Abstract
Myosteatosis refers to fat deposition within muscle and is linked to risk of cardiovascular disease and metabolic disorders. Though these comorbidities are common during and after therapy for acute lymphoblastic leukemia (ALL), little is known about tissue distribution, including myosteatosis, in this population. Using quantitative computed tomography, we assessed the impact of ALL therapy on bone, muscle, subcutaneous and muscle-associated (MA) fat in 12 adolescents and young adults (AYA) treated for ALL as compared to a healthy control group without ALL (n=116). AYA had a marked loss of muscle with a gain in MA fat between ALL diagnosis and end of induction. These changes persisted throughout intensive therapy. Lower bone and muscle and higher MA fat were also observed during and after treatment in comparison to controls. Altered lower extremity tissue distribution, specifically myosteatosis and sarcopenia, may contribute to functional declines and increased risk of metabolic disorders and cardiovascular diseases.
Keywords: adipose tissue, sarcopenia, pediatric leukemia, intermuscular fat, intramuscular fat
Introduction
In the United States more than 3,000 children and adolescents are diagnosed with acute lymphoblastic leukemia (ALL) each year [1]. Childhood leukemia therapy confers increased risk for obesity, sarcopenia, osteopenia, fractures, loss of lower extremity strength and sensation and impaired fitness [2, 3, 4]. We and others have demonstrated that adolescents and young adults (AYA) receiving treatment for ALL typically gain body fat and lose muscle mass during therapy [4, 5]. Late in therapy and in survivors of childhood ALL, changes in body composition develop concurrently with high rates of cardiovascular disease, dyslipidemia, metabolic disorders and diabetes [6, 7, 8, 9]. These risks are disproportionately present in AYA patients undergoing ALL therapy [6].
Despite the relatively high prevalence of these acute and long-term endocrine toxicities for AYA ALL patients, much of the specific pathophysiology connecting ALL therapy-associated changes in body composition to systemic and metabolic abnormalities remains unknown. Myosteatosis is a recently reported occurrence describing the presence of adipose tissue within skeletal muscle [10]. In other populations, myosteatosis is independently associated with insulin resistance, unfavorable lipid profiles, increased fracture risk and reduced strength [11, 12, 13, 14]. Myosteatosis has also been associated with lower skeletal muscle mass [15] and results in distinct metabolic and catabolic profiles compared to sarcopenia alone [16]. Research has previously shown through dual energy x-ray absorptiometry (DXA) and quantitative computed tomography (QCT) that patients treated for ALL suffer from sarcopenic obesity – loss of lean muscle and gain in total body fat – beginning within the first month of therapy (“induction”) [3, 4, 5, 17]. Additionally, this loss of muscle mass coupled with chemotherapy induced peripheral neuropathy and the associated reduced strength contribute to functional declines with consequences on quality of life [18, 19]. While AYA with ALL are at high risk for sarcopenic obesity and metabolic toxicities, the prevalence of myosteatosis during ALL therapy is entirely unknown. The purpose of this study was to more precisely define the distribution of adiposity gained during therapy to explore the presence, degree and timing of onset for ALL therapy-associated myosteatosis and sarcopenia in the lower extremities.
Materials and Methods
Study Population
Pre-adolescents, AYA adults 10-21 years of age newly diagnosed with National Cancer Institute/Rome High-Risk B-ALL (HR B-ALL) or T-cell ALL between 2011-2014 were enrolled in a prospective study of osteotoxicity and body composition [20]. Participants enrolled prior to May 2012 underwent serial imaging of the tibia by QCT and were potentially included in this analysis. Subsequent patients received limited imaging of the femur only due to a change in protocol/scanner precluding inclusion in this analysis [20]. All patients were treated following contemporary Children’s Oncology Group (COG) protocols. Induction therapy consisted of a “four-drug” regimen with vincristine, pegylated L-asparaginase, an anthracycline, and a glucocorticoid (either prednisone 60mg/m2/day for 28 days or dexamethasone 10mg/m2/day for 14 days). During the study period, therapy for both B-ALL and T-ALL at our institution followed the COG ALL HR-ALL regimen [21]. Demographic and baseline information included age, sex, self-reported ethnicity (Hispanic/non-Hispanic), height, weight, calculated Body Mass Index (BMI) and age-sex normalized BMI percentile (BMI%) using data from the Centers for Disease Control and Prevention [22]. BMI% was then dichotomized as overweight or obese (BMI%≥85) or normal/underweight (BMI%<85) and age as 15 and older or younger than 15 years. An external control group was identified from otherwise healthy youth ages 6.0-16.9 years who separately enrolled in a prospective bone and ambulation trial at our institution between 2010-2016 [23]. Eligibility criteria for this cohort included no chronic disease, no steroid exposure and no medications affecting growth and development. The subset of these subjects who were older than 10.0 years were used as the external control group in the current study to match the age range of the ALL study sample. All research studies were approved by the Institutional Review Board; the clinical trial in ALL was registered prior to enrolling the first patient (NCT01317940). Written informed consent and assent were obtained and documented for all participants.
Imaging and Analysis Protocols
Participants in both the ALL and control cohorts were imaged using identical protocols for whole-body DXA and three-dimensional QCT of the tibia, as described previously [3, 5, 23, 24, 25]. Participants in the ALL cohort underwent imaging within 96 hours from start of chemotherapy, again 28-35 days later (end of induction phase) and 7-9 months from diagnosis following completion of intensive chemotherapy (end of the “Delayed Intensification” phase) [5]. In summary, total body fat mass and percentage (BF%) were obtained using a fan-beam DXA densitometer, and QCT was obtained using contiguous 1-mm slices covering the entire tibia. To minimize operator-induced intra-patient (for serial imaging) and inter-patient variability in radiographic assessment, all imaging, DXA and QCT, was performed primarily by the same certified radiology technician with the same scanners (DXA: Delphi W, Hologic, Inc, Waltham, MA; QCT: Philips Gemini GXL, Philips Medical Systems Inc., Cleveland, OH) and QCT mineral reference phantom (Mindways Model 3 CT Calibration Phantom, Mindways Software, Inc., Austin, TX).
Tissue volumes for adipose, muscle and bone were computed along the entire length of both tibias using a custom MATLAB script (MathWorks, Inc., Natick, MA) [25]. Muscle-associated (MA) fat as assessed by QCT was utilized as a non-invasive surrogate marker for myosteatosis. The tibia was defined as extending from the proximal surface of the intercondylar eminence to the distal surface of the medial malleolus. Tissue volumes were quantified by a semi-automated, threshold-based method using previously validated attenuation ranges of [−190, −30], [−29, 150] and [151, 1000] Hounsfield units (HU) for adipose tissue, muscle and bone, respectively [26]. The boundary of the crural fascia was determined using Sobel operator based edge detection [27]. Subcutaneous (SC) fat tissue was defined as the adipose tissue found between the skin and the deep (crural) fascia [28]; MA fat tissue was then defined as total fat minus SC fat. Thus, MA fat included adipose within the crural fascia as well as that between muscles and between muscle and bone [28]. Bone marrow was masked and excluded, while all other tissues (skin, blood vessels, etcetera) have attenuations similar to muscle and adipose tissue and were therefore categorized into these two tissue types [29]. Tissue volumes for each leg were quantified separately with subsequent analysis using the sum of both legs for each participant.
Statistical Analyses
Dichotomous variables (ethnicity and sex) were compared between the ALL cohort at diagnosis and the external control cohort using chi square tests whereas continuous variables were compared using t-tests. Differences between the end of delayed intensification and the diagnosis and end of induction time-points in MA fat and MA fat % were calculated. Relationships between DXA fat measures (total body fat, total body fat percent) and QCT fat measures (MA fat and MA fat percent) were evaluated using correlations. Differences between groups (each ALL cohort visit and external control group) and among visits (within the ALL cohort) were assessed using a mixed model analysis of variance (ANOVA), with participant as a random effect and visit as a fixed effect to account for multiple visits within the ALL cohort. Pairwise comparison of means was then performed to examine pre-specified comparisons (each ALL cohort visit versus external control; each ALL visit compared to the others). Outcomes of interest were total body (DXA) and tissue volumes from the lower leg (QCT): bone, muscle, total adipose, SC adipose and MA adipose tissue volumes, along with each tissue volume expressed as a proportion of total volume. Both volume and proportion were assessed to provide insight into overall and relative differences. Effects of age group, sex and BMI percentile group on increase of MA fat and MA fat % across visits were evaluated using Mann-Whitney U tests. All statistical analyses were performed in STATA 14 (Stata-Corp, College Station, TX).
Results
Participant Characteristics and Baseline Comparisons
Of the 18 potential participants with ALL and QCT imaging of the tibia, 12 had sufficient serial imaging data for all three time points to be included in the current analysis. Two of 18 were excluded due to movement artifact during 1 scan, and 4 were excluded as they were only seen for the first 2 visits. Of 179 participants in the original external healthy control cohort, data from 116 participants were included in this study to match the ALL cohort; 61 were excluded as they were under 10 years of age, and 2 were excluded due to movement artifact. As described in Table 1, nearly all patients in the ALL-exposed group self-identified as being of Hispanic ethnicity (92%) as compared to a lower percentage in the external control group (60%); the latter reflects our expected institutional demographics. As anticipated, the cohorts were otherwise very similar at diagnosis with no significant differences in age, sex, BMI or BMI percentile.
Table 1.
Demographics and anthropometrics
| ALL Diagnosis (n=12) | Controls (n=116) | p-value | |
|---|---|---|---|
| Age (years) | 14.4 ± 2.8 (10.8, 18.8) | 13.6 ± 2.0 (10.0, 17.0) | 0.22 |
| Sex (male) | 5 (42%) | 62 (54%) | 0.55 |
| Hispanic | 11 (92%) | 70 (60%) | 0.06 |
| Height (cm) | 158.8 ± 15.5 (128.0, 187.0) | 159.0 ± 11.1 (132.3, 184.5) | 0.96 |
| Weight (kg) | 52.4 ± 16.9 (23.7, 84.1) | 55.5 ± 14.4 (25.4, 104.1) | 0.49 |
| BMI (kg/m2) | 20.2 ± 4.0 (13.6, 26.3) | 21.8 ± 4.7 (14.4, 38.4) | 0.28 |
| BMI Percentile | 51.2 ± 37.2 (0.3, 93.3) | 61.5 ± 31.3 (1.1, 99.4) | 0.29 |
| Obesity Classifications | 0.81 | ||
| Obese/ Overweight | 4 (33%) | 42 (37%) | |
| Normal/ Under | 8 (66%) | 74 (63%) | |
All values presented at mean ± standard deviation (minimum value, maximum value).
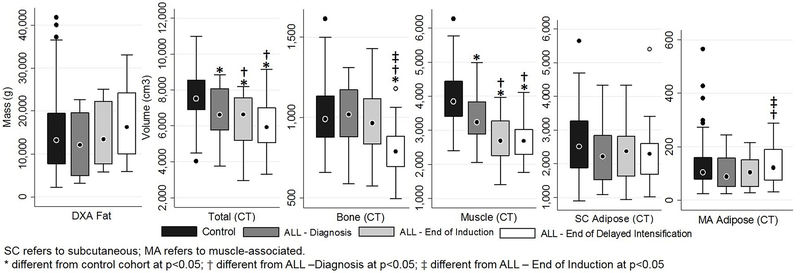
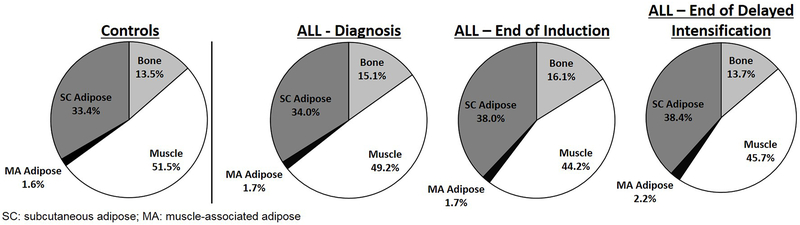
At the time of diagnosis, DXA-assessed total body fat mass and total body fat percent did not differ significantly between the ALL cohort and the control group (Tables 2 and 3). Additionally, at baseline, no significant differences in lower leg bone, total adipose, SC or MA fat volumes were observed between the ALL cohort and the control group; however, muscle (p=0.009) and total tissue (p=0.03) volumes were already significantly lower than the controls in the ALL group at diagnosis (Table 2; Figure 1). Proportionally, no differences were seen at baseline with the exception of bone, which was actually higher in the ALL group at diagnosis compared to controls (p=0.008) (Table 3; Figure 2).
Table 2.
Tissue volumes for ALL cohort visits and controls.
| ALL Diagnosis (n=12) | ALL End of Induction (n=12) | ALL End of Delayed Intensification (n=12) | Controls (n=116) | |
|---|---|---|---|---|
| DXA | ||||
| Total body fat (g) | 12530.1 ± 7550.1 (3152.0, 2270.0) | 14770.9 ± 7383.2† (5752.0, 25129.8) | 17727.3 ± 9385.0† ‡ (5877.1, 33120.9) | 14651.5 ± 8581.6 (2235.7, 41865.8) |
| CT | ||||
| Total (cm3) | 6744.7 ± 1545.0* (3757.7, 8859.6) | 6182.9 ± 1605.6* † (2946.2, 8184.7) | 6043.8 ± 1642.9* † (3319.3, 9153.2) | 7662.2 ± 1338.0 (4032.2, 10978.1) |
| Bone (cm3) | 1011.2 ± 210.2 (588.4, 1311.1) | 983.6 ± 242.9 (574.3, 1430.4) | 800.7 ± 193.3* † ‡ (496.0, 1181.5) | 1019.8 ± 194.0 (658.1, 1615.7) |
| Muscle (cm3) | 3307.3 ± 813.6* (2053.0, 4988.5) | 2730.0 ± 746.0* † (1405.9, 3959.2) | 2707.1 ± 639.6* † (1766.3, 4108.1) | 3918.9 ± 770.1 (2404.2, 6278.0) |
| Total Adipose (cm3) | 2426.4 ± 984.8 (1116.4, 4489.4) | 2469.0 ± 997.6 (966.1, 4488.4) | 2536.0 ± 1213.1 (1057.0, 5686.8) | 2723.5 ± 955.4 (928.1, 5894.2) |
| SC Adipose (cm3) | 2318.2 ± 936.8 (1091.2, 4325.2) | 2364.6 ± 948.8 (938.1, 4330.2) | 2397.8 ± 1139.8 (1025.6, 5406.8) | 2594.1 ± 895.7 (903.5, 5647.0) |
| MA Adipose (cm3) | 108.2 ± 69.3 (25.1, 243.7) | 104.4 ± 60.7 (28.0, 215.6) | 138.2 ± 85.9† ‡ (31.4, 288.8) | 129.3 ± 84.0 (24.7, 564.9) |
All values presented at mean ± standard deviation (minimum value, maximum value). SC refers to subcutaneous; MA refers to muscle-associated.
different from control cohort at p<0.05
different from ALL V1 at p<0.05
different from ALL V2 at p<0.05
Table 3.
Tissue proportions for ALL cohort visits and controls.
| ALL Diagnosis (n=12) | ALL End of Induction (n=12) | ALL End of Delayed Intensification (n=12) | Controls (n=116) | |
|---|---|---|---|---|
| DXA | ||||
| Total body fat % | 25.5 ± 10.4 (8.7, 40.0) | 32.6 ± 8.8† ‡ (19.2, 44.9) | 36.2 ± 8.5* † ‡ (20.0, 48.3) | 28.1 ± 10.6 (8.0, 49.3) |
| CT | ||||
| Bone (%) | 15.2 ± 2.1* (11.6, 19.8) | 16.2 ± 2.4* † (12.1, 19.7) | 13.6 ± 2.9† ‡ (8.8, 20.0) | 13.5 ± 2.1 (8.2, 19.0) |
| Muscle (%) | 49.5 ± 7.6 (36.3, 58.9) | 44.6 ± 6.9* † (30.4, 53.0) | 45.8 ± 7.0* † (29.1, 53.2) | 51.5 ± 7.0 (35.9, 67.2) |
| Total Adipose (%) | 35.3 ± 9.0 (21.4, 52.1) | 39.2 ± 8.9† (29.1, 57.4) | 40.5 ± 9.0* † (30.6, 62.1) | 35.1 ± 8.7 (16.2, 55.8) |
| SC Adipose (%) | 33.8 ± 8.8 (20.0, 50.2) | 37.6 ± 8.6† (27.1, 55.4) | 38.4 ± 8.6* † (28.3, 59.1) | 33.4 ± 8.4 (15.1, 53.5) |
| MA Adipose (%) | 1.5 ± 0.7 (0.60, 2.8) | 1.6 ± 0.65 (0.60, 2.9) | 2.1 ± 0.85* † ‡ (0.95, 3.9) | 1.6 ± 0.74 (0.61, 5.1) |
All values presented at mean ± standard deviation (minimum value, maximum value). SC refers to subcutaneous; MA refers to muscle-associated.
different from control cohort at p<0.05
different from ALL V1 at p<0.05
different from ALL V2 at p<0.05
Figure 1.
Whole-body DXA tissue mass and CT tissue volume in the lower legs of youth with ALL and a control comparison group.
Figure 2.
Tissue volume proportions in the lower legs of youth with ALL and a control comparison group.
Longitudinal evaluation of tissue composition during ALL therapy
During intensive pre-maintenance ALL therapy, pairwise comparisons show total tissue volume in the lower legs significantly decreased over time (p≤0.004) primarily due to early loss of muscle during induction (p<0.0001) and later loss of bone during delayed intensification (p<0.0001) (Tables 2–3; Figures 1–2). As is reflected in the parent study population [5], total body fat mass and percent increased throughout therapy in this sub-group, changing significantly from diagnosis to end of induction to end of delayed intensification (p≤0.02) (Tables 2 and 3). However, SC and total adipose volume in the legs did not significantly change during therapy and did not differ from controls at any visit; only MA adipose volume increased, primarily during the delayed intensification period (p=0.001). When evaluated as a proportion, total and SC adipose increased by the end of induction (p<0.0001) and were higher than controls at the end of delayed intensification (p=0.04) likely due to the decreased muscle and bone volume. The percent MA adipose also increased during delayed intensification due to both the decreased muscle and bone volume and the increased volume of MA fat (p=0.02).
The increases in MA fat volume and percent did not correlate significantly with the corresponding changes in total body fat (volume/mass: r=0.30, p=0.35; percent: r=−0.22, p=0.49). Age (under or over 15 years), sex and BMI category (overweight/obese vs. normal/underweight) did not affect MA fat volume (p≥0.23) or percent (p≥0.13). Though direct influence of ethnicity could not be evaluated due to the high prevalence of Hispanic ethnicity in the ALL cohort, a sub analysis with only the Hispanic participants in both groups yielded the same findings.
Discussion
This is the first study, to our knowledge, to report the early development of myosteatosis in AYA undergoing treatment for ALL. This early presence of even mild myosteatosis during pre-maintenance chemotherapy is concerning. These changes in overall body composition will likely be exacerbated by the years of steroid pulses, maintenance therapy and lower activity levels that follow [30, 31]. In this AYA cohort, the MA fat increased throughout therapy and the proportion of MA fat by the end of delayed intensification was significantly greater than that found in the healthy control group. The observed increases in MA fat were further exacerbated by concurrent muscle and bone loss in the lower extremities.
A higher risk of metabolic disorders, cardiovascular disease and fractures has been demonstrated in patients and survivors of ALL [6, 32, 33] along with the presence of sarcopenia during and after treatment [4, 17, 34]. In our cohort, a marked increase in MA fat with corresponding decreases in muscle and bone were observed during chemotherapy. Sarcopenia and myosteatosis are linked to adverse health outcomes, such as reduced insulin sensitivity, increased fracture risk and cardiovascular disease in several different populations; therefore, it is reasonable to infer that the altered lower extremity composition observed in this study may contribute to the increased risk and occurrence of metabolic disorders, cardiovascular disease and fractures previously observed in this population [11, 12, 35, 36, 37]. This sarcopenia and myosteatosis, which predominantly occur during treatment but do not decline once treatment is complete, may contribute to reduced quality of life and other comorbidities [38, 39]. Future research should explore the effects of myosteatosis during ALL therapy as well as the longer-term presence and consequences of this physiological occurrence. As this phenomenon becomes better understood, in those at risk or with metabolic comorbidities, it may be beneficial to include measurement of lean tissue in the lower extremities in addition to obesity measures.
Both muscle mass and strength have been shown to decline following ALL diagnosis and during chemotherapy treatment, while muscle quality has not been directly studied [2, 34]. This study highlights the presence of sarcopenia and myosteatosis in youth who undergo treatment for ALL. The loss of muscle volume in the lower leg occurs predominantly during treatment; whereas, similar to findings in previous work, loss of bone is slower, manifesting by the end of delayed intensification [3]. Patients in this study saw, on average, a 4%, and as much as a 10%, loss of muscle mass in the lower legs. This is comparable to what is lost in a 10 year period as an age-related decline in older adults [40]. Muscle loss, specifically in the lower legs, may help explain some functional declines seen during and post-treatment for ALL such as walking difficulties. Lack of strength and muscle mass, potentially from chemotherapy induced peripheral neuropathy, inactivity and increased myosteatosis, likely contribute to functional declines such as reduced push off power, slower speed and atypical timing of muscle co-activation in the calf during gait in youth with ALL [18]. Implementation of physical therapy and activity interventions early on can be important for sustaining and regaining function for activities of daily living, walking and overall quality of life [1, 41].
The development of both myosteatosis and sarcopenia in our cohort of AYA ALL may have significance beyond functional deficits alone. While sarcopenic obesity is related to poorer quality of life in survivors of childhood ALL, [34], sarcopenia during ALL therapy is also associated with a higher rate of severe adverse events during the critical induction therapy phase [4]. In addition, several studies relate sarcopenia and myosteatosis to poorer outcomes in adults with various cancers and even other diseases. Specifically, in colorectal cancer survivors, both sarcopenia and myosteatosis were found to be independent predictors of survival [42]; similar findings are described in gastrointestinal, lung and pancreatic cancer patients [43, 44, 45]. Future research is necessary to explore the broader implications of myosteatosis in AYA undergoing treatment for ALL as well as its impact on metabolic dysregulation and functional deficits in survivors; inclusion of functional and quality of life related outcomes will be essential components of future research contextualizing the effects of ALL therapy on myosteatosis and the long term implications. Furthermore, because this study is novel in assessment of therapy requiring steroids and myosteatosis and is specific to the ALL population, we do not know if the findings are unique to ALL. Other populations using steroids as part of medical therapy should also be evaluated for myosteatosis.
As a secondary analysis of two prospective cohorts, the study has limitations. First, only a small sample of AYA ALL patients with complete imaging was available. As the prospective body composition trial focused on intensive chemotherapy phases only, follow-up was relatively short and less than 1 year from diagnosis. Therefore, the contribution of the continued changes in body composition from ALL maintenance therapy could not be assessed in this pilot cohort. However, it is well documented that sarcopenia, reduced strength, increased fracture rate, increased risk of cardiovascular disease and metabolic disorders persist in survivors of ALL [46]. While the findings were significant and compelling, replication is therefore necessary in a larger, prospective cohort focused on myosteatosis both during the intensive chemotherapy captured here as well as later during maintenance therapy. Additionally, both groups, especially the ALL cohort, were predominantly Hispanic, which should be considered when interpreting study results. There is a higher prevalence of ALL in the Hispanic pediatric population compared to other ethnicities and a greater degree of neurotoxicity in Hispanic people treated for ALL making this subset of the ALL population important to study [47, 48]. However, we cannot ascertain if these results are generalizable to other ethnicities until larger studies with greater representation of other ethnicities are performed. It is also important to acknowledge that MA fat as measured by QCT is a non-invasive, surrogate marker for myosteatosis, not a direct measure of myolipids. This imaging surrogate is limited by QCT resolution to precisely detect edges of the different tissue types and visualization of the fascia and dermis. However, these limitations are balanced by the feasibility of an accurate imaging surrogate for myosteatosis, the incorporation of a large, healthy AYA control for comparison and the use of identical QCT and DXA protocols, equipment, and technicians in both cohorts to mitigate confounding from technical variability.
Through the opportunity afforded by concurrent imaging with QCT and DXA, we present a new hypothesis-generating finding of the early development of therapy-associated myosteatosis in AYA patients treated for ALL. Coupled with the well-characterized risk of cardiovascular and metabolic disorder components during ALL therapy and in ALL survivors, therapy-associated myosteatosis may serve as an important physiologic link to an improved understanding of these chronic morbidities. Further research is necessary to assess the prevalence, degree, chronicity and metabolic impact of therapy-associated myosteatosis during and after ALL maintenance therapy and in survivors.
Acknowledgements
The study team would like to thank Daniel Lorenzana and Kyle Chadwick for their assistance with data analysis.
This work was supported by a Translational Research Program grant from the Leukemia and Lymphoma Society (LLS-6249-11), Hyundai Hope on Wheels Foundation, the National Center for Advancing Translational Sciences/NIH (UL1TR000130) via the Southern California Clinical and Translational Science Institute, funds from The Saban Research Institute, NIH-NICHD grant 5R01HD059826 from the National Institutes of Health–Eunice Kennedy Shriver National Institute of Child Health and Human Development and NIH/NCI 1R01CA201444 from the National Institutes of Health National Cancer Institute.
Footnotes
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Ward E, DeSantis C, Robbins A, et al. Childhood and adolescent cancer statistics, 2014. CA: a cancer journal for clinicians. 2014. Mar-Apr;64(2):83–103. [DOI] [PubMed] [Google Scholar]
- 2.Ness KK, Kaste SC, Zhu L, et al. Skeletal, neuromuscular and fitness impairments among children with newly diagnosed acute lymphoblastic leukemia. Leukemia & lymphoma. 2015. April;56(4):1004–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orgel E, Mueske NM, Wren TA, et al. Early injury to cortical and cancellous bone from induction chemotherapy for adolescents and young adults treated for acute lymphoblastic leukemia. Bone. 2016. April;85:131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki D, Kobayashi R, Sano H, et al. Sarcopenia after induction therapy in childhood acute lymphoblastic leukemia: its clinical significance. International journal of hematology. 2017. December 12. [DOI] [PubMed] [Google Scholar]
- 5.Orgel E, Mueske NM, Sposto R, et al. Limitations of body mass index to assess body composition due to sarcopenic obesity during leukemia therapy. Leukemia & lymphoma. 2018. January;59(1):138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy E, Samoilenko M, Morel S, et al. Cardiometabolic Risk Factors in Childhood, Adolescent and Young Adult Survivors of Acute Lymphoblastic Leukemia - A Petale Cohort. Scientific reports. 2017. December 15;7(1):17684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warris LT, van den Akker EL, Bierings MB, et al. Acute Activation of Metabolic Syndrome Components in Pediatric Acute Lymphoblastic Leukemia Patients Treated with Dexamethasone. PloS one. 2016;11(6):e0158225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esbenshade AJ, Simmons JH, Koyama T, et al. Obesity and insulin resistance in pediatric acute lymphoblastic leukemia worsens during maintenance therapy. Pediatric blood & cancer. 2013. August;60(8):1287–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zareifar S, Haghpanah S, Shorafa E, et al. Evaluation of Metabolic Syndrome and Related Factors in Children Affected by Acute Lymphoblastic Leukemia. Indian journal of medical and paediatric oncology : official journal of Indian Society of Medical & Paediatric Oncology. 2017. Apr-Jun;38(2):97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miljkovic I, Zmuda JM. Epidemiology of myosteatosis. Current opinion in clinical nutrition and metabolic care. 2010. May;13(3):260–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. The American journal of clinical nutrition. 2000. April;71(4):885–92. [DOI] [PubMed] [Google Scholar]
- 12.Lang T, Cauley JA, Tylavsky F, et al. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010. March;25(3):513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schafer AL, Vittinghoff E, Lang TF, et al. Fat infiltration of muscle, diabetes, and clinical fracture risk in older adults. The Journal of clinical endocrinology and metabolism. 2010. November;95(11):E368–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mojtahedi MC, Valentine RJ, Arngrimsson SA, et al. The association between regional body composition and metabolic outcomes in athletes with spinal cord injury. Spinal cord. 2008. March;46(3):192–7. [DOI] [PubMed] [Google Scholar]
- 15.Tachi Y, Kozuka A, Hirai T, et al. Impact of myosteatosis on skeletal muscle volume loss in patients with chronic liver disease. J Gastroenterol Hepatol. 2018. February 27. [DOI] [PubMed] [Google Scholar]
- 16.Stretch C, Aubin JM, Mickiewicz B, et al. Sarcopenia and myosteatosis are accompanied by distinct biological profiles in patients with pancreatic and periampullary adenocarcinomas. PLoS One. 2018;13(5):e0196235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rayar M, Webber CE, Nayiager T, et al. Sarcopenia in children with acute lymphoblastic leukemia. Journal of pediatric hematology/oncology. 2013. March;35(2):98–102. [DOI] [PubMed] [Google Scholar]
- 18.Wright MJ, Twose DM, Gorter JW. Gait characteristics of children and youth with chemotherapy induced peripheral neuropathy following treatment for acute lymphoblastic leukemia. Gait & posture. 2017. October;58:139–145. [DOI] [PubMed] [Google Scholar]
- 19.Gocha Marchese V, Chiarello LA, Lange BJ. Strength and functional mobility in children with acute lymphoblastic leukemia. Medical and pediatric oncology. 2003. April;40(4):230–2. [DOI] [PubMed] [Google Scholar]
- 20.Orgel E, Mueske NM, Sposto R, et al. A randomized controlled trial testing an adherence-optimized Vitamin D regimen to mitigate bone change in adolescents being treated for acute lymphoblastic leukemia. Leukemia & lymphoma. 2017. October;58(10):2370–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen EC, Devidas M, Chen S, et al. Dexamethasone and High-Dose Methotrexate Improve Outcome for Children and Young Adults With High-Risk B-Acute Lymphoblastic Leukemia: A Report From Children’s Oncology Group Study AALL0232. J Clin Oncol. 2016. April 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital and health statistics Series 11, Data from the National Health Survey. 2002. May(246):1–190. [PubMed] [Google Scholar]
- 23.Horenstein RE, Shefelbine SJ, Mueske NM, et al. An approach for determining quantitative measures for bone volume and bone mass in the pediatric spina bifida population. Clinical biomechanics (Bristol, Avon). 2015. August;30(7):748–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mueske NM, Ryan DD, Van Speybroeck AL, et al. Fat distribution in children and adolescents with myelomeningocele. Developmental medicine and child neurology. 2015. March;57(3):273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorenzana DJ, Mueske NM, Ryan DD, et al. Quantitative Analysis of Lower Leg Adipose Tissue Distribution in Youth with Myelomeningocele. Journal of child neurology. 2016. July;31(8):979–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. Journal of applied physiology (Bethesda, Md : 1985). 1998. July;85(1):115–22. [DOI] [PubMed] [Google Scholar]
- 27.Maini R, Aggarwal H. Study and comparison of various image edge detection techniques. In J Image Processing. 2009. (3):1–12. [Google Scholar]
- 28.Shen W, Wang Z, Punyanita M, et al. Adipose tissue quantification by imaging methods: a proposed classification. Obesity research. 2003. January;11(1):5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamba R, McGahan JP, Corwin MT, et al. CT Hounsfield numbers of soft tissues on unenhanced abdominal CT scans: variability between two different manufacturers’ MDCT scanners. AJR American journal of roentgenology. 2014. November;203(5):1013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Withycombe JS, Smith LM, Meza JL, et al. Weight change during childhood acute lymphoblastic leukemia induction therapy predicts obesity: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2015. March;62(3):434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nayiager T, Barr RD, Anderson L, et al. Physical Activity in Long-term Survivors of Acute Lymphoblastic Leukemia in Childhood and Adolescence: A Cross-sectional Cohort Study. Journal of pediatric hematology/oncology. 2017. January;39(1):15–19. [DOI] [PubMed] [Google Scholar]
- 32.Baker KS, Chow EJ, Goodman PJ, et al. Impact of treatment exposures on cardiovascular risk and insulin resistance in childhood cancer survivors. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013. November;22(11):1954–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Sluis IM, van den Heuvel-Eibrink MM, Hahlen K, et al. Altered bone mineral density and body composition, and increased fracture risk in childhood acute lymphoblastic leukemia. The Journal of pediatrics. 2002. August;141(2):204–10. [DOI] [PubMed] [Google Scholar]
- 34.Marriott CJC, Beaumont LF, Farncombe TH, et al. Body composition in long-term survivors of acute lymphoblastic leukemia diagnosed in childhood and adolescence: A focus on sarcopenic obesity. Cancer. 2017. December 12. [DOI] [PubMed] [Google Scholar]
- 35.Kim JY, Hickner RC, Cortright RL, et al. Lipid oxidation is reduced in obese human skeletal muscle. American journal of physiology Endocrinology and metabolism. 2000. November;279(5):E1039–44. [DOI] [PubMed] [Google Scholar]
- 36.Sachs S, Zarini S, Kahn DE, et al. Intermuscular adipose tissue (IMAT) directly modulates skeletal muscle insulin sensitivity in humans. American journal of physiology Endocrinology and metabolism. 2019. January 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miljkovic I, Kuipers AL, Cauley JA, et al. Greater Skeletal Muscle Fat Infiltration Is Associated With Higher All-Cause and Cardiovascular Mortality in Older Men. The journals of gerontology Series A, Biological sciences and medical sciences. 2015. September;70(9):1133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iughetti L, Bruzzi P, Predieri B, et al. Obesity in patients with acute lymphoblastic leukemia in childhood. Italian journal of pediatrics. 2012. January 27;38:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vetsch J, Wakefield CE, Robertson EG, et al. Health-related quality of life of survivors of childhood acute lymphoblastic leukemia: a systematic review. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2018. January 25. [DOI] [PubMed] [Google Scholar]
- 40.Newman AB, Haggerty CL, Goodpaster B, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. Journal of the American Geriatrics Society. 2003. March;51(3):323–30. [DOI] [PubMed] [Google Scholar]
- 41.Marchese VG, Chiarello LA, Lange BJ. Effects of physical therapy intervention for children with acute lymphoblastic leukemia. Pediatric blood & cancer. 2004. February;42(2):127–33. [DOI] [PubMed] [Google Scholar]
- 42.Hopkins JJ, Reif RL, Bigam DL, et al. The Impact of Muscle and Adipose Tissue on Long-Term Survival in Patients With Stage I to III Colorectal Cancer. Diseases of the colon and rectum. 2019. February 14. [DOI] [PubMed] [Google Scholar]
- 43.Rollins KE, Tewari N, Ackner A, et al. The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clinical nutrition (Edinburgh, Scotland). 2016. October;35(5):1103–9. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Wang JP, Wang XL, et al. Computed tomography-quantified body composition predicts short-term outcomes after gastrectomy in gastric cancer. Current oncology (Toronto, Ont). 2018. October;25(5):e411–e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013. April 20;31(12):1539–47. [DOI] [PubMed] [Google Scholar]
- 46.Haddy TB, Mosher RB, Reaman GH. Late effects in long-term survivors after treatment for childhood acute leukemia. Clinical pediatrics. 2009. July;48(6):601–8. [DOI] [PubMed] [Google Scholar]
- 47.Lim JY, Bhatia S, Robison LL, et al. Genomics of racial and ethnic disparities in childhood acute lymphoblastic leukemia. Cancer. 2014. April 1;120(7):955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor OA, Brown AL, Brackett J, et al. Disparities in Neurotoxicity Risk and Outcomes among Pediatric Acute Lymphoblastic Leukemia Patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2018. October 15;24(20):5012–5017. [DOI] [PMC free article] [PubMed] [Google Scholar]