Abstract
In 2015, we identified a non-groupable clade of Neisseria meningitidis (Nm) that causes urethritis in men (the US_NmUC). Because repeat infection is common with Neisseria gonorrhoeae, we examined whether reinfection also occurs with the US_NmUC. We provide evidence that men are susceptible to repeat episodes of urethritis from the US_NmUC.
Keywords: Neisseria meningitidis, Neisseria gonorrhoeae, urethritis
Summary for Table of Contents
This study reports that similar to Neisseria gonorrhoeae, men can develop repeat episodes of symptomatic urethritis due to a non-groupable and monophyletic urethritis clade of Neisseria meningitidis (the US_NmUC).
INTRODUCTION
In 2015, a large cluster of symptomatic urethral infections caused by a non-groupable and monophyletic urethritis clade (UC) of Neisseria meningitidis (Nm) [the US_NmUC] emerged among primarily Black, heterosexual men seeking care at sexually transmitted disease (STD) clinics in Columbus, Ohio, Oakland County, Michigan, and other US sites.1–5 Subsequent multi-locus sequence typing and whole genome sequencing (WGS) analysis demonstrated that the US_NmUC belongs to the sequence type (ST)-11 clonal complex (cc11), 11.2 sub-lineage, and contains an insertion/deletion event at the capsule locus which abrogates serogroup C capsule expression and confers a non-groupable phenotype. The US_NmUC has also acquired genes from Neisseria gonorrhoeae (Ng), the cause of the common STD gonorrhea, including an intact and functional gonococcal denitrification pathway (norB-aniA locus).2–5 Similar to other reports of confirmed meningococcal urethritis,6,7 oral-genital contact (fellatio) is the most likely mode of transmission of the US_NmUC from the oropharynx to the male urethra.1,2
Repeat episodes of gonococcal urethritis are well documented, including infections with homologous strains.8,9 In order to determine whether the same phenomenon occurs with the US_NmUC, we examined men who presented for care at the STD clinic in Columbus, Ohio over a 40-month period to see if they experienced repeat episodes of urethritis due to the US_NmUC.
MATERIALS AND METHODS
For this analysis, we extracted demographic and clinical data from electronic medical records for men diagnosed with urethritis confirmed to be caused by the US_NmUC between 1 January 2015 and 30 April 2018. The process of urethral Nm screening and US_NmUC identification and characterization at the STD clinic in Columbus, Ohio has been previously described.1,2,4 Briefly, we collected urethral swabs for Gram stain analysis from men screened at the STD clinic, regardless of reported symptoms or behavioral risks. Swabs in which Gram-negative intracellular diplococci were visualized were cultured using media selective for Neisseria spp. Culture growth was confirmed to be Nm by Analytical Profile Index (API NH) (bioMérieux) and Nm-specific PCR. US_NmUC identification and characterization was performed by clade-specific PCR, WGS, and phylogenetic analysis. We examined demographic, clinical and epidemiological characteristics of all patients, comparing men with repeat episodes of urethritis due to the US_NmUC to those who experienced only one episode. We assessed categorical variables using exact tests (e.g. Fisher Exact test) for small sample sizes and compared medians of continuous variables using the Kruskal-Wallis test.
US_NmUC isolates from men with repeat episodes of urethritis underwent genomic analysis to determine sequence similarity between the first and second isolate. Genomic DNA was extracted with Qiagen genomic tip 20. Whole genome sequencing was done with the PacBio platform and then assembled with Canu using default settings. Mean coverages of all genomes were > 40X and the largest contig for each isolate was close to 2.2 Mb. A phylogenetic tree was constructed using the Tree Building Service at PATRIC (https://www.patricbrc.org/app/PhylogeneticTree). The option of “All Shared Proteins”, which discovers single-copy homology groups by BLAST and analyzes protein alignments by the RAxML program10, was applied with the genome of FAM18 (reference Nm serogroup C cc11 strain) as the out-group and the phylogenetic tree rendered with iTOL.11 Using the genome comparator tool in PubMLST and core genome allele coding sequences, we generated a concatenated sequence file from each genome to use for alignment and calculation of sequence identity and distance matrix of allelic polymorphism.12 The study was approved by the Ohio State University Institutional Review Board.
RESULTS
During the study period, we identified 133 episodes of urethritis caused by the US_NmUC among 128 unique men seeking STD care. More than half (57%), 73 men, returned at least once to the STD clinic during the follow-up period. Of these, 5 (7%) had a documented repeat episode of symptomatic urethritis in which US_NmUC was confirmed to be the cause (Table 1). Four of the men with repeat infections were non-Hispanic Black and one was White. They had a median age of 31 years (range: 23–39 years). All reported heterosexual orientation. All five reported oral-genital contact (two received fellatio; the remaining three reported “oral sex” but the type of contact was not defined) with at least one sex partner prior to the first episode of urethritis due to the US_NmUC; four out of five also reported oral-genital contact (three fellatio; one not defined) in the period before the second episode. Only one had documented prior meningococcal vaccination (he had received a quadrivalent MenACWY vaccine). The median duration of time between the onset of symptoms and presentation to the STD clinic, for both the first and second episodes of urethritis due to the US_NmUC, was 3 days (range: 1–6 days). The median duration of time between the first and second episode was 273 days (range: 83–576 days). Only one patient had an STD clinic visit in between his first and second episodes of urethritis. All five men received effective treatment with intramuscular ceftriaxone (250 mg) and oral azithromycin (1 gram) at each episode.1,2,13,14 Across all characteristics, men who experienced repeat episodes of urethritis due to the US_NmUC were similar to men who experienced only one episode (Table 1).
Table 1.
Patient characteristics at first episode of urethritis due to the US_NmUC, comparing men who experienced repeat US_NmUC infections (N=5) to men who did not have repeat infections (N=123), 2015–2018
| No repeat infection | Repeat infection | p-value* | |||
|---|---|---|---|---|---|
| Patient characteristics at first US_NmUC urethritis episode | N=123 | (%) | N=5 | (%) | |
| Race† | |||||
| Black | 105 | (85) | 4 | (80) | 0.56 |
| White | 14 | (11) | 1 | (20) | 0.47 |
| Pacific Islander | 2 | (2) | 0 | (0) | 1.00 |
| Asian | 1 | (1) | 0 | (0) | 1.00 |
| Native American | 1 | (1) | 0 | (0) | 1.00 |
| Other race, not specified | 5 | (4) | 0 | (0) | 0.65 |
| Missing | 1 | (1) | 0 | (0) | 1.00 |
| Ethnicity | 0.22 | ||||
| Hispanic | 9 | (7) | 0 | (0) | |
| Non-Hispanic | 110 | (89) | 4 | (80) | |
| Missing | 4 | (3) | 1 | (20) | |
| Marital Status | 0.71 | ||||
| Single | 87 | (71) | 4 | (80) | |
| Married/Partnered | 14 | (11) | 1 | (20) | |
| Divorced/Legally Separated | 10 | (8) | 0 | (0) | |
| Widowed | 1 | (1) | 0 | (0) | |
| Missing | 11 | (9) | 0 | (0) | |
| Education | 0.44 | ||||
| Elementary School | 1 | (1) | 0 | (0) | |
| Some high school | 9 | (7) | 0 | (0) | |
| High school graduate/GED | 60 | (49) | 1 | (20) | |
| Some college | 31 | (25) | 3 | (60) | |
| College graduate | 16 | (13) | 1 | (20) | |
| Missing | 6 | (5) | 0 | (0) | |
| Sexual Orientation | 0.95 | ||||
| Heterosexual | 115 | (94) | 5 | (100) | |
| Gay | 4 | (3) | 0 | (0) | |
| Bisexual | 3 | (2) | 0 | (0) | |
| Missing | 1 | (1) | 0 | (0) | |
| Meningococcal vaccination status | |||||
| MCV4 | 14 | (11) | 1 | (20) | 0.47 |
| MPSV4 | 0 | (0) | 0 | (0) | -- |
| MenB | 0 | (0) | 0 | (0) | -- |
| Missing | 109 | (89) | 4 | (80) | 0.47 |
| Any oral sex in the last 12 months | 0.58 | ||||
| Yes | 96 | (78) | 5 | (100) | |
| No | 25 | (20) | 0 | (0) | |
| Missing | 2 | (2) | 0 | (0) | |
| Frequency of condom use | 0.44 | ||||
| Always | 22 | (18) | 2 | (40) | |
| Sometimes | 94 | (76) | 3 | (60) | |
| Never | 7 | (6) | 0 | (0) | |
| HIV positive (self-reported or laboratory-confirmed) | 1 | (1) | 0 | (0) | 1.00 |
| Median | (range) | Median | (range) | p-value | |
| Age (years) | 28 | (16–75) | 31 | (23–39) | 0.68 |
| Number of sex partners, last 3 months | 2 | (1–15) | 2 | (1–2) | 0.12 |
p-values computed using exact tests for categorical variables or Kruskal-Wallis tests for continuous variables
Race categories total >100% because respondents can choose more than one race category
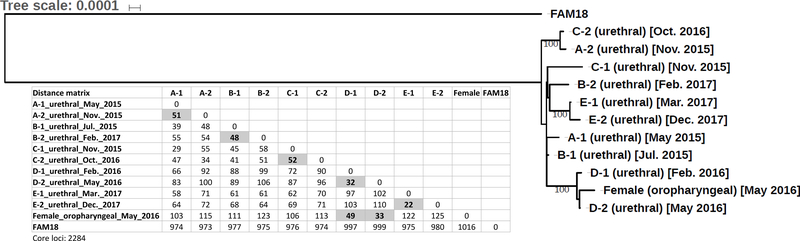
Phylogenetic analysis of WGS data demonstrated a high degree of sequence homology between all urethral US_NmUC isolates (A-1, A-2 to E-1, E-2) from the men with repeat infections (Figure 1). N. meningitidis is known to undergo high-frequency transformation and allelic exchange.14 When comparing the core genome of paired urethral isolates (initial and repeat) for each man who experienced reinfection, we observed an even higher degree of sequence identity: A-1 and A-2 (99.4%), B-1 and B-2 (99.9%), C-1 and C-2 (99.2%), D-1 and D-2 (99.5%), and E-1 and E-2 (100%). The comparison is based on 2284 core loci and paired isolates have allelic polymorphisms ranging from 22 (E-1 and E-2) to 52 (C-1 and C-2) alleles (Figure 1). Sex partner information was available only for one patient. During his clinical interview, this patient reported receiving fellatio from the same female partner prior to both his first (D-1 urethral isolate) and second (D-2 urethral isolate) episodes of symptomatic urethritis; 83 days elapsed between the two episodes. The female partner screened positive at the oropharynx for the US_NmUC [female (oropharyngeal) isolate]. No vaginal or rectal screening for Nm was performed. She was treated with intramuscular ceftriaxone (250 mg) and oral azithromycin (1 gram) shortly before the male patient presented with his second episode of symptomatic urethritis due to the US_NmUC. These epidemiologically linked US_NmUC isolates were also very closely related on phylogenetic analysis (Figure 1). When the female oropharyngeal US_NmUC isolate was compared to the urethral isolates of her male sex partner, we observed a core genome sequence identity of 99.5% (49 alleles) and 99.9% (33 alleles) with the first (D-1) and second (D-2) urethral US_NmUC isolates, respectively. This is in contrast to the more than 100 allele differences seen with other repeat urethral isolates (Figure 1). Neither the male patient nor his female sex partner returned to the STD clinic for evaluation during the remainder of the study period after both received treatment. Finally, the core genome sequence identity between the US_NmUC isolates (ten urethral and one oropharyngeal) and the cc11 reference isolate FAM18 was approximately 95%.
Figure 1.
Maximum likelihood phylogeny. Ten urethral US_NmUC isolates (5 pairs of initial and repeat infection) and one oropharyngeal US_NmUC isolate from a female partner were compared with an Nm serogroup C cc11 reference strain (FAM18) as the out-group. Single-copy homology groups were identified by BLAST and protein alignments analyzed by the RAxML program. The distance matrix of allelic polymorphism based on 2284 core loci analyzed by the genome comparator program of PubMLST is shown below the phylogenetic tree. The numbers of allelic differences in loci between paired urethral isolates and between the female oropharyngeal isolate and the two urethral isolates of her male sex partner (D-1 and D-2) are bolded and highlighted in gray shade.
DISCUSSION
The US_NmUC has primarily been associated with episodes of symptomatic urethritis that mimics gonorrhea.1–5 However, cases of invasive disease and neonatal conjunctivitis have also been reported.5,15 We provide evidence that, similar to gonococcal urethritis,8,9 repeat episodes of symptomatic urethritis caused by the US_NmUC are possible. These findings suggest that infection with the US_NmUC may not fully protect against future reinfection, even with isolates that show a very high degree of sequence identity.
This brief study has several limitations. First, many (43%) of the men diagnosed with urethritis due to the US_NmUC did not return to the STD clinic for later evaluation. If men were reinfected but presented at other venues for care (e.g., emergency departments or private providers), these infections are missing from the cases we evaluated. However, these missing data would lead the current findings to reflect an underestimate of total reinfections, and does not diminish the primary observations. There was also no post-treatment follow-up to document clearance of infection and resolution of symptoms in the men with repeat episodes of urethritis due to the US_NmUC. However, all received a ceftriaxone-based regimen at each episode of confirmed US_NmUC infection and none of them returned to the STD clinic with persistent symptoms after treatment. Furthermore, the short median duration of time between onset of symptoms and presentation to the STD clinic (3 days) and long median duration of time between episodes (273 days), suggest that subsequent infections were most likely due to reinfection rather than persistent infection. Another limitation relates to the lack of detailed sex partner information for most men; using the available data, we are unable to establish important epidemiological links between the men with repeat infections (e.g., whether any shared sex partners or had direct sexual contact). Such data are necessary to understand whether repeat infection isolates that shared a high degree of sequence identity could be due to a common source of infection. We are also unable to evaluate the risk of infection or reinfection with the US_NmUC after repeated exposure to an untreated sex partner. Regardless, observations from one patient suggest that re-exposure to an untreated sex partner can result in reinfection. We hypothesize that this patient’s female sex partner had asymptomatic oropharyngeal US_NmUC carriage for at least the period between his first and second episode of urethritis. Treatment with an antibiotic regimen that effectively eradicates meningococcal oropharyngeal carriage14 should be considered in sex partners of men diagnosed with urethritis due to the US_NmUC. Additionally, while immune correlates of protection are well-described for invasive meningococcal infection,16 we do not have data on the type of immune response elicited by urethral infection with the US_NmUC or whether vaccination impacts the risk of urethral infection. Finally, the number of men with repeat urethral infection due to the US_NmUC is small, and the patterns we observed may not be replicated in larger samples.
In summary, we find that similar to N. gonorrhoeae, symptomatic urethral reinfection with the US_NmUC can and does occur. Studies should evaluate not only the type of immune responses elicited by urethral US_NmUC infection, but also the risk of infection and reinfection following exposure to untreated sex partners.
Acknowledgments
Funding Source: This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) (R01AI127863 to A.N.T and J.A.B). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH/NIAID.
Footnotes
Author Disclosures: The authors have no disclosures to report.
REFERENCES
- 1.Bazan JA, Peterson AS, Kirkcaldy RD, et al. Notes from the Field: Increase in Neisseria meningitidis-Associated Urethritis Among Men at Two Sentinel Clinics – Columbus, Ohio, and Oakland County, Michigan, 2015. MMWR Morb Mortal Wkly Rep. 2016; 65: 550–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bazan JA, Turner AN, Kirkcaldy RD, et al. Large Cluster of Neisseria meningitidis Urethritis in Columbus, Ohio 2015. Clin Infect Dis. 2017; 65: 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toh E, Gangaiah D, Batteiger BE, et al. Neisseria meningitidis ST11 Complex Isolates Associated with Nongonococcal Urethritis, Indiana, USA, 2015–2016. Emerg Infect Dis. 2017; 23: 336–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tzeng YL, Bazan JA, Turner AN, et al. Emergence of a new Neisseria meningitidis clonal complex 11 lineage 11.2 clade as an effective urogenital pathogen. Proc Natl Acad Sci USA. 2017; 114: 4237–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Retchless AC, Kretz CB, Chang HY, et al. Expansion of a urethritis-associated Neisseria meningitidis clade in the United States with concurrent acquisition of N. gonorrhoeae alleles. BMC Genomics. 2018; 19: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urra E, Alkorta M, Sota M, et al. Orogenital transmission of Neisseria meningitidis serogroup C confirmed by genotyping techniques. Eur J Clin Microbiol Infect Dis. 2005; 24: 51–53. [DOI] [PubMed] [Google Scholar]
- 7.Jannic A, Mammeri H, Larcher L, et al. Orogenital Transmission of Neisseria meninigitidis Causing Acute Urethritis in Men Who Have Sex with Men. Emerg Infect Dis. 2019; 25: 175–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kissinger PJ, Reilly K, Taylor SN, et al. Early Repeat Chlamydia trachomatis and Neisseria gonorrhoeae Infections Among Heterosexual Men. Sex Transm Dis. 2009; 36: 498–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt KA, Schneider H, Lindstrom JA, et al. Experimental Gonococcal Urethritis and Reinfection with Homologous Gonococci in Male Volunteers. Sex Transm Dis. 2001; 28: 555–564. [DOI] [PubMed] [Google Scholar]
- 10.Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML Web servers. Syst Biol. 2008; 57: 758–771. [DOI] [PubMed] [Google Scholar]
- 11.Letunic I, Bork P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016; 44 (W1): W242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.This publication made use of the Neisseria Multi Locus Sequence Typing website (https://pubmlst.org/neisseria/) cited at the University of Oxford (Jolley et al. Wellcome Open Res. 2018, 3: 124 [version 1; referees: 2 approved]). The development of this site has been funded by the Wellcome Trust and European Union.
- 13.Hook EW III, Handsfield HH. Gonococcal infections in the adult In: Holmes KK, Sparling PF, Stamm WE, et al. , eds. Sexually Transmitted Diseases, 4th ed New York, NY: McGraw-Hill, 2008: 627–645. [Google Scholar]
- 14.Apicella MA, Stephens DS. Neisseria meningitidis In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, Updated Edition, 8th ed Philadelphia, PA: Saunders Elsevier, 2015: 2425–2445.e6 [Google Scholar]
- 15.Kretz CB, Bergeron G, Aldrich M, et al. Neonatal Conjunctivitis Caused by Neisseria meningitidis US Urethritis Clade, New York, USA, August 2017. Emerg Infect Dis. 2019; 25: 972–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McIntosh ED, Broker M, Wassil J, et al. Serum bactericidal antibody assays - The role of complement in infection and immunity. Vaccine. 2015; 33: 4414–4421. [DOI] [PubMed] [Google Scholar]