Abstract
Adoptive transfer of autologous polyclonal regulatory T cells (Tregs) is a promising option for reducing graft rejection in allogeneic transplantation. To gain therapeutic levels of Tregs there is a need to expand obtained cells ex vivo, usually in the presence of the mTOR inhibitor Rapamycin due to its ability to suppress proliferation of non-Treg T cells, thus promoting a purer Treg yield. Azithromycin is a bacteriostatic macrolide with mTOR inhibitory activity that has been shown to exert immunomodulatory effects on several types of immune cells. In this study we investigated the effects of Azithromycin, compared with Rapamycin, on Treg phenotype, growth, and function when expanding bulk, naïve, and memory Tregs. Furthermore, the intracellular concentration of Rapamycin in CD4+ T cells as well as in the culture medium was measured for up to 48 h after supplemented. Treg phenotype was assessed by flow cytometry and Treg function was measured as inhibition of responder T-cell expansion in a suppression assay. The concentration of Rapamycin was quantified with liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). Azithromycin and Rapamycin both promoted a FoxP3-positive Treg phenotype in bulk Tregs, while Rapamycin also increased FoxP3 and FoxP3+Helios positivity in naïve and memory Tregs. Furthermore, Rapamycin inhibited the expansion of naïve Tregs, but also increased their suppressive effect. Rapamycin was quickly degraded in 37°C medium, yet was retained intracellularly. While both compounds may benefit expansion of FoxP3+ Tregs in vitro, further studies elucidating the effects of Azithromycin treatment on Tregs are needed to determine its potential use.
Keywords: azithromycin, rapamycin, regulatory T cells, Tregs, mTOR
Introduction
Adoptive cell therapy with immunosuppressive regulatory T cells (Tregs) raises hope for clinical applications in transplantation settings and for prevention of immunological disorders1–4. Previous studies have shown promising results for prevention of graft-versus-host disease in allogeneic stem cell transplantation and in reducing beta cell destruction in newly onset type-1 diabetes1–3.
Currently, the effects of adoptive Treg therapy in liver and kidney transplantation are under study at several clinics worldwide4. The exact numbers of Tregs needed to reach therapeutic levels have not been defined; however, previous trials suggest dose ranges between 0.1 × 106 and 20 × 106 polyclonal natural Tregs (nTregs) per kilo of bodyweight1–3,5. In order to amass such cell numbers, in vitro expansion of nTregs is required prior to adoptive cell transfer6. However, Treg cultures risk contamination of T effector (Teff) cells and of Tregs converting to pro-inflammatory Th17 cells during expansion7–11. The ideal in vitro culture condition of Tregs would therefore promote fast expansion of stable functional Tregs while reducing non-Treg contamination.
Rapamycin (RAP) is a macrolide with immunosuppressive properties used in transplantation settings for the prevention of graft rejection12. It is also utilized for improving in vitro cultures of Tregs due to its ability to suppress proliferation of non-Treg T cells, thus promoting a purer Treg yield7,8,13,14. RAP works by binding the 12-Kda FK506- and-Rapamycin-binding protein (FKBP12), forming a complex that exerts an inhibitory effect on the mammalian target of Rapamycin (mTOR) kinase, which is involved in the regulation of dendritic, B-cell, and T-cell activation15,16. While both Treg and Teff cells are affected by the suppressive properties of RAP, the impact is less profound on Tregs15. Still, the growth inhibition of Tregs treated with RAP remains an issue, and therefore alternatives for improving Treg in vitro expansions are sought17–19. Apart from RAP, other macrolides such as Clarithromycin and especially Azithromycin (AZM) have also been recognized for their immunomodulatory effects20–22. AZM is widely used for treatment of soft tissue infections and respiratory tract diseases, and has favorable safety and tolerability qualities22–29. Interestingly, the drug shows immunomodulatory properties even in therapeutic dosages22–24,27. AZM is also known for high intracellular accumulation in peripheral blood mononuclear cells (PBMCs), polymorphonuclear cells, and fibroblasts, with pleotropic effects on their function21,22,30–33. Notably, the intracellular concentration of RAP in lymphocytes over time during expansion in vitro has not been analyzed before. A previous study by Ratzinger et al. suggests that AZM may, in similarity to RAP, suppress T-cell activation by modulation of the mTOR pathway, thus hampering CD4+ T-cell proliferation and cytokine secretion20,34. However, while the effects of AZM on bulk CD4+ T cells have been studied, the impact on Tregs has not been explored. The aim of the present study was to investigate if AZM is a possible alternative to RAP in enhancing the quality of in vitro expanded Tregs. We measured the effects of treating Treg cultures with AZM compared with RAP, in terms of expansive capacity, phenotype, and suppressive activity. In addition, we examined if the effects of AZM and RAP differ between expanded bulk, naïve CD45RA+, and memory CD45RA- Treg cultures. Furthermore, the degradation of RAP was examined by measuring intracellular and extracellular concentrations over time in vitro.
Materials and Methods
Blood Samples
Buffy coats were collected from healthy blood donors at the Uppsala University Hospital Blood Bank (Uppsala, Sweden) with approval from the Regional Ethics Committee (Dnr 2010/69). Female and male donors aged 18–65 were eligible for donation and a total of 27 donors were included in the study.
Cell Purification
PBMCs were isolated from buffy coats by separation over a Ficoll-Paque gradient (GE Healthcare, Chicago, Il, USA). Adherent cells were removed during 2 h incubation at 37°C in T175 flasks using standard RPMI-1640 medium modified with L-glutamine (Gibco, Thermo Fisher Scientific, Waltham, MA, USA), 1% 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 1% penicillin-streptomycin, 0,04% β-mercaptoethanol and 2% pooled human serum. T cells were purified from the non-adherent PBMCs by magnetic activated cell sorting (MACS) using either the CD4+CD25+ Regulatory T Cell Isolation Kit or the CD4+ T Cell Isolation Kit (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. Isolated Tregs were used for bulk Treg cell culture, while isolated CD4+ T cells were used for further Treg purification by flow cytometric sorting or cryopreserved for later use with CryoStor® cell cryopreservation media (Merck, Darmstadt, Germany), frozen with CoolCell (Corning, NY, USA) and stored at –70°C for up to 3 weeks.
Flow Cytometric Sorting
MACS-sorted CD4+ T cells were thawed in 37°C water baths and washed with RPMI-1640 medium containing 10% fetal bovine serum. Cells were stained for flow cytometric sorting using the following antibodies: CD4-Brilliant Violet 421, CD25-Phycoerythrin, CD127-Fluorescein Isothiocyanate, and CD45RA-Phycoerythrin Cyanin 7, all from BD Biosciences, San Jose, CA, USA. Sorting was performed on FACS Aria III and FACS Melody from BD Biosciences. Different populations were selected for sorting: Effector T cells CD4+CD25lowCD127+, bulk Tregs CD4+CD25highCD127low or naïve Tregs CD4+CD25highCD127lowCD45RA+ and memory Tregs CD4+CD25highCD127lowCD45RA–. The purified Tregs were used for cell culture and the Teffs were cryopreserved for later use as described above.
Flow Cytometric Analyses
The expression of Treg-associated markers Forkhead box P3 (FoxP3), Helios, L-selectin (CD62L), and Interleukin 1 receptor type I (IL-1RI) was analyzed and compared between the expanded Treg cultures. In addition to the previously mentioned antibodies for flow cytometric sorting, the following antibodies were used for analysis: IL-1RI-FITC (R&D Systems, Minneapolis, MN, USA), CD45-Allophycocyanin-Hilite 7 (BioLegend, San Diego, CA, USA), CD62L-Brilliant Violet 421 (BioLegend), CD25-Brilliant Violet 510 (BioLegend), FoxP3-Alexa Fluor 647 (BD Biosciences), CD4-Peridinin-Chlorophyll-Protein Cyanin 5.5 (BD Biosciences) and Helios-Phycoerythrin (BD Biosciences). Cells were prepared for intracellular staining using FoxP3 Staining Buffer Set (eBioscience, Thermo Fisher Scientific) according to the manufacturer’s instructions. All flow cytometric analyses were performed on a FACSVerse™ (BD Biosciences).
Cell Cultures
During preparatory studies we found a large reduction of CD4+ T-cell numbers at AZM concentrations of 50 ug/ml or more, which is in concordance with what has been previously reported20. An intermediate AZM concentration of 25 ug/ml was therefore chosen to avoid cell toxicity and still retain a possible inhibition of the mTOR pathway. RAP was used at concentration of 100 ng/ml, which is commonly used in the literature and known to prevent Teff expansion14. Tregs purified by MACS or flow cytometric sorting were cultured in RPMI-1640 medium with the previously mentioned supplements and the addition of 10% human serum and IL-2 500 IU/ml. Starting populations consisted of 80,000–100,000 cells per well using 96-well plates. Human T-Activator CD3/CD28 beads (Gibco, Thermo Fisher Scientific) were added during culture start at a bead:cell ratio of 4:1 for Tregs and 1:1 for Teffs. Rapamycin 100 ng/ml (LC-laboratories, Woburn, MA, USA) or the AZM trade name Zithromax 25 ug/ml (Pfizer, New York, NY, USA) were added at culture start and continuously every second day. Beads were removed after a week and replaced at a 1:1 bead:cell ratio. Cultures were split to further wells and larger culture plates as the expansions grew, scaling up to 12-well and 6-well plates. Cultures were harvested after 2 weeks of growth and cells were either used for phenotype analysis by flow cytometry or rested for 48 h with IL-2 100 IU/ml without AZM or RAP before use in suppression assays.
Suppression Assays
FACS-sorted CD4+CD25lowCD127+ T cell responders (Tresps) were thawed in 37°C water baths and washed with RPMI-1640 medium containing 10% fetal bovine serum. Cells were stained with CellTrace™ Violet (Molecular Probes, Thermo Fisher Scientific) at a 5 μM concentration and incubated at 37°C for 20 min. Stained cells were washed using RPMI-1640 medium with 10% human serum. Tresps were cultured in 96-well plates at starting populations of 50,000 cells together with expanded resting Tregs at Treg:Tresp ratios of 1:1, 1:2, and 1:5. Human T-Activator CD3/CD28 beads were added during culture start at a bead:Tresp ratio of 0.25:1. Tresps co-cultured with expanded Teffs, or Tresps cultured alone, were used as positive controls. Tresps cultured without the addition of beads were used as negative controls. The suppression assays were harvested after 72 h, stained with LIVE/DEAD® Fixable Near-IR Dead Cell Stain (Molecular Probes, Thermo Fisher Scientific) and analyzed by flow cytometry. The frequencies of live non-proliferating Tresps were calculated using the proliferation tool in FlowJo version 10.6.0 (FlowJo LLC, Ashland, OR, USA).
Quantification of Rapamycin
MACS-sorted CD4+ T cells were thawed and washed as previously described. RPMI-1640 medium was used with addition of the previously mentioned supplements plus 10% human serum and IL-2 at a concentration of 100 IU/ml. The medium was stored in 12-well plates at 4°C or incubated at 37°C with or without the addition of 1 × 106 CD4+ T cells per well. RAP was added to all wells at the start of the experiment at a concentration of 100 ng/ml. Medium, cells, and supernatants were collected at multiple times during 48 h. Supernatants and cell pellets were separated by centrifugation and the cells were washed with 4°C phosphate buffer saline. Both supernatants and cells pellets were stored at –70°C until analysis. RAP in cells, medium, and supernatants was measured with liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) using a Transcend II LX-2 TSQ Quantiva system (Thermo Fisher Scientific). HPLC-grade methanol was used as extraction reagent for the cell analysis and methanol:zinc sulfate 0.1 mol/l (2:1) for analysis of the cell medium and supernatant. The extraction solutions contained the internal standard 13C, D3-rapamycin. Following mixing on a multishaker and centrifugation, the extracts were transferred to the instrument autosampler and injected on an Accucore C8 column (50 × 2.1 mm, 2.6 µm; Thermo Fisher Scientific). The mobile phases consisted of UHPLC-MS grade (A) water and (B) methanol added 0.10% formic acid and 2.0 mmol/l ammonium acetate. The chromatographic starting condition was 50% B, and elution of the analyte and internal standard was performed with 90% B (flow rate 0.600 ml/min, temperature 75°C). Electrospray ionization combined with selective reaction monitoring was applied for the mass spectrometric detection of positively charged ammonium adducts (RAP m/z 931.6 to 864.5 and the internal standard m/z 935.6 to 864.5). The peak area ratio between analyte and internal standard was used as instrument response, and the quantification was based on calibrators with declared RAP concentrations (Recipe, Munich, Germany).
Statistical Analysis
The Wilcoxon signed-rank test was used for comparing data between two populations. Groups of three populations were compared using the Friedman test with Dunn’s multiple comparisons post hoc test. A p-value <0.05 was considered statistically significant. FlowJo version 10.6.0 was used for analyzing flow cytometric data. Graphpad Prism version 6 (Graphpad Software Inc., San Diego, CA, USA) was used for statistical computations and visualizations.
Results
Phenotype of Expanded Tregs
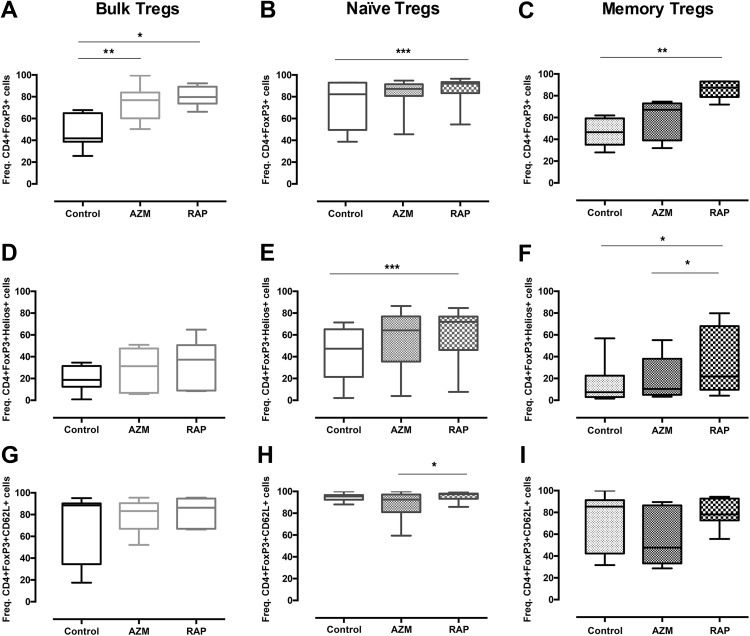
Bulk, naïve, and memory Tregs were analyzed for CD4+FoxP3 along with the common Treg-associated markers Helios and CD62L after 2 weeks of culture. RAP treatment presented higher frequencies of CD4+FoxP3+Helios+ cells among memory Tregs compared with AZM treatment (p = 0.0373) (Fig. 1f). In addition, RAP showed higher frequencies of CD4+FoxP3+CD62L+ cells in naïve Tregs compared with AZM (p = 0.0231) (Fig. 1h). In comparison to no treatment, RAP treatment presented higher frequencies of CD4+FoxP3+ cells in all three populations: bulk Tregs (p = 0.0226), naïve Tregs (p = 0.0009), and memory Tregs (p = 0.0015) (Fig. 1a–c). RAP also showed higher frequencies of CD4+FoxP3+Helios+ cells compared with no supplement for both naïve Tregs (p = 0.0003) and memory Tregs (p = 0.0179) (Fig. 1e,f). AZM presented higher frequencies of CD4+FoxP3+ cells compared with no supplement only in bulk Tregs (p = 0.0099) (Fig. 1a). The CD4+FoxP3+ frequencies were also analyzed prior to expansion: bulk Tregs 91.6% (±6.31 SD), naïve Tregs 90.9% (±5.15 SD), and memory Tregs 86.3% (±3.82 SD).
Fig 1.
Azithromycin and Rapamycin affect Treg phenotype. Graph showing the effect of Azithromycin and Rapamycin on the frequency of (a,b,c) CD4+FoxP3+ cells, (d,e,f) Helios+ of all CD4+FoxP3+ cells and (g,h,i) CD62L+ of all CD4+FoxP3+ cells in expanded bulk, naïve, and memory Tregs. Tregs enriched by FACS were stimulated with CD3/CD28 beads and cultured with no treatment or in the presence of Azithromycin or Rapamycin for 14 days. Cells were then stained for CD4, CD62L, FoxP3, and Helios and acquired by FACS. Bulk Tregs (n = 7), naïve Tregs (n = 12), and memory Tregs (n = 8). *, **, *** denotes p<0.05, p<0.01, p<0.001, respectively.
Expansion of Tregs
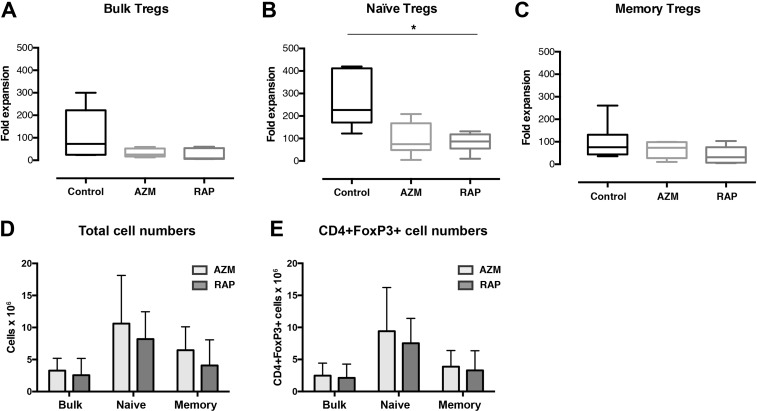
The expansion of Tregs was investigated after 2 weeks of culture. No significant differences in fold expansion were found between RAP and AZM treatment in either Treg sub-population. However, naïve Tregs treated with RAP presented lower fold expansion compared with no treatment (p = 0.0226) (Fig. 2b).
Fig 2.
The influence of Azithromycin and Rapamycin on Treg expansion. The graphs summarize fold expansion of (a) bulk, (b) naïve and (c) memory Tregs expanded with no treatment or in the presence of Azithromycin or Rapamycin. It also shows (d) total cell numbers and (e) CD4+FoxP3+ cell numbers at harvest. Tregs enriched by FACS were stimulated with CD3/CD28 beads and cultured for 14 days. Bulk Tregs (n = 5), naïve (n = 7), and memory Tregs (n = 6). * denotes p<0.05.
The Treg populations treated with AZM showed a trend of larger mean numbers of cells compared with the Treg populations treated with RAP: bulk Tregs 28.9%, naïve Tregs 29.4%, and memory Tregs 58.5% more cells than with RAP treatment (Fig. 2d). However, these differences in the mean number of cells did not reach statistical significance. The bulk Tregs treated with RAP showed a mean number of 2.55 (±2.61 SD) × 106 cells, compared with bulk Tregs treated with AZM which presented 3.27 (±1.91 SD) × 106 cells. A similar difference in mean cell numbers was found between naïve Tregs treated with RAP and AZM, which showed 8.19 (±4.27 SD) and 10.61 (±7.50 SD) × 106 cells, respectively. A larger difference was found between memory Tregs treated with RAP and AZM, which presented 4.07 (±3.99 SD) × 106 cells and 6.46 (±3.64 SD) × 106 cells, respectively.
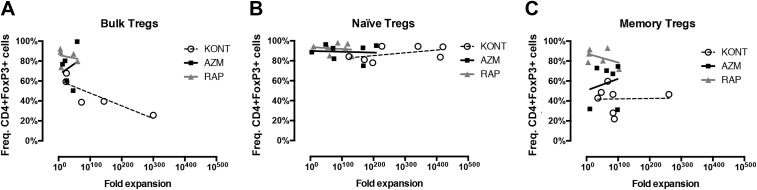
FoxP3 Expression in Relation to Expansion
To investigate the Treg preserving effects of AZM and RAP during expansion, both fold expansion and the frequencies of CD4+FoxP3+ cells were compared after 2 weeks of culture. Tregs cultured with AZM presented larger variations than RAP in the frequencies of CD4+FoxP3+ cells, for which we found no significant correlation with fold expansion. RAP-treated Tregs presented higher frequencies of CD4+FoxP3+ cells than no treatment for all three populations, though no correlation was found between FoxP3 positivity and fold expansion (Fig. 3a–c). Neither did Tregs cultured with no supplement present any correlation between CD4+FoxP3+ cell frequencies and fold expansion (Fig. 3a–c). Though no significant differences were found between AZM and RAP, the Treg populations treated with AZM showed a trend of larger mean numbers of CD4+FoxP3+ cells compared with the Treg populations treated with RAP: bulk Tregs 16.2%, naïve Tregs 25.0%, and memory Tregs 17.6% more CD4+FoxP3+ cells than with RAP treatment (Fig. 2e). The bulk Tregs treated with RAP showed a mean number of 2.12 (±2.14 SD) × 106 CD4+FoxP3+ cells, compared with bulk Tregs treated AZM which presented 2.46 (±1.96 SD) × 106 CD4+FoxP3+ cells. Naïve Tregs treated with RAP and AZM presented slightly larger differences in mean numbers, showing 7.52 (±3.89 SD) and 9.40 (±6.81 SD) × 106 CD4+FoxP3+ cells, respectively. Memory Tregs treated with RAP and AZM showed differences in mean numbers comparable to that of bulk Tregs, presenting 3.3 (±3.06 SD) and 3.88 (±2.50 SD) × 106 CD4+FoxP3+ cells, respectively.
Fig 3.
Treg fold expansion in relation to FoxP3 frequency. Graph showing the frequencies of CD4+FoxP3 positive cells in relation to fold expansion of (a) bulk, (b) naïve and (c) memory Tregs cultured with no treatment or in the presence of Azithromycin or Rapamycin. Tregs enriched by FACS were stimulated with CD3/CD28 beads and cultured for 14 days before harvest. Bulk Tregs (n = 5), naïve (n = 7), and memory Tregs (n = 6).
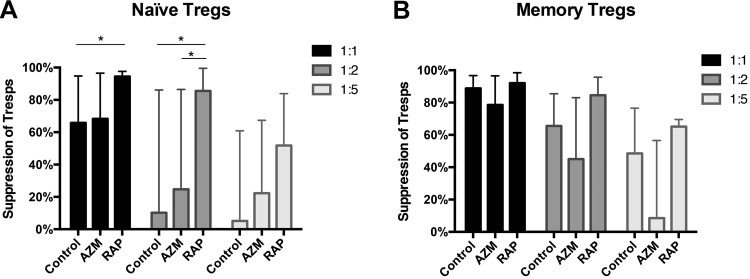
Suppression Assay Using Cultured Tregs
Tregs expanded with AZM, RAP, or no supplements were tested for their suppressive activity in an antigen-presenting cell independent suppression assay. CellTrace™ Violet-stained CD4+ T cells (Tresps) were co-cultured with Tregs for 72 h at different Treg:Tresp ratios. The data are presented as the frequencies of live non-proliferating Tresps. The Treg cultures suppressed Tresps in a dose-dependent manner. RAP-treated naïve Tregs showed higher rates of suppression than AZM-treated Tregs for 1:2 Treg:Tresp ratios (p = 0.0424) (Fig. 4a). Also, RAP-treated naïve Tregs presented higher rates of suppression than Tregs with no treatment for both 1:1 (p = 0.0117) and 1:2 (p = 0.0183) Treg:Tresp ratios (Fig. 4a). No significant differences in the rates of suppression were found among memory Tregs (Fig. 4b).
Fig 4.
Suppressive capacity of Tregs expanded with Azithromycin or Rapamycin. The graph summarizes the suppressive capacity of Tregs expanded with no treatment or in the presence of Azithromycin or Rapamycin. The rate of suppression is presented as the frequency of live non-proliferating autologous CD4+ T cell responders (Tresps) after co-culture with expanded (a) naïve or (b) memory Tregs at Treg:Tresp ratios of 1:1, 1:2 and 1:5. Expanded Tregs and Tresps were stimulated with CD3/CD28 beads and co-cultured for 3 days before analysis by FACS. Naïve Tregs (n = 7) and memory Tregs (n = 3).
CD4+FoxP3+IL-1RI Positivity in Expanded Tregs
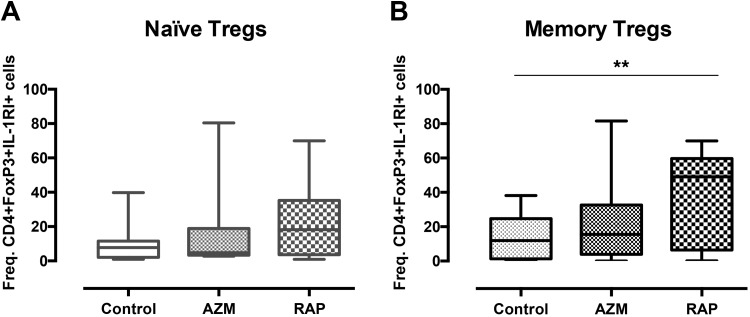
As IL-1RI positivity in Tregs has been associated with potential conversion to a pro-inflammatory phenotype, the frequency of Tregs positive for CD4+FoxP3+IL-1RI was analyzed after 2 weeks of culture. No differences were found between treatment with RAP and AZM. Memory Tregs treated with RAP showed higher frequency of cells positive for CD4+FoxP3+IL-1RI compared with no treatment (p = 0.0081) (Fig. 5b), while no differences were found for naïve Tregs (Fig. 5a).
Fig 5.
Rapamycin induces IL-1R1 expression. The graph present the frequencies of IL-1RI positive CD4+FoxP3+ cells among expanded (a) naïve and (b) memory Tregs cultured with no treatment or in the presence of Azithromycin or Rapamycin. Tregs enriched by FACS were stimulated with CD3/CD28 beads and cultured for 14 days. Cells were then stained for CD4, IL-1RI, and FoxP3 and acquired by FACS. Naïve Tregs (n = 12) and memory Tregs (n = 8). ** denotes p<0.01.
Quantification of Rapamycin
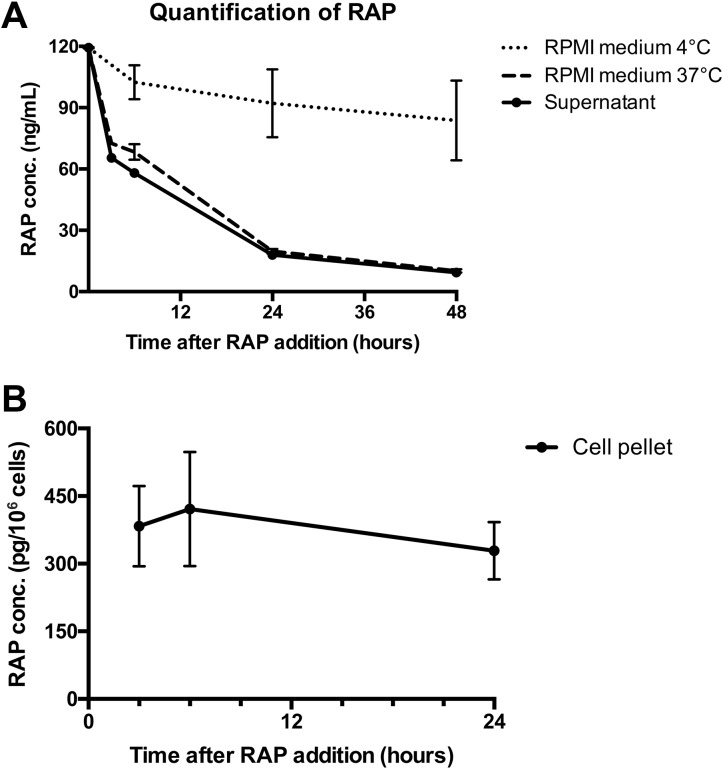
To investigate the degradation of RAP in CD4+ T cells and in culture medium, both the intracellular and extracellular concentrations were measured over time. The medium, cells, and supernatants were harvested at multiple times during 24- or 48-h culture and the concentration of RAP was analyzed by LC-MS/MS. Measurement of RAP concentration in 37°C medium showed a pattern of quick degradation during the first 24 h regardless of whether it was cultured with cells or not, while the degradation in 4°C medium progressed more slowly (Fig. 6a). The CD4+ T cells presented a mean intracellular concentration of 383.2 (± 89 SD) ρg/106 cells 3 h after the addition of RAP, which dropped to 328 (± 63.6 SD) ρg/pellet in 24 h (Fig. 6b).
Fig 6.
Rapamycin is retained in CD4+ T cells. Graph depicting the concentration of Rapamycin in RPMI medium, supernatant and cell pellet at multiple times during 48 h. Medium was stored at 4°C or incubated at 37°C with or without the addition of MACS-sorted CD4+ T cells. The concentration of Rapamycin was measured by LC-MS/MS. (a) Rapamycin concentration in 4°C medium, 37°C medium (n = 4) or supernatant (n = 8) measured five times during 48 h. (b) Rapamycin concentration in CD4+ T-cell pellets (n = 5) measured three times during 24 h.
Flow Cytometric Scatter Analysis
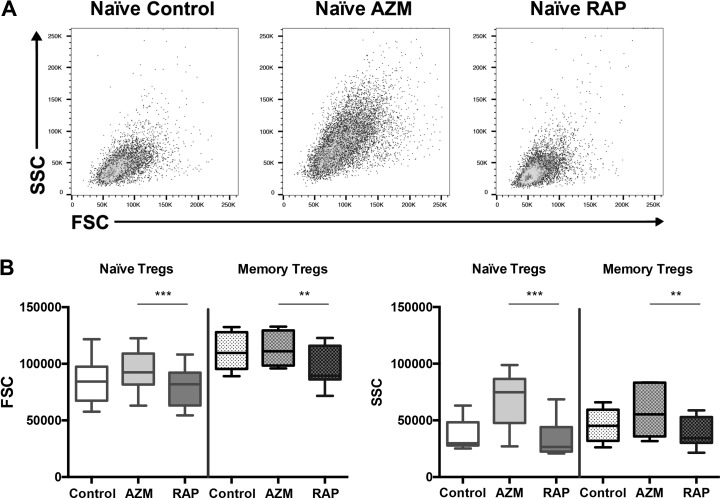
To measure the potential effect of AZM and RAP on cell size and internal cellular complexity, forward scatter (FSC) and side scatter (SSC) was compared between cell cultures after 2 weeks of expansion. Significant differences in FSC and SSC were found between Tregs cultured with RAP and AZM for both naïve Tregs (p = 0.0004, p = 0.0004) and memory Tregs (p = 0.004, p = 0.004) (Fig. 7b).
Fig 7.
Azithromycin increases Treg FSC and SSC compared with Rapamycin. Flow cytometric data showing forward-scatter (FSC) and side-scatter (SSC) properties of expanded Tregs. Cells enriched by FACS were stimulated with CD3/CD28 beads and cultured with no treatment or in the presence of Azithromycin or Rapamycin for 14 days. (A) Representative flow cytometric dot plots of cultured naïve Tregs. (B) FSC and SSC properties of cultured naïve (n = 10) and memory Tregs (n = 7). ** denotes p<0.01 and *** p<0.001.
Discussion
The main objective of this study was to investigate the possible effects of AZM compared with RAP on in vitro expanded Tregs. Compounds that improve the quality of Treg in vitro expansions are sought as Treg cultures risk contamination of Teffs and conversion of Tregs to pro-inflammatory Th17 cells7–11. Various agents such as RAP, transforming growth factor beta (TGF-beta), butyrate, and all-trans retinoic acid (ATRA) have presented Treg-enhancing capabilities in previous studies, though no consensus guidelines exist regarding optimal regimen for improving Treg in vitro expansions7,8,14,35–38. While the mTOR inhibitor RAP is commonly used for improving the quality of Treg cultures, the growth inhibition of RAP-treated Tregs is an issue17–19. AZM has known immunomodulatory properties and a previous study by Ratzinger et al. of AZM-treated CD4+ T cells suggests that AZM may, in similarity with RAP, suppress T-cell activation by modulation of the mTOR pathway20. Today AZM is widely used in the treatment of soft tissue infections and respiratory tract diseases, and has favorable safety and tolerability qualities that make the drug suitable for use in clinical applications22–29.
In this study we analyzed the impact of AZM compared with RAP on Treg phenotype, expansion, and function. The effects were studied in bulk, naïve, and memory Tregs due to the known heterogeneity of Tregs subpopulations39,40. We found that RAP treatment induced higher frequencies of FoxP3+Helios+ cells among memory Tregs than AZM treatment. Also, RAP-treated naïve Tregs were more suppressive compared with AZM or no treatment. Previous studies have shown that FoxP3+Helios+ Tregs maintain a stable phenotype and feature more immune-suppressive characteristics compared with FoxP3+Helios– Tregs41–44. This stability may be vital, as unstable Tregs can differentiate to pro-inflammatory Th17 cells during expansion, which could possibly be detrimental in clinical applications9–11. For bulk Tregs, both RAP and AZM treatment induced higher frequencies of FoxP3+ cells than cultures with no treatment. This trend was also evident among naïve and memory Tregs, but was only statistically significant for RAP treatment. Thus, further studies are required to elucidate how and to which degree AZM treatment affects FoxP3 expression. Also, analysis of the Treg-specific demethylated region, which is considered a signature of stable Tregs when demethylated, would be interesting for future studies in terms of determining whether AZM treatment induces a stable or transient Treg phenotype45.
Regarding FoxP3+IL-1RI expression, we found higher frequencies of positive cells among the memory Treg cultures than the naïve Treg cultures, which is in concordance with previous studies on memory Tregs and IL1-RI expression46,47. In this study, RAP treatment, but not AZM treatment, further increased memory Treg IL-1RI frequency compared with no treatment. Interestingly, IL-1RI expression in Helios– Tregs has been associated with secretion of the suppressive cytokine IL-10, but also with secretion of the inflammatory cytokine IL-17, which is known to increases when Tregs are stimulated in the presence of IL-146,47. The stability of the Treg lineage may be essential in clinical applications, and this notion suggests that memory Tregs and RAP treatment should be used with caution as IL-17 has been associated with multiple forms of inflammatory autoimmune pathologies48. According to our findings, culturing naïve Tregs may be preferred to minimize the frequencies of potentially inflammatory IL1-RI+ cells in Treg expansions. In addition, we found that naïve Treg cultures presented high frequencies of classic FoxP3+Helios+CD62L+ Tregs even with no treatment. Naïve Tregs may therefore be suitable for stable expansion of Tregs, which has been previously suggested in the literature40,49. However, naïve Tregs constitute only a minority of the Treg population in adults, and have been found to decrease with age while CD45RO memory Tregs increase50. Thus, the relatively small proportion of naïve Tregs may hinder amassment of large cell numbers for clinical applications, especially when dealing with elderly patients with lower numbers of naïve cells. We found that RAP-treated naïve Tregs expanded less than Tregs with no treatment, which is line with the literature17–19. Interestingly, our study did not show any differences in fold expansion for AZM-treated Tregs, compared with studies on CD4+ T cells by Ratzinger et al. and Lin et al., which present a dose-dependent negative effect of AZM treatment on expansion, viability and cytokine secretion20,34. While we did not analyze for viability or cytokine secretion in this study, our findings showed that the mean number of cells after expansion with AZM was 28–58% more compared with expansion with RAP. Although this trend did not reach statistical significance in our study, it may be of importance as Treg therapies are dose-dependent and require ex vivo expansion of Tregs to accumulate therapeutic levels6. Notably, even though RAP treatment may retain the purity of Treg expansions better than AZM treatment, the mean number of FoxP3+ cells acquired in our study was still 16–25% higher in Treg expansions treated with AZM compared with RAP. While the mechanisms of AZM that inhibits CD4+ T cells has not been elucidated, the study by Ratzinger et al. suggested that AZM may in similarity with RAP interfere with the mTOR pathway, though in an FKBP12-independent manner. As Tregs are more resistant to the suppressive effects of RAP compared with conventional CD4+ T cells, this resistance could potentially apply for AZM treatment of Tregs as well. Further research exploring how AZM affects Treg viability, expansion, phenotype, and cytokine secretion would be of interest, preferably in comparison with conventional CD4 T cells. Also, studies of AZM dosing and its potential effects on Tregs are needed. Notably, we found that memory Treg expansions treated with RAP or AZM from two different blood donors did not expand while their respective naïve Treg cultures thrived, which may indicate a lower resistance among memory Tregs for these compounds. Our analysis of RAP concentration in CD4+ T cells and cell medium showed stable intracellular levels of RAP and rapid degradation in 37°C cell medium within 24 h. Similarly, a previous study of AZM presented an intracellular accumulation of AZM in leukocytes that slowly degraded over days, while the concentration in plasma quickly dropped within hours32. However, considerable individual variations in AZM concentration were found in the study. Another pharmacological study of AZM concentration in PBMCs and polymorphonuclear leukocytes also showed high uptake of AZM with large individual variations, as well as inconsistent fluctuations of AZM concentration over time33. Notably, we found larger individual variations in the frequencies of FoxP3+ cells between donors treated with AZM compared with RAP treatment. Further research is required to elucidate the mechanisms behind intracellular accumulation of AZM and whether individual variations in uptake may affect Tregs differently.
In the flow cytometric analysis, Treg cultures treated with AZM showed significantly increased FSC and SSC in comparison with the cultures treated with RAP for both naïve and memory Tregs. These findings indicate that Tregs treated with AZM are both larger and show increased internal cellular complexity in comparison with Tregs treated with RAP. The internal complexity of AZM Tregs can possibly be explained by the known intracellular accumulation of AZM in lysosomes, which are highly efficient structures for scattering light33,51,52.
In conclusion, we found that RAP treatment induced a FoxP3+Helios+ phenotype and increased suppressive function, but may also inhibit Treg expansion. In comparison, AZM treatment promoted a FoxP3+ phenotype, but to a lesser extent than RAP and the AZM-treated Tregs are possibly less suppressive. While AZM treatment showed a trend of marginally more Treg expansion than RAP treatment, no significant differences were found in this regard. These findings imply that further elucidation of the biological effects and functional impact of AZM is required to determine if the compound may benefit Treg in vitro cultures for clinical applications.
Supplemental Material
Supplemental_material for Comparing the Effects of the mTOR Inhibitors Azithromycin and Rapamycin on In Vitro Expanded Regulatory T Cells by Marcus Bergström, Malin Müller, Marie Karlsson, Hanne Scholz, Nils Tore Vethe and Olle Korsgren in Cell Transplantation
Footnotes
Ethical Approval: This study was approved by the Regional Ethics Committee (Dnr 2010/69), Uppsala, Sweden.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Marcus Bergström  https://orcid.org/0000-0002-5774-6626
https://orcid.org/0000-0002-5774-6626
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, Del Papa B, Zei T, Ostini RI, Cecchini D, Aloisi T, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117(14):3921–3928. [DOI] [PubMed] [Google Scholar]
- 2. Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Derkowska I, Juscinska J, Owczuk R, Szadkowska A, Witkowski P, Mlynarski W, Jarosz-Chobot P, et al. Therapy of type 1 diabetes with CD4(+)CD25(high)CD127-regulatory T cells prolongs survival of pancreatic islets - results of one year follow-up. Clin Immunol. 2014;153(1):23–30. [DOI] [PubMed] [Google Scholar]
- 3. Trzonkowski P, Bieniaszewska M, Juscinska J, Dobyszuk A, Krzystyniak A, Marek N, Mysliwska J, Hellmann A. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol. 2009;133(1):22–26. [DOI] [PubMed] [Google Scholar]
- 4. Tang Q, Vincenti F. Transplant trials with Tregs: perils and promises. J Clin Invest. 2017;127(7):2505–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van der Net JB, Bushell A, Wood KJ, Harden PN. Regulatory T cells: first steps of clinical application in solid organ transplantation. Transpl Int. 2016;29(1):3–11. [DOI] [PubMed] [Google Scholar]
- 6. Tang Q, Bluestone JA. Regulatory T-cell therapy in transplantation: moving to the clinic. Cold Spring Harb Perspect Med. 2013;3(11):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177(12):8338–8347. [DOI] [PubMed] [Google Scholar]
- 8. Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol. 2007;178(1):320–329. [DOI] [PubMed] [Google Scholar]
- 9. Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10(9):1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112(6):2340–2352. [DOI] [PubMed] [Google Scholar]
- 11. Beriou G, Costantino CM, Ashley CW, Yang L, Kuchroo VK, Baecher-Allan C, Hafler DA. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113(18):4240–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morath C, Arns W, Schwenger V, Mehrabi A, Fonouni H, Schmidt J, Zeier M. Sirolimus in renal transplantation. Nephrol Dial Transplant. 2007;22(suppl 8):viii61–viii65. [DOI] [PubMed] [Google Scholar]
- 13. Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999;162(5):2775–2784. [PubMed] [Google Scholar]
- 14. Nikolaeva N, Bemelman FJ, Yong SL, van Lier RA, ten Berge IJ. Rapamycin does not induce anergy but inhibits expansion and differentiation of alloreactive human T cells. Transplantation. 2006;81(3):445–454. [DOI] [PubMed] [Google Scholar]
- 15. Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9(5):324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Limon JJ, Fruman DA. Akt and mTOR in B cell activation and differentiation. Front Immunol. 2012;3:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singh K, Kozyr N, Stempora L, Kirk AD, Larsen CP, Blazar BR, Kean LS. Regulatory T cells exhibit decreased proliferation but enhanced suppression after pulsing with sirolimus. Am J Transplant. 2012;12(6):1441–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Golovina TN, Mikheeva T, Suhoski MM, Aqui NA, Tai VC, Shan X, Liu R, Balcarcel RR, Fisher N, Levine BL, Carroll RG, et al. CD28 costimulation is essential for human T regulatory expansion and function. J Immunol. 2008;181(4):2855–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Golovina TN, Mikheeva T, Brusko TM, Blazar BR, Bluestone JA, Riley JL. Retinoic acid and rapamycin differentially affect and synergistically promote the ex vivo expansion of natural human T regulatory cells. Plos One. 2011;6(1):e15868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ratzinger F, Haslacher H, Poeppl W, Hoermann G, Kovarik JJ, Jutz S, Steinberger P, Burgmann H, Pickl WF, Schmetterer KG. Azithromycin suppresses CD4(+) T-cell activation by direct modulation of mTOR activity. Sci Rep. 2014;4:7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khan AA, Slifer TR, Araujo FG, Remington JS. Effect of clarithromycin and azithromycin on production of cytokines by human monocytes. Int J Antimicrob Agents. 1999;11(2):121–132. [DOI] [PubMed] [Google Scholar]
- 22. Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010;23(3):590–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wolter J, Seeney S, Bell S, Bowler S, Masel P, McCormack J. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax. 2002;57(3):212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anwar GA, Bourke SC, Afolabi G, Middleton P, Ward C, Rutherford RM. Effects of long-term low-dose azithromycin in patients with non-CF bronchiectasis. Respir Med. 2008;102(10):1494–1496. [DOI] [PubMed] [Google Scholar]
- 25. Hansen CR, Pressler T, Hoiby N, Johansen HK. Long-term, low-dose azithromycin treatment reduces the incidence but increases macrolide resistance in Staphylococcus aureus in Danish CF patients. J Cyst Fibros. 2009;8(1):58–62. [DOI] [PubMed] [Google Scholar]
- 26. Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA, Jr, Criner GJ, Curtis JL, Dransfield MT, Han MK, Lazarus SC, Make B, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Uzun S, Djamin RS, Kluytmans JA, Mulder PG, van’t Veer NE, Ermens AA, Pelle AJ, Hoogsteden HC, Aerts JG, van der Eerden MM. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2(5):361–368. [DOI] [PubMed] [Google Scholar]
- 28. Wong C, Jayaram L, Karalus N, Eaton T, Tong C, Hockey H, Milne D, Fergusson W, Tuffery C, Sexton P, Storey L, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):660–667. [DOI] [PubMed] [Google Scholar]
- 29. Daniel R. Azithromycin, erythromycin and cloxacillin in the treatment of infections of skin and associated soft tissues. European Azithromycin Study Group. J Int Med Res. 1991;19(6):433–445. [DOI] [PubMed] [Google Scholar]
- 30. Culic O, Erakovic V, Cepelak I, Barisic K, Brajsa K, Ferencic Z, Galovic R, Glojnaric I, Manojlovic Z, Munic V, Novak-Mircetic R, et al. Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects. Eur J Pharmacol. 2002;450(3):277–289. [DOI] [PubMed] [Google Scholar]
- 31. Hiwatashi Y, Maeda M, Fukushima H, Onda K, Tanaka S, Utsumi H, Hirano T. Azithromycin suppresses proliferation, interleukin production and mitogen-activated protein kinases in human peripheral-blood mononuclear cells stimulated with bacterial superantigen. J Pharm Pharmacol. 2011;63(10):1320–1326. [DOI] [PubMed] [Google Scholar]
- 32. Matzneller P, Krasniqi S, Kinzig M, Sorgel F, Huttner S, Lackner E, Muller M, Zeitlinger M. Blood, tissue, and intracellular concentrations of azithromycin during and after end of therapy. Antimicrob Agents Chemother. 2013;57(4):1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sampson MR, Dumitrescu TP, Brouwer KL, Schmith VD. Population pharmacokinetics of azithromycin in whole blood, peripheral blood mononuclear cells, and polymorphonuclear cells in healthy adults. CPT Pharmacometrics Syst Pharmacol. 2014;3:e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin SJ, Kuo ML, Hsiao HS, Lee PT. Azithromycin modulates immune response of human monocyte-derived dendritic cells and CD4+ T cells. Int Immunopharmacol. 2016;40:318–326. [DOI] [PubMed] [Google Scholar]
- 35. Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol. 2001;166(12):7282–7289. [DOI] [PubMed] [Google Scholar]
- 36. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–450. [DOI] [PubMed] [Google Scholar]
- 37. Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J Immunol. 2007;179(6):3724–3733. [DOI] [PubMed] [Google Scholar]
- 38. Schmidt A, Eriksson M, Shang MM, Weyd H, Tegner J. Comparative analysis of protocols to induce human CD4+Foxp3+ regulatory T cells by combinations of IL-2, TGF-beta, Retinoic Acid, Rapamycin and Butyrate. Plos One. 2016;11(2):e0148474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yuan X, Cheng G, Malek TR. The importance of regulatory T-cell heterogeneity in maintaining self-tolerance. Immunol Rev. 2014;259(1):103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hoffmann P, Eder R, Boeld TJ, Doser K, Piseshka B, Andreesen R, Edinger M. Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood. 2006;108(13):4260–4267. [DOI] [PubMed] [Google Scholar]
- 41. Elkord E, Abd Al Samid M, Chaudhary B. Helios, and not FoxP3, is the marker of activated Tregs expressing GARP/LAP. Oncotarget. 2015;6(24):20026–20036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim HJ, Barnitz RA, Kreslavsky T, Brown FD, Moffett H, Lemieux ME, Kaygusuz Y, Meissner T, Holderried TA, Chan S, Kastner P, et al. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science. 2015;350(6258):334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Getnet D, Grosso JF, Goldberg MV, Harris TJ, Yen HR, Bruno TC, Durham NM, Hipkiss EL, Pyle KJ, Wada S, Pan F, et al. A role for the transcription factor Helios in human CD4(+)CD25(+) regulatory T cells. Mol Immunol. 2010;47(7–8):1595–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thornton AM, Lu J, Korty PE, Kim YC, Martens C, Sun PD, Shevach EM. Helios(+) and Helios(-) Treg subpopulations are phenotypically and functionally distinct and express dissimilar TCR repertoires. Eur J Immunol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, Olek S, Hamann A, von Boehmer H, Huehn J. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008;38(6):1654–1663. [DOI] [PubMed] [Google Scholar]
- 46. Raffin C, Raimbaud I, Valmori D, Ayyoub M. Ex vivo IL-1 receptor type I expression in human CD4+ T cells identifies an early intermediate in the differentiation of Th17 from FOXP3+ naive regulatory T cells. J Immunol. 2011;187(10):5196–5202. [DOI] [PubMed] [Google Scholar]
- 47. Raffin C, Pignon P, Celse C, Debien E, Valmori D, Ayyoub M. Human memory Helios- FOXP3+ regulatory T cells (Tregs) encompass induced Tregs that express Aiolos and respond to IL-1beta by downregulating their suppressor functions. J Immunol. 2013;191(9):4619–4627. [DOI] [PubMed] [Google Scholar]
- 48. Tabarkiewicz J, Pogoda K, Karczmarczyk A, Pozarowski P, Giannopoulos K. The Role of IL-17 and Th17 lymphocytes in autoimmune diseases. Arch Immunol Ther Exp (Warsz). 2015;63(6):435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Canavan JB, Scotta C, Vossenkamper A, Goldberg R, Elder MJ, Shoval I, Marks E, Stolarczyk E, Lo JW, Powell N, Fazekasova H, et al. Developing in vitro expanded CD45RA+ regulatory T cells as an adoptive cell therapy for Crohn’s disease. Gut. 2016;65(4):584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Booth NJ, McQuaid AJ, Sobande T, Kissane S, Agius E, Jackson SE, Salmon M, Falciani F, Yong K, Rustin MH, Akbar AN, et al. Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. J Immunol. 2010;184(8):4317–4326. [DOI] [PubMed] [Google Scholar]
- 51. Parnham MJ, Erakovic Haber V, Giamarellos-Bourboulis EJ, Perletti G, Verleden GM, Vos R. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther. 2014;143(2):225–245. [DOI] [PubMed] [Google Scholar]
- 52. Marina OC, Sanders CK, Mourant JR. Correlating light scattering with internal cellular structures. Biomed Opt Express. 2012;3(2):296–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental_material for Comparing the Effects of the mTOR Inhibitors Azithromycin and Rapamycin on In Vitro Expanded Regulatory T Cells by Marcus Bergström, Malin Müller, Marie Karlsson, Hanne Scholz, Nils Tore Vethe and Olle Korsgren in Cell Transplantation