Abstract
Liver transplantation has been deemed the best choice for end-stage liver disease patients but immune rejection after surgery is still a serious problem. Patients have to take immunosuppressive drugs for a long time after liver transplantation, and this often leads to many side effects. Mesenchymal stem cells (MSCs) gradually became of interest to researchers because of their powerful immunomodulatory effects. In the past, a large number of in vitro and in vivo studies have demonstrated the great potential of MSCs for participation in posttransplant immunomodulation. In addition, MSCs also have properties that may potentially benefit patients undergoing liver transplantation. This article aims to provide an overview of the current understanding of the immunomodulation achieved by the application of MSCs in liver transplantation, to discuss the problems that may be encountered when using MSCs in clinical practice, and to describe some of the underlying capabilities of MSCs in liver transplantation. Cell–cell contact, soluble molecules, and exosomes have been suggested to be critical approaches to MSCs’ immunoregulation in vitro; however, the exact mechanism, especially in vivo, is still unclear. In recent years, the clinical safety of MSCs has been proven by a series of clinical trials. The obstacles to the clinical application of MSCs are decreasing, but large sample clinical trials involving MSCs are still needed to further study their clinical effects.
Keywords: mesenchymal stromal cells, liver transplantation, immunosuppression, tolerance, clinical trial
Introduction
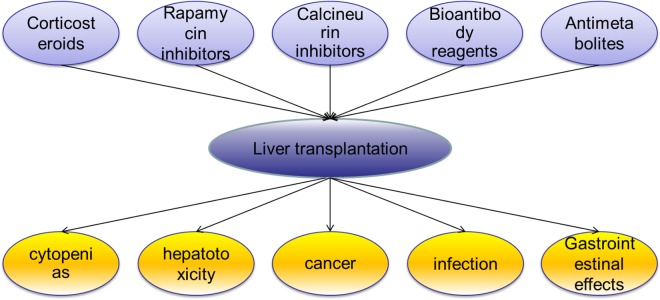
Liver transplantation has been deemed the best therapy for end-stage liver diseases, but recipients usually have to live with life-long immunosuppression1 (Figure 1). Although the standard pharmacological immunosuppressive treatment commonly used in clinical practice can achieve the favorable results of long grafts and patients survival rates, the side effects (Figure 1) caused by the treatment are significant2. In addition to the drug toxicities, the risks of malignancies and opportunistic infections have been reported to be increasing significantly3. Immunomodulatory cell therapy, as a complementary plan to standard pharmacological immunosuppression, appears to be a solution to this problem. In transplantation cases, the ultimate goal of immunomodulatory cell therapy is to prolong solid-organ allograft survival with reduced, or even no, usage of systemic pharmacological immunosuppression4.
Figure 1.
Common classes of immunosuppressive drugs in liver transplantation and the main side effects of immunosuppressive drugs.
Mesenchymal stem cells (MSCs), a subpopulation of multipotent and non-hematopoietic cells initially isolated from bone marrow and reported by Friedenstein et al. in 19705,6, are also called mesenchymal stromal cells and have been the focus of transplant scientists due to their great potential capacities for tissue repair and immunomodulation. Although no reports of large-scale clinical practices involving MSCs for liver transplantation have been found, and MSCs investigations remain mostly at the preclinical stage, the powerful immunomodulatory properties shown in recent reports make MSCs a promising candidate therapy in clinical liver transplantation. In addition, the effects of MSCs can be improved through gene modification and pretreatment, and the potential properties of MSCs show great promise in liver transplantation.
Research Status of MSCs
Cell therapy for immune rejection after organ transplantation is characterized mainly by its powerful immunomodulatory function. Although the immunomodulatory property seems to be a shared feature of all stromal cells including MSCs and fibroblasts, the characteristics of MSCs (such as easy cultivation, expansion, and storage in vitro) make them more appropriate for organ transplantation7–9. Based on these characteristics, MSCs have been studied for immunosuppression after transplantation of various organs, including the liver, kidney, skin, etc.
MSCs can be isolated from diverse sources, such as umbilical cord blood, peripheral blood, and adipose tissue, and all MSCs share many characteristics, including cell phenotype and immunomodulatory properties10–15. MSCs can also be isolated from some other species such as mice, rats, and rabbits, and great differences exist among them in many aspects, such as the mechanisms of their immunomodulatory properties16. Moreover, MSCs isolated from different human tissues do not have the identical properties. For example, all MSCs secrete different cytokines, which may affect their immunosuppressive effects17. A unique and definitive marker has not yet been found for MSCs isolated from various sources; however, it is known that they lack the expression of major histocompatibility complex class II and costimulatory molecules (including CD80, CD86, and CD40), and this characteristic appears to explain the low immunogenicity of MSCs18. Due to this low immunogenicity, in in vitro mixed lymphocyte reaction (MLR) experiments, even MSCs from third-party sources barely caused a lymphocyte response19,20. Thus, some authors believe that MSCs from the recipient, donor, and even third-party sources could be feasible in organ transplantation, although this is not yet widely accepted due to the lack of research evidence in the human body and the effects not being entirely satisfactory21.
In general, as the first tissue from which MSCs were isolated, bone marrow (BM) is currently the main source of MSCs for investigation. In clinical studies of organ transplantation, the most widely accepted practice is to choose MSCs isolated from the BM of the donor or the recipient; however, researchers are keen on seeking alternative sources of MSCs such as urine, placenta, and adipose tissue, due to the limited quantities and difficulty in isolating BM-MSCs. MSCs from other sources also have their own immunoregulatory characteristics. Although it is still unclear, and there is a lack of clinical evidence about which type of MSCs is most suitable for immunoregulation after liver transplantation, recent studies have revealed differences in MSCs from diverse sources. For instance, Rho et al. demonstrated that adipose-MSCs (A-MSCs) and BM-MSCs expressed high levels of IL-10 and TGF-β, and dermal skin-MSCs expressed high levels of IFN-γ22. The same dose of melatonin treatment for A-MSCs and BM-MSCs had different degrees of influence on viability23. Moreover, the function of MSCs from the same source may also be different. A study showed that aged BM-MSCs secrete more IL-6 and exerted stronger immunomodulatory effects than young BM-MSCs24. Considering that the differences among these MSCs may have different outcomes in patients undergoing liver transplantation, the above studies indicate that doctors could choose personalized medicine regimens based on MSCs according to the conditions of their patients in the future, though much research is still needed to achieve this goal.
MSC In Vitro Studies
The results of in vitro experiments have indicated the powerful immunomodulatory capacity of MSCs, which could influence almost all cells involved in the immune reaction (Table 1). For B cells, MSCs can inhibit B cell differentiation and proliferation, most likely by directly contact between MSCs and T cells, and by arresting B cells in the G0/G1 phase of the cell cycle25–28. For natural killer (NK) cells, MSCs can inhibit their activation and proliferation in a dose-dependent manner29,37. Secreted factors, including interferon-γ (IFN-γ), prostaglandin E2 (PGE2), transforming growth factor β (TGF-β), and indoleamine 2,3-dioxygenase (IDO), as well as cell-to-cell contact might be involved in this process29,30. Regarding dendritic cells (DCs), MSCs could influence their differentiation, maturation, and function by direct interaction and soluble mediators, such as prostaglandin E2 (PGE2) and IL-631. In detail, MSCs can interfere with the differentiation of DCs, maintain DCs in the immature stage and suppress pro-inflammatory cytokine release by DCs31,32. And, dependent on maturation inducers, coculture of MSCs with DCs could also upregulate the expression of PD-L1 in DCs, and results indicate that MSCs could induce tolerogenic DCs by manipulating the molecular phenotype of DCs33. Additionally, researchers have become gradually aware of the importance of exosomes from MSCs in immunoregulation, and have performed large scale studies38. The role of MSC-derived exosomes in liver transplantation will be discussed in the following sections.
Table 1.
The Effects of MSCs on Immune Cells.
| Immune cells | Effects | References |
|---|---|---|
| B-cells | MSCs could inhibit B-cell differentiation and proliferation, and arrest B cells in the G0/G1 phase of the cell cycle. | 25–28 |
| NK-cells | MSCs could inhibit NK cells activation and proliferation. in a dose-dependent manner | 29,30 |
| DCs | MSCs could maintain DCs in an immature stage, suppress pro-inflammatory cytokine release and upregulate the expression of PD-L1in DCs. | 31–33 |
| T-cells | MSCs could suppress T cells’ proliferation activation and differentiation through cell-cell contact that is mediated by PD-L1, and soluble molecules. | 34,35 |
| Kupffer cells | MSCs could reprogram Kupffer cells | 36 |
For investigations in organ transplantation, suppressing the function of T cells plays the most important role among the immunomodulatory properties of MSCs39. Previous studies have confirmed that MSCs can inhibit T cell activation and proliferation, promote T cell apoptosis in vitro, and regulate the T cell subset ratio by increasing Treg and Th2, as well as decreasing Th1 and Th17 cells40–42. Regarding the mechanism of T cell suppression, some authors believe that the inhibition of DC maturation and phenotype is closely involved43,44. However, if only CD4+ T cells (with phytohemagglutinin stimulation) and MSCs are present, the immunosuppressive effect of MSCs on T-cell proliferation still works45. Cell–cell contact was deemed to be important for this immunosuppressive property of MSCs. Specifically, cell–cell contact was found to be crucial for regulatory T cell induction, and direct contact through the programmed death (PD) 1 inhibitory pathway was considered to be involved in the inhibitory effects on T cell activation and proliferation34. As well as direct contact, soluble molecules are also thought to mediate T cell suppression because MSCs could still suppress T cell proliferation even without cell–cell contact35. Molecules possibly involved in human MSCs, as they are known, include transforming growth factor (TGF), hepatocyte growth factor (HGF), IDO, PGE2, insulin-like growth factor binding proteins, heme oxygenase-1 (HO), human histocompatibility antigen-G5 (HLA-G5), chemokine (C-C motif) ligand 2 (CCL2), IL-10, galectin-1, and galectin-335,46–55. All these molecules were found to be involved in T cell suppression. There have been some recent studies on the mechanism of interaction between cytokines derived from MSCs and T-cells. For instance, studies have shown that cytokines derived from MSCs could participate in the inhibition of T cells by regulating expression of the FoxP3 gene 34,38. However, the mechanism governing cytokine interactions between MSCs and FoxP3 is not completely clear, and these studies explained only part of the mechanisms linking cytokines and T-cells. Thus, the mechanism of the effects of MSC-derived cytokines on T cells is still the focus of research.
Macrophages have also been found to be involved in the immunosuppressive capacity of MSCs, and recently authors have begun to pay attention to MSC regulation of macrophages to achieve the goal of treating some specific diseases56. Németh et al. observed inhibition of the systemic inflammatory response and prolonged survival of rats after infusing MSCs into rats with sepsis57. Moreover, the immunoregulatory property of MSCs still existed after T cells, B cells, or NK cells were depleted, but the effect was eliminated when macrophages were depleted56. This result proved the critical role played by macrophages in MSC-mediated immunoregulation. This observation might be quite important in the application of MSCs in liver transplantation and we shall further discuss this issue below.
MSC In Vivo Studies
Concerning in vivo studies, MSCs have been found to play a role in various diseases, such as relieving illness in patients with systemic lupus erythematosus (SLE), ameliorating diabetes in a murine model, reducing inflammatory MRI parameters in multiple sclerosis (MS) patients, and preventing lethal graft-versus-host disease (GVHD) in clinical patients58-61. Although much knowledge has been accumulated in term of the immunoregulatory properties of MSCs, the exact mechanism remains undefined and unclear62. Moreover, because of the different conditions between in vivo and in vitro studies, research data in vitro could not comprehensively explain results in vivo. For example, due to lack of direct cell–cell interaction, the cell–cell contact that was deemed critical in MSC immunosuppression in vitro is no longer believed to be critical. Therefore, the mechanism of the immunoregulatory property of MSCs in vivo needs to be investigated further.
In transplantation, their immunosuppressive property and low immunogenicity could allow MSCs to induce immunosuppression in vivo without rejection, which makes MSCs suitable for organ transplantation. In animal experiments (Table 2), many successful cases of MSC application in organ transplantation, including heart, skin, kidney, and liver83–85, have been reported, supporting the use of MSCs in clinical patients. In clinical trials, since BM-MSCs were first used as immunomodulators in a patient with GVHD, several studies have supported the safety and feasibility of BM-MSC use in autologous and allogeneic organ transplantation through a large number of experiments86–88. In the past, researchers were generally concerned about the long-term complications of MSCs, such as tumors and infections. To date, all clinical studies on MSCs and liver transplantation that have been published have confirmed the safety and feasibility of use of MSCs in the human body89–92. Additionally, dozens of studies in other tissues have also proven the safety of MSC transplantation93. However, because the sample size of the previously described liver transplantation study was small and the follow-up period was short, the role of MSCs in cancer progression remains controversial, and more samples and longer-term clinical trials are still needed to support the application of MSCs in liver transplantation patients, especially those with hepatocellular carcinoma.
Table 2.
Liver Transplantation Trials Based on MSCs in Animal Models.
| Source of cells | Model | Injection dose | Mechanism | Conclusion | References |
|---|---|---|---|---|---|
| BM-MSCs | Guinea pig model | 1 × 107 | MSCs interfere with recipient humoral immunity. | MSCs alleviated hyperacute immune rejection in xenogeneic liver transplantation. | 63 |
| A-MSCs | Rat model | 2 × 106 | A- MSCs inhibited T-cell proliferation. | A-MSCs relieved acute rejection following orthotopic and relieved hepatic ischemia reperfusion injury liver transplantation in rats. | 64 |
| BM-MSCs | Rat model | 5 × 106 | MSCs pretreated with IFN-γ expressed higher level of PDL-1, MHC-I, MHC-II, and CD54 than MSCs without pretreatment. | IFN-γ enhances the immunosuppressive function of MSCs to protect liver allografts. | 65 |
| A-MSCs | Dog model | – | The stem cells transplanted differentiated into mature hepatocyte-like cells and secreted albumin in the hepatic tissue. | Autologous MSCs infusion through the portal vein prolonged the survival of the recipient dogs and promoted the recovery of damaged liver function. | 66 |
| HGF overexpressing MSCs | Rat model | 2-5 × 106 | HGF-overexpressing MSCs significantly alleviated acute rejection after liver transplantation and countered liver fibrosis through synergistic action with hepatic stellate cells. | HGF-overexpressing MSCs improved the survival rate of transplantation compared with BM-MSCs. | 67–69 |
| BM-MSCs | Rat model | 1-5 × 106 | MSCs regulated immune responses by increasing the Treg ratio and prompting Kupffer cell M2 polarization. | MSCs administered after liver transplantation prevented GVHD, and early injection after transplantation is recommended. | 36,70,71,72 |
| TGF-β overexpressing MSCs |
Rat model | 5× 106 | TGF-β overexpressing MSCs increased CD4 + Foxp3 + Helios- induced Tregs and decreased Th17cells. |
TGF-β overexpressing MSCs could significantly alleviate immune rejection. | 73 |
| HO-1 overexpressing MSCs | Rat model | 5 × 106-1×107 | HO-MSCs had stronger immune regulation than normal MSCs and could promote liver function recovery by regulating autophagy. | Heme oxygenase-1(HO-1) enhanced and prolonged the effect of MSCs on GVHD of
normal and reduced-size liver transplantation and promoted liver function
recovery. |
74–76 |
| BM-MSCs | Rat model | 0.5-3× 106/kg | BM-MSCs reduced ischemia-reperfusion injury, regulate T cell ratio and prevent Kupffer cell apoptosis. | BM-MSCs effectively improved the success rate of liver transplantation from donors after cardiac death. | 77,78 |
| BM-MSCs | Rat model | 2.4×106 | MSCs upregulates the expression of transcription factors AP-1, NF-κB, p-c-Jun and CyD1 in grafts. | MSCs prolonged survival in small-for-size liver grafts. | 79 |
| IL-10 overexpressing MSCs |
Rat model | 2.5×105 | IL-10 increases the immunoregulatory capacity of MSCs. | Compared with normal MSCs, IL-10 overexpressing MSCs had stronger effects on GVHD in liver transplantation. | 80 |
| A-MSCs | Rat model | – | MiR-27b inhabited the expression of CXCL12 in A-MSCs and thereby inducing T cell proliferation. | MiR-27b participated in immune suppression after liver transplantation mediated by MSCs. | 81 |
| CXCR4 over expressing MSCs | Rat model | 1×107 | CXCR4 overexpression enhanced the mobilization and engraftment of MSCs in liver transplantation. | CXCR4 over ex-pressing MSCs promoted early liver regeneration of small-for-size liver transplantation. | 82 |
Current clinical studies focus mainly on the safety and feasibility of MSCs, but some in vivo animal trials that improved the immunomodulatory capacity and homing ability of MSCs through phenotypic modification or pretreatment have been carried out and have acquired good results. For example, researchers have enhanced the efficiency of MSCs by expressing the TNF-αR-Fc and HO-1 genes in porcine islet xenotransplantation mouse models94. However, whether this treatment can benefit liver transplant recipients needs further study.
Current Status of Clinical Liver Transplantation
Since the first case of human orthotopic liver transplantation was described in 1963, liver transplantation has undergone 50 years of development, with the 1-year survival rate increasing from 25% to more than 85%34,95. However, the problem of immune rejection after surgery still exists, with more than 30% of liver transplant recipients still suffering varying degrees of rejection (Figure 2)96. Nevertheless, the incidence and severity of rejection after liver transplantation remains much lower than that in other solid organs, which makes the liver an “immunologically privileged organ”. From the perspective of physiology, given its exposure to numerous foreign materials from the gut, the liver is necessarily a tolerogenic organ, and the tolerance of the liver plays a key role in preventing potentially devastating inflammatory responses against both food and normal enteric bacteria97. Although the phenomenon of “liver tolerance” has been described for decades, the exact mechanism is yet not thoroughly understood. All the theories involving T cell anergy induction and regulatory DC induction cannot completely explain this phenomenon98. However, it is sure that the liver microenvironment, especially the environment around the hepatic sinusoid, plays a critical role in maintaining liver tolerance. Xia et al. described changes in the microenvironment status that could affect the state of liver tolerance99. On the other hand, changing and influencing the local immune microenvironment is considered to be an important MSC immunomodulatory property, which makes MSCs excellent candidate cells for promoting and maintaining liver graft tolerance.
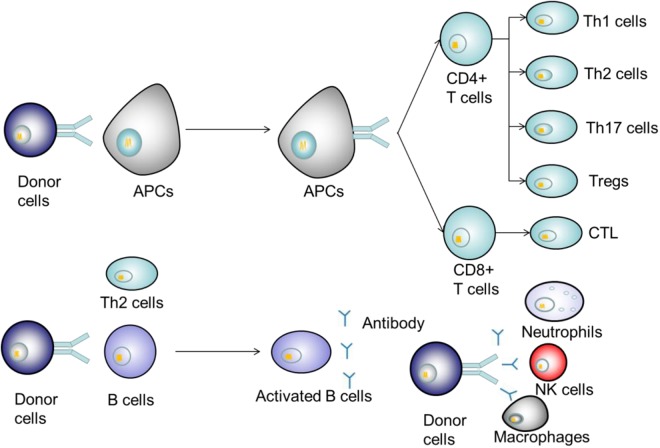
Figure 2.
Mechanism of immune rejection after liver transplantation. Donor antigens are recognized by antigen-presenting cells (APCs) and transferred to T cells. After activation, T cells differentiate into various effector T cells to secrete inflammatory factors or directly participate in immune rejection. B cells are activated with or without the help of Th2 cells after recognition of the donor antigens, secreting antibodies and cytokines that act on donor cells.
Although some immunocytes including NK cells, DCs, and T cells have already been deemed to be involved in the liver tolerance mechanism, some other factors should be considered for the explanation of liver tolerance, considering that those cells exist all over the body100. Kupffer cells (KCs)—the macrophages located in the hepatic sinusoid—account for 75%–80% of the macrophages in the whole body, and are known to be one of the key factors that make the microenvironment of the hepatic sinusoid different from that in other organs101. Several decades ago, Callery et al. reported that blocking KCs could inhibit the induction of liver tolerance, thus proving the critical role of KCs in liver tolerance102. However, the role of KCs in immunity is ambiguous because they could both prevent liver injury and aggravate liver injury under different conditions103. Our previous results and those of other studies demonstrated that switching KCs from pro-inflammatory to anti-inflammatory status could promote hepatic immune tolerance36,104. Considering the influence of MSCs on macrophages, and the similarities between KCs and macrophages, MSCs could possibly reprogram the immune status of KCs. In fact, we already have evidence indicating that the MSC-dependent reprogramming of KCs is crucial for liver transplant tolerance36. Given that macrophages might be one of the major target cells through which MSCs can induce their immunomodulatory effects, and that KCs (as they are essentially macrophages) are key factors in liver transplant tolerance induction, we believe that, compared with other solid-organ transplantation, liver transplantation might be more suitable for immunoregulation involving MSCs.
MSCs in Animal Models
Since animal models of solid-organ transplantation involving MSCs were reported in 2006 in heart transplantation, many reports have described using MSCs in liver transplantation105,106. Such studies have indicated that different MSC sources, including recipients, donors, and third-parties, could all lead to liver allograft tolerance. Although some investigation results from in vitro experiments have been verified in vivo [for example, MSCs overexpressing interleukin-10 (IL-10) could lead to liver transplant tolerance in rats more effectively than normal MSC], due to their complexity, in vivo investigations are quite different from in vitro studies, and more research is needed36,80. For example, the ratio of MSCs to T-cells or peripheral blood mononuclear cells (PBMCs), which has already been determined in in vitro studies, would mean a large number of cells in in vivo experiments, and such a large number of cells would easily cause pulmonary embolism107. Thus, the approach and dose of MSC infusion are quite important in vivo investigation. The timing and methods of cell administration (pretransplant versus posttransplant) are also crucial because the infusion scheme can determine the final migratory location of MSCs, thus affecting MSC stimulation and interactions within the local environments and their immunomodulatory capacity108,109. Although several studies have reported that MSC infusion after transplantation may lead to graft dysfunction and failure to prolong graft survival, more studies still recommended a posttransplant infusion scheme, because the posttransplant infusion scheme can promote intragraft MSC migration, which can improve MSC tissue repair properties110. Moreover, pretransplant infusion may limit MSC homing into grafts and promote their migration to the lungs, where they might lose viability after 24 h111.
In most animal liver transplantation studies, liver function improvements and amelioration of inflammation can be observed in the early posttransplant phase (7 days after surgery) while anesis of liver inflammation during this phase can be attributed to the control of rejection as well as the relief of ischemia reperfusion injury (IRI)110,112. In fact, MSCs have been regarded as a protective factor against IRI, and MSC-mediated IRI protection might be a consequence of their inherent immunosuppressive capacity, similar to inhibition of rejection113. For example, Zheng and his group proved that MSCs, through the release of anti-inflammatory factors, significantly alleviate hepatic IRI114. Moreover, researchers also believe that MSCs could stabilize neovascularization by inhibiting inflammation, which would improve the success rate of liver transplantation115. To sum up, in liver transplantation, the immunomodulatory capacity of MSCs could potentially be helpful to promote liver transplantation, reduce hepatic ischemia reperfusion injury, and control liver transplantation rejection.
MSCs in Clinical Trials
More than 10 years have passed since MSCs were first used as an immunomodulator in humans (in a patient with GVHD in 2004)116. To date, although several cases of MSC application in the clinic have been reported (Table 3), clinical trials with large sample sizes on liver transplantation involving MSCs are still insufficient. The reasons might lie partly in the following intrinsic constraints and obstacles in the application of MSC in clinical liver transplantation: (1) Ethical problems: after decades of development, clinical liver transplantation has become quite a safe and efficient therapy for end-stage liver diseases; thus, any alteration in clinical standards would bring about much ethical doubt. In addition, ethical issues have also contributed greatly to the delay in stem cells being brought into clinical trials in many countries or regions. (For instance, wide applications of stem cells in preclinical studies were prohibited in China until July 2015.) (2) Immune environment issues: to ensure the best survival of the liver graft, recipients generally have to be severely immunosuppressed in the induction period, which means that any additional cell therapy would encounter a condition of systemic immunosuppression and would usually meet high concentrations of pharmacological immunosuppressors that would probably influence the function of transplanted MSCs. (3) Sources and pretreatment of MSCs: although the low immunogenicity of MSCs is well known, MSCs cannot be completely immunoprivileged in vivo110. Moreover, it is well documented that the culture conditions and pretreatment can influence the immunomodulatory potentials of MSCs; therefore the source and pretreatment of MSCs that are chosen for clinical use need further study. (4) Timing, approach, and dosage of MSC infusion: as previously mentioned, each of these factors is quite important in MSC application; however, the experience of in vitro studies and animal experiments cannot inform clinical applications. Thus, more studies with larger animals (for example, pigs) with dose escalation should be conducted. (5) Safety concerns: due to a lack of knowledge, crucial safety concerns need to be considered, such as whether MSCs increase tumor incidence or opportunistic infections risk, similar to immunosuppressive drugs, and whether MSCs have the potential for maldifferentiation given that MSCs possess multilineage differentiation potential. On the other hand, however, due to the different types of liver diseases that warrant liver transplantation, these safety problems might not be critical in all clinical transplantation cases. For example, due to the potential risk of tumor promotion, MSCs may not be suitable for patients diagnosed with early stage liver cancer but might be feasible for the patients with liver cirrhosis.
Table 3.
Clinical Trials of Liver Transplantation Based on MSCs Carried Out Up to August 1, 2019. All Data from https://clinicaltrials.gov/ and PubMed.
| Study title | NCT number | Study start date | Transplantation schemes | Sample size | Follow-up period | References | Conclusion and effectiveness analysis |
|---|---|---|---|---|---|---|---|
| Safety and feasibility of multipotent adult progenitor cells for immunomodulation therapy after liver transplantation: A phase I study of the MiSOT study consortium | NCT01841632 | November 2011 | Multipotent adult progenitor
cells(MAPCs) Allotransplantation Dose:3× 108 -1.2×109 Per patient |
1 | 6 months | 91,117 | This first-in-human case study confirmed that intraportal and intravenous infusion of third-party MSCs after liver transplantation is clinically feasible. Due to the limitation of small sample size, large sample clinical trials are still needed to further verify safety and efficacy of third-party MSCs. |
| Human Mesenchymal Stem Cells Induce Liver | NCT01429038 | February 2012 | BM-MSCs Allotransplantation Dose:1.5-3×106/kg |
20 | 12 months | 89 | This phase I-II, open-label, clinical study shown that no severe opportunistic infections or neoplasms were found in patients treated with MSCs, which further confirmed the safety of MSCs for liver transplantation. However, two patients had recurrence of hepatocellular carcinoma and two high-risk patients developed asymptomatic CMV, although there was no evidence that MSCs were involved, which deserved our attention. Additionally, MSC injection schemes should be optimized to benefit patients. |
| Human Mesenchymal Stem Cells Induce Liver Transplant Tolerance | NCT01690247 | February 2012 | UC-MSCs Allotransplantation Dose: 1×106/kg |
27 | 3 months | 92 | Compared with the previous open trials, this study applied UC-MSCs and showed stronger immunomodulatory effects on liver transplantation patients, and no MSC-related complications occurred during follow up. This study supports the feasibility and clinical effects of MSCs in liver transplantation patients. |
| Therapeutic Strategy and the Role of Mesenchymal Stromal Cells for ABO Incompatible Liver Transplantation | NCT02706132 | February 2014 | – | – | – | – | Not yet published. |
| Mesenchymal Stem Cells Transplantation for Ischemic-type Biliary Lesions | NCT02223897 | February 2012 | UC-MSCs Allotransplantation Dose: 1×106/kg |
12 | 24 months | 90 | The study showed that MSC infusion in patients with ischemic biliary tract disease after liver transplantation reduced the need for interventional therapy improved graft survival compared with traditional therapy and further supported the feasibility of applying MSCs for liver transplantation. |
| MSC Therapy in Liver Transplantation | NCT02260375 | October 2014 | – | – | – | – | Not yet published. |
| Mobilization of Mesenchymal Stem Cells During Liver Transplantation | NCT02557724 | September 2015 | – | – | – | – | Not yet published. |
| Safety and Tolerance of Immunomodulating Therapy With Donor-specific MSC in Pediatric Living Donor Liver Transplantation | NCT02957552 | March 2017 | – | – | – | 118 | Not yet published. |
Despite the many constraints and obstacles, the application of MSCs in clinical use has not been stopped. From reports of a series of phase II clinical studies in Europe on the treatment of acute steroid-resistant GVHD, MSCs have shown substantially promising results116. These results not only suggest that MSCs might still be effective in GVHD even after the failure of immunosuppressive drugs, but also indicate the possibility of MSCs application in solid-organ transplantation. The MiSOT-I trial, performed by the Mesenchymal Stem Cells in Solid Organ Transplantation (MiSOT) organization, was initiated in 2013. To date, much feedback information regarding clinical research involving MSC application in liver and kidney transplantation has been summarized110. Such feedback, combined with other clinical trials of liver transplantation, includes mainly the following aspects: (1) The timing of MSC infusion: MSCs need to be used in the early posttransplantation phase because of their long-term pro-tolerogenic effects. Because of persistent chronic rejection, MSCs should be used posttransplantation if the function of the graft is stable or deteriorating. Moreover, the long-term effects of MSCs on chronic rejection are still expected. (2) Use of concomitant immunosuppression: Considering the existence of immunogenicity and drug targets of MSCs, the drug combinations used with MSCs may be a promising way to improve future clinical transplantation. (3) Sources of MSCs: all sources of MSC, including BM, adipose tissue and other human tissues share similarly high therapeutic potentials. Regarding immunogenicity, based on the outcomes of liver transplantation trials, MSCs were proven to be safe and effective. (4) Safety concerns: regarding the potential of MSC maldifferentiation, evidence for malignant transformation of MSCs was not found in all studies, but further attention is still needed. For the issue of opportunistic infections, four recent clinical trials of MSCs in liver transplantation showed no increased incidence of infection in patients after receiving MSCs89–92. However, given the small sample size and short follow-up period of the above-mentioned trials, and a clinical trial based by Reinders et al. on renal transplantation that described that three of six patients infused with MSCs had opportunistic but mild viral infections, the possibility of infection after MSC injection should still be taken seriously87. (5) Immunoregulation results: Although MSCs were proven to be effective in alloimmune reaction treatments by a large number of in vivo and in vitro experiments, a clinical trial on liver transplantation above mentioned lacked the protective effects of MSCs in patients with liver transplantation, and, after withdrawal of the immunosuppressive agents, immunological rejection occurred successively in MSC-treated patients and disappeared after resumption of the immunosuppressive agents89. This result was most likely due to the choice of immunosuppressants and MSCs; and an inappropriate application scheme of MSCs. Another clinical trial of liver transplantation showed that, in comparison to using immunosuppressive drugs alone, MSCs could better improve liver tissue, and were able to regulate the proportion of T lymphocyte subsets to promote immune tolerance during the acute rejection phase after liver transplantation. However, the long-term status of patients could not be observed, due to the short follow-up period of only 12 weeks92. For induction therapy, although no clinical trials related to liver transplantation have been reported, two clinical trials on kidney transplantation confirmed the safety of MSCs as induction therapy for organ transplantation119,120. Moreover, one of the above studies showed that MSCs were superior to IL-2 for induction therapy120.
Concerning liver transplantation, specifically, information on MSC application in clinical liver surgery is still lacking. The available information on MSC application in liver transplantation is encouraging; however, large-scale trials are still needed. Nevertheless, the experience of researchers in the design of investigations is still helpful and meaningful for subsequent studies.
MSCs Combined with Immunosuppressive Drugs in Liver Transplantation
At present, there is a consensus on the use of calcineurin inhibitors (CNIs) such as tacrolimus and cyclosporine, as immune maintenance drugs after liver transplantation, supplemented with antimetabolites such as acetazolamide (AZA) and mycophenolic acid (MPA), or m-TOR inhibitors to achieve a lower dose of CNI and alleviate side effects121. Glucocorticoids, interleukin-2 and antithymosin are recommended for induction therapy of liver transplantation121. Previous clinical studies on liver transplantation are also based on the application of MSCs combined with tacrolimus and mycophenolate mofetil (MMF) in the immune maintenance therapy of liver transplantation patients. Given the low immunogenicity of MSCs, and given that there are still targets on which immunosuppressive drugs act, this issue should be considered when developing a combination regimen of MSCs and immunosuppressive drugs122.
It was found that short-term exposure to immunosuppressive agents in vitro had no toxicity and did not induce apoptosis in MSCs, but long-term exposure to tacrolimus at high doses for 7 days could significantly promote the death of MSCs122. Even exposure to MPA and rapamycin at therapeutic doses inhibited the proliferation of MSCs122. These results suggested that short-term and low-dose immunosuppressive agents combined with MSCs seemed to achieve better results in patients undergoing liver transplantation. In addition, the added protective agents seemed to be effective in reducing the toxicity of immunosuppressants on MSCs. For example, combination with oxytocin counteracted the cytotoxicity of tacrolimus on MSCs123. Researchers also found that different kinds of immunosuppressants have heterogeneous interaction effects with MSCs. For CNIs, MSCs combined with tacrolimus demonstrated considerable coordination in immune regulation after organ transplantation. Kuo et al. found that MSCs plus tacrolimus improved graft survival in a miniature swine hind-limb model124. Suda and his group also showed that the combination of MSCs and tacrolimus could relieve ischemic brain damage in rats125. A prospective, nonrandomized study on kidney transplantation proved that the use of MSCs could offset the dose of tacrolimus used in patients without increasing the risk of rejection, and indicated that combined use with MSCs could decrease tacrolimus dosage and thus alleviate adverse reactions in organ transplantation patients126. However, the synergistic effects of tacrolimus combined with MSCs on immune regulation have been controversial in some in vitro studies. Poncelet et al. reported that MSCs cocultured with tacrolimus effectively increased the inhibitory effect on T cells127, but Buron et al. showed an antagonistic effect of MSCs and tacrolimus on T cell inhibition128. This may be attributed to the difference in the origin of the MSCs in the above-mentioned studies. Cyclosporine was also found to interact with MSCs. Some studies have shown that cyclosporine synergistically promotes immunosuppression with MSCs129. However, some studies have found that cyclosporine and MSCs have antagonistic effects, which may be attributed to the differences in experimental treatment methods, such as lymphocyte stimulation, and the basic condition of the experimental subjects128. Exhilaratingly, MMF/MPA showed better synergistic immunosuppressive action with MSCs than other immunosuppressive drugs in both in vitro and in vivo studies. For example, a study showed that MSCs combined with MMF could significantly increase heart transplant survival compared with single-use MSCs or MSCs combined with CNIs in a rat model130. In an in vitro study, MSCs combined with MPA showed stronger inhibitory effects on lymphocyte proliferation than CNIs, m-TOR inhibitors, and dexamethasone128. These studies seemed to suggest that MMF/MPA was more suitable for combined use with MSCs than other immunosuppressants. Additionally, both steroid hormones and m-TOR inhibitors also exhibited synergistic immunosuppressive effects with MSCs in animal models131,132. Given the potential role of MSCs in liver transplantation, these effects should be further studied and considered when formulating a compatibility program for liver transplantation patients132. Taken together, the above findings imply that multiple combination regimens based on MSCs could be designed as induction or maintenance therapies for liver transplant patients with different disease conditions in the future.
Potential Therapeutic Properties of MSCs in Liver Transplantation
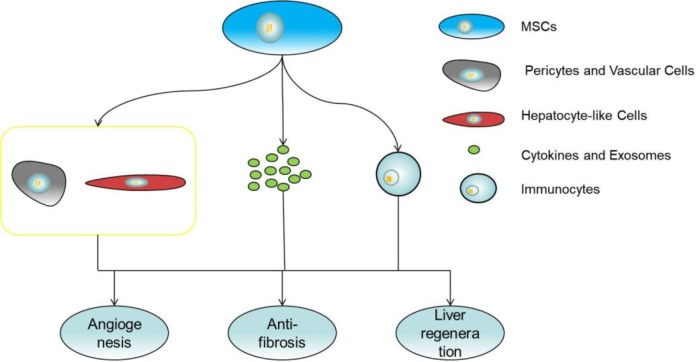
In addition to posttransplant immunosuppression, MSCs are also able to promote liver regeneration, angiogenesis, and anti-fibrosis, which are potentially helpful for liver transplantation in patients (Figure 3). For liver regeneration, studies found that MSCs could promote liver reparation after partial hepatectomy in mice with hepatic steatosis (HS)133. MSCs that increased angiogenesis in an animal model of acute liver failure and that relieve hepatic cirrhosis in animal and clinical trials were also found134–136. Cytokines and factors secreted by MSCs through the paracrine pathway appear to play a major role in these processes. For example, MSCs could secrete cytokines such as IL-6, hepatocyte growth factor (HGF), and IGFBP-2 to promote hepatocyte regeneration and inhibit hepatocyte apoptosis. Metalloproteinase (MMPs) and insulin growth factor-like-I (IGF-I) released by MSCs could effectively reduce collagen accumulation and counter liver fibrosis137,138. MSCs were able to secrete various angiogenic factors to promote angiogenesis. Molecules such as VEGF-A, VWF, SDF-1α, Cyr61, MMP2, and MMP9 are involved in this process139–142. In addition, MSCs can also participate in these processes by regulating the immune response and inhibiting the release of inflammatory factors143. Moreover, studies show that overexpression of related cytokines such as HGF, in MSCs can alleviate liver function damage, reduce liver fibrosis, and improve graft survival compared with normal MSCs67–69. These studies suggest that MSCs have potential therapeutic properties in liver transplantation, and looking for ways to enhance these properties of MSCs and thereby enhance the effect of MSCs on liver transplantation seems to be a promising approach but it still needs be further evaluated in clinical trials.
Figure 3.
MSCs promote liver regeneration, angiogenesis and count fibrosis by secreting cytokines and exosomes, regulating inflammation and differentiation.
Despite their immunomodulatory capacity as a multipotent stem cells, MSCs have multidirectional differentiation characteristics. In previous studies, researchers proved that MSCs could differentiate into pericytes and vascular cells in vivo139. It should be mentioned that MSCs have the potential to differentiate into hepatocyte-like cells both in vitro and vivo137,144. Hepatocyte-like cells derived from MSCs have gene expression profiles similar to those of hepatocytes and perform several hepatocyte-like functions that promote the recovery of damaged liver function. These studies show a potential mechanism for MSCs to help patients undergoing liver transplantation. However, given that the immune regulation of MSCs is impaired during the process of differentiating into hepatocyte-like cells, it seems unwise to only pursue the promotion of differentiation of MSCs into hepatocytes in the application of liver transplantation.
Exosomes, as a type of extracellular vesicle (EV) are defined as lipid bilayer particles 20–150 nm in diameter released by various cells145. Despite being initially regarded as a waste product of cell rejection, exosomes, which are rich in proteins, coding and non-coding RNA, and even DNA, are closely associated with many diseases and physiological processes, and have attracted the attention of researchers in recent years. Given that exosomes from MSCs display powerful physiological regulatory functions, have lower ethical considerations and immunogenicity compared with stem cell therapy, and have no increased risk of cancer, they seem to be a promising approach to acellular therapy. For liver transplantation, exosomes released from MSCs have been proven to have immune regulation, tissue repair and angiogenesis functions similar to those of source cells. One study showed that injecting exosomes derived from BM-MSCs into rats with GVHD every week could inhibit Th17 cells and Tregs, and enhance pathological conditions146. Liver fibrosis was also alleviated after transplantation of MSC-derived exosomes by reducing oxidative stress and inhibiting hepatocyte apoptosis in a liver injury model induced by CCL4147. Whole-body administration of exosomes derived from MSCs promoted liver repair and angiogenesis in damaged areas148,149. In addition, a study showed that injecting MSC-derived exosomes into mice with hepatocellular carcinoma could effectively inhibit tumor growth and enhance chemosensitivity due to the abundance of anti-tumor microRNAs in the exosomes150. This study indicated that exosomes derived from MSCs may be particularly suitable for liver transplantation patients with malignant tumors, though the actual effects still need to be measured further by clinical trials. Together, exosomes derived from MSCs can be used not only as a potential mechanism for explaining the effect of MSCs on liver transplantation, but also as a new tool that may be used to benefit patients undergoing liver transplantation at some future date.
Conclusions
According to the available information from investigations including in vitro, animal, and phase I and II clinical studies, MSC infusion is generally feasible and safe for liver transplantation, but we suggest the need for a cautious approach for certain types of patients, such as patients with tumors. The application of MSCs could promote immune tolerance in patients with liver transplantation; however, whether MSCs could replace immunosuppressive drugs is questionable.
Due to the heterogeneity of MSCs, it is clear that the immunoregulatory function of MSCs can be improved by choosing appropriate sources, providing feasible pretreatment, compatible with practicable immunosuppressants and gene modification; however, whether the above schemes are actually beneficial for liver transplant patients without increasing the risk of complications, and even help to completely replace immunosuppressive drugs, still requires further clinical trial verification. It is known that soluble molecules, exosomes and direct cell-to-cell contact are considered to be the main ways whereby MSCs exert their immunosuppressive effects, but the exact mechanisms of immune regulation are not yet fully understood. Additionally, exosomes show promising therapeutic prospects, but whether exosomes can replace cell therapy in liver transplantation needs further confirmation. The MSC characteristics of promoting liver regeneration, angiogenesis, and alleviating liver fibrosis are helpful for liver transplantation patients. Improving these properties of MSCs may better benefit liver transplantation patients. The ability of MSCs to undergo multidirectional differentiation is also involved in these processes. However, it remains unclear whether regulating the differentiation of MSCs can benefit liver transplant recipients.
In summary, MSC-based therapy is a promising treatment for liver transplantation, but the exact mechanisms and effects of MSCs on liver transplantation, as well as the problems that might be encountered in the clinical applications, still require further study; such studies will greatly aid the future clinical use of MSCs in liver transplantation.
Footnotes
Author Notes: Yu You and Di-guang Wen contributed equally to this work.
Author Contributions: YY, WDG, and LZJ performed research and wrote the first draft. GJP collected and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. LZJ is the guarantor.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by the National Science Foundation of China (No. 81170442, 81470899, 81702357), National Scholarship Foundation (No.201208505116), and Outstanding young talent fund of the second hospital of CQMU (2011).
ORCID iD: Zuo-jin Liu  https://orcid.org/0000-0003-1440-5693
https://orcid.org/0000-0003-1440-5693
References
- 1. Chascsa DM, Vargas HE. The gastroenterologist’s guide to management of the post-liver transplant patient. Am J Gastroenterol. 2018;113(6):819–28. [DOI] [PubMed] [Google Scholar]
- 2. Durand F, Levitsky J, Cauchy F, Gilgenkrantz H, Soubrane O, Francoz C. Age and liver transplantation. J Hepatol. 2019;70(4):745–58. [DOI] [PubMed] [Google Scholar]
- 3. Bottomley MJ, Harden PN. Update on the long-term complications of renal transplantation. Br Med Bull. 2013;106:117–34. [DOI] [PubMed] [Google Scholar]
- 4. Casiraghi F, Perico N, Cortinovis M, Remuzzi G. Mesenchymal stromal cells in renal transplantation: opportunities and challenges. Nat Rev Nephrol. 2016;12(4):241–53. [DOI] [PubMed] [Google Scholar]
- 5. Hinden L, Shainer R, Almogi-Hazan O, Or R. Ex Vivo induced regulatory human/murine mesenchymal stem cells as immune modulators. Stem Cells. 2015;33(7):2256–67. [DOI] [PubMed] [Google Scholar]
- 6. Lu H, Wang F, Mei H, Wang S, Cheng L. Human adipose mesenchymal stem cells show more efficient angiogenesis promotion on endothelial colony-forming cells than umbilical cord and endometrium. Stem Cells Int. 2018;2018:7537589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lim JY, Ryu DB, Lee SE, Park G, Min CK. Mesenchymal stem cells (MSCs) attenuate cutaneous sclerodermatous graft-versus-host disease (Scl-GVHD) through inhibition of immune cell infiltration in a mouse model. J Invest Dermatol. 2017;137(9):1895–904. [DOI] [PubMed] [Google Scholar]
- 8. Cortés-Araya Y, Amilon K, Rink BE, Black G, Lisowski Z, Donadeu FX, Esteves CL. Comparison of antibacterial and immunological properties of mesenchymal stem/stromal cells from equine bone marrow, endometrium, and adipose tissue. Stem Cells Dev. 2018;27(21):1518–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peng Y, Chen X, Liu Q, Zhang X, Huang K, Liu L, Li H, Zhou M, Huang F, Fan Z, Sun J, et al. Mesenchymal stromal cells infusions improve refractory chronic graft versus host disease through an increase of CD5+ regulatory B cells producing interleukin 10. Leukemia. 2015;29(3):636–46. [DOI] [PubMed] [Google Scholar]
- 10. Abumaree MH, Abomaray FM, Alshehri NA, Almutairi A, AlAskar AS, Kalionis B, Al Jumah MA. Phenotypic and functional characterization of mesenchymal stem/multipotent stromal cells from decidua parietalis of human term placenta. Reprod Sci. 2016;23(9):1193–207. [DOI] [PubMed] [Google Scholar]
- 11. Bourebaba L, Michalak I, Röcken M, Marycz K. Cladophora glomerata methanolic extract decreases oxidative stress and improves viability and mitochondrial potential in equine adipose derived mesenchymal stem cells (ASCs). Biomed Pharmacother. 2018;111:6–18. [DOI] [PubMed] [Google Scholar]
- 12. Sivan U, Jayakumar K, Krishnan LK. Matrix-directed differentiation of human adipose-derived mesenchymal stem cells to dermal-like fibroblasts that produce extracellular matrix. J Tissue Eng Regen Med. 2016;10(10):E546–58. [DOI] [PubMed] [Google Scholar]
- 13. Bana N, Sanooghi D, Soleimani M, Hayati Roodbari N, Alavi Moghaddam S, Joghataei MT, Sayahpour FA, Faghihi F. A comparative study to evaluate myogenic differentiation potential of human chorion versus umbilical cord blood-derived mesenchymal stem cells. Tissue Cell. 2017;49(4):495–502. [DOI] [PubMed] [Google Scholar]
- 14. Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, Wang Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14(8):493–507. [DOI] [PubMed] [Google Scholar]
- 15. Fujita Y, Kawamoto A. Stem cell-based peripheral vascular regeneration. Adv Drug Deliv Rev. 2017;120:25–40. [DOI] [PubMed] [Google Scholar]
- 16. Ren G, Su J, Zhang L, Zhao X, Ling W, L’huillie A, Zhang J, Lu Y, Roberts AI, Ji W, Zhang H, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27(8):1954–62. [DOI] [PubMed] [Google Scholar]
- 17. Du WJ, Chi Y, Yang ZX, Li ZJ, Cui JJ, Song BQ, Li X, Yang SG, Han ZB, Han ZC. Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta. Stem Cell Res Ther. 2016;7(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rajeshkumar B, Agrawal P, Rashighi M, Saidi RF. Mesenchymal stem cells and co-stimulation blockade enhance bone marrow engraftment and induce immunological tolerance. Int J Organ Transplant Med. 2015;6(2):55–60. [PMC free article] [PubMed] [Google Scholar]
- 19. Barberini DJ, Aleman M, Aristizabal F, Spriet M, Clark KC, Walker NJ, Galuppo LD, Amorim RM, Woolard KD, Borjesson DL. Safety and tracking of intrathecal allogeneic mesenchymal stem cell transplantation in healthy and diseased horses. Stem Cell Res Ther. 2018;9(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–43. [DOI] [PubMed] [Google Scholar]
- 21. Kaundal U, Bagai U, Rakha A. Immunomodulatory plasticity of mesenchymal stem cells: a potential key to successful solid organ transplantation. J Transl Med. 2018;16(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ock SA, Baregundi Subbarao R, Lee YM, Lee JH, Jeon RH, Lee SL, Park JK, Hwang SC, Rho GJ. Comparison of immunomodulation properties of porcine mesenchymal stromal/stem cells derived from the bone marrow, adipose tissue, and dermal skin tissue. Stem Cells Int. 2016;2016:9581350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rafat A, Mohammadi Roushandeh A, Alizadeh A, Hashemi-Firouzi N, Golipoor Z. Comparison of the melatonin preconditioning efficacy between bone marrow and adipose-derived mesenchymal stem cells. Cell J. 2019;20(4):450–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhuang Y, Li D, Fu J, Shi Q, Lu Y, Ju X. Comparison of biological properties of umbilical cord-derived mesenchymal stem cells from early and late passages: immunomodulatory ability is enhanced in aged cells. Mol Med Rep. 2015;11(1):166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Che N, Li X, Zhou S, Liu R, Shi D, Lu L, Sun L. Umbilical cord mesenchymal stem cells suppress B-cell proliferation and differentiation. Cell Immunol. 2012;274(1–2):46–53. [DOI] [PubMed] [Google Scholar]
- 26. Rosado MM, Bernardo ME, Scarsella M, Conforti A, Giorda E, Biagini S, Cascioli S, Rossi F, Guzzo I, Vivarelli M, Dello Strologo L, et al. Inhibition of B-cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cells Dev. 2015;24(1):93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–72. [DOI] [PubMed] [Google Scholar]
- 28. Vitacolonna M, Schubert M, Herbert N, Taubert I, Singh R, Ho A, Zöller M. Improved T and B cell recovery by the transfer of slowly dividing human hematopoietic stem cells. Leuk Res. 2010;34(5):622–30. [DOI] [PubMed] [Google Scholar]
- 29. Thomas H, Jäger M, Mauel K, Brandau S, Lask S, Flohé SB. Interaction with mesenchymal stem cells provokes natural killer cells for enhanced IL-12/IL-18-induced interferon-gamma secretion. Mediators Inflamm. 2014;2014:143463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J Immunol Res. 2015;2015:394917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Park M, Kim YH, Ryu JH, Woo SY, Ryu KH. Immune suppressive effects of tonsil-derived mesenchymal stem cells on mouse bone-marrow-derived dendritic cells. Stem Cells Int. 2015;2015:106540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jo H, Eom YW, Kim HS, Park HJ, Kim HM, Cho MY. Regulatory dendritic cells induced by mesenchymal stem cells ameliorate dextran sodium sulfate-induced chronic colitis in mice. Gut Liver. 2018;12(6):664–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khosravi M, Bidmeshkipour A, Moravej A, Hojjat-Assari S, Naserian S, Karimi MH. Induction of CD4+CD25+Foxp3+ regulatory T cells by mesenchymal stem cells is associated with RUNX complex factors. Immunol Res. 2018;66(1):207–18. [DOI] [PubMed] [Google Scholar]
- 34. Ma Y, Wang Z, Zhang A, Xu F, Zhao N, Xue J, Zhang H, Luan X. Human placenta-derived mesenchymal stem cells ameliorate GVHD by modulating Th17/Tr1 balance via expression of PD-L2. Life Sci. 2018;214:98–105. [DOI] [PubMed] [Google Scholar]
- 35. Khosravi M, Bidmeshkipour A, Cohen JL, Moravej A, Hojjat-Assari S, Naserian S, Karimi MH. Induction of CD4+CD25+FOXP3+ regulatory T cells by mesenchymal stem cells is associated with modulation of ubiquitination factors and TSDR demethylation. Stem Cell Res Ther. 2018;9(1):273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. You Y, Zhang J, Gong J, Chen Y, Li Y, Yang K, Liu Z. Mesenchymal stromal cell-dependent reprogramming of Kupffer cells is mediated by TNF-α and PGE2 and is crucial for liver transplant tolerance. Immunol Res. 2015;62(3):292–305. [DOI] [PubMed] [Google Scholar]
- 37. Li Y, Qu YH, Wu YF, Liu L, Lin XH, Huang K, Wei J. Bone marrow mesenchymal stem cells suppressing activation of allogeneic cytokine-induced killer/natural killer cells either by direct or indirect interaction. Cell Biol Int. 2015;39(4):435–45. [DOI] [PubMed] [Google Scholar]
- 38. Rong X, Liu J, Yao X, Jiang T, Wang Y, Xie F. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res Ther. 2019;10:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li CL, Leng Y, Zhao B, Gao C, Du FF, Jin N, Lian QZ, Xu SY, Yan GL, Xia JJ, Zhuang GH, et al. Human iPSC-MSC-derived xenografts modulate immune responses by inhibiting the cleavage of caspases. Stem Cells. 2017;35(1):1719–32. [DOI] [PubMed] [Google Scholar]
- 40. Laing AG, Fanelli G, Ramirez-Valdez A, Lechler RI, Lombardi G, Sharpe PT. Mesenchymal stem cells inhibit T-cell function through conserved induction of cellular stress. PLoS One. 2019;14(3):e0213170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li Y, Wang F, Guo R, Zhang Y, Chen D, Li X, Tian W, Xie X, Jiang Z. Exosomal sphingosine 1-phosphate secreted by mesenchymal stem cells regulated Treg/Th17 balance in aplastic anemia. IUBMB Life. 2019;71(9):1284–92. [DOI] [PubMed] [Google Scholar]
- 42. Liu H, Liu SY, Qiu XY, Deng ZH, Jin Y. Influence of TNF-α on the immunomodulatory property of laryngeal mucosa mesenchymal stromal cells’ [in Chinese]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019;54(3):203–08. [DOI] [PubMed] [Google Scholar]
- 43. Chiesa S, Morbelli S, Morando S, Massollo M, Marini C, Bertoni A, Frassoni F, Bartolomé ST, Sambuceti G, Traggiai E, Uccelli A. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc Natl Acad Sci U S A. 2011;108(42):17384–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ghosh T, Barik S, Bhuniya A, Dhar J, Dasgupta S, Ghosh S, Sarkar M, Guha I, Sarkar K, Chakrabarti P, Saha B, et al. Tumor-associated mesenchymal stem cells inhibit naïve T cell expansion by blocking cysteine export from dendritic cells. Int J Cancer. 2016;139(9):2068–81. [DOI] [PubMed] [Google Scholar]
- 45. Kim SH, Jung J, Cho KJ, Choi JH, Lee HS, Kim GJ, Lee SG. Immunomodulatory effects of placenta-derived mesenchymal stem cells on t cells by regulation of FoxP3 expression. Int J Stem Cells. 2018;11(2):196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Crain SK, Robinson SR, Thane KE, Davis AM, Meola DM, Barton BA, Yang VK, Hoffman AM. Extracellular vesicles from wharton’s jelly mesenchymal stem cells suppress CD4 expressing T cells through transforming growth factor beta and adenosine signaling in a canine model. Stem Cells Dev. 2019;28(3):212–26. [DOI] [PubMed] [Google Scholar]
- 47. Liang C, Jiang E, Yao J, Wang M, Chen S, Zhou Z, Zhai W, Ma Q, Feng S, Han M. Interferon-γ mediates the immunosuppression of bone marrow mesenchymal stem cells on T-lymphocytes in vitro. Hematology. 2018;23(1):44–49. [DOI] [PubMed] [Google Scholar]
- 48. He JG, Xie QL, Li BB, Zhou L, Yan D. Exosomes derived from IDO1-Overexpressing rat bone marrow mesenchymal stem cells promote immunotolerance of cardiac allografts [published online ahead of print October 12, 2018]. Cell Transplant. 2018;963689718805375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miyagawa I, Nakayamada S, Nakano K, Yamagata K, Sakata K, Yamaoka K, Tanaka Y. Induction of regulatory T cells and its regulation with insulin-like growth factor/insulin-like growth factor binding protein-4 by human mesenchymal stem cells. J Immunol. 2017;199(5):1616–25. [DOI] [PubMed] [Google Scholar]
- 50. Wang Y, Wang JL, Ma HC, Tang ZT, Ding HR, Shi XL. Mesenchymal stem cells increase heme oxygenase 1-activated autophagy in treatment of acute liver failure. Biochem Biophys Res Commun. 2019;508(3):682–89. [DOI] [PubMed] [Google Scholar]
- 51. An JH, Song WJ, Li Q, Kim SM, Yang JI, Ryu MO, Nam AR, Bhang DH, Jung YC, Youn HY. Prostaglandin E2 secreted from feline adipose tissue-derived mesenchymal stem cells alleviate DSS-induced colitis by increasing regulatory T cells in mice. BMC Vet Res. 2018;14(1):354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nahar S, Nakashima Y, Miyagi-Shiohira C, Kinjo T, Toyoda Z, Kobayashi N, Saitoh I, Watanabe M, Noguchi H, Fujita J. Cytokines in adipose-derived mesenchymal stem cells promote the healing of liver disease. World J Stem Cells. 2018;10(11):146–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee HK, Kim HS, Kim JS, Kim YG, Park KH, Lee JH, Kim KH, Chang IY, Bae SC, Kim Y, Hong JT, et al. CCL2 deficient mesenchymal stem cells fail to establish long-lasting contact with T cells and no longer ameliorate lupus symptoms. Sci Rep. 2017;7:41258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Reesink HL, Sutton RM, Shurer CR, Peterson RP, Tan JS, Su J, Paszek MJ, Nixon AJ. Galectin-1 and galectin-3 expression in equine mesenchymal stromal cells (MSCs), synovial fibroblasts and chondrocytes, and the effect of inflammation on MSC motility. Stem Cell Res Ther. 2017;8(1):243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hong JW, Lim JH, Chung CJ, Kang TJ, Kim TY, Kim YS, Roh TS, Lew DH. Immune tolerance of human dental pulp-derived mesenchymal stem cells mediated by CD4+CD25+FoxP3+ regulatory T-cells and induced by TGF-β1 and IL-10. Yonsei Med J. 2017;58(5):1031–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Willis GR, Fernandez-Gonzalez A, Reis M, Mitsialis SA, Kourembanas S. Macrophage immunomodulation: the gatekeeper for mesenchymal stem cell derived-exosomes in pulmonary arterial hypertension. Int J Mol Sci. 2018;19(9):E2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nature Medicine. 2009;15(1):42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liang J, Zhang H, Kong W, Deng W, Wang D, Feng X, Zhao C, Hua B, Wang H, Sun L. Safety analysis in patients with autoimmune disease receiving allogeneic mesenchymal stem cells infusion: a long-term retrospective study. Stem Cell Res Ther. 2018;9(1):312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Daneshmandi S, Karimi MH, Pourfathollah AA. TGF-β engineered mesenchymal stem cells (TGF-β/MSCs) for treatment of Type 1 diabetes (T1D) mice model. Int Immunopharmacol. 2017;44:191–96. [DOI] [PubMed] [Google Scholar]
- 60. Llufriu S, Sepúlveda M, Blanco Y, Marín P, Moreno B, Berenguer J, Gabilondo I, Martínez-Heras E, Sola-Valls N, Arnaiz JA, Andreu EJ, et al. Randomized placebo-controlled phase II trial of autologous mesenchymal stem cells in multiple sclerosis. PLoS One. 2014;9(12):e113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Blazar BR, MacDonald K, Hill GR. Immune regulatory cell infusion for graft-versus-host disease prevention and therapy. Blood. 2018;131(24):2651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang H, Chen Z, Bie P. Bone marrow-derived mesenchymal stem cells as immunosuppressants in liver transplantation: a review of current data. Transfus Med Rev. 2012;26(2):129–41. [DOI] [PubMed] [Google Scholar]
- 63. Wang JW, Liu YB, Xu B, Li JT, Qian HR, Zhang M, Peng SY. The study on immunomodulation of donor mesenchymal stem cells on discordant liver xenotransplantation [in Chinese]. Zhonghua Wai Ke Za Zhi Zhonghua Wai Ke Za Zhi. 2005;43(19):1254–58. [PubMed] [Google Scholar]
- 64. Wan CD, Cheng R, Wang HB, Liu T. Immunomodulatory effects of mesenchymal stem cells derived from adipose tissues in a rat orthotopic liver transplantation model. Hepatobiliary Pancreat Dis Int. 2008;7(1):29–33. [PubMed] [Google Scholar]
- 65. Hong ZF, Huang XJ, Yin ZY, Zhao WX, Wang XM. Immunosuppressive function of bone marrow mesenchymal stem cells on acute rejection of liver allografts in rats. Transplant Proc. 2009;41(1):403–9. [DOI] [PubMed] [Google Scholar]
- 66. Pan MX, Hou WL, Zhang QJ, Gong DH, Cheng Y, Jian GD, Gao Y. Infusion of autologous mesenchymal stem cells prolongs the survival of dogs receiving living donor liver transplantation [in Chinese]. Nan Fang Yi Ke Da Xue Bao. 2009;29(9):1783–86. [PubMed] [Google Scholar]
- 67. Yu Y, Lu L, Qian X, Chen N, Yao A, Pu L, Zhang F, Li X, Kong L, Sun B, Wang X. Antifibrotic effect of hepatocyte growth factor-expressing mesenchymal stem cells in small-for-size liver transplant rats. Stem Cells Dev. 2010;19(6):903–14. [DOI] [PubMed] [Google Scholar]
- 68. Yu Y, Yao AH, Chen N, Pu LY, Fan Y, Lv L, Sun BC, Li GQ, Wang XH. Mesenchymal stem cells over-expressing hepatocyte growth factor improve small-for-size liver grafts regeneration. Mol Ther. 2007;15(7):1382–89. [DOI] [PubMed] [Google Scholar]
- 69. Zhu J, Chen Y. Effect of human hepatocyte growth factor gene-modified bone marrow mesenchymal stem cells on immunological rejection after allograft liver transplantation in rats [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011;25(7):871–76. [PubMed] [Google Scholar]
- 70. Sun Z, Li T, Wen H, Wang H, Ji W, Ma Y. Immunological effect induced by mesenchymal stem cells in a rat liver transplantation model. Exp Ther Med. 2015;10(2):401–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Xia X, Chen W, Ma T, Xu G, Liu H, Liang C, Bai X, Zhang Y, He Y, Liang T. Mesenchymal stem cells administered after liver transplantation prevent acute graft-versus-host disease in rats. Liver Transpl. 2012;18(6):696–706. [DOI] [PubMed] [Google Scholar]
- 72. Wang Y, Zhang A, Ye Z, Xie H, Zheng S. Bone marrow-derived mesenchymal stem cells inhibit acute rejection of rat liver allografts in association with regulatory T-cell expansion. Transplant Proc. 2009. 41(10):4352–56. [DOI] [PubMed] [Google Scholar]
- 73. Tang J, Yang R, Lv L, Yao A, Pu L, Yin A, Li X, Yu Y, Nyberg SL, Wang X. Transforming growth factor-β-expressing mesenchymal stem cells induce local tolerance in a rat liver transplantation model of acute rejection. Stem Cells. 2016;34(11):2681–92. [DOI] [PubMed] [Google Scholar]
- 74. Shen ZY, Wu B, Liu T, Yang Y, Yin ML, Zheng WP, Zhang BY, Song HL. Immunomodulatory effects of bone marrow mesenchymal stem cells overexpressing heme oxygenase-1: protective effects on acute rejection following reduced-size liver transplantation in a rat model. Cell Immunol. 2017,313:10–24. [DOI] [PubMed] [Google Scholar]
- 75. Wang R, Shen Z, Yang L, Yin M, Zheng W, Wu B, Liu T, Song H. Protective effects of heme oxygenase-1-transduced bone marrow-derived mesenchymal stem cells on reduced size liver transplantation: role of autophagy regulated by the ERK/mTOR signaling pathway. Int J Mol Med. 2017;40(5):1537–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wu B, Song HL, Yang Y, Yin ML, Zhang BY, Cao Y, Dong C, Shen ZY. Improvement of liver transplantation outcome by heme oxygenase-1-transduced bone marrow mesenchymal stem cells in rats. Stem Cells Int. 2016;2016:9235073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sasajima H, Miyagi S, Kakizaki Y, Kamei T, Unno M, Satomi S, Goto M. Cytoprotective effects of mesenchymal stem cells during liver transplantation from donors after cardiac death in rats. Transplant Proc. 2018;50(9):2815–20. [DOI] [PubMed] [Google Scholar]
- 78. Tian Y, Wang J, Wang W, Ding Y, Sun Z, Zhang Q, Wang Y, Xie H, Yan S, Zheng S. Mesenchymal stem cells improve mouse non-heart-beating liver graft survival by inhibiting Kupffer cell apoptosis via TLR4-ERK1/2-Fas/FasL-caspase3 pathway regulation. Stem Cell Res Ther. 2016;7(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang W, Du Z, Yan J, Ma D, Shi M, Zhang M, Peng C, Li H. Mesenchymal stem cells promote liver regeneration and prolong survival in small-for-size liver grafts: involvement of C-Jun N-terminal kinase, cyclin D1, and NF-κB. PLoS One. 2014;9(12):e112532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Niu J, Yue W, Song Y, Zhang Y, Qi X, Wang Z, Liu B, Shen H, Hu X. Prevention of acute liver allograft rejection by IL-10-engineered mesenchymal stem cells. Clin Exp Immunol. 2014;176(3):473–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chen KD, Goto S, Hsu LW, Lin TY, Nakano T, Lai CY, Chang YC, Weng WT, Kuo YR, Wang CC, Cheng YF, et al. Identification of miR-27b as a novel signature from the mRNA profiles of adipose-derived mesenchymal stem cells involved in the tolerogenic response. PLoS One. 2013;8(4):e60492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Du Z, Wei C, Yan J, Han B, Zhang M, Peng C, Liu Y. Mesenchymal stem cells overexpressing C-X-C chemokine receptor type 4 improve early liver regeneration of small-for-size liver grafts. Liver Transpl. 2013;19(2):215–225. [DOI] [PubMed] [Google Scholar]
- 83. Jia Z, Li F, Zeng X, Lv Y, Zhao S. The effects of local administration of mesenchymal stem cells on rat corneal allograft rejection. BMC Ophthalmol. 2018;18(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Treacy O, O’Flynn L, Ryan A. E, Morcos M, Lohan P, Schu S, Wilk M, Fahy G, Griffin MD, Nosov M, Ritter T. Mesenchymal stem cell therapy promotes corneal allograft survival in rats by local and systemic immunomodulation. Am J Transplant. 2014;14(9):2023–36. [DOI] [PubMed] [Google Scholar]
- 85. Zhang Y, Yu Z, Jiang D, Liang X, Liao S, Zhang Z, Yue W, Li X, Chiu SM, Chai YH, Liang YC, et al. iPSC-MSCs with High Intrinsic MIRO1 and Sensitivity to TNF-α Yield Efficacious Mitochondrial Transfer to Rescue Anthracycline-Induced Cardiomyopathy. Stem Cell Reports. 2016;7(4):749–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Reinders ME, de Fijter JW, Roelofs H, Bajema IM, de Vries DK, Schaapherder AF, Claas FH, van Miert PP, Roelen DL, van Kooten C, Fibbe WE, et al. Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: results of a phase I study. Stem Cells Transl Med. 2013;2(2):107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Perico N, Casiraghi F, Introna M, Gotti E, Todeschini M, Cavinato RA, Capelli C, Rambaldi A, Cassis P, Rizzo P, Cortinovis M, et al. Autologous mesenchymal stromal cells and kidney transplantation: a pilot study of safety and clinical feasibility. Clin J Am Soc Nephrol. 2011;6(2):412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Reinders ME, Dreyer GJ, Bank JR, Roelofs H, Heidt S, Roelen DL, Zandvliet ML, Huurman VA, Fibbe WE, van Kooten C, Claas FH, et al. Safety of allogeneic bone marrow derived mesenchymal stromal cell therapy in renal transplant recipients: the neptune study. J Transl Med. 2015;13:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Detry O, Vandermeulen M, Delbouille MH, Somja J, Bletard N, Briquet A, Lechanteur C, Giet O, Baudoux E, Hannon M, Baron F, et al. Infusion of mesenchymal stromal cells after deceased liver transplantation: a phase I-II, open-label, clinical study. J Hepatol. 2017;67(1):47–55. [DOI] [PubMed] [Google Scholar]
- 90. Zhang YC, Liu W, Fu BS, Wang GY, Li HB, Yi HM, Jiang N, Wang G, Zhang J, Yi SH, Li H, et al. Therapeutic potentials of umbilical cord-derived mesenchymal stromal cells for ischemic-type biliary lesions following liver transplantation. Cytotherapy. 2017;19(2):194–99. [DOI] [PubMed] [Google Scholar]
- 91. Soeder Y, Loss M, Johnson CL, Hutchinson JA, Haarer J, Ahrens N, Offner R, Deans RJ, Van Bokkelen G, Geissler EK, Schlitt HJ, et al. First-in-human case study: multipotent adult progenitor cells for immunomodulation after liver transplantation. Stem Cells Transl Med. 2015;4(8):899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Shi M, Liu Z, Wang Y, Xu R, Sun Y, Zhang M, Yu X, Wang H, Meng L, Su H, Jin L, et al. A pilot study of mesenchymal stem cell therapy for acute liver allograft rejection. Stem Cells Transl Med. 2017;6(12):2053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Blau HM, Daley GQ. Stem cells in the treatment of disease. N Engl J Med. 2019;380(18):1748–60. [DOI] [PubMed] [Google Scholar]
- 94. Lee HS, Song S, Shin DY, Kim GS, Lee JH, Cho CW, Lee KW, Park H, Ahn C, Yang J, Yang HM, et al. Enhanced effect of human mesenchymal stem cells expressing human TNF-αR-Fc and HO-1 gene on porcine islet xenotransplantation in humanized mice. Xenotransplantation. 2018;25(1): e12342. [DOI] [PubMed] [Google Scholar]
- 95. Lee SG, Moon DB, Hwang S, Ahn CS, Kim KH, Song GW, Jung DH, Ha TY, Park GC, Jung BH. Liver transplantation in Korea: past, present, and future. Transplant Proc. 2015;47(3):705–08. [DOI] [PubMed] [Google Scholar]
- 96. Levitsky J, Feng S. Tolerance in clinical liver transplantation. Hum Immunol. 2018;79(5): 283–87. [DOI] [PubMed] [Google Scholar]
- 97. Branco JC, Morbey A, Martins A, Barroso E. Tolerance after liver transplantation: where are we. Liver Transpl. 2018;24(9):1303–04. [DOI] [PubMed] [Google Scholar]
- 98. Huang H, Lu Y, Zhou T, Gu G, Xia Q. Innate immune cells in immune tolerance after liver transplantation. Front Immunol. 2018;9:2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Xia S, Guo Z, Xu X, Yi H, Wang Q, Cao X. Hepatic microenvironment programs hematopoietic progenitor differentiation into regulatory dendritic cells, maintaining liver tolerance. Blood. 2008;112(8):3175–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fahrner R, Dondorf F, Ardelt M, Settmacher U, Rauchfuss F. Role of NK, NKT cells and macrophages in liver transplantation. World J Gastroenterol 2016;22(27):6135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17(5):306–21. [DOI] [PubMed] [Google Scholar]
- 102. Callery MP, Kamei T, Flye MW. Kupffer cell blockade inhibits induction of tolerance by the portal venous route. Transplantation. 1989;47(6):1092–94. [PubMed] [Google Scholar]
- 103. Sun YY, Li XF, Meng XM, Huang C, Zhang L, Li J. Macrophage phenotype in liver injury and repair. Scand J Immunol. 2017;85(3):166–74. [DOI] [PubMed] [Google Scholar]
- 104. Chen Y, Liu Z, Liang S, Luan X, Long F, Chen J, Peng Y, Yan L, Gong J. Role of Kupffer cells in the induction of tolerance of orthotopic liver transplantation in rats. Liver Transpl. 2008;14(6):823–36. [DOI] [PubMed] [Google Scholar]
- 105. Zhou HP, Yi DH, Yu SQ, Sun GC, Cui Q, Zhu HL, Liu JC, Zhang JZ, Wu TJ. Administration of donor-derived mesenchymal stem cells can prolong the survival of rat cardiac allograft. Transplant Proc. 2006;38(9):3046–51. [DOI] [PubMed] [Google Scholar]
- 106. Sun C, Zhang J, Yin DL, Li K, Wang Q, Xie YH, Li W. Role of bone marrow mesenchymal stem cells with CTLA4Ig and CD40LIg gene modification in rejection reaction after liver transplantation [in Chinese]. Zhonghua Gan Zang Bing Za Zhi. 2018;26(1):54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mäkelä T, Takalo R, Arvola O, Haapanen H, Yannopoulos F, Blanco R, Ahvenjärvi L, Kiviluoma K, Kerkelä E, Nystedt J, Juvonen T, et al. Safety and biodistribution study of bone marrow-derived mesenchymal stromal cells and mononuclear cells and the impact of the administration route in an intact porcine model. Cytotherapy. 2015;17(4):392–402. [DOI] [PubMed] [Google Scholar]
- 108. Braid LR, Wood CA, Wiese DM, Ford BN. Intramuscular administration potentiates extended dwell time of mesenchymal stromal cells compared to other routes. Cytotherapy. 2018;20(2):232–44. [DOI] [PubMed] [Google Scholar]
- 109. Park WS, Ahn SY, Sung SI, Ahn JY, Chang YS. Strategies to enhance paracrine potency of transplanted mesenchymal stem cells in intractable neonatal disorders. Pediatr Res. 2018;83(1–2):214–22. [DOI] [PubMed] [Google Scholar]
- 110. Franquesa M, Hoogduijn MJ, Reinders ME, Eggenhofer E, Engela AU, Mensah FK, Torras J, Pileggi A, van Kooten C, Mahon B, Detry O, et al. Mesenchymal stem cells in solid organ transplantation (MiSOT) fourth meeting: lessons learned from first clinical trials. Transplantation. 2013;96(3):234–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Obermajer N, Popp FC, Johnson CL, Benseler V, Dahlke MH. Rationale and prospects of mesenchymal stem cell therapy for liver transplantation. Curr Opin Organ Transplant. 2014;19:60–4. [DOI] [PubMed] [Google Scholar]
- 112. Saidi RF, Rajeshkumar B, Shariftabrizi A, Bogdanov AA, Zheng S, Dresser K, Walter O. Human adipose-derived mesenchymal stem cells attenuate liver ischemia-reperfusion injury and promote liver regeneration. Surgery. 2014;156(5):1225–31. [DOI] [PubMed] [Google Scholar]
- 113. Bai M, Zhang L, Fu B, Bai J, Zhang Y, Cai G, Bai X, Feng Z, Sun S, Chen X. IL-17A improves the efficacy of mesenchymal stem cells in ischemic-reperfusion renal injury by increasing Treg percentages by the COX-2/PGE2 pathway. Kidney Int. 2018;93(4):814–25. [DOI] [PubMed] [Google Scholar]
- 114. Zheng J, Li H, He L, Huang Y, Cai J, Chen L, Zhou C, Fu H, Lu T, Zhang Y, Yao J, Yang Y, Yang Y. Preconditioning of umbilical cord-derived mesenchymal stem cells by rapamycin increases cell migration and ameliorates liver ischaemia/reperfusion injury in mice via the CXCR4/CXCL12 axis. Cell Prolif. 2019;52(2):e12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Bronckaers A, Hilkens P, Martens W, Gervois P, Ratajczak J, Struys T, Lambrichts I. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol Ther. 2014;143(2):181–96. [DOI] [PubMed] [Google Scholar]
- 116. Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–41. [DOI] [PubMed] [Google Scholar]
- 117. Popp FC, Fillenberg B, Eggenhofer E, Renner P, Dillmann J, Benseler V, Schnitzbauer AA, Hutchinson J, Deans R, Ladenheim D, Graveen CA, et al. Safety and feasibility of third-party multipotent adult progenitor cells for immunomodulation therapy after liver transplantation--a phase I study (MISOT-I). J Transl Med. 2011;9:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hartleif S, Schumm M, Döring M, Mezger M, Lang P, Dahlke MH, Riethmüller J, Königsrainer A, Handgretinger R, Nadalin S, Sturm E. Safety and tolerance of donor-derived mesenchymal stem cells in pediatric living-donor liver transplantation: the MYSTEP1 study. Stem Cells Int. 2017;2017:2352954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Sun Q, Huang Z, Han F, Zhao M, Cao R, Zhao D, Hong L, Na N, Li H, Miao B, Hu J. Allogeneic mesenchymal stem cells as induction therapy are safe and feasible in renal allografts: pilot results of a multicenter randomized controlled trial. J Transl Med. 2018;16(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, Sun X, Chen J, Yang S, Cai J, Gao X, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA. 2012;307(11):1169–77. [DOI] [PubMed] [Google Scholar]
- 121. Charlton M, Levitsky J, Aqel B, O'Grady J, Hemibach J, Rinella M, Fung J, Ghabril M, Thomason R, Burra P, Little EC, et al. International liver transplantation society consensus statement on immunosuppression in liver transplant recipients. Transplantation. 2018;102(5):727–43. [DOI] [PubMed] [Google Scholar]
- 122. Hoogduijn MJ, Crop MJ, Korevaar SS, Peeters AM, Eijken M, Maat LP, Balk AH, Weimar W, Baan CC. Susceptibility of human mesenchymal stem cells to tacrolimus, mycophenolic acid, and rapamycin. Transplantation. 2008;86(9):1283–91. [DOI] [PubMed] [Google Scholar]
- 123. Sir G, Goker Bagca B, Yigitturk G, Cavusoglu T, Biray Avci C, Gunduz C, Uyanikgil Y. Antagonistic effect of oxytocin and tacrolimus combination on adipose tissue - derived mesenchymal stem cells: antagonistic effect of oxytocin and tacrolimus. Biomed Pharmacother. 2018;97:1173–81. [DOI] [PubMed] [Google Scholar]
- 124. Kuo YR, Chen CC, Chen YC, Chien CM. Recipient adipose-derived stem cells enhance recipient cell engraftment and prolong allotransplant survival in a miniature swine hind-limb model. Cell Transplant. 2017;26(8):1418–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ikeguchi R, Kakinoki R, Ohta S, Oda H, Yurie H, Kaizawa Y, Mitsui H, Aoyama T, Toguchida J, Matsuda S. Recipient bone marrow-derived stromal cells prolong graft survival in a rat hind limb allotransplantation model. Microsurgery. 2017;37(6):632–40. [DOI] [PubMed] [Google Scholar]
- 126. Pan GH, Chen Z, Xu L, Zhu JH, Xiang P, Ma JJ, Peng YW, Li GH, Chen XY, Fang JL, Guo YH, et al. Low-dose tacrolimus combined with donor-derived mesenchymal stem cells after renal transplantation: a prospective, non-randomized study. Oncotarget. 2016;7(11):12089–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Poncelet AJ, Nizet Y, Vercruysse J, Hiel AL, Saliez A, Gianello P. Inhibition of humoral response to allogeneic porcine mesenchymal stem cell with 12 days of tacrolimus. Transplantation. 2008;86(11):1586–95. [DOI] [PubMed] [Google Scholar]
- 128. Buron F, Perrin H, Malcus C, Héquet O, Thaunat O, Kholopp-Sarda MN, Moulin FT, Morelon E. Human mesenchymal stem cells and immunosuppressive drug interactions in allogeneic responses: an in vitro study using human cells. Transplant Proc. 2009;41(8):3347–52. [DOI] [PubMed] [Google Scholar]
- 129. Maccario R, Moretta A, Cometa A, Montagna D, Comoli P, Locatelli F, Podestà M, Frassoni F. Human mesenchymal stem cells and cyclosporin a exert a synergistic suppressive effect on in vitro activation of alloantigen-specific cytotoxic lymphocytes. Biol Blood Marrow Transplant. 2005;11(12):1031–32. [DOI] [PubMed] [Google Scholar]
- 130. Eggenhofer E, Renner P, Soeder Y, Popp FC, Hoogduijn MJ, Geissler EK, Schlitt HJ, Dahlke MH. Features of synergism between mesenchymal stem cells and immunosuppressive drugs in a murine heart transplantation model. Transpl Immunol. 2011;25(2-3):141–47. [DOI] [PubMed] [Google Scholar]
- 131. Lee HK, Kim KH, Kim HS, Kim JS, Lee JH, Ji A, Kim KS, Lee TY, Chang IY, Bae SC, Hong JT, et al. Effect of a combination of prednisone or mycophenolate mofetil and mesenchymal stem cells on lupus symptoms in MRL.Faslpr mice. Stem Cells Int. 2018;2018:4273107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Duan W, Yu X, Ma D, Yang B, Li Y, Huang L, Liu L, Chen G, Xu D, Ding Y. Mesenchymal stem cells in combination with low-dose rapamycin significantly prolong islet allograft survival through induction of regulatory T cells. Biochem Biophys Res Commun. 2018;506(3):619–25. [DOI] [PubMed] [Google Scholar]
- 133. Ezquer F, Bahamonde J, Huang YL, Ezquer M. Administration of multipotent mesenchymal stromal cells restores liver regeneration and improves liver function in obese mice with hepatic steatosis after partial hepatectomy. Stem Cell Res Ther. 2017;8(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lanthier N, Lin-Marq N, Rubbia-Brandt L, Clément S, Goossens N, Spahr L. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: phase 2 trial. Hepatology. 2016;64(6):2185–97. [DOI] [PubMed] [Google Scholar]
- 135. Xu L, Wang S, Sui X, Wang Y, Su Y, Huang L, Zhang Y, Chen Z, Chen Q, Du H, Zhang Y. Mesenchymal stem cell-seeded regenerated silk fibroin complex matrices for liver regeneration in an animal model of acute liver failure. ACS Appl Mater Interfaces. 2017;9(17):14716–23. [DOI] [PubMed] [Google Scholar]
- 136. Jang YO, Kim SH, Cho MY, Kim KS, Park KS, Cha SK, Kim MY, Chang SJ, Baik SK. Synergistic effects of simvastatin and bone marrow-derived mesenchymal stem cells on hepatic fibrosis. Biochem Biophys Res Commun. 2018;497(1):264–71. [DOI] [PubMed] [Google Scholar]
- 137. Zhang GZ, Sun HC, Zheng LB, Guo JB, Zhang XL. In vivo hepatic differentiation potential of human umbilical cord-derived mesenchymal stem cells: therapeutic effect on liver fibrosis/cirrhosis. World J Gastroenterol. 2017;23(46):8152–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Fiore EJ, Bayo JM, Garcia MG, Malvicini M, Lloyd R, Piccioni F, Rizzo M, Peixoto E, Sola MB, Atorrasagasti C, Alaniz L, et al. Mesenchymal stromal cells engineered to produce IGF-I by recombinant adenovirus ameliorate liver fibrosis in mice. Stem Cells Dev. 2015;24(6):791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Jiang YC, Wang XF, Xu YY, Qiao YH, Guo X, Wang DF, Li Q, Turng LS. Polycaprolactone nanofibers containing vascular endothelial growth factor-encapsulated gelatin particles enhance mesenchymal stem cell differentiation and angiogenesis of endothelial cells. Biomacromolecules. 2018;19(9):3747–53. [DOI] [PubMed] [Google Scholar]
- 140. Kuchroo P, Dave V, Vijayan A, Viswanathan C, Ghosh D. Paracrine factors secreted by umbilical cord-derived mesenchymal stem cells induce angiogenesis in vitro by a VEGF-independent pathway. Stem Cells Dev. 2015;24(4):437–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Park YS, Hwang S, Jin YM, Yu Y, Jung SA, Jung SC, Ryu KH, Kim HS, Jo I. CCN1 secreted by tonsil-derived mesenchymal stem cells promotes endothelial cell angiogenesis via integrin αv β3 and AMPK. J Cell Physiol. 2015;230(1):140–49. [DOI] [PubMed] [Google Scholar]
- 142. Boomsma RA, Geenen DL. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One. 2012;7(4):e35685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Zagoura D, Trohatou O, Makridakis M, Kollia A, Kokla N, Mokou M, Psaraki A, Eliopoulos AG, Vlahou A, Roubelakis MG. Functional secretome analysis reveals Annexin-A1 as important paracrine factor derived from fetal mesenchymal stem cells in hepatic regeneration. EBioMedicine. 2019;45:542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Guo Y, Chen B, Chen LJ, Zhang CF, Xiang C. Current status and future prospects of mesenchymal stem cell therapy for liver fibrosis. J Zhejiang Univ Sci B. 2016;17(11):831–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Barile L, Vassalli G. Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63–78. [DOI] [PubMed] [Google Scholar]
- 146. Lai P, Chen X, Guo L, Wang Y, Liu X, Liu Y, Zhou T, Huang T, Geng S, Luo C, Huang X, et al. A potent immunomodulatory role of exosomes derived from mesenchymal stromal cells in preventing cGVHD. J Hematol Oncol. 2018;11(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Jiang W, Tan Y, Cai M, Zhao T, Mao F, Zhang X, Xu W, Yan Z, Qian H, Yan Y. Human umbilical cord MSC-derived exosomes suppress the development of CCl4-induced liver injury through antioxidant effect. Stem Cells Int. 2018;2018:6079642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Huang JH, Yin XM, Xu Y, Xu CC, Lin X, Ye FB, Cao Y, Lin FY. Systemic administration of exosomes released from mesenchymal stromal cells attenuates apoptosis, inflammation, and promotes angiogenesis after spinal cord injury in rats. J Neurotrauma. 2017;34(24):3388–96. [DOI] [PubMed] [Google Scholar]
- 149. Chinnici CM, Pietrosi G, Iannolo G, Amico G, Cuscino N, Pagano V, Conaldi PG. Mesenchymal stromal cells isolated from human fetal liver release soluble factors with a potential role in liver tissue repair. Differentiation. 2019;105:14–26. [DOI] [PubMed] [Google Scholar]
- 150. Lou G, Song X, Yang F, Wu S, Wang J, Chen Z, Liu Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015;8:122. [DOI] [PMC free article] [PubMed] [Google Scholar]