Letter to the Editor
GATA2 is a zinc finger transcription factor that plays a critical role in hematopoiesis, lymphangiogenesis, and leukemogenesis [1]. GATA2 haploinsufficiency can occur with somatic or germline aberrations of a single allele. GATA2 somatic variants in myeloid progenitor cells are associated with distinct acute myeloid leukemia (AML) subtypes, including acute erythroid leukemia and AML with normal cytogenetics harboring bi-allelic CEBPA variants [2], blast phase chronic myelogenous leukemia [3], high risk myelodysplastic syndromes (MDS), and MDS-EB (MDS with excess blasts) [4]. Germline GATA2 haploinsufficiency leads to a range of variably penetrant autosomal dominant disorders, including B/NK/dendritic cell immunodeficiency, familial MDS/AML, monocytopenia and mycobacterial infection (DCML deficiency, MonoMac) syndrome, and primary lymphedema or Emberger Syndrome (ES) [1]. Severe morbidity or mortality arises from opportunistic infections such as atypical mycobacterial, disseminated viral or fungal infections, HPV-driven warts and malignancy, and pulmonary alveolar proteinosis (PAP) from macrophage dysfunction. Hematological manifestations develop in approximately 80% of individuals with GATA2 haploinsufficiency, and can present as clonal cytopenias, aplastic anemia, MDS, or AML [5]. Myeloid clonal evolution in individuals with germline GATA2 haploinsufficiency is commonly associated with acquisition of additional somatic mutation(s) such as ASXL1, RUNX1, SETBP1, and NRAS [6].
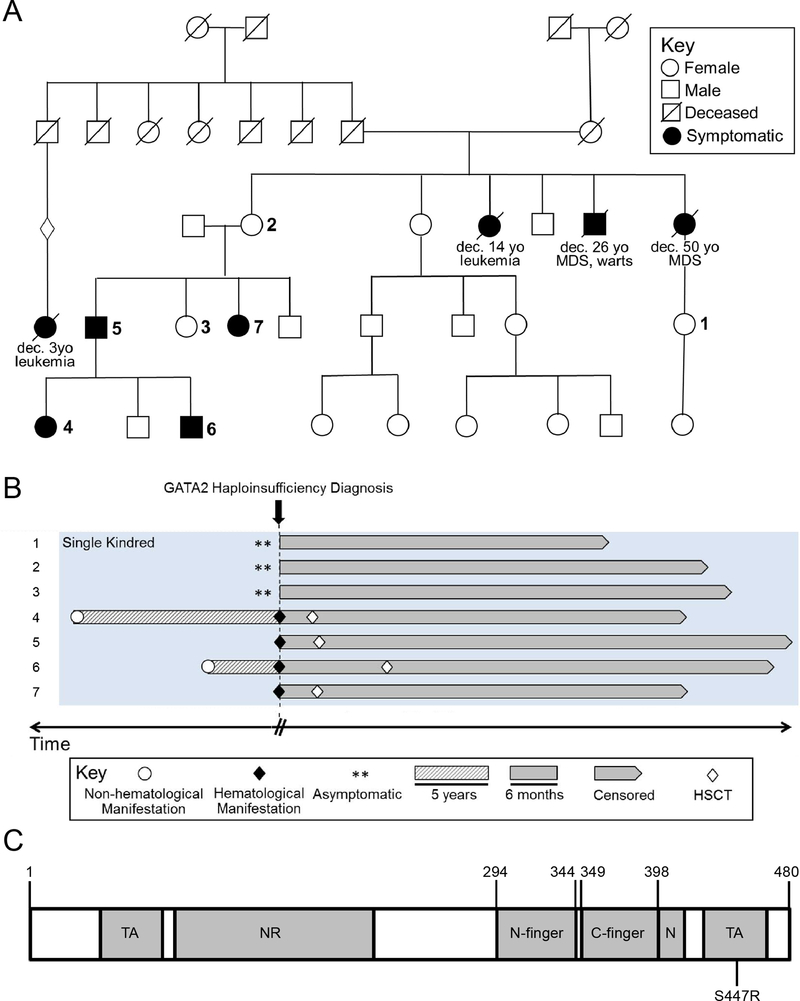
In this paper we expand on our previously described GATA2 haploinsufficiency cohort focusing on a seven-member kindred harboring an atypical missense mutation in the C-terminal domain (c.1339A>C, p.S447R) (Figure 1A, Table 1) [1]. This study was approved by the IRB at Mayo Clinic in Rochester, MN. Patient data were extracted from our institutional database of individuals with germline GATA2 haploinsufficiency. Sanger and next generation sequencing (NGS) were used to characterize pathogenic variants in GATA2, as previously described [1]. The timing of patients’ earliest clinical manifestations were estimated based on patient history, and onset of hematological manifestations was defined by blood and bone marrow histology showing clonal cytogenetic abnormalities, clonal cytopenias, marrow failure, MDS, or AML. In 3 of 7 patients a myeloid-specific NGS panel (single base substitutions and insertion/deletion events: accuracy>99%; reproducibility 100% and sensitivity 5–10% variant allele fraction with a minimum depth coverage of 250X) was obtained to identify somatic variants. Relevant transplant outcome measure was GVHD relapse-free survival (GRFS). Overall survival was calculated from the time of GATA2 haploinsufficiency diagnosis. Standard statistical methods were performed using JMP Software (SAS Institute Inc., Cary, NC).
Figure 1.
A) Family tree of kindred of interest. Large bold numbers correspond to number indicated in the Swimmer plot below. B) Swimmer plot demonstrating clinical evolution of the seven-member kindred over time. C) Diagram depicting functional domains of GATA2 protein. Above the diagram key amino acid residues are numbered. Below the diagram the kindred’s S447R relative location is annotated. TA, transactivation domain; NR, negative regulatory domain; N-finger, N-terminal zinc finger; C-finger, C-terminal zinc finger; N, nuclear localization signal
Table 1.
Clinical presentation, treatment, and outcomes of 7-member GATA2 (c.1339A>C, p.S447R) kindred
| Kindred | Age (Dx) | Sex | Earliest Clinical Manifestation | Bone Marrow Findings (Dx) | Cytogenetics at Evolution | Time to Clonal Evolution (mo.) | Somatic Mutations at Evolution | Allele Burden of Somatic Mutations | HSCT Donor & Conditioning | GRFS (mo.) | Overall Survival | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 35 | F | Asymptomatic | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 41.7 | Alive, Observation |
| 2 | 56 | F | Asymptomatic | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 54.3 | Alive, Observation |
| 3 | 30 | F | Suboptimal NK cell function | Mildly hypercellular | N/A | N/A | N/A | N/A | N/A | N/A | 57.2 | Alive, Observation |
| 4 | 16 | F | Warts | MDS-MLD | 46,XX[20] | 156 | Not Done | N/A | M-MUD, MA | 47.6 | 51.9 | Alive, Remission |
| 5 | 37 | M | AML | 60% blasts with dyserythropoiesis, dysgranulopoiesis | 46,XY,+1,der(1;21)(q10;q10)[9] 47,XY,+8[4] 46,XY[7] |
* | ASXL1, DNMT3A, ETV6 | 43% | M-MUD, NMA | 10.1 | 64.9 | Alive, Relapse, Remission |
| 6 | 9 | M | Warts, Lymphedema | Aplastic Anemia | 46,XY[20] | 72 | ASXL1 | 12% | MUD, MA | 48.9 | 62.6 | Alive, Lymphedema |
| 7 | 31 | F | MDS-MLD | MDS-MLD | 46,XX[20] | * | ASXL1 | 23% | MUD, MA | 46.9 | 51.7 | Alive, Remission |
Dx, at diagnosis. Genomic variant information including evidence of and somatic status in cancer, significant protein domains, and known pathogenicity was further annotated using public databases: the Catalogue of Somatic Mutations in Cancer – COSMIC. M-MUD, mismatched unrelated donor. MUD, matched unrelated donor. MA, myeloablative conditioning. NMA, nonmyeloablative conditioning. GRFS, GVHD-free relapse-free survival. Time to clonal evolution was calculated from earliest GATA2 syndrome symptoms. “*” denotes cases where myeloid transformation was the earliest symptom.
The seven-member kindred of interest illustrates incomplete penetrance and variable expressivity of GATA2 germline variants (Figure 1A). Two asymptomatic patients (#1 and 2), followed annually with a complete blood count (CBC) and a comprehensive physical examination, have no clinical features associated with GATA2 haploinsufficiency to date. Multiple bone marrow assessments in both patients have been normocellular without morphological or cytogenetic evidence for myeloid transformation. Furthermore, as B cell lymphopenia has proven very common in GATA2 deficiency, even in patients with normal CBCs, serial quantitative B and NK cell assessments in both these patients were surprisingly normal [7]. Patient #3 has suboptimal NK cell cytotoxic function with bone marrow assessments revealing mild hypercellularity for age, without dysplasia or somatic variants. Four patients developed myeloid clonal evolution including aplastic anemia (#6), MDS-MLD (multi lineage dysplasia) (#4 and 7) and AML (#5). Aplastic anemia in patient #6 was associated with somatic ASXL1 mutation acquisition with variant allele frequency (VAF) of 12%. Patient #6 developed MDS-MLD 13 years after diagnosis though NGS was not performed. Similarly, patient #7 developed MDS-MLD with normal cytogenetics and ASXL1 mutation (VAF 23%). Only patient #5 developed AML with somatic mutations involving ASXL1, DNMT3A and ETV6. Interestingly, we demonstrate a significant variability in the non-hematologic manifestations in these kindred. Only patients #4 and 6 demonstrated recurrent HPV driven-warts and only patient #6 developed lymphedema (ES).
Since first described, GATA2 haploinsufficiency remains a challenge to treat given varied clinical presentations and syndrome-related limitations to the only potential cure, allogenic HCT. As in our cohort, although myeloablative regimens are more likely to eradicate aggressive clones when hematologic malignancies are present, they may be less tolerated in patients with disseminated opportunistic infections and comorbidities. Patient #5 relapsed 10 months following HSCT with extramedullary disease, presenting with right eye proptosis. An orbital mass biopsy demonstrated a myeloid sarcoma with a PET-CT revealing involvement of the ninth right rib, left lateral thigh and right buccal space. After treatment with external beam radiation therapy to the orbit and high dose cytarabine, the patient remains in remission 48 months later. He was the only kindred who underwent non-myeloablative conditioning due to comorbidities. Of the other 3 patients who underwent HSCT, all received myeloablative conditioning with transplant soon after detection of clonal myeloid evolution. Currently, timing of HCT and choice of conditioning regimen depends on severity of the syndrome and anticipated tolerability.
Management difficulties of GATA2 haploinsufficiency are rooted in challenges delineating the genotype-phenotype relationship. If prediction of clinical consequences from the specific variant was possible, more personalized management and monitoring frequency for clonal evolution would be possible. To this end, we largely look to protein structure to define function, and by extension, we may determine structural consequences of the variant to predict functional corollaries. GATA2 has several well-characterized functional domains including two transactivation domains, negative regulatory domain, nuclear localization signal, and N-terminal and C-terminal zinc fingers (N-finger and C-finger respectively) (Figure 1B). Most commonly germline GATA2 variants are truncating (typically frameshift) followed closely by missense variants. Truncating variants almost exclusively occur upstream of the C-finger while most missense variants are located either within the C-finger or immediate vicinity. Missense variants in the N-finger are exceedingly uncommon [8–11]. Taken together, this suggests C-finger disruption is critical for the GATA2 haploinsufficiency phenotype. However, the S447R variant in our cohort is unique given its significant distance from the C-finger (Figure 1B) raising the question how this mutation confers similar clinical manifestations.
Given most variants affect the GATA2 C-finger, hypothesized mechanistic consequences revolve around disruption of GATA2-DNA interaction. Testing recurrent C-finger variants demonstrated impaired transcription, transactivation and reduced binding of GATA2 to intronic cis-elements important for autoregulation [5,12,13]. This led to the hypothesis that C-finger variants confer a dominant-negative effect causing haploinsufficiency. However, not all missense variants are likely interchangeable as function and regulatory mechanisms of GATA2 are diverse and there is a paucity of mechanistic studies testing the variety of GATA2 mutations observed. Given GATA2 has two zinc finger domains with slightly different sequence preference, each can bind DNA independently and both domains can bind cooperatively in the context of two proximal GATA sites. Long-range regulation of gene expression can be mediated by distinct zinc finger domains binding separate DNA molecules. Furthermore, each domain can independently or cooperatively interact with other proteins forming homodimers or heterodimers [14]. Post-translational modifications also augment GATA2 activity with putative phosphorylation, acetylation and SUMOylation sites. In our cohort, given transition of a serine residue, one may initially hypothesize this represents disruption of a phosphorylation site given GATA2 is a downstream target of MAPK and AKT [14]. However, in silico analysis (NetPhos 3.1) does not support this hypothesis. More likely consequences of S447R include augmentation of the C-terminal transactivation domain in which it is located or disruption of a region important for ubiquitination (amino acids 412–480) altering steady-state GATA2 levels [14]. Taken together, these complexities illustrate how different germline variants may result in heterogeneous phenotypes.
Recent studies question the haploinsufficiency paradigm entirely. Katsumura et al. showed common GATA2 variants disrupt DNA binding. While mutant GATA2 had impaired erythroid differentiation promotion, relative to wildtype GATA2 it had enhanced promotion of myeloid differentiation and proliferation [13]. Similarly, common variants were found to enhance binding to hematopoietic differentiation factor PU.1 relative to wildtype GATA2 [5]. Therefore, missense variants likely do not simply abrogate GATA2 function, and gain-of-function consequences must be considered contributing to varied clinical presentations.
However, this does not explain differential expressivity, incomplete penetrance, and variable symptom onset in our kindred. Familial cohorts could prove invaluable to understand these differences. Broadly, such variability is likely to be epistatic and/or epigenetic. While susceptibility/protective loci have not been identified, there are some studies characterizing epigenetic regulation of GATA2 activity [15]. Nonetheless, penetrance is typically high based on one cohort with diverse variants having probability of 8% remaining symptom-free at 40 years [10]. To our knowledge, only one other study has identified a germline variant (c.1341C>A) resulting in the same protein change as this kindred, p.S447R. This patient was diagnosed at age 12.9 years with MDS-RCC (refractory cytopenia of childhood) and karyotype with monosomy 7 [11]. If included in our cohort, three individuals (37.5%) remain asymptomatic without clonal hematopoiesis at age > 40 years. Though there is an order of magnitude difference in patients between cohorts, our kindred may harbor epistatic or epigenetic mechanisms limiting penetrance, and epigenomic and transcriptomic studies may be useful in these cohorts.
Our cohort shares recurrent somatic mutations (ASXL1, DNMT3A) and karyotype abnormalities (monosomy 7) with other patients harboring different germline GATA2 variants, and who have undergone myeloid clonal evolution [16]. Such recurrent findings suggest GATA2 variants may result in common evolutionary trajectories. Ultimately, additional targeted mechanistic studies comparing germline variants and their effects on the transcriptome and epigenome are necessary to elucidate not only the complex genotype-phenotype relationship but also events in evolution to MDS/AML. With systematic, mechanistic and longitudinal studies of GATA2 haploinsufficiency, we may more precisely manage this complex disease.
Acknowledgements:
Current publication is supported in part by grants from the “The Gerstner Family Career Development Award” and the Mayo Clinic Center for Individualized Medicine, Mayo Clinic, Rochester, MN, USA”.
This publication was supported by CTSA Grant Number KL2 TR000136 from the National Center for Advancing Translational Science (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Disclosures: The authors do not have any funding sources or conflicts of interest to disclose.
References
- [1].Mir MA, Kochuparambil ST, Abraham RS, et al. Spectrum of myeloid neoplasms and immune deficiency associated with germline GATA2 mutations. Cancer Med. 2015;4:490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fasan A, Eder C, Haferlach C et al. GATA2 mutations are frequent in intermediate-risk karyotype AML with biallelic CEBPA mutations and are associated with favorable prognosis. Leukemia. 2013;27:482–485. [DOI] [PubMed] [Google Scholar]
- [3].Zhang SJ, Ma LY, Huang QH, et al. Gain-of-function mutation of GATA-2 in acute myeloid transformation of chronic myeloid leukemia. Proc Natl Acad Sci USA. 2008;105:2076–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Theis F, Corbacioglu A, Gaidzik VI, et al. Clinical impact of GATA2 mutations in acute myeloid leukemia patients harboring CEBPA mutations: a study of the AML study group. Leukemia. 2016;30:2248–2250. [DOI] [PubMed] [Google Scholar]
- [5].Chong CE, Venugopal P, Stokes PH, et al. Differential effects on gene transcription and hematopoietic differentiation correlate with GATA2 mutant disease phenotypes. Leukemia. 2018;32:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang X, Muramatsu H, Okuno Y, et al. GATA2 and secondary mutations in familial myelodysplastic syndromes and pediatric myeloid malignancies. Haematologica. 2015;100:e398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Novakova M, Zaliova M, Sukova M, et al. Loss of B cells and their precursors is the most constant feature of GATA-2 deficiency in childhood myelodysplastic syndrome. Haematologica. 2016;101:707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hahn CN, Chong C-E, Carmichael CL, et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet. 2011;43:1012–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hsu AP, Sampaio EP, Khan J, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. 2011;118:2653–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Donadieu J, Lamant M, Fieschi C, et al. Natural history of GATA2 deficiency in a survey of 79 French and Belgian patients. Haematologica. 2018;103:1278–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wlodarski MW, Hirabayashi S, Pastor V, et al. Prevalence, clinical characteristics, and prognosis of GATA2-related myelodysplastic syndromes in children and adolescents. Blood. 2016;127:1387–1397. [DOI] [PubMed] [Google Scholar]
- [12].Cortes-Lavaud X, Landecho MF, Maicas M, et al. GATA2 germline mutations impair GATA2 transcription, causing haploinsufficiency: Functional analysis of the p.Arg396Gln mutation. J Immunol. 2015;194:2190–2198. [DOI] [PubMed] [Google Scholar]
- [13].Katsumura KR, Mehta C, Hewitt KJ, et al. Human leukemia mutations corrupt but do not abrogate GATA-2 function. Proc Natl Acad Sci USA. 2018;115:E10109–E10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vicente C, Conchilla A, Garcia-Sanchez MA and Odero MD. The role of the GATA2 transcription factor in normal and malignant hematopoiesis. Crit Rev Oncol Hematol. 2012;82:1–17. [DOI] [PubMed] [Google Scholar]
- [15].Celton M, Forest A, Gosse G, et al. Epigenetic regulation of GATA2 and its impact on normal karyotype acute myeloid leukemia. Leukemia. 2014;28:1617–1626. [DOI] [PubMed] [Google Scholar]
- [16].McReynolds LJ, Yang Y, Wong HY, et al. MDS-associated mutations in germline GATA2 mutated patients with hematologic manifestations. Leuk Res. 2019;76:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]