Abstract
Purpose:
Patients undergoing a hematopoietic stem cell transplantation (HCT) have varied symptoms during their hospitalization. This study examined whether daily symptom reporting (with electronic patient-reported outcomes [PROs]) in an inpatient bone marrow transplant clinic reduced symptom burden on post-transplant days +7, +10, and +14.
Methods:
A prospective, single-institution1:1 pilot randomized, two-arm study recruited HCT patients. HCT inpatients (N=76) reported daily on 16 common symptoms using the PRO version of the Common Terminology for Adverse Events (PRO-CTCAE). Fisher’s exact test was used to examine differences in the proportion of patients reporting individual symptoms. Multivariable linear regression modeling was used to examine group differences in peak symptom burden, while controlling for symptom burden at baseline, age, comorbidity, and transplantation type (autologous or allogeneic).
Results:
HCT patients receiving the PRO intervention also experienced lower peak symptom burden (average of 16 symptoms) at days +7, +10, and +14 (10.4 vs 14.5, p =0.03).
Conclusions:
Daily use of electronic symptom reporting to nurses in an inpatient bone marrow transplant clinic reduced peak symptom burden and improved individual symptoms during the two weeks post-transplant. A multi-site site trial is warranted to demonstrate the generalizability, efficacy, and value of this intervention.
Keywords: symptoms, electronic symptom reporting, patient-reported outcomes, autologous stem cell transplantation, allogeneic stem cell transplantation, cancer
Introduction
Hematopoietic stem cell transplantation (HCT) often results in multi-week hospitalizations with a focus to cure disease and/or prolong life in patients with hematologic malignancies. Short and long-term treatment-related toxicities can lead to significant symptom burden during the peri-transplant period1. Toxicities are often a consequence of high-dose chemotherapy or radiation received as “conditioning” prior to stem cell infusion, during which patients are closely monitored for infection and other complications.2 Prevalent symptoms during the peri-transplant period include fatigue3, nausea4, vomiting4, diarrhea5, mucositis6, pain7, and sleep disturbance8, among others9. Because symptom burden is high and may fluctuate daily, clinical teams may under-recognize symptom burden and miss opportunities to better manage symptoms during inpatient stays.
Symptom burden in the early weeks after allogeneic or autologous transplantation is well documented and may be associated with long-term complications including persistent QOL impairments and treatment-related mortality9. The time to peak symptom burden is similar for most patients undergoing either autologous or allogeneic transplantation (typically 7–14 days post-chemotherapy), but type and intensity of pre-transplant conditioning may affect the severity and distribution of symptoms4. Moreover, little data currently exists about clinical and symptom management of hospitalized patients with HCT and there are likely missed opportunities to address these symptoms during hospitalization. Thus, there is a need to explore common treatment-related symptoms that may contribute to increased medical and nursing care of these patients.
In previous HCT research, there is only limited information on the feasibility and value of using standardized electronic patient-reported outcomes (PRO) symptom collection with automated feedback to the medical team.10–12 Existing literature has focused on graft vs. host disease13 and HCT-treatment related complications14 with little attention to symptoms and symptom monitoring during hospitalization. In our qualitative work, providers and nurses found value in electronic assessment of symptoms during inpatient clinical care and reported that communication about symptoms had improved.15 Emerging work highlights the value of collecting and using PROs to change clinical care.16–18 Developing an intervention to understand symptom burden and symptom reporting better in this population is an initial step to improved outcomes.
This pilot randomized trial tested whether daily use of electronic symptom reporting results automatically fed back to nurses in an inpatient bone marrow transplant clinic reduced peak symptom burden and improved individual symptoms more than usual care at post-transplant days +7, +10, and +14. Symptom burden was anticipated to peak between post-transplantation +7 and +14 4 We defined “peak symptom burden” as the average of the available (not missing) symptom burden scores on days +7, +10, and +14. We hypothesized that integrating an electronic PRO system within the care delivery of hospitalized HCT patients would be associated with improved peri-transplant symptoms in the intervention group compared to the usual care group.
Materials and Methods
Study Design and Sample Size
This prospective, single institution 1:1 pilot randomized, two-arm study recruited patients between May 2015 and June 2017 (ClinicalTrials.gov identifier: NCT 02574897). Adults with any hematological malignancy admitted for preparative chemotherapy for HCT were recruited at the North Carolina Cancer Hospital, the primary treatment facility of the University of North Carolina Lineberger Comprehensive Cancer Center. Eligible patients were adults 18–75 years old, who were within three days of being admitted for scheduled inpatient transplant care and had an expected hospital stay of at least two weeks. Participants also needed the ability to access and effectively navigate an email account. All study participants provided written informed consent. The study protocol was approved by the UNC Institutional Review Board.
To determine sample size, we conducted a power analysis. A sum symptom score of 16 for the control arm, a score of 10 for the intervention arm, and a common standard deviation of 10 (based on previous work and the possible score range)19. The original planned sample size was 120 (60 per arm), and this led to 77% power to detect a difference in peak symptom burden between the intervention and control groups, using a two-sided, two-sample t-test with an alpha of 0.05. However, we re-adjusted the sample size due to limited personnel staff to 76 (38 per arm), noticing that the actual standard deviation should be around 9, when we accrued 60 patients.
The control group had 76% myeloablative and 24% reduced intensity or non-myeloablative conditioning type, whereas the intervention group had 79% myeloablative and 21% reduced intensity or non-myeloablative conditioning. Majority of the sample, 78% had myeloablative conditioning. The graft versus host disease prophylaxis at this study site was tacrolimus and methotrexate.
Study Procedures
Two-hundred and forty-nine patients were screened for participation in the study through electronic abstraction. Fifty did not meet study eligibility criteria (Figure 1). An additional 123 were eligible based on initial screening but not recruited, most often because of study personnel limitations (Figure 1). In total, 76 patients were recruited and randomized 1:1 into either the PRO or usual care groups, stratified by autologous vs allogeneic transplant, and by myeloablative vs reduced intensity conditioning. The HCT inpatients randomly assigned to the PRO group (N=38) had their PRO results automatically fed back to nurses on a daily basis, whereas their counterparts in the usual care group did not have this feedback mechanism after they reported the PRO symptoms.
Figure 1.
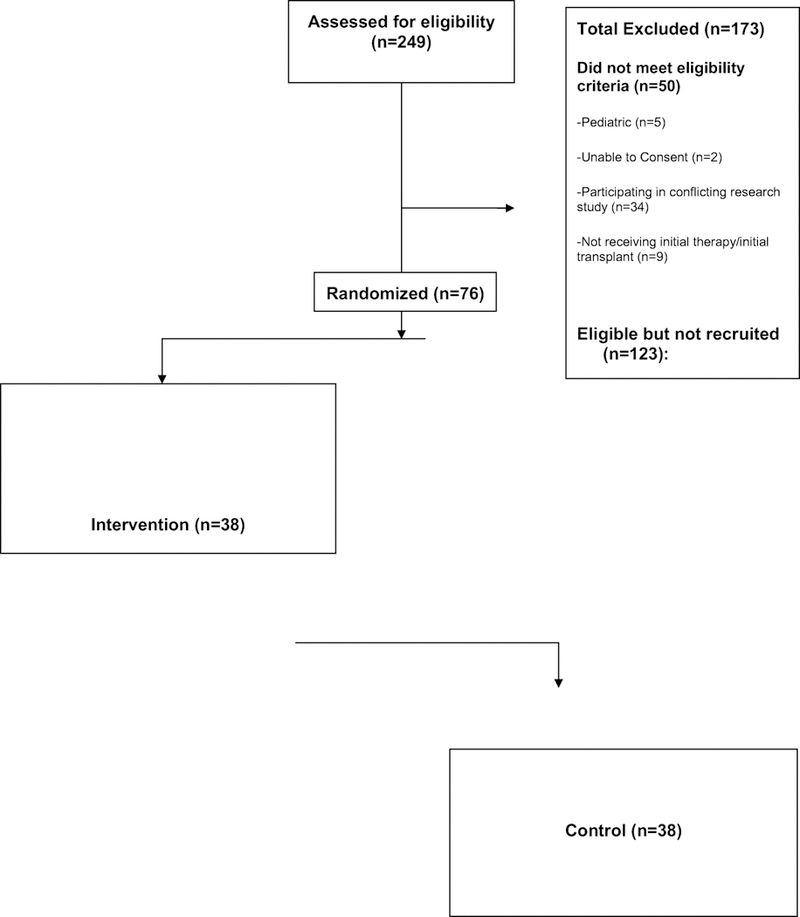
Screening process in choosing eligible patients
After providing baseline demographic and patient-reported measures, patients were registered within the electronic symptom reporting system to receive PRO surveys daily for the PRO group and at days +7, +10, +14 for the standard care group, throughout the study period. Screening, enrollment, randomization, and study orientation were conducted by a research coordinator who was not a member of the clinical care team.
Patients were instructed to complete a PRO survey by either using a tablet provided by the study or on their personal tablet or smartphone. Patients were asked to do this once per day until discharge and nurses were asked to remind the patient every 12 hours. For those patients randomized to the PRO intervention arm of the study, the nurse would receive an email containing the symptom report when patients completed the survey. The nurse was then asked to print the report and present it at morning rounds. The report was discussed by providers and guided conversations with patients during rounds to help direct symptom related supportive care. Patients in the usual care group only completed the PRO assessment; they did not receive follow-up and feedback from the nurses or other providers in responding to their symptoms. The PRO surveys, email alerts and symptoms reports were generated and distributed via the University of North Carolina Patient-Reported Outcomes Core survey system.
Study Measures
PRO-CTCAE Survey.
The patient-reported outcome version of the Common Terminology for Adverse Events (CTCAE), developed by the National Cancer Institute (NCI), is a tool for patients to self-report symptomatic adverse events 19–12. The PRO-CTCAE item library assesses different attributes (e.g., frequency, severity, interference, presence of condition) of 81 symptoms that are represented in both the CTCAE (version 4) and the Medical Dictionary for Regulatory Activities (MedDRA) adverse event lexicons. Questions were comprehensively developed and validated in diverse samples of patients receiving anti-cancer therapy16.
Previous item response theory analyses for the PRO-CTCAE indicate that individual items can be selected to form custom questionnaires,.22 and thus we chose 16 items assessing common symptoms after HCT. In hospitalized HCT patients, based on the clinical expertise of the research team and literature.1,12,23 These 16 symptoms included shortness of breath, pain, vomiting, diarrhea, nausea, mouth/throat sores, constipation, cough, insomnia, heartburn, rash, decreased appetite, fatigue, anxiety, and depression.
The PRO-CTCAE recall period was changed from the previous 7 days to a 24-hour recall period to match the daily assessment schedule. Each symptom was scored from 0–4, where 0 means not present and 4 means severe. The aggregate symptom score was defined as the “symptom burden” for each day, which is the sum of the severity of each symptom in one day. The total score ranged from 0–64, and higher scores indicated greater symptom burden.
Symptom burden was anticipated to peak between post-transplantation +7 and +14.4 We defined “peak symptom burden” as the average of the available (not missing) symptom burden scores on days +7, +10, and +14.
Hematopoietic Cell Transplantation-Comorbidity Index (HCT-CI).
This is a comorbidity index that comprises 17 categories of organ impairment for recipients of HCT24. We used this measure to determine their comorbidity index at baseline, for use as a control variable in multivariate analyses. The following categories received a score of 1 point: arrhythmia, cardiovascular, inflammatory bowel disease, diabetes, cerebrovascular disease, psychiatric disorder, hepatic comorbidity, obesity, and infection. Rheumatological comorbidity, peptic ulcer, renal comorbidity, and pulmonary comorbidity receive a score of 2. Prior malignancy and heart valve disease received a score of 3. Two comorbidity categories (pulmonary and hepatic) had 2 different levels of severity based on laboratory data. Scores ranged from 0–3. With higher overall HCT-CI scores comes an increased likelihood for more HCT related complications24.
Intervention and Attention Control
Patients assigned to the intervention arm completed the PRO-CTCAE items as a part of the baseline/admission visit and daily until discharged. The survey was available to be completed from 12noon-10pm. A daily report containing the most recent HCT patient reported symptom scores was generated on a study web page. Each morning, an email containing a link to the page was sent to the nurse (unblinded) caring for that patient(s) on the intervention arm of the study. Nurses used their institutional login to access patient symptom reports. These reports showed the most severe symptoms at the top. The nurse printed and presented the report at morning rounds and used it to guide conversations with patients and help direct plan of care with patients and help direct plan of care. In addition to reporting their symptoms using PRO-CTCAE daily, patients also reported their symptoms to the nurse throughout the day.
Patients in the attention control arm completed PRO-CTCAE surveys as a part of the baseline/admission visit, and at days +7, +10, and +14 during their hospitalization. These survey results were not sent to the study team or BMT medical team of the patient. In addition to reporting their symptoms using PRO-CTCAE at days +7, +10, and +14, patients also reported their symptoms to the nurse throughout the day.
Statistical Analysis
Baseline patient, disease, and treatment-related variables were reported using descriptive statistics (mean, standard deviation, and percentages) for all subjects, and by intervention arm. Individual symptom items were on a 0–4 scale, with higher scores indicating greater symptom burden. Fisher’s exact test was used to examine differences in the proportion of patients reporting individual symptoms. Symptom burden was anticipated to peak between transplantation and days +7, +10, and +14, and thus we also calculated “peak symptom burden” as the average of 16 symptoms on those days. Multivariable linear regression modeling was used to examine group differences in peak symptom burden, while controlling for symptom burden at baseline, age, comorbidity, and transplantation type (autologous or allogeneic).
Four patients died during the study period and were not included in the analysis due to a lack of peak symptom burden data. Missing events were evenly distributed across the two arms, and only listwise deletion was employed to deal with missing data in the above analyses.
All tests were two-sided at significance level of 0.05, unless otherwise specified. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) software.
Results
Participant Sample
Patients included 76 adults (38 intervention and 38 control), with a mean age of 51.2 years (SD 13.6, range 19–75). Most (74%) were female, non-Hispanic white (70%), had a college or advanced degree (59%), and were married/partnered (69%) (Table 1). Hematopoietic cell transplantation-comorbidity index (HCT-CI) scores at baseline ranged from 2–7. The most common cancer diagnoses were leukemia, multiple myeloma, and lymphoma. Baseline characteristics between arms were similar in age, gender, and comorbidities (Table 1).
Table 1.
Demographic Characteristics Clinical Characteristics
| Control (N=38) | Intervention (N=38) | All | |
|---|---|---|---|
| Age (years) mean(SD) | 51.1(13.7) | 51.3 (13.6) | 51.2 (13.6) |
| Gender | |||
| Male n(%) | 12 (31.6) | 8 (21.1) | 20 (26.3) |
| Female n(%) | 26 (68.4) | 30 (78.9) | 56 (73.7) |
| Race | |||
| White n(%) | 29 (78.0) | 28 (76.0) | 57 (77.0) |
| African American n(%) | 8 (22.0) | 6 (16.0) | 14 (18.9) |
| Pacific Islander n(%) | 0 (0.0) | 1 (3.0) | 1 (14) |
| Other n(%) | 0 (0.0) | 2 (5.0) | 2 (2.7) |
| Ethnicity | |||
| Hispanic or Latino n(%) | 1 (2.6) | 2 (5.4) | 3 (4.0) |
| Non-Hispanic n(%) | 37 (97.4) | 35 (94.6) | 72 (96.0) |
| Education, n=54 | |||
| Missing | 13 ( 34.2) | 7 ( 18.9) | 20 ( 26.7) |
| High school graduate/GED n(%) | 12 ( 31.6) | 10 (27.0) | 22 ( 29.3) |
| College Degree n(%) | 8 ( 21.1) | 12 ( 32.4) | 20 ( 26.7) |
| Advanced Degree n(%) | 5 (13.2) | 8 ( 21.6) | 13 ( 17.3) |
| Household Income, n=64 | |||
| Missing | 12 ( 31.6) | 7 ( 18.9) | 19 ( 25.3) |
| <20,000 n(%) | 2 ( 5.3) | 2 ( 5.4) | 4 ( 5.3) |
| 20,001–40,000 n(%) | 0 ( 0.0) | 7 ( 18.9) | 7 ( 9.3) |
| 40,001–60,000 | 8 ( 21.1) | 4 ( 10.8) | 12 ( 16.0) |
| 60,001–80,000 n(%) | 0 ( 0.0) | 1 ( 2.7) | 1 ( 13) |
| 80,001–100,000 n(%) | 3 ( 7.9) | 6 ( 16.2) | 9 ( 12.0) |
| >100,000 n(%) | 7 ( 18.4) | 6 ( 16.2) | 13 ( 17.3) |
| Prefer Not to Say n(%) | 6 ( 15.8) | 4 ( 10.8) | 10 ( 13.3) |
| Marital Status | |||
| Single/Never married n(%) | 5 (13.2) | 5 ( 13.5) | 10 ( 13.3) |
| Married/Partnered n(%) | 26 ( 68.4) | 26 ( 70.3) | 52 ( 69.3) |
| Separated/Divorced n(%) | 6 ( 15.8) | 6 ( 16.2) | 12 ( 16.0) |
| Widowed n(%) | 1 ( 2.6) | 0 ( 0.0) | 1 ( 13) |
| Type of Transplant | |||
| Autologous n(%) | 22 ( 57.9) | 23 ( 60.5) | 45 ( 59.2) |
| Allogeneic n(%) | 16 ( 42.1) | 15 ( 39.5) | 31 ( 40.8) |
| Height (cm) | 172.0 (9.3) | 176.1 (8.7) | 174.1 (9.1) |
| Weight (kg) | 96.8 (24.3) | 89.7 (23.0) | 93.0 (22.7) |
| Body Mass Index | 32.7 (7.8) | 28.8 (6.2) | 30.7 (7.3) |
| Length of Stay (days) | 21.7 (15.0) | 21. 7 (8.1) | 21.7 (11.9) |
Patient-reported symptoms
Unadjusted analysis:
Univariate analyses (Wilcoxon tests or Chi-square tests) showed that patient characteristics, including age, gender, race, symptom burden, disease status or intervention (condition, transplant type) were all evenly distributed between the two arms. On day +7, 92% of patients in the intervention group reported fatigue vs. 100% in usual care (p=.045). (Table 2). There were no group differences for pain, appetite, diarrhea, and other symptoms. On day +10, there was a large difference in the proportion of patients reporting vomiting. No patients in the intervention group reported vomiting vs. 36% in usual care (p=.002). There were no group differences on other symptoms. On day +14, there were no significant differences in symptoms among the arms. HCTs patients receiving the PRO intervention also experienced lower peak symptom burden (average of 16 symptoms) at days +7, +10, and +14 (10.4 vs 14.5, p =0.03). (Table 2).
Table 2.
Commonly Reported Symptoms (Proportions in intervention vs control), days +7, +10, and +14
| Symptoms | Day 7 | Day 10 | Day 14 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Usual Care |
PROs | p-value | Usual Care |
PROs | p-value | Usual Care |
PROs | p-value | |
| Fatigue | 100% | 92% | 0.045 | 100% | 79% | 0.103 | 93% | 83% | 0.540 |
| Pain | 75% | 57% | 0.518 | 86% | 61% | 0.216 | 53% | 54% | 0.311 |
| Vomiting | 36% | 26% | 0.704 | 36% | 0% | 0.002 | 33% | 13% | 0.306 |
| Appetite | 93% | 83% | 0.687 | 93% | 79% | 0.439 | 87% | 79% | 0.338 |
| Diarrhea | 86% | 77% | 0.421 | 57% | 64% | 0.500 | 67% | 63% | 0.738 |
Note: P-values are based on Fisher’s exact test with symptoms being categorical outcomes.
Peak symptom burden at baseline was significantly lower in the intervention group (M=10.4, SD = 6.2), compared to the control group (M = 14.5, SD = 7.5), (p=0.03). (Table 3). Figures 2 illustrate changes over time between the autologous and allogeneic transplantation and control and intervention groups for days +7, +10, and +14. Overall, the severity of symptoms were greater in the autologous group vs allogeneic HCT across days +7, +10, and 14, specifically fatigue, pain, vomiting, appetite, and diarrhea. The intervention group had lower symptom scores in the above stated symptoms compared to the control group at days +7, +10 and 14. Day +7 fatigue and day +10 vomiting were statistically significant (Table 2).
Table 3.
Linear mixed effects model analysis of Symptom Burden Over Time
| Parameter | Estimate | Standard Error |
t Value | Pr > |t| |
|---|---|---|---|---|
| Peak Symptom Burden (intervention) | 10.4 | - | - | - |
| Peak Symptom Burden (control ) | 14.5 | - | - | - |
| Intercept | −2.15 | 6.46 | −0.33 | 0.74 |
| Control (Intervention as ref) | 4.69 | 2.19 | 2.14 | 0.04 |
| Baseline Burden | 0.54 | 0.18 | 3.00 | 0.006 |
| Age | 0.01 | 0.12 | 0.09 | 0.93 |
| Autologous | 0.62 | 2.30 | 0.27 | 0.79 |
| (Allogeneic as reference) | ||||
| Myeloablative | 2.15 | 3.48 | 0.62 | 0.54 |
| (Reduced Intensity Conditioning 2 as reference) | ||||
| Comorbidites (HCT-CI) | 1.82 | 1.25 | 1.45 | 0.16 |
| Days | 0.31 | 1.15 | 0.27 | 0.79 |
Figure 2.
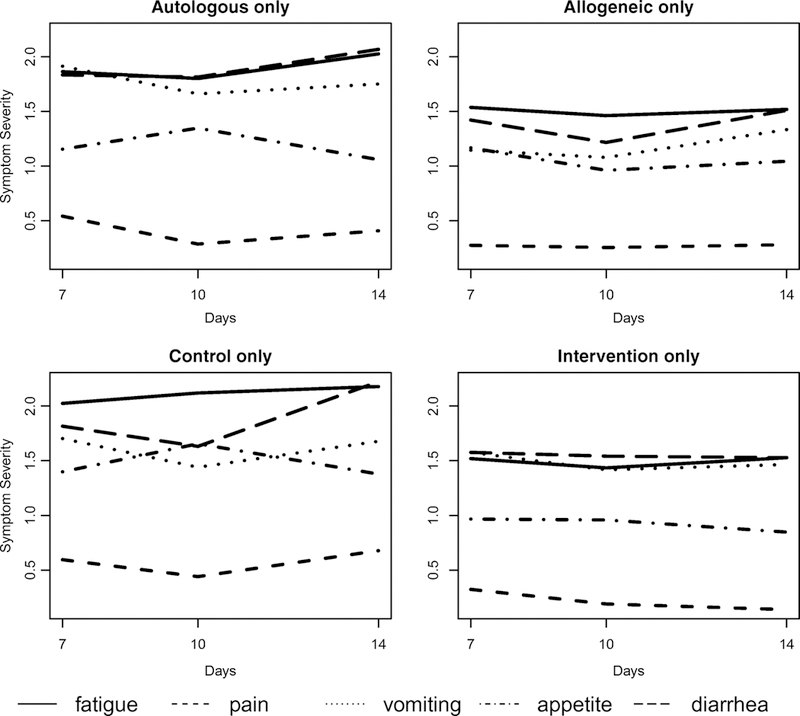
Peak Symptom Burden Groups
Upper left: Mean symptom severities at days 7 (n=40), 10 (n=25), and 14 (n=20) of patients under ‘Autologous’ transplant.
Upper right: Mean symptom severities at days 7 (n=23), 10 (n=17), and 14 (n=19) of patients under ‘Allogenic’ (right) transplant.
Lower left: Mean symptom severities at days 7 (n=28), 10 (n=14), and 14 (n=15) of patients under ‘Control’ (no feedback).
Lower right: Mean symptom severities at days 7 (n=35), 10 (n=28), and 14 (n=24) of patients under ‘Intervention’ (with feedback)
Multivariate analysis:
After controlling for possible confounders including age, transplant type, conditioning type, HCT-CI, and symptom burden at baseline, the control group still had higher (difference = 4.38) average peak symptom burden compared to that of the intervention group, but the difference was only marginally significant (p-value = 0.08). (Table 3).
Discussion
Little is known about PROs in HCT inpatients, and our study presents an in-depth investigation of HCT patients’ symptoms that are typically at their highest peak at days +7, +10, and +14 post-transplant. This single site, pilot randomized trial showed that HCT inpatients who electronically self-reported their symptoms on a daily basis and received feedback from the clinical care team had improved symptom scores compared to the attention control group. To date, no other studies have assessed daily self-reporting of symptoms of inpatient HCT patients with reports to the medical team. A definitive trial using larger sample size from multi-sites is warranted to examine generalizability of findings, efficacy and value of the findings.
We focused on peak symptom burden +7, +10 and +14 days post-SCT as a measure of the aggregate symptomatic experience because synergies between side effects can worsen the overall symptomatic experience;25,26 we focused on peak symptom burden (day +7, +10 and +14) as a measure of the aggregate symptomatic experience. For example, vomiting was higher in the usual care group at day +10 compared to the intervention group. We have little explanation for this significant difference. Routine symptom assessment and feedback from providers in response to the symptoms can lead to a better appreciation of peak symptom burden in regular practice and may stimulate the development of interventions that can reduce symptoms and improve health related QOL. In addition to receiving the HCT, patients take medications for GVHD prophylaxis including Tacrolimus and Methotrexate which may impact their symptom burden during hospitalization. As oncology nurses are integral team members in the management of transplant symptoms, nursing-directed interventions are a logical place to start for peri-transplant symptom burden management.
Our work is similar to that of Wood et al9, who found that it was feasible and acceptable to collect electronic PROs routinely on patients during and beyond the HCT period. Bevans et al23 found prevalent transplant symptoms of fatigue and decreased appetite in the first 100 days following an allogeneic HCT. Fatigue was particularly notable in all studies, and this has been found in several prior studies to be a persistent and bothersome symptom during and post transplantation12,25,26. With this information, early identification and management of fatigue in the transplant period may represent an opportunity to reduce long-term fatigue in transplant survivors through physical activity interventions and clustering care to conserve their energy. survivors through physical activity interventions and clustering care to conserve their energy.
Lastly, our findings support ongoing efforts to implement routine symptom monitoring into the care of all oncology patients. Basch et al17 demonstrated that routine electronic symptom reporting and intervention in patients receiving outpatient care for advanced solid tumors was associated with decreased health care utilization, improved QOL, and prolonged survival. Nipp et al18 also found that hospitalized patients with cancer were able to complete the symptom assessments. Adults receiving inpatient care for HCT have different trajectories of symptom burden than those in outpatient care (e.g., receiving chemotherapy for solid tumors). Similarly, trial endpoints for inpatient care (transplant) need to be carefully considered. Basch et al16,17 found decreased emergency room use among outpatient cancer patients, but this endpoint is not applicable to inpatient settings. Trial endpoints for inpatient oncology care would likely include survival, symptom trajectories, function, quality of life, and days hospitalized. Other measures of acuity care include transfer to the medical intensive care unit (MICU) and use of rapid response teams but me useful endpoints if the sample was larger. HCT trials would also need to continue to assess these trial endpoints in the early post-transplant period. Currently, daily symptom reporting is not standard of care in the U.S. and warrants further research before it becomes a routine component of inpatient care.
While this study is unique in its innovative acquisition and use of electronic patient reported symptoms in the inpatient setting, it has a few limitations. First, it was undertaken at a single comprehensive cancer center, thereby limiting its generalizability Second, we had 30% missing data from electronic symptom reporting and this may be due to high symptom burden. While the omissions were evenly distributed between the two arms, this is a significant logistical challenge that should be addressed before undertaking a larger study. Third, gender was evenly distributed between arms, but men were significantly underrepresented. One plausible explanation is that an NCI funded exercise study was being undertaken concurrently with this trial in an overlapping patient population. That study competed with this one and since more males engaged in the exercise study, a higher number of female HSCT recipients were available to participate in this trial. Fourth, although relevant symptoms were identified through expert opinion and extant literature, the aggregate PRO-CTCAE symptom score used in this study has not been specifically validated. not been specifically validated.
In conclusion, our findings suggest that routine daily electronic symptom monitoring and intervention in patients hospitalized for HCT may lead to reductions in peak symptom burden and improve individual symptoms during inpatient hospitalization. A multi-site trial is warranted to examine the generalizability and overall value of this intervention during inpatient hospitalization. Further studies are also needed to examine daily PRO monitoring (or daily patient reported symptoms) during the post-transplant period. If this approach proves successful, integration of symptom reporting within the electronic health record could support dissemination of this approach throughout the practice of HCT
Acknowledgments
This study was supported by a 5K12CA120780-07 (Bryant), University Cancer Research Fund UL1RR025747 (Bryant and Wood), and T32 NR00709 (Hirschey). The University of North Carolina’s Patient-Reported Outcomes Core is supported in part by the University Cancer Research Fund and the Lineberger Comprehensive Cancer Center core grant (P30-CA-016086).
Dr. Bill Wood has funding support from Pfizer, Genetech, Koneksa Health, and Best Doctors.
I do not have a financial relationship with the organization that sponsored the research.
Footnotes
I have full control of all primary data and will agree to allow the journal to review the data if requested.
Conflict of Interest
Contributor Information
Ashley Leak Bryant, School of Nursing, The University of North Carolina at Chapel Hill, Carrington Hall, CB #7460, Chapel Hill, NC 27599-7460.
Erin Coffman, Department of Nutrition, Gillings School of Global Public Health, University of North Carolina at Chapel Hill.
Brett Phillips, Hemophilia and Thrombosis Center, Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill.
Xianming Tan, UNC Lineberger Biostatistics Core, The University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7460.
Elizabeth Bullard, North Carolina Cancer Hospital, UNC Hospitals.
Rachel Hirschey, School of Nursing, University of North Carolina at Chapel Hill.
Joshua Bradley, North Carolina Cancer Hospital, UNC Hospitals.
Antonia V. Bennett, Department of Health Policy and Management, The University of North Carolina at Chapel Hill.
Angela M. Stover, Department of Health Policy and Management, Gillings School of Global Public Health, University of North Carolina at Chapel Hill.
Lixin Song, School of Nursing, University of North Carolina at Chapel Hill.
Thomas C Shea, Division of Hematology/Oncology, The University of North Carolina at Chapel Hill.
William A. Wood, Division of Hematology/Oncology, The University of North Carolina at Chapel Hill.
References
- 1.Bevans MF, Mitchell SA, Barrett AJ, et al. Function, adjustment, quality of life and symptoms (FAQS) in allogeneic hematopoietic stem cell transplantation (HSCT) survivors: a study protocol. Health and quality of life outcomes. 2011; 9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood. 2014;124(3):344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang Y, Zhou M, Wang F, Wu Z. Exercise for physical fitness, fatigue and quality of life of patients undergoing hematopoietic stem cell transplantation: a meta-analysis of randomized controlled trials. Japanese journal of clinical oncology. 2018;48(12):1046–1057. [DOI] [PubMed] [Google Scholar]
- 4.Nakagaki M, Barras M, Curley C, Butler JP, Kennedy GA. A randomized trial of olanzapine versus palonosetron versus infused ondansetron for the treatment of breakthrough chemotherapy-induced nausea and vomiting in patients undergoing hematopoietic stem cell transplantation. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2017;25(2):607–613. [DOI] [PubMed] [Google Scholar]
- 5.Liu D, Yan C, Xu L, et al. Diarrhea during the conditioning regimen is correlated with the occurrence of severe acute graft-versus-host disease through systemic release of inflammatory cytokines. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16(11):1567–1575. [DOI] [PubMed] [Google Scholar]
- 6.Bowen JM, Wardill HR. Advances in the understanding and management of mucositis during stem cell transplantation. Current opinion in supportive and palliative care. 2017;11(4):341–346. [DOI] [PubMed] [Google Scholar]
- 7.Ma JD, El-Jawahri AR, LeBlanc TW, Roeland EJ. Pain Syndromes and Management in Adult Hematopoietic Stem Cell Transplantation. Hematology/oncology clinics of North America. 2018;32(3):551–567. [DOI] [PubMed] [Google Scholar]
- 8.Boonstra L, Harden K, Jarvis S, et al. Sleep disturbance in hospitalized recipients of stem cell transplantation. Clinical journal of oncology nursing. 2011;15(3):271–276. [DOI] [PubMed] [Google Scholar]
- 9.Wood WA, Deal AM, Abernethy A, et al. Feasibility of frequent patient-reported outcome surveillance in patients undergoing hematopoietic cell transplantation. Biology of Blood and Marrow Transplantation. 2013;19(3):450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stover A, Irwin DE, Chen RC, et al. Integrating Patient-Reported Outcome Measures into Routine Cancer Care: Cancer Patients’ and Clinicians’ Perceptions of Acceptability and Value. EGEMS (Washington, DC). 2015;3(1):1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green AK, Reeder-Hayes KE, Corty RW, et al. The project data sphere initiative: accelerating cancer research by sharing data. The oncologist. 2015;20(5):464–e420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood WA, Deal AM, Abernethy A, et al. Feasibility of frequent patient-reported outcome surveillance in patients undergoing hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013;19(3):450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCurdy SR, Kanakry CG, Tsai HL, et al. Development of Grade II Acute Graft-Versus-Host Disease is Associated with Improved Survival after Myeloablative HLA-Matched Bone Marrow Transplantation using Single-Agent Post-Transplant Cyclophosphamide. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atilla E, Atilla PA, Bozdag SC, Demirer T. A review of infectious complications after haploidentical hematopoietic stem cell transplantations. Infection. 2017;45(4):403–411. [DOI] [PubMed] [Google Scholar]
- 15.Bryant AL, Coffman EM, Bullard E, et al. Experiences of Inpatient Bone Marrow Transplantation Nurses and Providers Using Electronic Symptom Reporting. Journal of oncology practice. 2018;14(8):e496–e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basch E, Deal AM, Dueck AC, et al. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. Jama. 2017;318(2):197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basch E, Deal AM, Kris MG, et al. Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. J Clin Oncol. 2016;34(6):557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nipp RD, El-Jawahri A, Ruddy M, et al. Pilot Randomized Trial of an Electronic Symptom Monitoring Intervention for Hospitalized Patients with Cancer. Ann Oncol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basch E, Reeve BB, Mitchell SA, et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). Journal of the National Cancer Institute. 2014;106(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hay JL, Atkinson TM, Reeve BB, et al. Cognitive interviewing of the US National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2014;23(1):257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and Reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA oncology. 2015;1(8):1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atkinson TM, Hay JL, Dueck AC, et al. What Do “None,” “Mild,” “Moderate,” “Severe,” and “Very Severe” Mean to Patients With Cancer? Content Validity of PRO-CTCAE Response Scales. J Pain Symptom Manage. 2018;55(3):e3–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bevans MF, Mitchell SA, Marden S. The symptom experience in the first 100 days following allogeneic hematopoietic stem cell transplantation (HSCT). Support Care Cancer. 2008;16(11):1243–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esser P, Kuba K, Scherwath A, et al. Stability and Priority of Symptoms and Symptom Clusters Among Allogeneic HSCT Patients Within a 5-Year Longitudinal Study. J Pain Symptom Manage. 2017;54(4):493–500. [DOI] [PubMed] [Google Scholar]
- 26.Kroemeke A, Sobczyk-Kruszelnicka M, Kwissa-Gajewska Z. Everyday life following hematopoietic stem cell transplantation: decline in physical symptoms within the first month and change-related predictors. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2018;27(1):125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]


