Abstract
Protein arginine methyltransferase 5 (PRMT5) is implicated in various types of human cancer and tumor development, especially in lung cancer. Nevertheless, it is still unclear whether suppression of PRMT5 could promote lung cancer cell apoptosis and chemosensitivity induced by resveratrol, and the underlying molecular mechanism remains completely unknown. Here, we showed that PRMT5 was overexpressed in human lung cancer tissues and different types of lung cancer cell lines. Moreover, we constructed PRMT5 stable knockdown cell lines (A549 and ASCT-a-1) and investigated the roles of PRMT5 and the related signaling pathway in lung cancer cell apoptosis induced by resveratrol. Our results indicated that inhibition or down-regulation of PRMT5 by GSK591, a PRMT5-specific inhibitor, or shRNAs markedly enhanced cell apoptosis and chemosensitivity stimulated by resveratrol. Further investigation showed that inhibition or down-regulation of PRMT5 further reduced Akt/GSK3β phosphorylation and the downstream targets cyclin D1 and E1 expression upon resveratrol treatment. Our findings suggest that PRMT5 is a pivotal mediator for human lung cancer cell death induced by resveratrol, which also reveals that PRMT5 may serve as a new therapeutic target for the treatment of human lung cancer.
Keywords: PRMT5, Akt, GSK3β, cyclin D1, cyclin E1, lung cancer, resveratrol
Introduction
Lung cancer is the most deadly human cancer worldwide, with a highly lethal and aggressive malignant tumor. Two main subtypes of human lung cancer are identified: non-small cell lung carcinoma (NSCLC) and small cell lung carcinoma1. The most common type of lung cancer is adenocarcinoma, which constitutes around 40% of all lung cancer cases. NSCLC is the most frequently diagnosed cancer and the primary cause of human death in China. So far, the primary therapeutic methods for lung cancer include surgery, radiotherapy, chemotherapy, immunotherapy, and combination treatment. Although these methods have their advantages and disadvantages for the treatment of NSCLC, the therapeutic effects are still poor, and the mortality rate of patients with NSCLC remains high. Based on the currently available therapeutic technologies, most patients with NSCLC cannot be cured. Therefore, identification of new therapeutic targets for NSCLC is urgently needed.
It has been shown that protein arginine methyltransferase 5 (PRMT5) was crucially involved in the regulation of chromatin remodeling, gene expression, cell cycle progression, cell proliferation, protein functions, and metabolism2,3. PRMT5 belongs to the type II arginine methyltransferases, and it catalyzes the formation of symmetric dimethylarginine of protein substrates at the arginine residues. PRMT5 was found to directly methylate arginine residues and led to symmetric dimethylation of histone H3 and H4, which in turn remodeled chromatin structure and regulated gene transcription4,5. Recently, increasing evidence has shown that PRMT5 is ectopically expressed in many human cancers, such as breast cancer, lymphoma, leukemia, and lung cancer, and PRMT5 also plays a vital role in the regulation of tumor cell proliferation and transformation6–9. In addition, PRMT5 was found to methylate the promoters of forkhead box O transcription (FOXO) factors and epidermal growth factor receptors (EGFR) to protect cancer cells against apoptosis and promote cell growth10,11. Previous research has reported that knockdown of PRMT5 suppressed tumor cell growth and cell cycle progression via regulation of the PI3-K/Akt signaling axis12. Nevertheless, it is still unclear whether PRMT5 could promote lung cancer cell apoptosis and chemosensitivity induced by resveratrol, and the underlying molecular mechanism remains completely unknown.
In the current study, we found that PRMT5 was highly expressed in human lung cancer tissues and different types of human lung cancer cell lines. Moreover, inhibition or silencing of PRMT5 by a specific inhibitor (GSK591) distinctly and further reduced cell viability and promoted cell apoptosis induced by resveratrol. Furthermore, inhibition or down-regulation of PRMT5 promoted lung cancer cell death and chemosensitivity through regulation of the Akt/GSK3β signaling axis upon resveratrol stimulation. Our findings provide new insight into the role of PRMT5 in lung cancer cell death induced by resveratrol. Our results also indicate that PRMT5 may serve as a novel therapeutic target for the treatment of human lung cancer.
Material and Methods
Chemicals
Trans-resveratrol was purchased from Sigma-Aldrich (Cat#R5010, St. Louis, MO, USA). GSK591, a specific inhibitor of PRMT5, was purchased from Sigma-Aldrich (Cat# SML1751, St Louis, MO, USA). The study was approved by the Ethical Committee of Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (LWEC201603).
Cell Culture
The following cell lines were used in our study: ASTC-a-1 and A549 cells were cultured in Dulbecco’s Modified Eagle’s Medium (1:1) (DMEM, Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% (v/v) FBS (GIBCO, Co. Ltd., Grand Island, NY, USA), 50 units/ml penicillin, and 50 µg/ml streptomycin. PC14, H69, and IMR90 (primary human fetal lung fibroblast cells) cells were cultured in RPMI 1640 medium (Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA), containing 10% FBS, 50 units/ml penicillin and 50 µg/ml streptomycin. Cells were maintained in a humidified atmosphere incubator at 37°C containing 5% CO2 and 95% air.
Constructs and Generation of Lentivirus
Human PRMT5 shRNA knockdown lentiviral plasmids were described previously and generated using pLVTHM vector9. PRMT5 knockdown targeting sequences are: shRNA-1, 5’GGATAAAGCTGTATGCTGT3’; shRNA2: 5’ GCCATCTATAAATGTCTGCTA 3’. In order to generate the lentivirus containing PRMT5 shRNAs, the PRMT5-shRNA plasmids and helper plasmids MD2G (Addgene, Cambridge, MA, USA) and helper plasmid PAX2 (Addgene, Cambridge, MA, USA) were co-transfected into HEK293 T cells. After 24 h, the medium was replaced with fresh medium, and then the medium was harvested after 48 h post-transfection. The viral titers were predetermined before experiments. In our studies, the same number of viral particles was used.
Generation of PRMT5 Stable Knockdown Cell Lines
In order to generate PRMT5 stable knockdown cell lines, ASTC-a-1 and A549 cells were seeded into 60 mm dishes for 24 h before transduction. The lentivirus containing human PRMT5-shRNAs or scramble-shRNA was added into the indicated cells and cultured for 48 h. The cells were then selected with puromycin (1ug/ml, Sigma, St Louis, MO, USA), and the non-infected cells were killed. The stable down-regulation of PRMT5 cell lines was subjected to the next experiments.
Gene Expression Analysis
Total RNA was extracted from human lung cancer tissues and adjacent normal tissues and ASTC-a-1, A549, PC14, H69, and IMR90 cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. An equal amount of RNA was reverse transcribed, and gene expression was measured by quantitative real-time PCR (qRT-PCR) using SYBR green fluorescent Dye (Bio-Rad, Hercules, CA, USA) with an ABI7300 real-time PCR instrument (Applied Biosystems, Foster City, CA, USA). The following primers were used: human PRMT5 forward: 5’ CCTGTGGAGGTGAACACAGT 3’ and reverse: 5’AGAGGATGGGAAACCATGAG3’; GAPDH, forward: 5’ GAAGGTGAAGGTCGGAGTCAACG3’ and reverse: 5’ TGCCATGGGTGGAATCATATTGG 3’. GAPGH served as control. Finally, the relative mRNA expression level was calculated by ΔΔ-Ct method.
Immunoblotting Analysis
For immunoblotting analysis, proteins were extracted from human lung cancer tissues, adjacent normal tissues, A549, ASTC-a-1, PC14, H69, and IMR90 cells with lysis buffer (20 mM Tris, PH 7.4, 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1 mM sodium orthovanadate, 50 mM sodium fluoride, 1% Triton X-100, 0.1% SDS and 100 mM phenylmethylsulfonyl fluoride). Cell lysates were mixed with 5× Laemmli sample buffer (2% SDS) and placed in a heat block (100°C) for 10 min. Proteins were separated in 8–15% SDS-polyacrylamide gels and transferred to PVDF membranes. The membranes were washed three times for 10 min with TBST and blocked for 1 h at room temperature with 5% non-fat milk. The membranes were incubated with PRMT5 (cat#sc-376937, Santa Cruz Biotechnology, Santa Cruz, CA, USA), cyclin D1 (cat#2978, Cell Signaling Technology, Danvers, MA, USA), cyclin E1 (cat#20808, Cell Signaling Technology, Danvers, MA, USA), Akt (cat#4691, Cell Signaling Technology, Danvers, MA, USA), p-Thr308-Akt (cat#13038, Cell Signaling Technology, Danvers, MA, USA), p-Ser473-Akt (cat#4060, Cell Signaling Technology, Danvers, MA, USA), GSK3β (cat#12456, Cell Signaling Technology, Danvers, MA, USA), p-Ser9-GSK3β (cat#5558, Cell Signaling Technology, Danvers, MA, USA), cleaved caspase-3 (cat#9661, Cell Signaling Technology, Danvers, MA, USA), cleaved PARP (cat#5625, Cell Signaling Technology, Danvers, MA, USA), and Actin (cat#sc-47778, Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibodies at 4°C overnight. The membranes were labeled with goat anti-mouse conjugated to horseradish peroxidase (HRP) or goat anti-rabbit conjugated to HRP secondary antibodies (Santa Cruz Biotechnology, cat#sc-2004 and sc-2005). Data were analyzed using LI-COR Image Studio Software (LI-COR, Biosciences, Lincoln, NE, USA).
Cell Viability Assay
For cell viability assay, ASTC-a-1 and A549 cells were seeded into 96-well plates (3000 cells/well). Cell counting kit-8 (CCK-8; Dojindo Molecular Technologies, Rockville, MD, USA) was used for measuring cell viability under different conditions according to the manufacturer’s protocol. Cell viability was determined by absorbance at 450 nm using an Infinite 200 plate reader (TECAN, Mönnedorf, Switzerland). To determine the effect of PRMT5 inhibitor GSK591 on cell viability induced by resveratrol, ASTC-a-1 and A549 cells were seeded into 96-well plates (3000 cells/well) and treated with or without vehicle, GSK591, and resveratrol at the indicated concentration and the cell viability was detected by CCK-8.
Statistical Analysis
All assays were repeated independently three times. Data are represented as mean ± SEM. Statistical analysis was performed with unpaired two-tailed Student’s t-test. Differences were considered statistically significant at p < 0.05.
Results
PRMT5 is Overexpressed in Lung Cancer Cells and Tissues
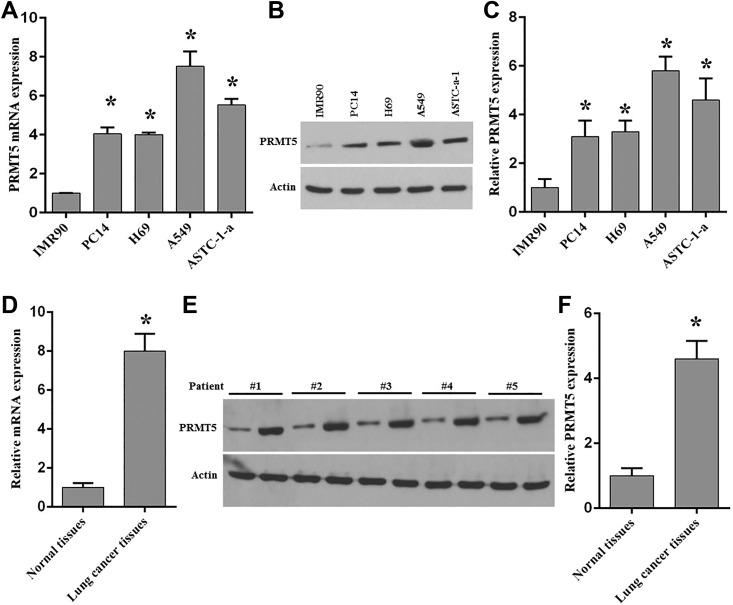
In order to explore the functions of PRMT5 in human lung cancer death induced by chemotherapy agents, we firstly evaluated the expression level of PRMT5 in human lung cancer cell lines and tumors from patients. Previous studies showed that PRMT5 was highly expressed in many human cancer cells and human cancer tissues9. To validate whether PRMT5 is overexpressed in human lung cancer cells and tissues, we measured the mRNA expression level of PRTM5 in various lung cancer cell lines. As seen in Fig. 1A, ectopic expression of PRMT5 mRNA was seen in the lung cancer cell lines compared with normal lung fibroblast cells (IMR90 cells). To further confirm this result, the protein expression level of PRMT5 was detected in these cell lines. As seen in Figs. 1B and 1C, PRMT5 protein expression level was highly expressed in the lung cancer cell lines compared with IMR90 cells. These findings suggest that PRTM5 plays an essential role in human lung tumorigenesis. Subsequently, PRMT5 mRNA and protein expression levels were analyzed in human lung cancer tissues and adjacent normal tissues from patients. As seen in Figs. 1D–1F, we found that PRMT5 mRNA and protein expression levels were distinctly elevated in tumor tissues compared with the adjacent normal tissues. All these results indicate that PRMT5 is a critical regulator in human lung cancer development. Next, the function of PRMT5 was evaluated in lung cancer cell apoptosis induced by resveratrol.
Fig. 1.
Ectopic expression of PRMT5 in human lung cancer cell lines and tissues.
(A) mRNA expression of PRMT5 was detected by qRT-PCR in various lung cancer cell lines compared with fetal lung fibroblast cells (IMR90). *p < 0.05 vs. IMR90 cells. (B) PRMT5 protein expression was detected by immunoblotting in the indicated cell lines. Representative pictures are shown. (C) PRMT5 protein expression level was quantified in the indicated cell lines. *p < 0.05 vs. IMR90 cells. (D) mRNA expression of PRMT5 was measured by qRT-PCR in human lung cancer tissues and adjacent normal tissues. *p< 0.05 vs. normal tissues. (E) PRMT5 protein expression was measured by immunoblotting in human lung cancer tissues and adjacent normal tissues. Representative pictures are shown. (F) PRMT5 protein expression level was quantified in cancer tissues and adjacent normal tissues. *p< 0.05 vs. normal tissues.
Inhibition of PRMT5 Promotes Lung Cancer Cell Apoptosis Induced by Resveratrol
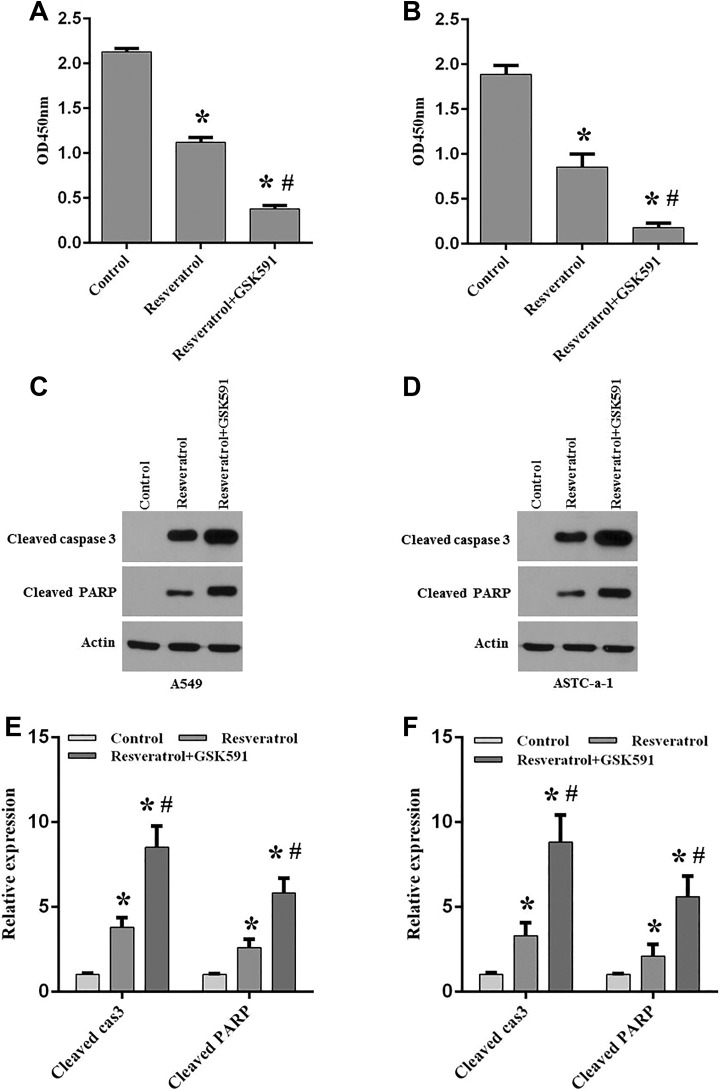
It has been shown that PRMT5 was involved in human cancer development and regulated cancer cell proliferation and growth12. Nevertheless, it is still unclear whether PRMT5 participates in lung cancer cell apoptosis induced by chemotherapy agents. To this end, A549 and ASTC-a-1 cells were treated with GSK591, a PRMT5-specific inhibitor, and then these cells were stimulated with or without resveratrol, and the cell viability was assessed. As seen in Figs. 2A and 2B, we found that cell viability was markedly reduced by resveratrol stimulation compared with the control group. Surprisingly, this effect was further augmented when cells were treated with GSK591 compared with resveratrol treatment only, indicating that blocking PRMT5 promotes lung cancer cell death induced by resveratrol. Next, the apoptotic effectors cleaved caspase-3, and its downstream target PARP was detected in A549 and ASTC-a-1 cells upon treatment of GSK591 and resveratrol. As seen in Figs. 2C–2F, we found that cleaved caspase-3 and the downstream target cleaved PARP was significantly increased in A549 and ASTC-a-1 cells induced by resveratrol compared with the control group. When cells were treated with GSK591 and resveratrol, the cleaved caspase-3 and cleaved PARP was further enhanced compared with resveratrol only. Altogether, these findings indicate that PRMT5 is implicated in lung cancer cell apoptosis induced by resveratrol and that blocking PRMT5 promotes chemosensitivity induced by resveratrol.
Fig. 2.
Blocking PRMT5 promotes lung cancer cell apoptosis induced by resveratrol.
A549 and ASTC-a-1 cells were treated with PRMT5 inhibitor GSK591 (100 nM) for 5 days, and then these cells were stimulated with or without resveratrol (100 μM) for 24 h. Cell viability and apoptotic markers were assessed (A, B) Cell viability was measured by CCK-8 assay in A549 and ASTC-a-1 cells under different treatments. *p< 0.05 vs. control group. (C, D) Cleaved caspase 3 and the downstream target cleaved PARP was detected by immunoblotting in A549 and ASTC-a-1 cells upon different treatments. Representative pictures are shown. (E, F) The protein expression level of cleaved caspase 3 and cleaved PARP was quantified in A549 and ASTC-a-1 cells. *p < 0.05 vs. control group; # p < 0.05 vs. resveratrol group.
Silencing PRMT5 Promotes Lung Cancer Cell Death Induced by Resveratrol
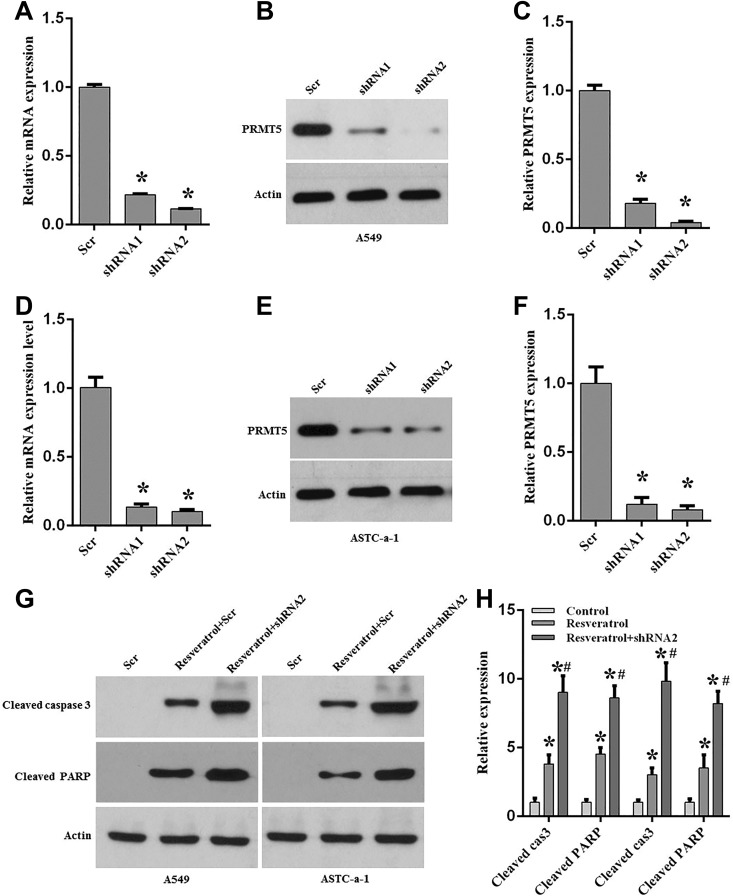
To further confirm our results obtained above, we generated the PRMT5 stable knockdown cell lines using lentivirus containing PRMT5-shRNAs or scramble-shRNA, and the apoptotic markers were detected upon resveratrol treatment. Firstly, PRTM5 knockdown efficiency was determined in A549 and ASTC-a-1 cells. As seen in Figs. 3A–3F, PRMT5 mRNA and the protein expression level were dramatically reduced both in A549 and ASTC-a-1 cells compared with scramble control, indicating that PRMT5 was successfully down-regulated, and these cells were used in the next experiments. In order to validate whether silencing of PRMT5 could promote cell apoptosis induced by resveratrol, A549 and ASTC-a-1 cells containing PRMT5-shRNA2 or scramble-shRNA were stimulated with or without resveratrol, and the apoptotic markers cleaved caspase 3 and downstream target cleaved PARP were detected by immunoblotting. As seen in Figs. 3G and 3H, cleaved caspase 3 and cleaved PARP were significantly increased upon resveratrol treatment both in A549 and ASTC-a-1 cells compared with scramble control. Strikingly, when PRMT5 was down-regulated by shRNA, cleaved caspase 3 and cleaved PARP were further enhanced induced by resveratrol compared with resveratrol treatment only. Collectively, these findings indicate that down-regulation of PRMT5 promotes lung cancer cells apoptosis and chemosensitivity induced by resveratrol.
Fig. 3.
Down-regulation of PRMT5 enhances lung cancer cell apoptosis induced by resveratrol.
A549 and ASCT-a-1cells were infected with lentivirus containing scramble-shRNA (Scr-shRNA), PRMT5-shRNA1 or shRNA2, and then the cells were selected with puromycin. The stable knockdown cells were treated with or without resveratrol (100 μM) for 24 h, and the indicated experiments were performed. (A) mRNA expression of PRMT5 was detected by qRT-PCR. *p < 0.05 vs. Scr. (B) PRMT5 protein expression level was determined by immunoblotting. Representative pictures are shown. (C) PRMT5 protein expression level was quantified in A549 cells. *p < 0.05 vs. Scr. (D–F). Similar results were observed from ASTC-a-1 cells. *p < 0.05 vs. Scr. (G) Cleaved caspase 3 and PARP were detected by immunoblotting in A549 and ASTC-a-1 cells upon different treatments. Representative pictures are shown. *p < 0.05 vs. Scr. (H) Quantitative analysis of cleaved caspase 3 and cleaved PARP in A549 and ASTC-a-1 cells. *p < 0.05 vs. Scr.
PRMT5/Akt/GSK3β Signaling Axis Regulates Lung Cancer Cell Apoptosis Upon Resveratrol Treatment
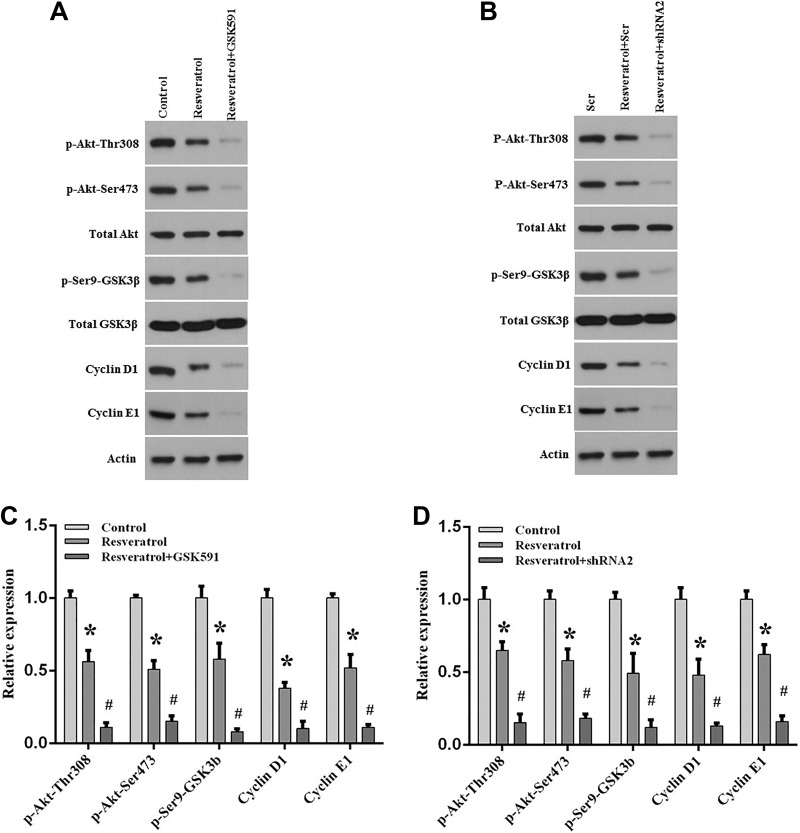
Our results showed that inhibition or silencing of PRMT5 not only enhances lung cancer cell apoptosis but also promotes chemosensitivity induced by resveratrol. Subsequently, we investigated how PRMT5 contributed to those effects. Previous studies have shown that Akt, also named PKB, was involved in cancer cell growth and tumor progression. Recently, several studies reported that PRMT5 interacted and regulated Akt activation in mouse liver cells2, lung cancer cells7, and lymphoma cells13. Nevertheless, it is still unclear whether PRMT5 promotes lung cancer cell death and chemosensitivity via regulation of Akt activity upon resveratrol treatment. To test this hypothesis, A549 cells were treated with PRMT5 inhibitor GSK591, and then the cells were stimulated with or without resveratrol. As seen in Figs. 4A and B, we found that Akt phosphorylation level at Thr308 and Ser473 was dramatically decreased upon resveratrol treatment, which also reduced the critical downstream target GSK3β phosphorylation level (Ser9), and cyclin D1 and cyclin E1 expression level. Interestingly, this effect was further enhanced when PRMT5 was suppressed by GSK591, indicating that inhibition of PRMT5 promoted lung cancer cell death via regulation of the Akt/GSK3/cyclin D/E signaling pathway induced by resveratrol. To further confirm this result, the PRMT5 stable knockdown cells were used. A549 cells that were depleted of PRMT5 were treated with or without resveratrol. As seen in Figs. 4C and D, we found that the phosphorylation level at Thr308 and Ser473 of Akt and the phosphorylation level at Ser9 of GSK3β were distinctly reduced upon resveratrol treatment, which also decreased the expression level of cyclin D1 and cyclin E1. Similar to the above results, silencing of PRMT5 by shRNA further lowered the phosphorylation level of Akt and GSK3β, and the expression level of cyclin D1 and cyclin E1 induced by resveratrol compared with resveratrol treatment only. Altogether, these findings suggest that inhibition or silencing of PRMT5 enhanced lung cancer cell apoptosis and chemosensitivity via Akt/GSK3β signaling axis induced by resveratrol.
Fig. 4.
PRMT5 regulates lung cancer cell death via Akt/GSK3β signaling axis stimulated by resveratrol.
A549 cells were stimulated with PRMT5 inhibitor GSK591 or infected with PRMT5-shRNA2, and then the cells were treated with or without resveratrol (100 μM) for 24 h. (A, B) The indicated proteins were detected by immunoblotting. Representative pictures are shown. (C, D) The indicated protein expression levels were quantified in A549 cells. *p < 0.05 vs. control group; # p < 0.05 vs. resveratrol group.
Discussion
Previous studies have shown that NSCLC is a critical disease involving multiple gradually accumulated epigenetic and genetic alterations14. These changes cause suppression of tumor repressor and activation of oncogenes, respectively, finally leading to NSCLC. Accumulating evidence suggests that PRMT5 is an oncoprotein and plays a crucial role in many human cancers through mediating various signaling pathways, which are often involved chromatin remodeling, gene modification and transcription, and protein methylation15. To date, it has been clear that dysfunction of PRMT5 has played a part in carcinogenesis and tumor progression, including in gastric cancer, breast cancer, prostate cancer, and lung cancer, especially in NSCLC. Resveratrol is the most widely used sensitizer agent to treat NSCLC. Several studies have shown that resveratrol induced tumor cell death and prevented cell proliferation and growth in breast cancer, prostate cancer, colon cancer, and lung cancer16,17. Nevertheless, so far, it is still unclear whether PRMT5 is implicated in lung cancer cell death and chemosensitivity induced by resveratrol, and the related signaling pathways are also completely unknown.
In the current study, we revealed that PRMT5 was not only overexpressed in different human lung cancer cell lines but also highly expressed in human lung cancer tissues (Fig. 1). In addition, our results uncovered that prevention or silencing of PRMT5 by a specific inhibitor (GSK591) or shRNAs significantly promoted lung cancer cell apoptosis induced by resveratrol (Figs. 2 and 3). Further investigation showed that blocking or down-regulation of PRMT5 markedly decreased Akt/GSK3β phosphorylation at Thr308/Ser473 and Ser9, respectively, and also dramatically reduced downstream targets cyclin D1/E1 expression upon resveratrol treatment (Fig. 4). Taken together, our findings suggest the possibility that targeting the PRMT5/Akt/GSK3β signaling axis promotes human lung cancer cell death and chemosensitivity induced by resveratrol, which strongly indicates that blocking PRMT5 activity or down-regulation of PRMT5 expression level could be a useful therapeutic strategy for human lung cancer.
The PI3-K/Akt signaling pathway plays a pivotal role in the regulation of many cellular processes, including cell cycle, cell proliferation, growth, survival, metabolism, and gene transcription18. Dysfunction of this signaling axis will lead to metabolic, cardiovascular, and neurological diseases, and cause different types of human cancer as well19. Recent studies have shown that PRMT5 interacted with Akt and regulated Akt activation, which controlled cancer cell replication and growth7. Moreover, Akt is activated by PRMT5 through hyperphosphorylation of PI3-K and hypophosphorylation of PTEN12 and co-localization of Akt7. These investigations mainly focused on the relationship between PRMT5 and Akt and related mechanisms. Although PRMT5 regulation of Akt activity is evident and validated by many studies, it remains unknown whether blocking or silencing of PRMT5 could promote lung cancer cell death and enhance chemosensitivity induced by resveratrol. In the present study, we found that inhibition of PRMT5 activity or down-regulation of PRMT5 not only further promoted lung cancer cell apoptosis (elevated cleaved caspase 3 and PARP) but also prevented the Akt/GSK3β signaling axis (decreased phosphorylation of Akt/GSK3β), which enhanced chemosensitivity upon resveratrol treatment. Our findings indicate that PRMT5 promotes the death of lung cancer cells via the Akt/GSK3β signaling pathway induced by resveratrol.
Once Akt is activated, numerous downstream targets can be phosphorylated. Among them, GSK3β is the most crucial target of Akt, which can be phosphorylated by Akt at Ser 9 and its activity inhibited. The previous study showed that GSK3β regulated Mcl-1 stability and promoted cell apoptosis by reactive oxygen species burst via the mitochondrial apoptotic pathway20. Also, GSK3β negatively regulated cell cycle progression by direct phosphorylation of cyclin D1 and controlled cyclin E1degradation21,22. In our study, we showed that inhibition or down-regulation of PRMT5 markedly reduced cyclin D1 and E1 expression levels induced by resveratrol. That is the reason why blocking PRMT5 can further prevent cell cycle progression under resveratrol stimulation. All those observations indicated that targeting the PRMT5/Akt/GSK3β signaling axis promotes human lung cancer cell death, which further leads to increase chemosensitivity induced by resveratrol. Collectively, our findings not only confirm that the PRMT5/Akt/GSK3β signaling pathway regulates apoptosis of lung cancer cells and cell cycle progression, but also indicate that PRMT5 is an essential upstream mediator for human lung cancer cell proliferation.
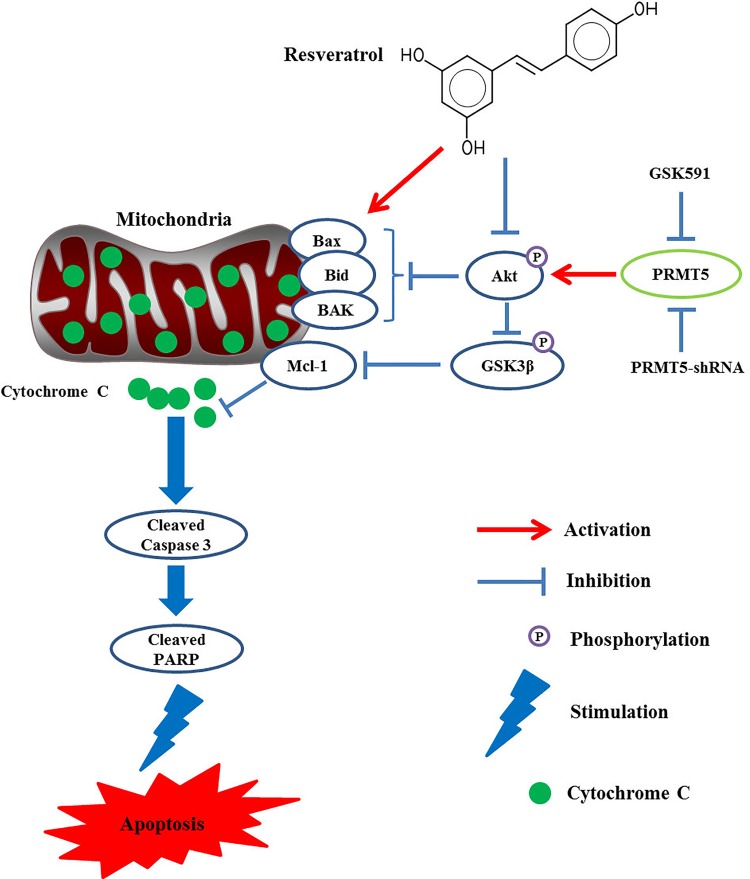
In conclusion, our results revealed that PRMT5 was highly expressed in human lung cancer cells and tumor tissues. Inhibition or down-regulation of PRMT5 further promoted lung cancer cell death upon resveratrol stimulation. Moreover, our data also showed that inhibition or silencing of PRMT5 further reduced Akt/GSK3β phosphorylation and downstream targets cyclin D1/E1 expression induced by resveratrol, which demonstrated a novel molecular signaling axis (PRMT5/Akt/GSK3β) mediating lung cancer cell apoptosis and chemosensitivity induced by resveratrol (Fig. 5). All these findings strongly suggest that PRMT5 is an important therapeutic target for the treatment of human lung cancer. More importantly, the new insights into the inhibition of PRMT5 provide some new evidence for the enhancement of therapeutic effect by chemotherapy agents in human lung cancer.
Fig. 5.
Schematic representation of the proposed model: blocking PRMT5 promotes lung cancer cell death via Akt/GSK3β signaling pathway induced by resveratrol.
Footnotes
Ethical Approval: The study was approved by the Ethical Committee of Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (LWEC201603).
Statement of Human Rights: All procedures in this study were conducted in accordance with the *Ethical Committee of Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (LWEC201603)* approved protocols.
Statement of Informed Consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This work was supported by the Shanghai Medical guided science and technology projects (No.164411973400), Special Scientific Research Project of Gansu Province Health and Family Planning Commission (GWGL2014-13) and Health Industry Clinical Research Project of Shanghai Health and Family Planning Commission (201840222).
ORCID iD: Yonghua Zheng  https://orcid.org/0000-0002-7799-9612
https://orcid.org/0000-0002-7799-9612
References
- 1. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–454. [DOI] [PubMed] [Google Scholar]
- 2. Huang L, Liu J, Zhang XO, Sibley K, Najjar SM, Lee MM, Wu Q. Inhibition of protein arginine methyltransferase 5 enhances hepatic mitochondrial biogenesis. J Biol Chem. 2018;293(28):10884–10894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shailesh H, Zakaria ZZ, Baiocchi R, Sif S. Protein arginine methyltransferase 5 (PRMT5) dysregulation in cancer. Oncotarget. 2018;9(94):36705–36718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pal S, Vishwanath SN, Erdjument-Bromage H, Tempst P, Sif S. Human SWI/SNF-associated PRMT5 methylates histone H3 arginine 8 and negatively regulates expression of ST7 and NM23 tumor suppressor genes. Mol Cell Biol. 2004;24(21):9630–9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao Q, Rank G, Tan YT, Li H, Moritz RL, Simpson RJ, Cerruti L, Curtis DJ, Patel DJ, Allis CD, Cunningham JM, et al. PRMT5-mediated methylation of histone H4R3 recruits DNMT3A, coupling histone and DNA methylation in gene silencing. Nat Struct Mol Biol. 2009;16(3):304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li Y, Chitnis N, Nakagawa H, Kita Y, Natsugoe S, Yang Y, Li Z, Wasik M, Klein-Szanto AJ, Rustgi AK, Diehl JA. PRMT5 is required for lymphomagenesis triggered by multiple oncogenic drivers. Cancer Discov. 2015;5(3):288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang S, Ma Y, Hu X, Zheng Y, Chen X. Targeting PRMT5/Akt signalling axis prevents human lung cancer cell growth. J Cell Mol Med. 2019;23(2):1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tarighat SS, Santhanam R, Frankhouser D, Radomska HS, Lai H, Anghelina M, Wang H, Huang X, Alinari L, Walker A, Caligiuri MA, et al. The dual epigenetic role of PRMT5 in acute myeloid leukemia: gene activation and repression via histone arginine methylation. Leukemia. 2016;30(4):789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chiang K, Davies CC. Linking PRMT5 to breast cancer stem cells: new therapeutic opportunities? Mol Cell Oncol. 2018;5(3):e1441628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamagata K, Daitoku H, Takahashi Y, Namiki K, Hisatake K, Kako K, Mukai H, Kasuya Y, Fukamizu A. Arginine methylation of FOXO transcription factors inhibits their phosphorylation by Akt. Mol Cell. 2008;32(2):221–231. [DOI] [PubMed] [Google Scholar]
- 11. Hsu JM, Chen CT, Chou CK, Kuo HP, Li LY, Lin CY, Lee HJ, Wang YN, Liu M, Liao HW, Shi B, et al. Crosstalk between Arg 1175 methylation and Tyr 1173 phosphorylation negatively modulates EGFR-mediated ERK activation. Nat Cell Biol. 2011;13(2):174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wei TY, Juan CC, Hisa JY, Su LJ, Lee YC, Chou HY, Chen JM, Wu YC, Chiu SC, Hsu CP, Liu KL, et al. Protein arginine methyltransferase 5 is a potential oncoprotein that upregulates G1 cyclins/cyclin-dependent kinases and the phosphoinositide 3-kinase/AKT signaling cascade. Cancer Sci. 2012;103(9):1640–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu F, Guo H, Bates PD, Zhang S, Zhang H, Nomie KJ, Li Y, Lu L, Seibold KR, Wang F, Rumball I, et al. PRMT5 is upregulated by B-cell receptor signaling and forms a positive-feedback loop with PI3K/AKT in lymphoma cells [published online ahead of print May 23, 2019]. Leukemia. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ansari J, Shackelford RE, El-Osta H. Epigenetics in non-small cell lung cancer: from basics to therapeutics. Transl Lung Cancer Res. 2016;5(2):155–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xiao W, Chen X, Liu L, Shu Y, Zhang M, Zhong Y. Role of protein arginine methyltransferase 5 in human cancers. Biomed Pharmacother. 2019;114:108790. [DOI] [PubMed] [Google Scholar]
- 16. Rauf A, Imran M, Butt MS, Nadeem M, Peters DG, Mubarak MS. Resveratrol as an anti-cancer agent: a review. Crit Rev Food Sci Nutr. 2018;58(9):1428–1447. [DOI] [PubMed] [Google Scholar]
- 17. Takashina M, Inoue S, Tomihara K, Tomita K, Hattori K, Zhao QL, Suzuki T, Noguchi M, Ohashi W, Hattori Y. Different effect of resveratrol to induction of apoptosis depending on the type of human cancer cells. Int J Oncol. 2017;50(3):787–797. [DOI] [PubMed] [Google Scholar]
- 18. Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell. 2017;169(3):381–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tokunaga E, Oki E, Egashira A, Sadanaga N, Morita M, Kakeji Y, Maehara Y. Deregulation of the Akt pathway in human cancer. Curr Cancer Drug Targets. 2008;8(1):27–36. [DOI] [PubMed] [Google Scholar]
- 20. Huang L, Wu S, Xing D. High fluence low-power laser irradiation induces apoptosis via inactivation of Akt/GSK3beta signaling pathway. J Cell Physiol. 2011;226(3):588–601. [DOI] [PubMed] [Google Scholar]
- 21. Liu SL, Liu Z, Zhang LD, Zhu HQ, Guo JH, Zhao M, Wu YL, Liu F, Gao FH. GSK3beta-dependent cyclin D1 and cyclin E1 degradation is indispensable for NVP-BEZ235 induced G0/G1 arrest in neuroblastoma cells. Cell Cycle. 2017;16(24):2386–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12(22):3499–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]