complicating acute myocardial infarction. Am Heart J 1993; 126: 946–955. [DOI] [PubMed] [Google Scholar]
- 23.Willecke F, Bode C, Zirlik A. Successful therapy of ventricular rupture by percutaneous intrapericardial instillation of fibrin glue: a case report. Case Rep Vasc Med 2013; 2013: 412341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai IC, Lin MC, Jan SLet al. Multidetector row computed tomography for percutaneous closure of a left ventricular free wall rupture after myocardial infarction. J Am Coll Cardiol Cardiovasc Interv 2015; 8: e75–e76. [DOI] [PubMed] [Google Scholar]
- 25.Reardon MJ, Carr L, Diamond Aet al. Ischemic left ventricular free wall rupture: prediction, diagnosis, and treatment. Ann Thorac Surg 1997; 64: 1509–1513. [DOI] [PubMed] [Google Scholar]
- 26.Padro JM, Mesa JM, Silvestre Jet al. Subacute cardiac rupture: repair with a sutureless technique. Ann Thorac Surg 1993; 55: 20–24. [DOI] [PubMed] [Google Scholar]
- 27.Almdahl SM, Hotvedt R, Larsen Uet al. Postinfarction rupture of left ventricular wall repaired with a glued-on pericardial patch. Scand J Thorac Cardiovasc Surg 1993; 27: 105–107. [DOI] [PubMed] [Google Scholar]
- 28.Vanezis AP, Qadery R, Wasil Met al. An unusual presentation of left ventricular free wall rupture following a silent myocardial infarction. BJMP 2009; 2: 41–43. [Google Scholar]