Short abstract
Background
Most of the knowledge about people with multiple sclerosis (PwMS) in France comes from cohorts, which may suffer from recruitment bias or from the unique registry located in Lorraine, East France.
Objective
To describe use of care in the French population of PwMS, over 2010–2015.
Methods
All PwMS in the French national health data system (97% of the general population covered) were included. Demographics, and use of care were described (visits with general practitioners (GPs), neurologists, nurses, physiotherapists and hospitalisations). A focus on the neurological follow-up was also conducted.
Results
A total of 112,415 PwMS were identified (sex ratio F:M = 2.4, median age 46), of whom 5005 died during follow-up. The median numbers of visits with GPs and neurologists were 6.6 and 1.3 respectively per patient-year. Moreover, 53,457 (47.6%) received multiple sclerosis (MS) treatments; about 13% of patients had no neurological follow-up, and 81.8% had at least one hospitalisation.
Conclusions
For the first time in France, this exhaustive dataset offered the opportunity to provide objective figures regarding care practices for MS at the national level, without any selection bias. It also allowed description of patients with MS according to their neurological follow-up, especially those who were absent from cohorts led by neurologists.
Keywords: Multiple sclerosis, care-seeking, administrative database, neurologists, hospital admissions, France
Introduction
France is a high-prevalence area for multiple sclerosis (MS).1 The most recent estimation from 31 December, 2012 was 151.2 per 100,000 inhabitants, with 99,123 people with MS (PwMS).2 This figure came from a four-criterion algorithm applied to the French national health data system (Système National des Données de Santé; SNDS),3 and was used to measure healthcare expenditure related to MS in France.4 Since 2010, this database has covered almost 66 million inhabitants (97% estimated coverage of the general French population), without any socio-economic or demographic restrictions.3 These individual and longitudinal data are prospectively recorded and exhaustive, offering a great opportunity for epidemiological studies as a complement to cohorts, which may only provide a partial vision of PwMS due to potential recruitment bias.
This database was made possible because of the French healthcare system, which is a universal service provided to each citizen irrespective of wealth, age or social status. It is composed of a fully integrated network of public hospitals, private hospitals (also called clinics), healthcare professionals (HCPs) and other medical service providers. Primary healthcare is based on a network of private general practitioners (GPs) that in 2017 had a ratio of 1 GP per 760 inhabitants.5,6 In addition to primary care, specialised care is available in both public and private settings. In 2017, there were 2450 neurologists in France, amongst whom 67% worked in public hospitals and 33% worked out-of-hospital (private practice).5,6 In the last decade, MS expert centres, Centre de ressources et de compétences pour la sclérose en plaques (CRC SEP), gathering multidisciplinary teams including both medical and paramedical HCPs, have emerged in university public hospitals (one per region, amounting to 23 for the whole territory).7 In the French health system, each medical procedure is paid by the patient and then reimbursed by health insurance. A specific status, ‘long-term disease’ (LTD), available upon request, allows for 100% reimbursement of both outpatient and hospital care for a list of 30 diseases, MS being one of them.
Studies on care-seeking for MS in France have focused on an economical perspective, quantifying MS costs for society,4,8 rather than on the level of care consumption of PwMS.9 However, describing the use of care by PwMS, especially the respective roles of GPs and neurologists, as well as the distinction between public and private settings, and the level of hospitalisation related or not to MS would be helpful to identify inequalities, optimise management of MS and define an appropriate care pathway for this chronic disease. Moreover, as opposed to our previous study that was based on only a sample of the French population,9 the SNDS gives access to the entire French population.
In this context, our main objective was to describe the level of care-seeking in the exhaustive population of PwMS in France over the 2010–2015 period using the French national health data system. A secondary objective was to describe the demographical and clinical characteristics of this population.
Materials and methods
Data source
Since 2006, the SNDS has compiled all out-of-hospital reimbursed care consumption (e.g. consultations and home visits with private HCPs, drugs dispensed, and paramedical care).3 Regarding consultations and paramedical care, the date, the specialty of the HCP and the type of care are available.3,9 Drugs are identified using CIP13 (Code Identifiant de Présentation), which is a unique French 13-number identifier for each presentation of a pharmaceutical medicine. This dataset is individually linked to in-hospital data (private and public) from medicine, surgery and obstetrics wards (MSO) and rehabilitation wards (REHAB).3 The latter contains the start and end dates of hospitalisations (including one-day hospitalisations) and the diagnoses established at discharge and coded using the International Classification of Diseases, 10th version (ICD-10).10 Outpatient consultations, that is, consultations held in hospitals, are also included, and assigned by default to GP when medical specialty cannot be determined.
Each patient can be identified by their unique ID that remains with them throughout their lifetime. It implies that if a patient has emigrated during the study period and subsequently returned to France, his/her care consumption could be retrieved and linked to previous data. However, only a small amount of information about the patients themselves is available: gender, birth year, death date, health insurance scheme (general scheme, agricultural workers, self-employed workers and other schemes) and LTD status (according to ICD-10 codes), and its corresponding starting year, if applicable.3 An individual’s CMU (Couverture Maladie Universelle) status,11 which is the universal health insurance run by the general scheme, is also available. The CMU provides access to social protection for people considered to be on a low income, taking the number of patients in the household and the size of the city of residence into consideration. In addition to this individual-level status, the socio-economic level of the area of residence for each patient was estimated using the FDep social deprivation index,12 which is a variable available at city level and categorised in quintiles.
Ethical and data access approvals for the present study were obtained in accordance with French legislation.
Study population
The following criteria, adapted from the most recent French MS prevalence study,2 were used to identify MS cases over the period 2010–2015: (i) at least one reimbursement for an MS-specific disease-modifying therapy (DMT) (beta-interferon, glatiramer acetate, fingolimod, natalizumab, teriflunomide, or dimethyl fumarate); or (ii) an active LTD for MS; or (iii) at least one admission in MSO or REHAB hospitals with a discharge diagnosis of MS, coded ‘G35’. The date of MS identification was defined as the earliest date between: date of the admission into LTD status for MS (potentially anterior to 1 January 2010), the first date of hospitalisation for MS and the first date of DMT prescription. The study population was thus formed of prevalent PwMS, that is, cases present in January 2010 (MS identification date anterior to January 2010), but also of incident cases over the study period, that is, PwMS who were identified between 1 January, 2010 and 31 December, 2015. Therefore, the inclusion date for each PwMS was defined as 1 January, 2010 for prevalent cases, and date of MS identification (comprised within 2010–2015) for incident cases. PwMS were followed up from their inclusion date until their death or 31 December, 2015. People who had no reimbursement over the study period were excluded as this probably corresponded to people who left the covered regimens or who no longer lived in France. To estimate some clinical parameters at inclusion, data was extracted for the years 2009–2015.
Outcomes
The following data was considered: home and outpatient visits with GPs, neurologists, and paramedical encounters with nurses and physiotherapists. Because of uncertainty about the HCP’s specialty in outpatient visits, those performed in hospitals by GPs where grouped with the neurologists’ visits. Hospitalisations in MSO or REHAB wards were analysed separately. Moreover, the ways in (hospital, home, emergency) and out (hospital, home, death) of the MSO ward were quantified. Hospitalisations corresponding to DMT injections and sessions were quantified then excluded from all analyses. All-cause admissions were considered together first, then separately for MS-related (main diagnosis ‘G35’) and non-MS-related admissions.
Neurological follow-up was split into four categories: follow-up with private neurologists only, follow-up with public neurologists only (performed in hospitals, including CRC SEP), mixed follow-up (private and public), and absence of neurological follow-up. The characteristics of the patients were presented according to these categories.
Patients were considered treated if they had at least one prescription dispensed of an MS-specific DMT over the 2010–2015 study period, that is, beta-interferon, glatiramer acetate, teriflunomide, fingolimod, natalizumab and dimethyl fumarate. Neither mitoxantrone, nor cyclophosphamide were available in the database, as they are not recorded in hospital data. Initiations of a DMT as well as DMT stops could occur over the study period. The Charlson comorbidity index, adapted for French administrative databases,13 was calculated in the year before the inclusion date amongst people with sufficient follow-up and then categorised as a four-level score (0, 1–2, 3–4, ≥5).
Statistical analysis
An age–sex pyramid was drawn for the study population and other characteristics were described using proportions or medians and the interquartile range. For each type of care, two kinds of indicators were computed: the number of visits per patient-year and the proportion of patients receiving this care at least once over the study period. In addition, a global parameter summarising the total number of consultations and outpatient visits, regardless of the medical specialty, was computed. Regarding hospitalisations, the median length of stay was calculated and the most frequent hospitalisation diagnoses were presented in a sunburst diagram. The characteristics of PwMS were compared according to their type of neurological follow-up. No statistical tests were realised because of the exhaustiveness of the dataset.
The PwMS who died over the study period were described and their cause of death was approximated using the main diagnosis of the hospital stay for those who died in hospital, and was represented with a sunburst diagram. A classification proposed by our team14 was then used to determine whether death was MS-related.
All analyses were conducted using R (v.3.4.3).15
Results
Characteristics of the population
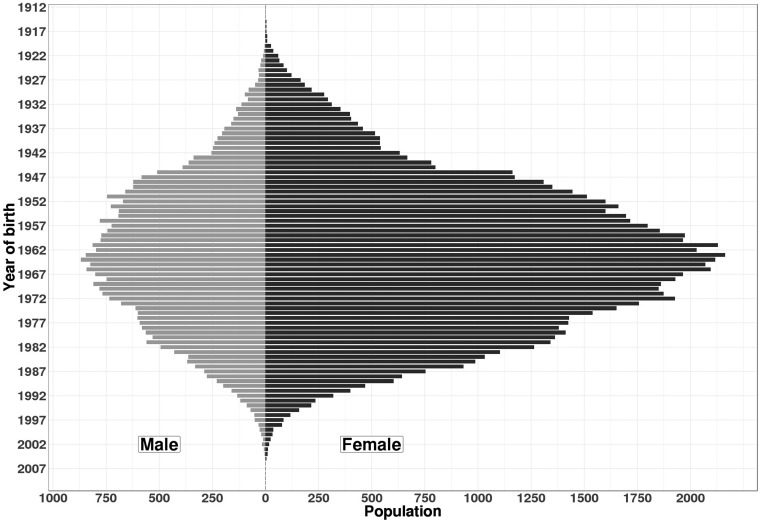
Overall, 112,415 patients were identified as having MS in France over the period 2010–2015. The sex ratio F:M was 2.4 and the median age in 2010 was 46 (36–57). The age–sex distribution is presented in Figure 1, and additional characteristics in Table 1. Amongst the 103,455 (92.0%) patients with a Charlson index available, one-fifth (n = 19,818; 19.2%) had at least one comorbid condition, chronic pulmonary disease being the most frequent (n = 6923; 6.2%). At least one MS treatment was identified for 53,457 (47.6%) PwMS.
Figure 1.
Age–sex pyramid of patients with MS in France identified over 2010–2015 (N = 112,415).
Table 1.
Characteristics of the 112,415 patients with MS and of the 5005 deaths observed over the 2010–2015 study period.
| Overall population (N = 112,415) | |
| Women, n (%) | 79,735 (70.9%) |
| Year of birth* | 1964 (1953–1974) |
| Age at MS identification* (years) | 40 (31–50) |
| Approximated MS duration at inclusion*,a (years) | 2.5 (0.0–9.8) |
| Study follow-up duration*,b (years) | 6.0 (3.9–6.0) |
| Deaths, n (%) | 5005 (4.5%) |
| Health insurance scheme, n (%) | |
| General scheme excluding CMU beneficiaries | 98,359 (87.5%) |
| Agricultural workers | 4148 (3.7%) |
| Self-employed workers | 4242 (3.8%) |
| CMU beneficiaries | 4258 (3.8%) |
| Other schemes | 1408 (1.3%) |
| Charlson comorbidity indexc, n (%) | |
| Missing | 8960 (8.0%) |
| 0 | 83,637 (74.4%) |
| 1–2 | 17,159 (15.3%) |
| 3–4 | 2156 (1.9%) |
| ≥5 | 503 (0.4%) |
| Deprivation index of the city of residence in 2009, n (%) | |
| Missing | 1520 (1.4%) |
| 1st quintile (most favoured) | 21,422 (19.1%) |
| 2nd quintile | 22,193 (19.7%) |
| 3rd quintile | 22,318 (19.9%) |
| 4th quintile | 22,479 (20.0%) |
| 5th quintile (most deprived) | 22,483 (20.0%) |
| Patients dying over the study period (n = 5005) | |
| Women, n (%) | 3057 (61.1%) |
| Age at death* (years) | 67.0 (58.0–78.0) |
| Age at MS identification* (years) | 53.0 (42.0–65.0) |
| Time from LTD admission* (n = 3711) (years) | 14.0 (7.0–21.0) |
| Charlson comorbidity indexc, n (%) | |
| Missing | 209 (4.2%) |
| 0 | 2708 (54.1%) |
| 1–2 | 1523 (30.4%) |
| 3–4 | 396 (7.9%) |
| ≥5 | 169 (3.4%) |
| Place of death, n (%) | |
| Unknown | 93 (1.9%) |
| At hospital | 3102 (62.0%) |
| At home | 1903 (38.0%) |
| Cause of death of patients dying at hospital (N = 3102)d, n (%) | |
| MS-related | 1025 (33.0%) |
| Non-MS-related | 2077 (67.0%) |
| Total number of access to care in the previous year | |
| All medical specialties (private or public)* | 14.0 (7.0–22.0) |
| Of whom visits to GP* | 10.0 (4.0–16.0) |
| At least one hospitalisation in MSO, n (%) | |
| All diagnoses | 4263 (85.2%) |
| MS-related | 716 (14.3%) |
| Length of stay in MSOe (days) | |
| All diagnoses* | 21.0 (8.0–43.0) |
| MS-related* | 7.0 (2.0–19.0) |
MS = multiple sclerosis; CMU = universal health insurance (Couverture Maladie Universelle); GP = general practitioner; MSO = medicine, surgery or obstetrics.
*Median (q1–q3). aTime from MS identification until inclusion date; btime from inclusion date until end of follow-up; cbased on data from the 12 months preceding study entry if available; daccording to the algorithm presented in Kingwell et al.14; eonly for patients having at least one hospitalisation.
Use of healthcare services
The results show a high use of health resources (Table 2), with a median number of visits to an HCP of 16.3 (10.2–26.9) per patient-year. The predominant place was attributed to GPs with 6.6 (3.7–12.0) visits per patient-year. Moreover, over the 6 years, 71.2% and 75.1% of PwMS had at least one annual visit with a GP or a neurologist, respectively. Over the study period, 40,247 (35.7%) PwMS went to a CRC SEP at least once. The presence of comorbidities was correlated with increased care-seeking, both medical and paramedical, and both in and out-of-hospital, as indicated in Table 2.
Table 2.
Care-seeking of the MS population over the 2010–2015 period (N = 112,415) and according to the Charlson comorbidity index (data missing for 8960 patients).
| TotalN = 112,415 | Charlson index <3n = 100,796 | Charlson index ≥3n = 2659 | |
|---|---|---|---|
| At least one visit to, n (%) | |||
| GP | 109,963 (97.8%) | 99,341 (98.6%) | 2616 (98.4%) |
| Private neurologist | 62,097 (55.2%) | 56,912 (56.5%) | 1096 (41.2%) |
| Public neurologista | 78,367 (69.7%) | 70,538 (70.0%) | 1989 (74.8%) |
| Private or public neurologista | 98,058 (87.2%) | 88,582 (87.9%) | 2225 (83.7%) |
| Nurse | 94,664 (84.2%) | 86,420 (85.7%) | 2437 (91.7%) |
| Physiotherapist | 61,332 (54.6%) | 56,633 (56.2%) | 1952 (73.4%) |
| Number of visits per patient-year | |||
| GP* | 6.6 (3.7–12.0) | 6.8 (3.8–12.2) | 11.0 (6.2–20.2) |
| Private neurologist* | 0.2 (0.0–1.7) | 0.2 (0.0–1.7) | 0.0 (0.0–0.7) |
| Public neurologist*,a | 0.4 (0.0–1.2) | 0.3 (0.0–1.2) | 0.5 (0.0–1.4) |
| Private or public neurologist*,a | 1.3 (0.4–2.7) | 1.3 (0.4–2.7) | 1.0 (0.3–2.4) |
| Nurse* | 3.3 (0.5–14.2) | 3.5 (0.7–14.8) | 16.9 (3.2–128.3) |
| Physiotherapist* | 1.3 (0.0–26.5) | 1.7 (0.0–28.5) | 14.3 (0.0–73.9) |
| All medical specialties (private or public)* | 16.3 (10.2–26.9) | 16.8 (10.8–27.2) | 25.7 (15.9–44.9) |
| At least one hospitalisation in MSO, n (%) | |||
| All diagnoses | 92,007 (81.8%) | 82,548 (81.9%) | 2537 (95.3%) |
| MS-related | 44,125 (39.3%) | 39,364 (39.1%) | 873 (32.8%) |
| Length of stay in MSOb (days per patient-year) | |||
| All diagnoses* | 2.3 (0.8–5.9) | 2.2 (0.8–5.7) | 8.7 (3.2–23.6) |
| MS-related* | 1.1 (0.5–2.5) | 1.1 (0.5–2.5) | 1.5 (0.6–3.7) |
| At least one hospitalisation in REHAB, n (%) | |||
| All diagnoses | 27,236 (24.2%) | 24,801 (24.6%) | 1213 (45.6%) |
| MS-related | 20,117 (17.9%) | 18,664 (18.5%) | 558 (21.0%) |
| Length of stay in REHABb (days per patient-year) | |||
| All diagnoses* | 7.7 (3.2–17.8) | 7.3 (3.2–17.3) | 13.0 (5.2–31.7) |
| MS-related* | 6.3 (2.7–14.7) | 6.3 (2.5–14.5) | 8.2 (3.8–18.0) |
| Received MS-specific DMT at least once, n (%) | 53,455 (46.7%) | 48,440 (48.1%) | 520 (19.6%) |
Charlson comorbidity index <3 and ≥3 correspond to the PwMS having a Charlson index based on data from the 12 months preceding their entry in the study, if available, strictly lower than 3 or greater than 3, respectively.
MS = multiple sclerosis; GP = general practitioner; MSO = medicine, surgery or obstetrics; REHAB = rehabilitation.
*Median (q1–q3). aPublic neurologists and public GPs altogether; bonly for patients having at least one hospitalization.
Neurological follow-up
About 38% of patients had a mixed neurological follow-up (private and public), about 32% had a follow-up with public neurologists only, about 17% had a follow-up with private neurologists only, while the remaining 13% had an absence of neurological follow-up (Table 3). Patients without any neurological follow-up were older and had a longer disease duration. Moreover, patients followed up by public neurologists generally lived in the most deprived areas; conversely, those followed up by private neurologists lived in some of the most wealthiest areas. We also noted that 3119 patients (5.8% of treated patients) did not visit a neurologist but were treated, even though DMTs can only be prescribed by this medical specialty.
Table 3.
Comparison of patient characteristics according to the type of neurological follow-up (N = 112,415).
| Private only | Public only | Mixed | Absence | |
|---|---|---|---|---|
| 19,691 (17.5%) | 35,961 (32.0%) | 42,406 (37.7%) | 14,357 (12.8%) | |
| Women, n (%) | 14,509 (73.7%) | 24,591 (68.4%) | 30,596 (72.2%) | 10,039 (69.9%) |
| Age at MS identification* (years) | 41 (33–50) | 40 (31–50) | 39 (30–48) | 44 (34–55) |
| Year of birth* | 1963 (1954–1972) | 1964 (1953–1975) | 1966 (1956–1976) | 1957 (1946–1968) |
| Approximated MS duration at inclusion*,a (years) | 3.2 (0.0–9.6) | 2.3 (0.0–10.1) | 1.5 (0.0–8.4) | 5.7 (0.0–13.6) |
| Study follow-up duration*,b (years) | 6.0 (4.3–6.0) | 6.0 (3.8–6.0) | 6.0 (3.8–6.0) | 6.0 (4.0–6.0) |
| Received MS-specific DMT at least once, n (%) | 10,866 (55.2%) | 15,157 (42.1%) | 24,313 (57.3%) | 3119 (21.7%) |
| Health insurance scheme, n (%) | ||||
| General scheme excluding CMU beneficiaries | 17,401 (88.4%) | 30,930 (86.0%) | 37,531 (88.5%) | 12,497 (87.0%) |
| Agricultural workers | 786 (4.0%) | 1492 (4.1%) | 1242 (2.9%) | 628 (4.4%) |
| Self-employed workers | 778 (3.9%) | 1618 (4.5%) | 1258 (3.0%) | 588 (4.1%) |
| CMU beneficiaries | 540 (2.7%) | 1336 (3.7%) | 1964 (4.6%) | 418 (2.9%) |
| Other schemes | 186 (0.9%) | 585 (1.6%) | 411 (1.0%) | 226 (1.6%) |
| Charlson comorbidity indexc, n (%) | ||||
| Missing | 1411 (7.2%) | 3162 (8.8%) | 2678 (6.3%) | 1709 (11.9%) |
| 0 | 15,664 (79.5%) | 25,558 (71.1%) | 32,464 (76.6%) | 9951 (69.3%) |
| 1–2 | 2380 (12.1%) | 6139 (17.1%) | 6404 (15.1%) | 2263 (15.8%) |
| 3–4 | 193 (1.0%) | 893 (2.5%) | 728 (1.7%) | 342 (2.4%) |
| ≥5 | 43 (0.2%) | 236 (0.7%) | 132 (0.3%) | 92 (0.6%) |
| Visits to GP per patient-year* | 5.7 (3.2–10.1) | 6.7 (3.5–12.2) | 7.5 (4.3–13.1) | 5.2 (2.3–10.5) |
| Visits to neurologists per patient-year*,d | 1.9 (0.7–3.0) | 0.8 (0.3–1.7) | 2.4 (1.4–3.8) | – |
| All medical specialties per patient-year* (private or public) | 15.2 (9.8–24.0) | 15.7 (9.7–26.2) | 19.0 (12.5–30.4) | 11.7 (6.3–20.8) |
MS = multiple sclerosis; CMU = universal health insurance (Couverture Maladie Universelle); DMT = disease-modifying therapy; GP = general practitioner.
*Median (q1–q3). aTime from MS identification until inclusion date; btime from inclusion date until end of follow-up; cbased on data from the 12 months preceding study entry, if available; dprivate and public neurologists, or public GPs.
Hospital stays
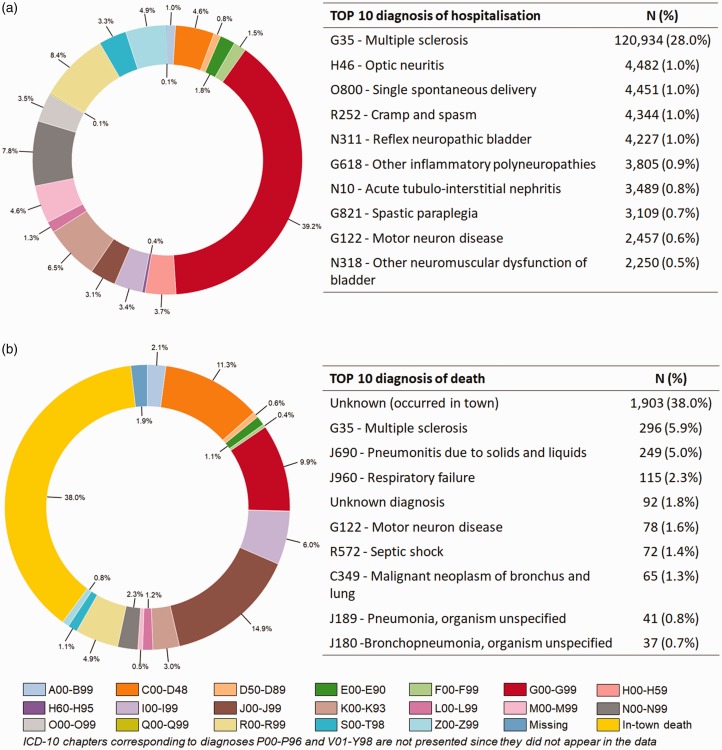
On the whole, 1,002,809 hospital admissions in MSO were extracted for 2010–2015. Injections of a DMT for MS (461,595; 46.0%) were excluded first, of which 249,816 (54.1%) were natalizumab injections (in 7529 patients); the remainder (211,779; 45.9%) were unspecified but were probably corticosteroid injections or mitoxantrone infusions. Secondly, 109,973 (11.0%) hospital stays were excluded because they corresponded to recurring therapeutic sessions outside MS (such as dialysis or cancer chemotherapy). After these exclusions (431,241 hospitalisations remaining), a large proportion of hospitalisations was not related to MS (310,225; 71.9%). The top 10 ICD-10 hospitalisations are presented in Figure 2. Moreover, we found a lower proportion of admissions through the emergency ward in MS-related than in non-MS-related admissions (9.1% versus 22.0%) (see Figure S1 in supplementary material). Deaths occurred more frequently during non-MS-related (0.8%) than in MS-related admissions (0.2%).
Figure 2.
Diagnoses related to (a) hospitalisations in MSO ward (except sessions and treatment injections) (N = 431,610) and (b) hospitalisations ending in death (n = 5005) over the 2010-2015 period.
Deaths
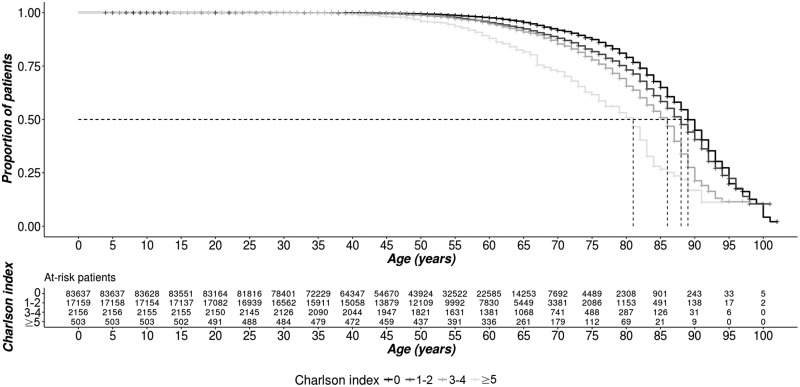
Over the 6-year study period, 5005 deaths occurred. As summarised in Table 1, the median age at death was 67.0 and almost half (43.5%) had a Charlson index score equal to or greater than 1, which is much higher than in the total population (19.2%). The association between the Charlson index score and the probability of dying was illustrated through Kaplan–Meier curves (Figure 3). Care-seeking with GPs represented the most important part of consultations in the year prior to death (75.0% in median) and most hospitalisations were unrelated to MS.
Figure 3.
Survival according to the Charlson comorbidity index (N = 112,415).
Causes of death were summarised using a sunburst diagram (Figure 2). The most common causes of death were diseases of the respiratory system (24.0%), and neoplasms including cancers, representing 18.2% of deaths. About one-third of in-hospital deaths could be considered as MS-related.
Discussion
To our knowledge, this is the first study conducted on the entire population of PwMS in France that provides a precise description of this population and an overview of care practices for MS at national level. The previous studies came either from the French health insurance system restricted to salaried workers,2,4 or from the French Observatory for MS (OFSEP; Observatoire Français de la Sclérose En Plaques)16 in which data collection is mainly based upon the CRC SEP or from the Lorraine registry.17–19 The use of SNDS allowed access to patients without any neurological follow-up or followed exclusively in private settings, which represented 14,357 patients (12.8%) and 19,691 (17.5%), respectively.
We found a notable level of visits to HCPs with 16.3 consultations per patient-year, significantly higher than the 6.5 visits per year reported in the French general population.20 These observations are in accordance with previous French9 and Canadian21,22 studies showing a higher level of care-seeking in PwMS compared with the general population. In the present study, GPs were the most frequently visited HCPs with a median of 6.6 consultations per patient-year. This central role is in line with the fact that MS is a chronic disease with many different symptoms and has an impact on daily life. Moreover, MS is frequently associated with comorbid conditions, which are more prevalent than in the general population23 and their prevalence increases care consumption,24,25 as confirmed in the present study by the annual number of visits with GPs increasing to 11.0.
One-third of PwMS were identified as having at least one visit to a CRC SEP during the study period, which corresponded with a previous French study.19 This result highlights the risk of recruitment bias in hospital-based databases, favouring active or severe MS and people with a high level of care-seeking19,26 or more complicated needs.19,26
Concerning hospitalisations, the low number of admissions through the emergency ward (9.1%) probably reflects that the majority of MS-related admissions were planned; the emergency entrances were likely to be related to episodes of relapse or disease worsening. Regarding mortality data, MS was the principal cause of death in 9.5% of in-hospital deaths, which also corresponds with the results from the literature.27 However, this result is probably underestimated since the cause of death was missing for one-third of the population, that is, the ones dying out-of-hospital. We did not find any specific distribution of MS in the five categories of the social deprivation index.
Neurological follow-up appeared to be mainly mixed and public, which is not surprising as there are twice as many neurologists in public hospitals than in private offices28 and due to the fact that CRC SEP are located in university hospitals. Because the medical specialty is not always mentioned in hospital data, we have considered hospital visits to GPs as visits to public neurologists. This strong assumption may thus lead to an overestimation of the level of care consumption with neurologists, but we do think that it may introduce a smaller bias than excluding all the outpatient visits with GPs. Indeed, in France, most of the MS expert neurologists are based in hospitals and account for a large part of care of PwMS.7 We also found that about 13% of patients had no neurological follow-up at all over the 6-year study period. In our opinion, this figure is plausible as it may correspond to several categories of patient: people with evolved MS, that is, highly disabled; elderly people living in nursing homes; and those who are mainly followed by GPs and symptom specialists. An unexpected result was the percentage of patients in this group receiving DMT; this may reflect either data entry errors or misuse of medications. Ongoing work is being carried out on the use of DMTs, with the aim of exploring therapeutic sequences over time and evaluating the impact of the arrival of new drugs on the market.
The use of the SNDS offered us the opportunity to describe the nearly exhaustive population of PwMS living in France. Indeed, 97% of the general French population is covered,3 which means that no selection or recruitment bias is anticipated, as already mentioned. This represents a significant advantage over other health insurance systems, such as those in the United States (Medicaid, Medicare), and over other French MS data sources, such as the OFSEP cohort. The latter is a network of French expert centres that in 2016 had an estimated MS patient coverage of 46.3% [44.8–47.8]29 and is not therefore considered to reflect the variety of French care practices. Moreover, administrative data are automatically and systematically collected in the SNDS, and therefore, not dependent from the completion of a database by neurologists or research assistants. This system thus drives the risk of having missing data close to zero. However, no clinical data are available in the SNDS as it is primarily an economic database. For instance, we did not have the date of MS onset, MS clinical form, relapse occurrence, or the disability level; data that is reliably available in the OFSEP database.16 Finally, complex administrative data need significant expertise and the use of several hypotheses to build the appropriate epidemiological indicators.
Due to the absence of neurologist-based data collection, there is a risk of false-positive cases in the dataset, that is, people being misclassified as PwMS. Indeed, we used an algorithm previously developed in France,2 but which has not yet been validated. Overall, 26.7% of the PwMS population was identified using a single source. In our opinion, LTD status and DMT prescription are robust criteria, but the criterion based on hospitalisations may raise issues due to the risk of errors in data entry, and the risk of a suspected MS diagnosis that is not confirmed later in the patient’s history. This may apply especially to patients with only one hospitalisation (7586 cases; 6.7%). Nonetheless, regarding case ascertainment, the risk of false negatives, that is, of MS patients not having been identified, is very low; indeed, this would mean that they did not use any care related to MS for more than six consecutive years, which seems highly improbable. For this reason, we chose to exclude people that had no care consumption over the study period (391 cases; 0.3%), which may have corresponded to people who were not covered by health insurance or to benign MS or misdiagnosis.
To conclude, the present study confirms that the SNDS is a useful data source for epidemiological and public health purposes. It is one of the largest datasets of MS worldwide. However, given its aforementioned limitations, our intention is now to link this dataset to the OFSEP cohort to combine the respective advantages of both data sources.
Supplementary Material
Acknowledgements
The authors acknowledge the Institut National des Données de Santé and the Commission Nationale Informatique et Libertés for use of data (Authorization DE-2017-026). The authors also thank Maximilien Genard-Walton for proofreading the manuscript.
Contributor Information
J. Roux, Univ Rennes, EHESP, REPERES (Pharmacoepidemiology and health services research-EA 7449), Rennes, France Univ Rennes, CHU Rennes, Inserm, CIC (Centre d’Investigation Clinique de Rennes), Rennes, France.
A. Guilleux, Univ Rennes, EHESP, REPERES (Pharmacoepidemiology and health services research-EA 7449), Rennes, France
M. Lefort, Univ Rennes, EHESP, REPERES (Pharmacoepidemiology and health services research-EA 7449), Rennes, France Univ Rennes, CHU Rennes, Inserm, CIC (Centre d’Investigation Clinique de Rennes), Rennes, France.
E. Leray, Univ Rennes, EHESP, REPERES (Pharmacoepidemiology and health services research-EA 7449), Rennes, France; Univ Rennes, CHU Rennes, Inserm, CIC (Centre d’Investigation Clinique de Rennes), Rennes, France.
Conflict of Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the French National Agency for Medicines and Health Products Safety (Agence Nationale de Sécurité du Médicament).
ORCID iD
References
- 1.MS International Federation. Atlas of MS, http://www.msif.org/about-us/advocacy/atlas/ (2015, accessed 1 July 2019).
- 2.Foulon S, Maura G, Dalichampt M, et al. Prevalence and mortality of patients with multiple sclerosis in France in 2012: a study based on French health insurance data. J Neurol 2017; 264: 1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions: from the système national d’information interrégimes de l’Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Rev Epidemiol Sante Publique 2017; 65: S149–S167. [DOI] [PubMed] [Google Scholar]
- 4.Lefeuvre D, Rudant J, Foulon S, et al. Healthcare expenditure of multiple sclerosis patients in 2013: a nationwide study based on French health administrative databases. Mult Scler J Exp Transl Clin 2017; 3: 2055217317730421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mourgues J-M. Atlas of the medical demography in France on 1 January 2017. Paris: Conseil National de L’Ordre des Medecins, 2018. [Google Scholar]
- 6.Institut National de la Statistique et des Études Économiques. Population estimates on 1 January 2018 by regions, departments, sex, and age from 1975 to 2018. Paris: Institut national de la statistique et des études économiques, https://www.insee.fr/fr/statistiques/1893198 (2018, accessed 1 July 2019). [Google Scholar]
- 7.Derache N, Dufay A, Lebarbey C. Organization of care for multiple sclerosis in France. Rev Neurol (Paris) 2018; 174: 475–479. [DOI] [PubMed] [Google Scholar]
- 8.Fromont A, Lehanneur M-N, Rollot Fet al. Cost of multiple sclerosis in France. Rev Neurol (Paris) 2014; 170: 432–439. [DOI] [PubMed] [Google Scholar]
- 9.Roux J, Grimaud O, Leray E. Use of state sequence analysis for care pathway analysis: the example of multiple sclerosis. Stat Methods Med Res 2019; 28: 1651–1663. [DOI] [PubMed] [Google Scholar]
- 10.Agence Technique de l’Information sur l’Hospitalisation (ATIH). International Classification of Diseases, 10th version (ICD-10) for the PMSI in France, 2015.
- 11.Fonds de financement de la protection complémentaire de la couverture universelle du risque maladie. Aid schemes to support healthcare access, 2018.
- 12.Rey G, Jougla E, Fouillet A, and Hémon D. Ecological association between a deprivation index and mortality in France over the period 1997 - 2001: Variations with spatial scale, degree of urbanicity, age, gender and cause of death. BMC Public Health 2009; 9(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bannay A, Chaignot C, Blotière PO, et al. The best use of the Charlson comorbidity index with electronic health care database to predict mortality. Med Care 2016; 54: 188–194. [DOI] [PubMed] [Google Scholar]
- 14.Kingwell E, Leray E, Zhu F, et al. Multiple sclerosis: effect of beta interferon treatment on survival. Brain 2019; 142: 1324–1333. [DOI] [PubMed] [Google Scholar]
- 15.R Core Team. R: A Language and Environment for Statistical Computing, http://www.r-project.org/ (2015, accessed 1 July 2019).
- 16.Vukusic S, Casey R, Rollot F, et al. Observatoire Français de la Sclérose en Plaques (OFSEP): a unique multimodal nationwide MS registry in France. Mult Scler 2018:135245851881560. [DOI] [PubMed] [Google Scholar]
- 17.El Adssi H, Debouverie M, Guillemin F, et al. Estimating the prevalence and incidence of multiple sclerosis in the Lorraine region, France, by the capture-recapture method. Mult Scler 2012; 18: 1244–1250. [DOI] [PubMed] [Google Scholar]
- 18.Chartier N, Epstein J, Soudant M, et al. Clinical follow-up of 411 patients with relapsing and progressive multiple sclerosis 10 years after discontinuing mitoxantrone treatment: a real-life cohort study. Eur J Neurol 2018; 25: 1439–1445. [DOI] [PubMed] [Google Scholar]
- 19.Debouverie M, Laforest L, Van Ganse E, et al. Earlier disability of the patients followed in multiple sclerosis centers compared to outpatients. Mult Scler 2009; 15: 251–257. [DOI] [PubMed] [Google Scholar]
- 20.Organisation for Economic Cooperation and Development (OECD) Health Statistics. Health care utilisation, http://www.oecd-ilibrary.org/social-issues-migration-health/data/oecd-health-statistics/oecd-health-data-health-care-utilisation_data-00542-en (2016, accessed 1 July 2019).
- 21.Pohar SL, Jones CA, Warren S, et al. Health status and health care utilization of multiple sclerosis in Canada. Can J Neurol Sci 2007; 34: 167–174. [DOI] [PubMed] [Google Scholar]
- 22.Marrie RA, Yu N, Wei Y, et al. High rates of physician services utilization at least five years before multiple sclerosis diagnosis. Mult Scler J 2013; 19: 1113–1119. [DOI] [PubMed] [Google Scholar]
- 23.Capkun G, Dahlke F, Lahoz R, et al. Mortality and comorbidities in patients with multiple sclerosis compared with a population without multiple sclerosis: an observational study using the US Department of Defense administrative claims database. Mult Scler Relat Disord 2015; 4: 546–554. [DOI] [PubMed] [Google Scholar]
- 24.Marrie RA, Elliott L, Marriott J, et al. Comorbidity increases the risk of hospitalizations in multiple sclerosis. Neurology 2014; 84: 350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKay KA, Marrie RA, Fisk JD, et al. Comorbidities are associated with altered health services use in multiple sclerosis: a prospective cohort study. Neuroepidemiology 2018; 51: 1–10. [DOI] [PubMed] [Google Scholar]
- 26.McKay KA, Tremlett H, Zhu F, et al. A population-based study comparing multiple sclerosis clinic users and non-users in British Columbia, Canada. Eur J Neurol 2016; 23: 1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harding K, Anderson V, Williams O, et al. A contemporary study of mortality in the multiple sclerosis population of south east Wales. Mult Scler Relat Disord 2018; 25: 186–191. [DOI] [PubMed] [Google Scholar]
- 28.Direction de la recherche, des études de l’évaluation et des statistiques (DREES). DATA.DREES - Etudes et statistiques, http://www.data.drees.sante.gouv.fr/ReportFolders/reportFolders.aspx (2018, accessed 1 July 2019).
- 29.Casey R, Rollot F, Clanet M, et al. Observatoire Français de la Sclérose en Plaques (OFSEP): a powerful epidemiological tool. Mult Scler J 2017; 23: 85–426. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.