Abstract
Introduction
Recent evidence demonstrated that prehospital plasma in patients at risk of hemorrhagic shock was safe for ground transport and resulted in a 28-day survival benefit for air medical transport patients. Whether any beneficial effect of prehospital plasma varies across injury mechanism remains unknown.
Methods
We performed a secondary analysis using a harmonized dataset derived from two recent prehospital plasma randomized trials. Identical inclusion/exclusion criteria and primary/secondary outcomes were employed for the trials. Prehospital time, arrival shock parameters and 24-hour transfusion requirements were compared across plasma and control groups stratified by mechanism of injury. Stratified survival analysis and Cox hazard regression were performed to determine the independent survival benefits of plasma across blunt and penetrating injury.
Results
Blunt patients had higher injury severity, were older and had a lower GCS. Arrival indices of shock and coagulation parameters were similar across blunt and penetrating injury. The percentage of patients with a prehospital time less than 20 mins was significantly higher for penetrating patients relative to blunt injured patients (28.0% vs 11.6%, p<0.01). Stratified Kaplan-Meier curves demonstrated a significant separation for blunt injured patients (n=465, p=0.01) with no separation demonstrated for penetrating injured patients (n=161, p=0.60) Stratified Cox hazard regression verified, after controlling for all important confounders, that prehospital plasma was associated with a 32% lower independent hazard for 28 day mortality in blunt injured patients (HR 0.68, 95% CI 0.47–0.96, p= 0.03) with no independent survival benefit found in penetrating patients (HR 1.16, 95%CI 0.4–3.1,p=0.78).
Conclusion
A survival benefit associated with prehospital plasma at 24 hours and 28 days exists primarily in blunt injured patients with no benefit shown in penetrating trauma patients. No detrimental effects attributable to plasma are demonstrated in penetrating injury. These results have important relevance to military and civilian trauma systems.
Keywords: Prehospital, Plasma, Blunt Mechanism of Injury
Introduction
The management of severe traumatic injury and hemorrhage has significantly evolved over the last decade with treatment priorities focusing on prevention of coagulopathy thru minimization of crystalloid infusion and early blood component-based resuscitation after arrival at definitive care.(1–4) Despite these beneficial changes, the majority of deaths due to hemorrhage continue to occur in the first hours after arrival, highlighting the importance of potentially beneficial resuscitation strategies during the early phase of care, as close to the time of injury as feasible.(5, 6)
Early blood transfusion during the prehospital phase of care has previously been demonstrated to be associated with a survival benefit in both military and civilian settings.(7–9) Most recently, results from two recent prehospital clinical trials demonstrated the safety of early prehospital plasma for ground transport and a significant survival benefit of those transported via air medical transport.(10, 11) Understanding those injured cohorts who benefit most from such prehospital interventions is of utmost importance allowing these early blood product resources to be provided to the most appropriate population. Patients significantly injured via blunt or penetrating mechanisms are both at risk of hemorrhage and poor outcome but their demographics, injury characteristics, management strategies and response to injury vary.(12–16)
Whether the beneficial effect of prehospital plasma varies across blunt and penetrating injury mechanisms remains unknown. Our overall objective was to characterize prehospital plasma outcomes across mechanism of injury using harmonized data obtained from these two recently completed prehospital plasma clinical trials. We hypothesized that the safety and beneficial effects of prehospital plasma would be consistent across blunt and penetrating mechanism of injury.
Methods
The current analysis is a predefined secondary analysis using data derived from two recently published studies, the Control of Major Bleeding After Trauma (COMBAT) Trial(10) and the Prehospital Air Medical Plasma (PAMPer) trial.(11) These trials were purposefully harmonized prior to commencement of enrollment to address questions that could not be answered by either trial individually. Harmonization was performed allowing experimental treatment groups, inclusion/exclusion criteria, adverse events, and methods to account for patient transport time to be equivalent across the two trials. Inclusion criteria were hypotension (SBP < 90mmHg) and tachycardia (HR >108) or severe hypotension (SBP < 70mmHg) without the tachycardia requirement at any time period in the prehospital environment. Common exclusion criteria included prisoner status, known pregnancy, isolated penetrating injury to the head, asystole or cardiopulmonary resuscitation (> 5 mins), known objection to blood products or wearing opt-out bracelets. For both trials, plasma was administered prior to other resuscitative fluids once the patient met all inclusion and no exclusion criteria. The FDA, Office of Research Protections of US Army Medical Research and Materiel Command and Institutional Review Board at all participating institutions approved exception from informed consent requirements, after consultation with community members and after public notification regarding the trial took place.
There were differences in the two prehospital plasma trials. The COMBAT was a single center clinical trial. Enrolled patients were transported by ground ambulance directly from the scene to a level 1 trauma center. Patients enrolled were administered either two units of thawed AB plasma or received ground transport standard care. Standard care was goal-directed crystalloid resuscitation using 0.9% saline. Randomization and enrollment were performed at the level of the ambulance. The PAMPer trial was a multicenter, cluster-randomized trial involving injured patients who were transported by air medical transport to a level 1 trauma center, either directly from the scene or from a referring hospital. Patients enrolled in PAMPer received two units of either group AB or group A with a low anti-B antibody titer (<1:100) thawed plasma or received standard air medical care. Standard care consisted of goal-directed, crystalloid-based resuscitation on the basis of hemodynamic status for air transport teams at 14 of the 27 participating air medical bases. Air transport teams at the other 13 participating air medical bases also carried 2 units of universal donor RBC on all flights. If a patient remained hypotensive after the plasma infusion or had obvious bleeding, transfusion of RBCs then proceeded according to the local protocol. Randomization was at the level of the air medical base for 1-month time periods.
The primary outcome for the current secondary analysis was 28-day mortality. Secondary outcomes of interest included 24-hour mortality; prehospital transport time; presenting indices of shock and coagulopathy, units of in-hospital blood components administered within 24-hours. All analyses were carried out in the intention-to-treat randomized patients used in both published studies.
We first evaluated the treatment effect of prehospital plasma on 28-day mortality across blunt and penetrating mechanism of injury using a generalized estimating equations (GEE) model.(17) The plasma and injury mechanism interaction was assessed for statistical significance, accounting for intra-trial cluster effects and multiple cofounders.
We then performed Kaplan-Meier survival analysis comparing prehospital plasma patients versus standard care patients overall and across blunt and penetrating mechanisms of injury for both 24-hour and 28-day mortality using log rank comparison.
To verify these unadjusted findings, we then performed a multivariate analysis of survival with the use of a Cox proportional-hazard model, to evaluate the treatment effect (plasma vs. standard care) with adjustment for stratification factors and other possible confounding factors on 24-hour and 28-day survival. The model was generated for the primary outcome in patients with blunt injury. All covariates statistically significant on univariate analysis (demographics, abbreviated injury severity scores (AIS) and presenting initial vital signs) were assessed. In the final model, only covariates with a p-value <0.1 and/or that altered the hazard ratio (HR) for the treatment of interested by >5% were utilized to prevent over fitting of the model. All regression models passed the proportional-hazards assumption on the basis of Schoenfeld residuals.(18) The identical model was utilized for all Cox-regression analyses.
For the blood component transfusion comparisons, in order to account for the Poisson distribution of units of blood product transfusions within the first 24-hours following arrival, groups were compared with a univariate, negative binomial regression which is more appropriate than standard non-parametric comparison. These comparisons were performed for the harmonized cohort stratified by mechanism of injury as well as by randomized (plasma vs. standard care) group when stratified by injury type.
Descriptive statistics characterized the demographics and injuries of the patients and outcomes of interest. Categorical variables were presented as frequencies and percentages and tested using the Chi-square test. Prehospital transport time was defined as time in minutes from arrival on scene to arrival at the emergency department of the trauma center (ED). Continuous variables were expressed as means and standard deviations (SDs) or medians and interquartile ranges (IQRs) and were tested using the t-test or Mann-Whitney test as appropriate. Statistical significance was determined at the P <0.05 level (2-sided). All data were analyzed using STATA version 10.0 and SAS, version 9.4 (SAS Institute Inc.).
Results
In this harmonized prehospital plasma study cohort (PAMPer-501 patients, COMBAT-125 patients; total n=626), patients were severely injured with a median injury severity of 22 (IQR 12,34), a mean prehospital systolic blood pressure of 80mmHg (80±31mmHg), a median GCS of 6 (IQR 3,15) and an overall mortality of 24.8%. From the primary published studies, there was excellent randomization across plasma and standard care arms for both studies. Importantly, in the PAMPer study, patients in the standard care arm were more likely to receive prehospital packed red blood cells and higher volumes of crystalloid prior to arrival, due to the absence of plasma resuscitation capabilities in the standard care arm.
Just under 75% of injuries for the study cohort were due to a blunt mechanism of injury (n=465) with the remaining resulting from penetrating injury (n=161). Importantly there were ten patients who suffered both blunt and penetrating injuries and these were including in the penetrating subgroup. The majority of blunt injuries were secondary to motor vehicle collisions while penetrating injuries were almost equally divided across firearm injury and stabbings. (Table 1.) There were important differences in the study cohort across those who suffered blunt versus penetrating mechanisms of injury. Blunt injured patients were more commonly from the PAMPer study while penetrating injury represented the most common mechanism for the COMBAT study. Blunt injured patients were older, had higher injury severity overall and greater head, chest and extremity abbreviated injury scores. Blunt injured patients had a lower arrival systolic blood pressure and lower Glasgow Coma Score (GCS). Penetrating patients were more racially diverse and had significantly shorter prehospital times.
Table 1.
Injury characteristics for harmonize study cohort patients stratified by blunt and penetrating mechanism of injury; caption (Abbreviations- interquartile range, IQR; body mass index, BMI; injury severity score, ISS; abbreviated injury score, AIS; Glasgow coma scale, GCS)
| Blunt (n= 465) | Penetrating (n=161) | p-value | |
|---|---|---|---|
| Classification of Mechanism of Injury | |||
| Motor Vehicle | 247 (53.1%) | ||
| Motorcycle | 85 (18.3%) | ||
| Pedestrian/cyclist | 44 (9.5%) | ||
| Fall | 38 (8.2%) | ||
| Other | 51 (11.0%) | ||
| Firearm | 77 (47.8%) | ||
| Stabbing | 69 (41.0%) | ||
| Other | 15 (9.3%) | ||
| Full Cohort, n (%) | 465 (74.3%) | 161 (25.7%) | |
| COMBAT | 59 (47.2%) | 66 (52.8%) | |
| PAMPer | 406 (81.0%) | 95 (19.0%) | |
| Age, median (IQR) | 45 (28, 61) | 35 (26, 49) | <0.001 |
| Male, n (%) | 326 (70.1%) | 141 (87.6%) | <0.001 |
| Race, n (%) | |||
| White | 418 (89.9%) | 108 (68.4%) | <0.001 |
| Black | 28 (6.0%) | 45 (28.5%) | |
| Other/Unknown | 19 (4.1%) | 5 (3.1%) | |
| Hispanic, n (%) | 33 (7.5%) | 31 (20.5%) | <0.001 |
| BMI, mean (SD) | 30.2 (11.9) | 27.7 (6.8) | 0.040 |
| ISS, median (IQR) | 24 (17, 34) | 14 (6, 25) | <0.001 |
| AIS ≥3, n (%) | |||
| Head | 203 (43.7%) | 15 (9.3%) | <0.001 |
| Face | 16 (3.4%) | 6 (3.7%) | 0.870 |
| Chest | 271 (58.3%) | 54 (33.5%) | <0.001 |
| Abdomen | 133 (28.6%) | 38 (23.6%) | 0.220 |
| Extremities | 168 (36.1%) | 25 (15.5%) | <0.001 |
| Skin | 8 (1.7%) | 9 (5.6%) | 0.009 |
| Prehospital Interval | |||
| Minutes, median (IQR) | 39.3 (28.4, 50.2) | 30.6 (19.7, 43.7) | <0.001 |
| ≤20 Minutes, n (%) | 54 (11.6%) | 45 (28.0%) | <0.001 |
| Arrival Vital Signs, median (IQR) | |||
| Heart Rate | 107 (89, 124) | 105 (91 121) | 0.500 |
| Systolic Blood Pressure | 98 (78, 119) | 106 (80, 128) | 0.034 |
| GCS | 3 (3, 15) | 14 (3, 15) | 0.004 |
| Mortality 24hr (%) | 18.7% | 11.8% | <0.001 |
| Mortality 28-day (%) | 29.2% | 12.4% | <0.001 |
Upon arrival at the definitive trauma center, patients demonstrated no clinically significant differences in presenting hemoglobin or standard coagulation assays. (Table 2.) Blood gas comparison demonstrated that blunt injured patients were more likely acidotic and thromboelastography (TEG) differences were limited to non-clinically significant lysis at 30 minutes (LY30) measurements. There were missing laboratory values for a proportion of patients however, the missingness did not vary across mechanism of injury.
Table 2.
Arrival laboratory measurements of resuscitation, shock parameters and coagulopathy across mechanism of injury; caption (Abbreviations - complete blood count, CBC; hemoglobin, HGB; international normalized ratio, INR; prothrombin time, PT; partial pressure of carbon dioxide, pCO2; maximum amplitude, MA; lysis at 30 minutes, LY30; activated clotting time, ACT)
| Median (IQR) | Blunt | Penetrating | p |
| 465 | 161 | ||
| CBC and Coagulation | |||
| HGB | 11.6 (9.7, 13.3) | 11.5 (10.0, 13.5) | 0.350 |
| INR | 1.3 (1.1, 1.5) | 1.3 (1.1, 1.5) | 0.580 |
| PT | 14.8 (13.25, 17.5) | 14.4 (13.3, 16.5) | 0.440 |
| Arterial Blood Gas | |||
| pH | 7.28 (7.20, 7.34) | 7.31 (7.25, 7.37) | 0.028 |
| Base Excess | −8.2 (−11.3, −6.0) | −10 (−15.9, −6.0) | 0.129 |
| Thromboelastogram | |||
| R time | 0.8 (0.6, 0.8) | 0.8 (0.7, 0.8) | 0.720 |
| Kappa | 1.7 (1.2, 2.7) | 1.8 (1.2, 2.6) | 0.840 |
| αAngle | 70.4 (62.0, 74.9) | 70.2 (63.5, 74.7) | 0.880 |
| MA | 58.3 (49.7, 64.5) | 59.1 (49.6, 63.0) | 0.500 |
| LY30 | 0.3 (0.0, 2.0) | 1.2 (0.0, 3.5) | 0.006 |
| ACT | 113 (105, 136) | 121 (105, 128) | 0.310 |
For the full harmonized study cohort, patients who were randomized to the plasma arm of both studies had significantly lower 24-hour (13.5% vs. 20.1%, p=0.028) and 28-day mortality rates (20.9% vs. 28.6%, p= 0.026) as compared to those patients randomized to standard care prehospital resuscitation. When we compared plasma versus standard care arms stratified by mechanism of injury we found statistically significant differences in the blunt injury subgroup, without significant differences found in the penetrating group. (Table 3.) Based upon this, we then tested to determine if the survival benefit of prehospital plasma was affected or altered by mechanism of injury. After accounting for intra-trial clustering and differences across blunt and penetrating injury, we tested for and found a significant interaction between randomization group and mechanism of injury (p<0.001).
Table 3.
Twenty-four hour and 28-day mortality in plasma versus standard care stratified by injury mechanism.
| Blunt (n = 465) | p-value | Penetrating (n = 161) | p-value | |||
|---|---|---|---|---|---|---|
| Standard Care | Plasma | Standard Care | Plasma | |||
| 24-hour | 58 (25.8%) | 29 (15.2%) | 0.010 | 8 (10.4%) | 11 (13.23%) | 0.595 |
| 28-day | 86 (34.1%) | 50 (23.5%) | 0.012 | 8 (10.4%) | 12 (14.3%) | 0.454 |
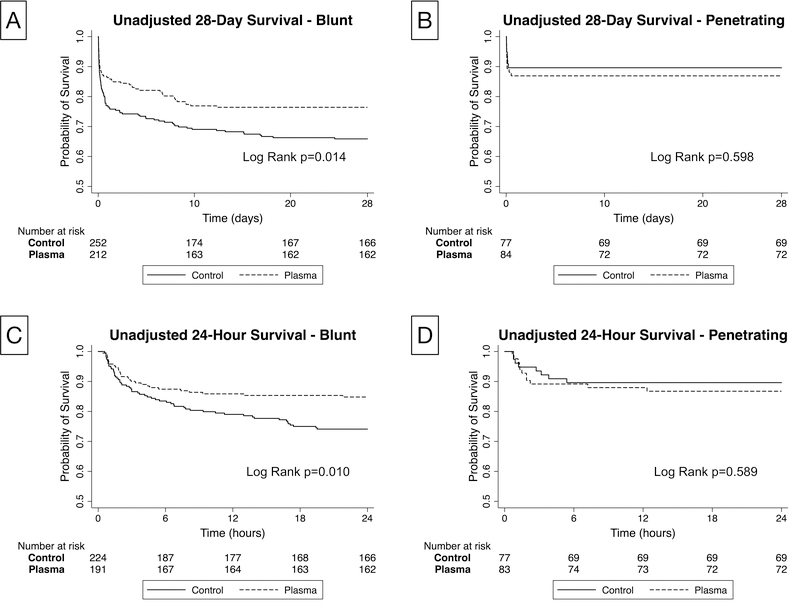
We then performed survival analysis with Kaplan-Meier survival analysis for 24-hour and 28-day mortality to determine when survival differences occurred for each mechanism of injury subgroup. (Figure 1.) This analysis revealed a significant and early separation starting around 3 hours from randomization that persisted out to 28 days for blunt injured patients. (log rank p=0.01) There was no significant separation apparent for those patients with penetrating injury. (log rank p=0.59)
Figure 1.
Kaplan-Meier Survival Analysis comparing plasma and standard care arms across blunt and penetrating mechanism of injury; caption (Unadjusted Kaplan Meier Curves for 28-day (A, B) and 24-hour (C, D) survival for those with blunt (A, C) and penetrating injury (B, D) comparing prehospital standard of care (control) to plasma with associated log rank testing)
Multivariate analysis of survival with the use of a Cox proportional-hazard model verified that after adjusting for all clinically and statistically significant covariates that prehospital plasma was independently associated with a survival benefit at 24 hours (HR 0.59, 95%CI 0.370 – 0.947, p=0.029) and at 28 days (HR 0.68, 95%CI 0.472 – 0.965, p=0.031) in the blunt injured subgroup. (Table 4.) When the penetrating subgroup was similarly analyzed, no significant association with survival or mortality was found for the plasma variable at 24 hours (HR 1.16, 95%CI 0.430 – 3.103, p=0.775) or at 28 days (HR 1.16, 95%CI 0.430 – 3.103, p=0.775).
Table 4.
Multivariate Cox-hazard regression model in blunt injured patients for 24-hour and 28-day mortality
| HR | 95% CI | p-value | |
|---|---|---|---|
| Blunt 24-hour | |||
| Plasma (vs. standard care) | 0.59 | 0.370 – 0.947 | 0.029 |
| Age | 1.01 | 0.999 – 1.023 | 0.074 |
| ISS | 1.00 | 0.987 – 1.019 | 0.751 |
| Initial GCS | 0.77 | 0.700 – 0.837 | <0.001 |
| PAMPer (vs. COMBAT) | 1.29 | 0.627 – 5.137 | 0.276 |
| Blunt 28-day | |||
| Plasma (vs. standard care) | 0.68 | 0.472 – 0.965 | 0.031 |
| Age | 1.02 | 1.007 – 1.029 | 0.001 |
| ISS | 1.02 | 1.001 – 1.029 | 0.031 |
| Initial GCS | 0.84 | 0.801 – 0.883 | <0.001 |
| PAMPer (vs. COMBAT) | 2.35 | 0.980 – 5.628 | 0.055 |
Finally, we compared 24-hour blood and blood component across randomization groups when stratified by mechanism of injury using univariate, negative binomial regression. (Table 5.) After taking into account the Poission distribution of blood transfusion, prehospital plasma was associated with a 24% reduction in the risk of total blood transfusion (incident risk ratio-IRR 0.76) as compared to standard care patients in those who suffered blunt injury. Similarly, prehospital plasma was associated with significant risk reductions for red blood cell and platelet transfusion in blunt injury. (IRR 0.77, 0.52, respectively) Importantly, no significant association between prehospital plasma and blood or component transfusion was found in those patients with penetrating injury.
Table 5.
Comparison of blood and blood component transfusion across plasma and standard care patients stratified by mechanism of injury; caption (unadjusted negative binomial regression evaluating the incidence rate ratio (IRR) of transfusion requirements within the first 24-hours)
| 24 Hour Transfusions | Blunt | Penetrating | ||||
|---|---|---|---|---|---|---|
| IRR | 95% CI | p | IRR | 95% CI | p | |
| Total | 0.76 | 0.59 – 0.98 | 0.035 | 0.79 | 0.44–1.38 | 0.400 |
| RBC | 0.77 | 0.61 – 0.98 | 0.033 | 0.87 | 0.46 – 1.65 | 0.671 |
| Plasma | 0.88 | 0.64 – 1.21 | 0.443 | 0.74 | 0.43 – 1.27 | 0.270 |
| Platelets | 0.52 | 0.30 – 0.91 | 0.020 | 0.58 | 0.24 – 1.34 | 0.198 |
Discussion
Initiating the principles of damage control resuscitation during the prehospital phase of care, as close to the time of injury as feasible, has great potential to improve outcomes in those patients at high risk of hemorrhage and mortality. Plasma initiated in the prehospital environment has been demonstrated to be safe and result in a survival benefit in patients with longer prehospital transport times.(10, 11) The current results derived from two a priori harmonized clinical trials demonstrate that the survival benefit resulting from prehospital plasma is most apparent in those with blunt mechanism of injury. These disparities in response to plasma across blunt and penetrating injury are independent of apparent differences in injury severity and study cohort characteristics and are disparate from the benefits demonstrated for in-hospital plasma.(4) Importantly, plasma was not associated with any harm in those with penetrating injury and the penetrating cohort is large due to the harmonization of the two studies.
The original trials were not randomized or powered to compare mechanism of injury and the response to prehospital plasma. With the given sample of penetrating injury, an effect size of HR=0.28 would be required to find a statistically significant difference between treatment groups in penetrating trauma. Therefore, although the harmonized dataset provides a larger number of penetrating injuries to characterize the relationship, the sample size is too small to find a statistically significant difference and is underpowered to rule out an effect of prehospital plasma in penetrating injury. Assuming a similar range for the hazard ratio for 28-day survival (0.5–0.7), a sample size of 527–1,988 would be required to be appropriately powered.
There were differences in presenting shock severity and early measurements of coagulopathy across the blunt and penetrating injury cohorts that are clinically insignificant and unlikely to explain these disparate findings. Overall, mortality across blunt and penetrating injury was significantly lower for penetrating patients and occurred within the first hours of admission. The current analysis also demonstrated that transfusion requirements were significantly lower in those patients who received prehospital plasma as compared to standard care in those with blunt injury, without such findings in penetrating patients. These results suggest that the risks of death and poor outcome may be different across mechanism of injury and the benefits of plasma may vary accordingly. The plasma outcome benefits may in part be due to reduced transfusion requirements in those at highest risk of mortality.
The current results demonstrate that prehospital plasma was not associated with a beneficial survival effect in penetrating as compared to blunt injured patients. However, these results are limited to the injured cohorts analyzed in these two randomized trials. The benefits of prehospital plasma in penetrating injury may be more apparent in those penetrating patients with longer prehospital times and in those with combined injuries, such as the military setting.
The current findings are robust, yet blunt injured patients were significantly different relative to those with penetrating mechanism. They were older, had more significant injuries including traumatic brain injury, had longer prehospital transit times and higher overall mortality. However, even when controlling for these factors in the statistical models the survival benefit of plasma in blunt injury alone persisted. The benefits of damage control resuscitation during the in-hospital phase of care have not previously been shown to vary across mechanism of injury. The benefits of plasma, such as the prevention of coagulopathy during the in-hospital phase of care, may play less of a role when given in smaller volumes and in the prehospital environment. The inflammatory benefits of plasma including endothelial cell protection may be most pertinent to multisystem blunt injury rather than penetrating mechanism.(19–21) The higher percentage of penetrating trauma in urban environments and the attributable shorter transport times may limit the benefit of plasma relative to current prehospital resuscitation. The early time course of mortality for patients who suffer penetrating injury may not allow the benefits of plasma to be appreciated. There may be a survival bias when enrolling penetrating injured patients as those who may benefit the most from prehospital plasma may be unable to be enrolled. Alternatively, those with penetrating injury have been previously shown to benefit from ‘permissive hypotension’ or ‘controlled resuscitation’.(22, 23) Any benefit of prehospital plasma resuscitation in patients who suffer penetrating injury may oppose the benefits of hypotensive or controlled prehospital resuscitation and mitigate any overall outcome benefit. These disparate findings of prehospital plasma across blunt and penetrating have significant relevance to both military and civilian practice and provides the impetus to determine the underlying mechanisms responsible and promote further research to verify those injured cohorts who are most likely to benefit from prehospital resuscitation interventions.
Limitations
There are limitations to this secondary analysis. Although the two studies were harmonized a priori and derived from two prospective randomized clinical trials, there were important differences in the study cohorts and the protocols followed. Most important was the differences in prehospital transport time and mortality risk between the two studies. Although we controlled for relevant differences via a robust statistical approach, the potential of residual confounding exists. The enrolled number of patients in the two clinical trials were different and the results from the current secondary analysis may be primarily driven by the clinical trial with the larger enrolled population.(10, 11)
There may be important differences across blunt and penetrating injuries that cannot be accounted for that may alter the current findings presented. Although the penetrating cohort was derived from combining both studies, the overall cohort may still be to small too provide a complete understanding of the relationship of prehospital plasma in penetrating patients. Further dividing penetrating injury into stab wounds and missile injuries limited the sample size even further. We attempted to determine if there was any interaction with the type of penetrating injury (stab vs. firearm) but mortality was too rare of an event for adequate modeling. It may be that subgroups of penetrating injury derive benefit from prehospital plasma but the current study was unable to demonstrate any association. Although all data was collected prospectively, the acuity of these patients upon presentation limited the collection of time sensitive data, including but not limited to laboratory tests resulting in missingness. Although the missingness did not vary across any of the groups that were compared, missing data represents a significant limitation in interpreting the laboratory data and TEG data.
Conclusion
In conclusion, the survival benefit associated with prehospital plasma exists primarily in blunt injured patients with no appreciable benefit demonstrated in penetrating trauma patients for the current harmonized cohort of injured patients. An associated reduced transfusion requirement for plasma patients relative to standard care was also found in blunt injury alone. No detrimental effects attributable to plasma are demonstrated in penetrating injury. These results have important relevance to military and civilian trauma systems. It remains unknown if prehospital plasma is beneficial in penetrating patients in different prehospital environments such as prolonged field care situations, with specific types of penetrating injuries or in those with particular injury severity. Using data derived from two civilian randomized prehospital plasma trials, 24 hour and 28-day survival benefit of prehospital plasma is principally demonstrated in blunt injured patients only.
Acknowledgments
Funding: This work was supported by the US Department of Defense (USAMRAA, W81XWH-12-2-0028) and (USAMRAA, W81XWH-12-2-0023)
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare and have received no financial or material support related to this manuscript
Level of Evidence- I
Bibliography
- 1.Cotton BA, Reddy N, Hatch QM, LeFebvre E, Wade CE, Kozar RA, Gill BS, Albarado R, McNutt MK, Holcomb JB. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg. 2011;254(4):598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris T, Davenport R, Mak M, Brohi K. The Evolving Science of Trauma Resuscitation. Emerg Med Clin North Am. 2018;36(1):85–106. [DOI] [PubMed] [Google Scholar]
- 3.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, Alarcon LH, Bai Y, Brasel KJ, Bulger EM, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox EE, Holcomb JB, Wade CE, Bulger EM, Tilley BC, Group PS. Earlier Endpoints are Required for Hemorrhagic Shock Trials Among Severely Injured Patients. Shock. 2017;47(5):567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhee P, Joseph B, Pandit V, Aziz H, Vercruysse G, Kulvatunyou N, Friese RS. Increasing trauma deaths in the United States. Ann Surg. 2014;260(1):13–21. [DOI] [PubMed] [Google Scholar]
- 7.Brown JB, Cohen MJ, Minei JP, Maier RV, West MA, Billiar TR, Peitzman AB, Moore EE, Cuschieri J, Sperry JL, et al. Pretrauma center red blood cell transfusion is associated with reduced mortality and coagulopathy in severely injured patients with blunt trauma. Ann Surg. 2015;261(5):997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown JB, Sperry JL, Fombona A, Billiar TR, Peitzman AB, Guyette FX. Pre-trauma center red blood cell transfusion is associated with improved early outcomes in air medical trauma patients. J Am Coll Surg. 2015;220(5):797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shackelford SA, Del Junco DJ, Powell-Dunford N, Mazuchowski EL, Howard JT, Kotwal RS, Gurney J, Butler FK Jr., Gross K, Stockinger ZT. Association of Prehospital Blood Product Transfusion During Medical Evacuation of Combat Casualties in Afghanistan With Acute and 30-Day Survival. JAMA. 2017;318(16):1581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore HB, Moore EE, Chapman MP, McVaney K, Bryskiewicz G, Blechar R, Chin T, Burlew CC, Pieracci F, West FB, et al. Plasma-first resuscitation to treat haemorrhagic shock during emergency ground transportation in an urban area: a randomised trial. Lancet. 2018;392(10144):283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperry JL, Guyette FX, Brown JB, Yazer MH, Triulzi DJ, Early-Young BJ, Adams PW, Daley BJ, Miller RS, Harbrecht BG, et al. Prehospital Plasma during Air Medical Transport in Trauma Patients at Risk for Hemorrhagic Shock. N Engl J Med. 2018;379(4):315–26. [DOI] [PubMed] [Google Scholar]
- 12.Rowell SE, Barbosa RR, Diggs BS, Schreiber MA, Trauma Outcomes G, Holcomb JB, Wade CE, Brasel KJ, Vercruysse G, MacLeod J, et al. Effect of high product ratio massive transfusion on mortality in blunt and penetrating trauma patients. J Trauma. 2011;71(2 Suppl 3):S353–7. [DOI] [PubMed] [Google Scholar]
- 13.Rowell SE, Barbosa RR, Diggs BS, Schreiber MA, Trauma Outcomes G, Holcomb JB, Wade CE, Brasel KJ, Vercruysse G, MacLeod J, et al. Specific abbreviated injury scale values are responsible for the underestimation of mortality in penetrating trauma patients by the injury severity score. J Trauma. 2011;71(2 Suppl 3):S384–8. [DOI] [PubMed] [Google Scholar]
- 14.Oyo-Ita A, Chinnock P, Ikpeme IA. Surgical versus non-surgical management of abdominal injury. Cochrane Database Syst Rev. 2015(11):CD007383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adam N, Sorensen V, Skinner R. Not all intestinal traumatic injuries are the same: a comparison of surgically treated blunt vs. penetrating injuries. Injury. 2015;46(1):115–8. [DOI] [PubMed] [Google Scholar]
- 16.Forristal C, Van Aarsen K, Columbus M, Wei J, Vogt K, Mal S. Predictors of Hypothermia upon Trauma Center Arrival in Severe Trauma Patients Transported to Hospital via EMS. Prehosp Emerg Care. 2019:1–8. [DOI] [PubMed] [Google Scholar]
- 17.Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157(4):364–75. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z, Reinikainen J, Adeleke KA, Pieterse ME, Groothuis-Oudshoorn CGM. Time-varying covariates and coefficients in Cox regression models. Ann Transl Med. 2018;6(7):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofmann N, Zipperle J, Jafarmadar M, Ashmwe M, Keibl C, Penzenstadler C, Ponschab M, Jafarmadar B, Redl H, Bahrami S, et al. Experimental Models of Endotheliopathy: Impact of Shock Severity. Shock. 2018;49(5):564–71. [DOI] [PubMed] [Google Scholar]
- 20.Johansson PI, Henriksen HH, Stensballe J, Gybel-Brask M, Cardenas JC, Baer LA, Cotton BA, Holcomb JB, Wade CE, Ostrowski SR. Traumatic Endotheliopathy: A Prospective Observational Study of 424 Severely Injured Patients. Ann Surg. 2017;265(3):597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu F, Chipman A, Pati S, Miyasawa B, Corash L, Kozar RA. Resuscitative Strategies to Modulate The Endotheliopathy of Trauma: From Cell to Patient. Shock. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bickell WH, Wall MJ Jr., Pepe PE, Martin RR, Ginger VF, Allen MK, Mattox KL. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331(17):1105–9. [DOI] [PubMed] [Google Scholar]
- 23.Schreiber MA, Meier EN, Tisherman SA, Kerby JD, Newgard CD, Brasel K, Egan D, Witham W, Williams C, Daya M, et al. A controlled resuscitation strategy is feasible and safe in hypotensive trauma patients: results of a prospective randomized pilot trial. J Trauma Acute Care Surg. 2015;78(4):687–95; discussion 95–7. [DOI] [PMC free article] [PubMed] [Google Scholar]