Abstract
Background.
Nutrient-mediated release of cholecystokinin and glucagon-like peptide-1 (GLP-1) regulate gastric emptying (GE) via duodenogastric feedback mechanisms; GLP-1 also regulates postprandial insulin secretion. Some patients with functional upper gastrointestinal symptoms have impaired glucose tolerance during enteral dextrose infusion. Our hypothesis was that variants in CCK, GLP-1, and TCF7L2 (transcription factor 7-like 2 locus), which is associated with greatest genetic risk for development of type 2 diabetes mellitus, are associated with GE and independently with glucose tolerance. Our aims were to evaluate the associations between these GE, glucose tolerance and these SNPs.
Methods.
Genetic variants, scintigraphic GE of solids, plasma glucose, insulin, and GLP-1 during enteral dextrose infusion (75gm over 2 hours), were measured. GE and enteral dextrose infusion were respectively evaluated in 44 (27 controls and 17 patients with functional dyspepsia or nausea) and 42 (28 controls, 14 patients) participants; of these, 51 participants consented to assessment of single nucleotide polymorphisms (SNPs). Four functional SNPs were studied: rs6923761 and rs1042044 at GLP-1 receptor, rs7903146 (TCF7L2), and rs1800857 (CCK receptor).
Results.
Gastric emptying was normal in 38, rapid in 4, and delayed in 2 participants; 38 had normal and 4 had impaired glucose tolerance. The T allele at rs7903146 (TCF7L2) was non-significantly associated (p=0.14) with faster GE. The associations between SNPs and demographic variables, GE thalf, glucose tolerance and plasma GLP1 levels were not significant.
Conclusions.
There is a trend toward an association between faster GE and the diabetes-associated allele at rs7903146 in TCF7L2. However, these SNPs were not associated with plasma glucose or GLP1 concentrations during enteral dextrose infusion.
Keywords: dumping, functional dyspepsia, insulin secretion, glucose tolerance
BACKGROUND
Some patients with functional upper gastrointestinal symptoms (i.e, non ulcer dyspepsia [NUD], nausea and/or vomiting, and postprandial abdominal bloating and/or distention) have rapid gastric emptying (GE).(1–5) Among patients who do not have a disease (e.g., diabetes mellitus) or gastric surgery that is associated with rapid GE, the rapid GE is deemed idiopathic.(6–8) Conceivably, disturbances of one or more mechanisms that normally regulate GE, such as impaired gastric accommodation, exaggerated gastric contractility, or impaired duodeno-gastric feedback mechanisms, may cause rapid GE in these patients.(2, 9, 10)
In the small intestine, nutrients evoke neurohumorally-mediated duodenogastric feedback reflexes that inhibit gastric emptying by modulating gastric and pyloric motor activity.(7) Cholecystokinin (CCK) and glucagon-like peptide-1 (GLP-1), which are released in response to nutrients in the small intestine, delay GE by increasing gastric accommodation and/or inhibiting antral contractility and/or increasing pyloric tone. Rapid GE or dumping induces an exaggerated release of these and other hormones (e.g., insulin and glucagon) that at least partly cause the gastrointestinal and vasomotor symptoms of dumping syndrome. (8) However, in one study, we observed that one-third of non-diabetic patients with NUD had impaired glucose tolerance (IGT) during duodenal infusion of glucose (75 gm) at a controlled rate over 2 hours.(9) These patients had IGT despite increased plasma GLP-1 levels during duodenal glucose infusion. Since the nutrients were delivered to the small intestine, the IGT is not secondary to rapid GE. Rather, because GLP-1 partly mediates the postprandial release of insulin and also modulates gastric emptying, we postulated that an impaired response to GLP-1, possibly related to genetic variation at the GLP-1 receptors, may explain IGT and rapid GE in these patients.
Hence, the overall objectives of this sub-study were to evaluate the associations between glucose tolerance and insulin release and selected polymorphisms in genes that control CCK, GLP-1, and other genes that predispose to T2DM and GE among patients with upper gastrointestinal symptoms. The specific aims were to (i) evaluate the associations between single nucleotide polymorphisms (SNPs) in the genes of the CCK and GLP1 receptors and GE, (ii) better understand the mechanisms of hyperglycemia by comparing the insulin secretion rate in patients with normal and impaired glucose tolerance, and (iii) assess the associations between insulin secretion rate and the genes of GLP-1 receptor and TCF7L2 which are associated with regulation of glycemia.
MATERIALS AND METHODS
Study Design
The original study was designed to evaluate hormonal responses to enteral dextrose infusion in 35 healthy asymptomatic controls and 30 patients with functional upper gastrointestinal (GI) symptoms (dyspepsia or nausea and vomiting) by Rome III criteria; gastric emptying of solids was also evaluated in 25 healthy controls and 30 patients.(9) From that cohort, 34 controls (23 women, age, 41 [3] years, mean [SE]; BMI 26.4 (0.7) kg/m2) and 17 patients (11 women, 45 [5] years, BMI 26.2 (1.2) kg/m2) consented to genotyping and are included in this paper. Among all participants, exclusion criteria were age <18 or >70 years; diabetes mellitus; a structural disorder affecting the GI tract; clinically significant systemic (eg, cardiorespiratory) disease that may interfere with study objectives or pose safety concerns, or both; medications that can affect GI motility; GI surgery other than appendectomy, cholecystectomy, hysterectomy, tubal ligation, or inguinal hernia repair; or a hemoglobin level <12.9 g/dL in men and <11.5 g/dL in women. All women of child-bearing potential had to have a negative pregnancy test within 48 hours of study participation. The Mayo Clinic Institutional Review Board approved the study. All participants signed informed consent.
Assessment of Dyspepsia Symptoms
Dyspepsia symptoms and quality of life (QOL) were evaluated with the Nepean Dyspepsia Index questionnaire (11, 12). The Hospital Anxiety and Depression Scale (HADS) questionnaire documented anxiety and depression (13).
Gastric and Small-Bowel Transit
On average, the assessments (i.e., gastric emptying and enteral nutrient infusion) were separated by 8 days. Gastric emptying of a solid meal (296 kcal; 32% protein, 35% fat, and 33% carbohydrate) consisting of 2 eggs labeled with technetium Tc 99m sulfur colloid (1 mCi) served on 1 slice of bread with milk (240 mL; 1%) was evaluated with scintigraphy.(14) Rapid and delayed gastric emptying were defined relative to 10th through 90th percentile values in controls from the present study. Rapid gastric emptying was defined as emptying >90th percentile value at 60 or 120 minutes (>31.4% and >71.2%, respectively for women and >40% and >82% for men) or half-time (t50) less the 10th percentile value (i.e., <89 and <73.2 minutes for women and men, respectively). Delayed gastric emptying was defined as emptying <10th percentile value at 2 (i.e., <25% for women and <28% for men) or 4 hours (i.e., <76% for women and <77% for men) or t50 longer than the 90th percentile value (i.e., >180 and >165 minutes for women and men, respectively).
Enteral Nutrient Infusions
Through a 8-Fr nasoduodenal feeding tube (Abbott Nutrition) the tip of which was placed with fluoroscopic guidance in the second part of the duodenum, dextrose (Limeondex, 75gm diluted to 222 ml, 300 kcal, Therma Fisher Scientific Inc) was administered over 2 hours at the rate at which glucose is absorbed after oral ingestion. (15) (16–18)
Glycemia and Enteral Hormonal Measurements
During enteral nutrient infusion, blood samples for measurement of plasma glucose, C-peptide, glucagon, GLP1, CCK, and PYY were collected at 5-minute intervals for 30 minutes, at 10-minute intervals from 30 to 60 minutes, and at 15-minute intervals from 60 to 120 minutes. Arterialized venous plasma samples were obtained from a retrograde hand or forearm vein and were placed in a Perspex hot box heated to 55 °C. These samples were placed in ice, centrifuged at 4°C, separated, and stored at −20°C until assayed. Glucose was measured by the Hitachi 912 assay (Roche Diagnostics). An enzyme-linked immunosorbent assay (ELISA) (Linco Research, Inc) was used to measure biologically active GLP1(7–36 amide, 7–37) concentrations. The CCK immunoassay (Alpco Diagnostics) assesses most biological active forms with nearly equimolar potency.(19) Glucagon was measured with a direct, double-antibody. radioimmunoassay (Linco Research, Inc). C-peptide levels were measured with a 2-site immunometric (sandwich) assay using electrochemiluminescence detection (Cobas e411; Roche Diagnostics).
Genotyping
Genomic DNA was isolated from whole blood by use of the QIAamp DNA Blood Maxi Kit (Qiagen, Valencia, CA), and stored at −80°C until genotyping. Candidate genotypes were analyzed with established PCR-based methods i.e., rs7903146 (TCF7L2),(20) rs1800857,(21) and with Taqman assays (catalog # C___2491143_10) (catalog C__25615272_20) (Applied Biosystems, Grand Island, NY) for GLP1R SNPs rs1042044 and rs6923761. Following polymerase chain reaction amplification, end reactions were analyzed by using ABI 7300 Real-Time PCR System by Sequence Detection Software (Applied Biosystems, Grand Island, NY). The PCR products were treated with ExoSAP-IT For PCR Product Cleanup (Affymetrix, Santa Clara, CA) and sequenced at the Mayo Clinic Genome Facility on an ABI PRISM™ 3730xl DNA Analyzers (Applied Biosystems, Grand Island, NY).
The SNPs evaluated in this study have been linked with the control of blood glucose (i.e., GLP1 and TCFL2) and/or hormones (i.e., CCK) that regulate gastric emptying. At the GLP1R gene, 2 coding variants (i.e., rs6923761 and rs1042044) have been associated with altered GLP1R function. Subjects who are homozygous for the major allele of rs6923761 secreted significantly more insulin than subjects containing at least one copy of the minor allele.(22) At rs1042044, the homozygous minor allele is linked to increased morning cortisol levels in children.(23)
The diabetes-associated T allele of the intronic SNP rs7903146 located within the transcription factor 7 like 2 (TCF7L2) gene affects insulin secretion and β-cell function,(24) and is the strongest genetic association with type 2 diabetes mellitus,(25) increasing the risk of type 2 diabetes by about 50%.(26) Also germane to this study, the T allele was previously shown to be associated with reduced fasting gastric volume and faster GE of liquids.(20) Finally, for the CCK receptor gene, we evaluated the intronic SNP rs1800857, where the wild T allele is associated with slow GE (27) and with constipation-predominant and mixed irritable bowel syndrome compared with healthy controls.(28)
Insulin Secretion Rate
Insulin secretion rate was calculated by deconvolution from C-peptide concentrations using a two-compartment model of C-peptide kinetics, where the relevant kinetic indices were estimated using anthropometric data as previously described (29) and recently validated independently.(30)
Data and Statistical Analysis
The associations between SNPs and physiological variables employed a dominant model in which homozygotes for the major allele were compared with homozygotes for the minor allele grouped with the heterozygotes. The associations between these SNPs and gastric emptying summarized as normal versus rapid and separately normal versus delayed was evaluated with Fischer’s exact test. The Wilcoxon rank sum test evaluated the relationship between the t50 and SNPs. Linear regression models evaluated the association between each SNP (predictor variables) to the area under the curve (AUC) for glucose and separately ISR (dependent variables). All analyses were performed with SAS (SAS Institute, Cary, NC) The data are summarized as Mean ± SD.
RESULTS
Baseline Characteristics and Study Flow
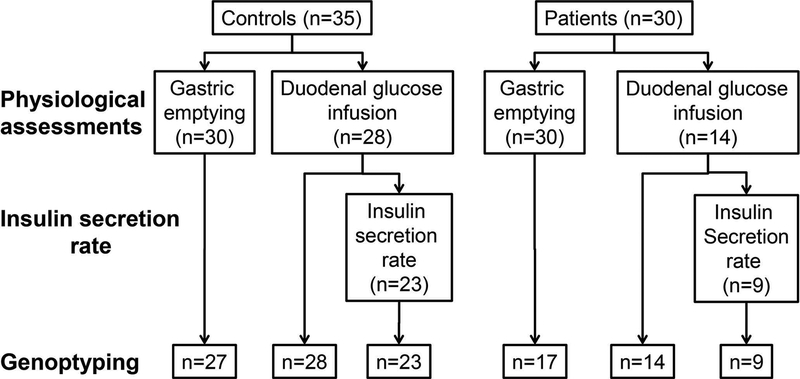
Among 17 patients, 15 and 2 satisfied Rome III criteria for functional dyspepsia and nausea respectively. Of 51 participants (34 controls and 17 patients) in whom genotyping was performed, gastric emptying was evaluated in 44 (27 controls and 17 patients) and the enteral dextrose infusion was performed in 42 participants (28 controls and 14 patients) (Figure 1). Gastric emptying and enteral dextrose infusion were both evaluated in 36 participants (22 controls and 14 patients).
Figure 1. Experimental Design.
The physiological assessments were performed in 35 controls and 30 patients. The insulin secretion rate could be accurately estimated in 23 controls and 9 patients.
On a scale from 0 (worst symptom severity) to 13 (no symptoms), the Nepean Dyspepsia Index mean symptom severity score was 12.9 ± 0.2 in controls and 11.3 ± 0.8 in patients (P<.0001). The QOL scores were 99.8 ± 1.0 in controls and 50.3 ± 18.1 in patients (P<.0001), on a scale from 0–100 where lower scores represent poorer QOL.
Distribution of SNPs and Associations with Demographic Features
For rs 1800857 (CCK), 40 participants were homozygotes for the major allele and 11 were heterozygotes. For rs 7903146 (TCF7L2), 28 and 3 participants were homozygous respectively for the major and minor alleles; the remainder (n=20) were heterozygotes. For rs 1042044 (GLP-1 receptor), there were 27 heterozyogotes and 24 homozygotes - 16 for the major and 9 for the minor allele. For rs 6923761 (GLP-1), there were 33 homozygotes - 27 for the major and 6 for the minor allele – and 18 heterozygotes. Associations between the SNPs and demographic variables (i.e. age, sex, and BMI) and with participant status (ie, control or patient) were not significant. Table 1 shows the distribution of demographic features and gastric emptying values for major and minor alleles only among participants in whom gastric emptying was evaluated.
Table 1.
Associations between SNPs, Demographic Features, and Gastric Emptying
| SNP (gene) | Allele | N | Age | Females (%) | BMI Kg/m2 |
GE thalf solids (min) | GE thalf liquids (min) | GE at 60 minutes (%) | GE at 120 minutes (%) | GE at 240 minutes (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| rs1800857 (CCK) | Major | 34 | 42 ± 17 | 68 | 26 ± 4 | 113 ± 35 | 28 ± 26 | 27 ± 15 | 60 ± 19 | 95 ± 9 |
| Minor | 10 | 45 ± 16 | 64 | 29 ± 5 | 122 ± 39 | 24 ± 11 | 20 ± 10 | 53 ± 19 | 92 ± 14 | |
| Rs 7903146 (TCF7L2) | Major | 24 | 45 ± 17 | 68 | 27 ± 5 | 122 ± 37 | 32 ± 28 | 21 ± 9 | 53 ± 19 | 92 ± 12 |
| Minor | 20 | 39 ± 16 | 65 | 26 ± 4 | 105 ± 31 | 21 ± 14 | 30 ± 16 | 64 ± 19 | 97 ± 6 | |
| rs1042044 (GLP1 receptor) | Major | 12 | 41 ± 18 | 73 | 27 ± 4 | 116 ± 39 | 19 ± 10 | 22 ± 10 | 58 ± 19 | 95 ± 9 |
| Minor | 32 | 43 ± 16 | 64 | 26 ± 5 | 114 ± 35 | 31 ± 26 | 26 ± 15 | 58 ± 19 | 96 ± 8 | |
| rs6923761 (GLP1) | Major | 23 | 41 ± 16 | 63 | 26 ± 5 | 117 ± 38 | 29 ± 28 | 27 ± 16 | 58 ± 20 | 95 ± 5 |
| Minor | 21 | 44 ± 18 | 71 | 26 ± 4 | 113 ± 33 | 26 ± 18 | 23 ± 9 | 59 ± 18 | 94 ± 11 |
Gastric emptying and associations with SNPs
Gastric emptying of solids was normal in 38 participants (ie, 27 controls and 11 patients). The remaining 6 patients had rapid (4 patients) or delayed GE (2 patients). Gastric emptying of liquids was normal in 27 controls and 14 patients; 2 had rapid and 1 had delayed GE (data not shown).
For TCF7L2, the minor allele was associated with numerically shorter GE thalf (i.e., faster GE) for solids (p=0.14) and liquids (p=0.15) compared to the major allele (Tables 1 and 2). However, differences were not significant. The other 3 SNPs were not associated with faster (Wilcoxon rank sum test) or with rapid GE (Fisher’s exact test).
Table 2.
Distribution of SNPs in Participants with Normal, Rapid and Delayed Gastric Emptying1
| SNP (gene) | Allele | N | GE solids | ||
|---|---|---|---|---|---|
| Normal | Rapid | Delayed | |||
| rs1800857 (CCK) | Major | 34 | 30 (88%) | 3 (9%) | 1 (3%) |
| Minor | 10 | 8 (80%) | 1 (10%) | 1(10%) | |
| Rs 7903146 (TCF7L2) | Major | 24 | 21 (88%) | 1 (4%) | 2 (8%) |
| Minor | 20 | 17 (85%) | 3 (15%) | 0 | |
| rs1042044 (GLP1 receptor) | Major | 12 | 10 (84%) | 1(8%) | 1(8%) |
| Minor | 32 | 28 (88%) | 3 (9%) | 1 (3%) | |
| rs6923761 (GLP1) | Major | 23 | 19 (83%) | 3 (13%) | 1 (4%) |
| Minor | 21 | 19 (90%) | 1 (5%) | 1 (5%) | |
Because the normal values for GE were derived from healthy controls, all healthy controls had normal GE.
Enteral dextrose infusion and associations with SNPs
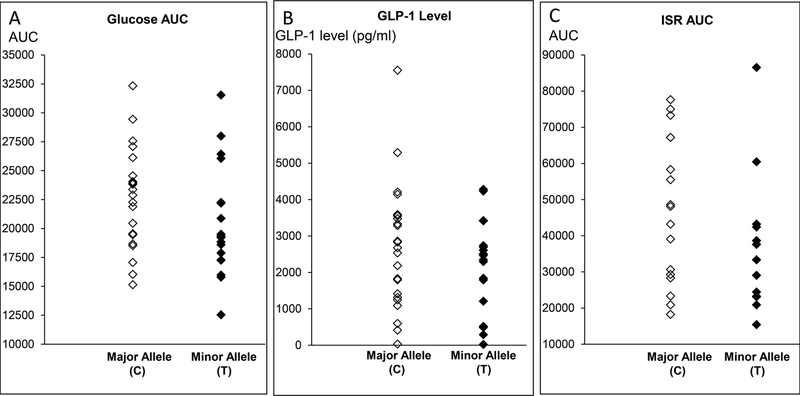
During the enteral dextrose infusion, the blood glucose concentration (area under the curve) exceeded the 90th percentile values in controls in 4 of 14 (29%) patients, indicative of impaired glucose tolerance. The insulin secretion rate could be reliably estimated in 32 participants (ie, 23 controls and 9 patients). The plasma glucose, GLP-1 levels, and insulin secretion rate were not significantly associated with any of the selected SNPs (Table 3, Figure 2).
Table 3.
Distribution of SNPs versus Glycemia, GLP1 Levels, and Insulin Secretion Rates during Enteral Dextrose Infusion
| SNP (gene) | Allele | Glucose | GLP1 | Insulin Secretion rate | |
|---|---|---|---|---|---|
| rs1800857 (CCK) | Major | N | 34 | 33 | 25 |
| Values | 19553 (18509, 24038) | 2690 (1826, 3476) | 37602 (24432, 49146) | ||
| Minor | N | 9 | 9 | 7 | |
| Values | 20450 (19353, 23935) | 1410 (1208, 1838) | 43211 (36204, 63796) | ||
| Rs 7903146 (TCF7L2) | Major | N | 23 | 23 | 18 |
| Values | 22265 (19484, 24295) | 2675 (1361, 3511) | 45627 (29598, 64942) | ||
| Minor | N | 20 | 19 | 14 | |
| Values | 19338 (17572, 22221) | 2468 (1499, 3072) | 33329 (23535, 43008) | ||
| rs1042044 (GLP1 receptor) |
Major | N | 13 | 12 | 10 |
| Values | 21383 (19543, 24089) | 2004 (1234, 3367) | 35978 (29598, 45835) | ||
| Minor | N | 30 | 30 | 22 | |
| Values | 19479 (18037, 23869) | 2641 (1791, 3415) | 42784 (23580, 57585) | ||
| rs6923761 (GLP1) | Major | N | 22 | 21 | 17 |
| Values | 19504 (18059, 23749) | 2704 (1952, 3421) | 43167 (29021, 60434) | ||
| Minor | N | 21 | 21 | 15 | |
| Values | 20663 (19180, 24089) | 2004 (1234, 2975) | 33329 (25773, 52037) |
Values are Median (Q1, Q3)
Figure 2. Comparison of plasma glucose (panel A), GLP-1 levels (panel B), and insulin secretion rate (panel C) versus major and minor alleles at rs 7903146 (TCF7L2).
DISCUSSION
Our study shows that the T allele at rs7903146 (TCF7L2) is associated with numerically faster GE of liquids and solids in patients with NUD. This is consistent with prior observations among 62 normal weight, overweight, or obese individuals previously evaluated at our institution, which showed the T allele at the TCF7L2 rs7903146 polymorphism was significantly associated with faster GE of liquids but not solids.(20) TCF7L2, a member of the Wnt signaling pathway, is involved in β-cell development during embryogenesis and also protects mature β-cells.(31) The T allele at the TCF7L2 rs7903146 polymorphism is associated with impaired glucose-stimulated and incretin-induced insulin secretion (32–34) and conversion of proinsulin to insulin.(35) Glucose-stimulated insulin secretion is partly mediated by GLP-1. The T allele at the TCF7L2 rs7903146 polymorphism is nominally associated (p=0.06) with smaller fasting gastric volumes.(20) Taken together, these data are consistent with the hypothesis that among people with the T allele, lower gastric volumes predispose to higher gastric pressure and faster GE. This hypothesis can be tested in future studies by studying whether the TCF7L2 rs7903146 polymorphism predicts the effects of GLP-1 on fasting and postprandial gastric volumes in healthy people, NUD, and diabetes mellitus. A separate but related question is whether this polymorphism is associated with rapid GE in patients with type 2 DM.(6)
A second mechanism of interest because of its effects on gastric functions and glycemic control is GLP-1 which increases fasting and postprandial gastric volumes; the latter is partly mediated by nitric oxide.(36) However, GE of solids, gastric accommodation, and fasting and postprandial plasma GLP-1 levels measured over 120 minutes during enteral dextrose infusion were not significantly associated with GLP-1 polymorphisms.
None of the other SNPs were associated with GE. While the associations between the two SNPs in GLP-1 and GE have not been evaluated previously, the major (T) allele at rs1800857 (CCK) was associated, albeit weakly, with delayed GE among patients with constipation-predominant irritable bowel syndrome.(27) The SNPs rs6923761 and rs1042044 in the GLP-1 gene which are associated respectively with GLP1-induced insulin secretion (22) and cortisol levels were not associated with abnormal GE in this study.
Peripheral insulin concentrations represent the end result of insulin secretion and hepatic extraction of insulin. Hence we used a validated approach (i.e., deconvolution of C-peptide concentrations) to measure insulin secretion rates. When caloric appearance into the duodenum is controlled by enteral infusion (i.e., not affected by differences in GE among patients), insulin secretion rate did not differ between groups. However it is possible that accelerated gastric emptying, which is associated with lower plasma insulin concentrations in type 2 diabetes mellitus, might unmask an inherent defect in the beta-cell response.(6) Also, we only measured insulin secretion. The gold-standard measurement of beta-cell function in a given individual expresses insulin secretion as a function of the prevailing insulin action.(37) It is therefore possible that our methodology missed significant between-group differences in beta-cell function.
The lack of associations between these selected SNPs and biological variables (gastric emptying, glucose and GLP1 during enteral dextrose infusion) tested in this study may reflect a type 2 error. A post hoc analysis of our dataset suggests that for the TCF7L2 rs7903146 polymorphism, assuming a SD of 49 minutes and a sample size of 40 participants we had 80% power (two-sample t test, two-sided α = 0.05) to detect a difference of 44 minutes in the GE of solids between the major and minor alleles. A sample size of 263 participants would be necessary to determine if the observed difference of 17 minutes between the major and minor alleles is statistically significant. One hundred and twenty three participants would be required to detect the minimum clinically-important difference defined as 0.5 times the baseline SD (i.e., 49 minutes) i.e., 25 minutes.(38)
There were several strengths and limitations of this prospective study. Gastric emptying, and measurements during enteral dextrose infusion were obtained with state-of-the-art techniques and compared in healthy controls and patients with NUD. For all SNPs, the observed minor allele frequency was comparable to previous studies, i.e., observed (published) were 0.11 (versus 0.13) for rs1800857, 0.27 (0.28) for rs7903146, 0.43 (0.40) for rs1042044, and 0.30 (0.37) for rs6923761. However, the pilot study was under-powered to detect clinically-significant differences between groups.
In conclusion, the current results provide the preliminary data for future studies evaluating the relationships between genetic polymorphisms, gastric emptying, and glycemia during enteral glucose infusion.
KEY POINTS.
For reasons unclear, some patients with functional upper gastrointestinal symptoms have impaired glucose tolerance during enteral glucose infusion.
This study evaluated the associations between selected genetic polymorphisms in CCK, GLP-1, and TCF7L2 and gastric emptying (GE), glucose tolerance, and insulin release in such patients.
While studied polymorphisms were not associated with plasma glucose, GLP-1 levels, and insulin secretion rates, a trend toward an association between faster GE and a diabetes-associated allele in TCF7L2 was observed.
This study serves as a pilot for future studies evaluating the associations between genetic polymorphisms, GE, and glycemia.
ACKNOWLEDGEMENTS
AEB designed the study
KF, PC, and ML conducted the studies
BA and AD analyzed the data
MC, AV, BA, and AEB interpreted the findings and wrote the manuscript
All authors reviewed and approved the final submitted version.
FUNDING
This work was supported in part by USPHS NIH Grant P01 DK68055 to Dr. Bharucha
Footnotes
DISCLOSURES
The authors have no conflicts of interest.
REFERENCES
- 1.Lawal A, Barboi A, Krasnow A, Hellman R, Jaradeh S, Massey BT. Rapid gastric emptying is more common than gastroparesis in patients with autonomic dysfunction. Am J Gastroenterol 2007; 102: 618–623. [DOI] [PubMed] [Google Scholar]
- 2.Bharucha AE, Manduca A, Lake DS, et al. Gastric Motor Disturbances In Patients With Idiopathic Rapid Gastric Emptying. Neurogastroenterol Motil 2010; 23: 617–e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hejazi RA, Patil H, McCallum RW. Dumping syndrome: establishing criteria for diagnosis and identifying new etiologies. Dig Dis Sci 2010; 55: 117–123. [DOI] [PubMed] [Google Scholar]
- 4.Loavenbruck A, Iturrino J, Singer W, et al. Disturbances of gastrointestinal transit and autonomic functions in postural orthostatic tachycardia syndrome. Neurogastroenterol Motil 2015; 27: 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez Cifuentes J, Radetic M, Lopez R, Gabbard S. Clinical Predictors of Rapid Gastric Emptying in Patients Presenting with Dyspeptic Symptoms. Dig Dis Sci 2019; 13: 13. [DOI] [PubMed] [Google Scholar]
- 6.Frank JW, Saslow SB, Camilleri M, Thomforde GM, Dinneen S, Rizza RA. Mechanism of accelerated gastric emptying of liquids and hyperglycemia in patients with type II diabetes mellitus. Gastroenterology 1995; 109: 755–765. [DOI] [PubMed] [Google Scholar]
- 7.Camilleri M Integrated upper gastrointestinal response to food intake. Gastroenterology 2006; 131: 640–658. [DOI] [PubMed] [Google Scholar]
- 8.Tack J, Arts J, Caenepeel P, De Wulf D, Bisschops R. Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nature Reviews Gastroenterology & Hepatology 2009; 6: 583–590. [DOI] [PubMed] [Google Scholar]
- 9.Bharucha AE, Camilleri M, Burton DD, et al. Increased nutrient sensitivity and plasma concentrations of enteral hormones during duodenal nutrient infusion in functional dyspepsia. Am J Gastroenterol 2014; 109: 1910–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SY, Acosta A, Camilleri M, et al. Gastric Motor Dysfunction in Patients With Functional Gastroduodenal Symptoms. Am J Gastroenterol 2017; 112: 1689–1699. [DOI] [PubMed] [Google Scholar]
- 11.Talley NJ, Haque M, Wyeth JW, et al. Development of a new dyspepsia impact scale: the Nepean Dyspepsia Index. Aliment Pharmacol Ther 1999; 13: 225–235. [DOI] [PubMed] [Google Scholar]
- 12.Talley NJ, Verlinden M, Jones M. Validity of a new quality of life scale for functional dyspepsia: a United States multicenter trial of the Nepean Dyspepsia Index. Am J Gastroenterol 1999; 94: 2390–2397. [DOI] [PubMed] [Google Scholar]
- 13.Zigmond A, Snaith R. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 14.Desai A, O’Connor M, Neja B, et al. Reproducibility of gastric emptying assessed with scintigraphy in patients with upper GI symptoms. Neurogastroenterol Motil 2018: e13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alzaid AA, Dinneen SF, Turk DJ, Caumo A, Cobelli C, Rizza RA. Assessment of insulin action and glucose effectiveness in diabetic and nondiabetic humans. J Clin Invest 1994; 94: 2341–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Firth RG, Bell PM, Marsh HM, Hansen I, Rizza RA. Postprandial hyperglycemia in patients with noninsulin-dependent diabetes mellitus. Role of hepatic and extrahepatic tissues. J Clin Invest 1986; 77: 1525–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMahon MM, Schwenk WF, Haymond MW, Rizza RA. Underestimation of glucose turnover measured with [6–3H]- and [6,6–2H]- but not [6–14C]glucose during hyperinsulinemia in humans. Diabetes 1989; 38: 97–107. [DOI] [PubMed] [Google Scholar]
- 18.Butler PC, Rizza RA. Contribution to postprandial hyperglycemia and effect on initial splanchnic glucose clearance of hepatic glucose cycling in glucose-intolerant or NIDDM patients. Diabetes 1991; 40: 73–81. [PubMed] [Google Scholar]
- 19.Rehfeld JF. Accurate measurement of cholecystokinin in plasma.[see comment]. Clin Chem 1998; 44: 991–1001. [PubMed] [Google Scholar]
- 20.Vazquez-Roque MI, Camilleri M, Vella A, Carlson P, Laugen J, Zinsmeister AR. Association of TCF7L2 allelic variations with gastric function, satiation, and GLP-1 levels. Clinical and translational science 2011; 4: 183–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camilleri CE, Carlson PJ, Camilleri M, et al. A study of candidate genotypes associated with dyspepsia in a U.S. community. Am J Gastroenterol 2006; 101: 581–592. [DOI] [PubMed] [Google Scholar]
- 22.Sathananthan A, Man CD, Micheletto F, et al. Common genetic variation in GLP1R and insulin secretion in response to exogenous GLP-1 in nondiabetic subjects: a pilot study. Diabetes Care 2010; 33: 2074–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheikh HI, Dougherty LR, Hayden EP, Klein DN, Singh SM. Glucagon-like peptide-1 receptor gene polymorphism (Leu260Phe) is associated with morning cortisol in preschoolers. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34: 980–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Florez JC, Jablonski KA, Bayley N, et al. TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med 2006; 355: 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helgason A, Palsson S, Thorleifsson G, et al. Refining the impact of TCF7L2 gene variants on type 2 diabetes and adaptive evolution. Nat Genet 2007; 39: 218–225. [DOI] [PubMed] [Google Scholar]
- 26.Cauchi S, Froguel P. TCF7L2 genetic defect and type 2 diabetes. Current Diabetes Reports 2008; 8: 149–155. [DOI] [PubMed] [Google Scholar]
- 27.Cremonini F, Camilleri M, McKinzie S, et al. Effect of CCK-1 antagonist, dexloxiglumide, in female patients with irritable bowel syndrome: a pharmacodynamic and pharmacogenomic study. Am J Gastroenterol 2005; 100: 652–663. [DOI] [PubMed] [Google Scholar]
- 28.Park SY, Rew JS, Lee SM, et al. Association of CCK(1) Receptor Gene Polymorphisms and Irritable Bowel Syndrome in Korean. Journal of neurogastroenterology and motility 2010; 16: 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992; 41: 368–377. [DOI] [PubMed] [Google Scholar]
- 30.Varghese RT, Dalla Man C, Laurenti MC, et al. Performance of individually measured vs population-based C-peptide kinetics to assess beta-cell function in the presence and absence of acute insulin resistance. Diabetes, Obesity & Metabolism 2018; 20: 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin T Current Understanding on Role of the Wnt Signaling Pathway Effector TCF7L2 in Glucose Homeostasis. Endocr Rev 2016; 37: 254–277. [DOI] [PubMed] [Google Scholar]
- 32.Saxena R, Gianniny L, Burtt NP, et al. Common single nucleotide polymorphisms in TCF7L2 are reproducibly associated with type 2 diabetes and reduce the insulin response to glucose in nondiabetic individuals. Diabetes 2006; 55: 2890–2895. [DOI] [PubMed] [Google Scholar]
- 33.Lyssenko V, Lupi R, Marchetti P, et al. Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest 2007; 117: 2155–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villareal DT, Robertson H, Bell GI, et al. TCF7L2 variant rs7903146 affects the risk of type 2 diabetes by modulating incretin action. Diabetes 2010; 59: 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y, Park SY, Su J, et al. TCF7L2 is a master regulator of insulin production and processing. Hum Mol Genet 2014; 23: 6419–6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andrews CN, Bharucha AE, Camilleri M, et al. Nitrergic contribution to gastric relaxation induced by glucagon-like peptide-1 (GLP-1) in healthy adults. American Journal of Physiology - Gastrointestinal & Liver Physiology 2007; 292: G1359–1365. [DOI] [PubMed] [Google Scholar]
- 37.Cobelli C, Dalla Man C, Toffolo G, Basu R, Vella A, Rizza R. The oral minimal model method. Diabetes 2014; 63: 1203–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol 2003; 56: 395–407. [DOI] [PubMed] [Google Scholar]