Abstract
The Developmental Origins of Disease hypothesis has spurred increased interest in how prenatal exposures affect lifelong health, while mechanisms such as epigenetics may explain the multigenerational influences on health. Such factors are not well captured within conventional epidemiologic study designs. We explored the feasibility of collecting information on the offspring and grand-offspring of participants in a long-running study. The Bogalusa Heart Study is a study, begun in 1973, of life-course cardiovascular health in a semirural population (65% white and 35% black). Female participants who had previously provided information on their pregnancies were contacted to obtain contact information for their daughters aged 12 and older. Daughters were then contacted to obtain reproductive histories, and invited for a clinic or lab visit to measure cardiovascular risk factors. 274 daughters of 208 mothers were recruited; 81% (223) had a full clinic visit, 19% (51) a phone interview only. 45% of the daughters were black, 55% white. Mean and median age at interview was 27, with 15% under the age of 18. The strongest predictors of participation were black race, recent maternal participation in the parent study, and living in or near Bogalusa. Simple correlations for cardiovascular risk factors across generations were between r=0.19 (systolic blood pressure) and r=0.39 (BMI, LDL). It is feasible to contact the children of study participants even when participants are adults, and initial information on the grandchildren can also be determined in this manner.
Keywords: transgenerational, developmental origins of health and disease, methods, epidemiologic, cardiovascular health, birth outcomes
Introduction
The trajectory for a person’s health may be set before they are born. Early life experiences, even those in utero, have lasting consequences for health,1–4 which have been demonstrated for conditions ranging from cardiovascular disease to osteoporosis2, 5. Prenatal and perinatal environment permanently influence offspring health through biological mechanisms which are only partially understood, but may include microbiome transfer6, 7 or epigenetic changes8, 9. Although genome-wide association studies have identified hundreds of genetic variants associated with complex human diseases and traits, these explain only a small proportion of the variability in phenotypes, leading many to question how the remaining, “missing” heritability can be explained10.
Animal studies on genetically similar organisms indicate the possibility of transgenerational inheritance relevant to perinatal, metabolic, and cardiovascular health (The term “transgenerational” is used inconsistently, especially in human/clinical studies; in this article, we will use “transgenerational” to refer to research on 3 generations or more.) These studies have demonstrated multigenerational and/or transgenerational effects of nutrition, smoking, and environmental pollutants11–15, with the offspring and grandoffspring demonstrating altered phenotypes. In particular, nutrient and growth restriction (and sometimes overprovision) in generation 1 have been demonstrated to lead to changes in glucose and insulin metabolism in generation 3 in rats16–19, mice20, and swine12.
However, very few human studies have been able to examine possible multigenerational effects on important outcomes like low birthweight, obesity, or blood pressure. In particular, few studies start in early life and run long-term. While mother-child dyads are commonly recruited for some studies21–23, and adult children of existing cohorts have been recruited in other situations24–26, rarely are studies established in a way that allow for multiple generations to be followed starting prior to pregnancy/reproduction in the first generation. We conducted a study to examine the feasibility of establishing a two- and three-generation study building on the Bogalusa Heart Study (BHS). Because of our particular interest in reproductive outcomes, we focused on daughters of BHS women.
Method
The Bogalusa Heart Study (BHS) is a series of studies of cardiovascular health in a semirural, biracial population (65% white and 35% black), founded by Dr. Gerald Berenson. The first exams were conducted in 1973, of children aged 3–18, which were followed by 9 surveys of children aged 5–17 years and 9 of young adults aged 18–44 through 1995; these surveys have been combined to create the BHS study population (Table 1). 1803 women participated in the Bogalusa Babies study was a study of reproductive health and pregnancy outcomes nested in BHS; women were interviewed by phone or in the clinic about their pregnancy history. The Bogalusa Daughters project gathered data on female offspring ages 12 and older of participants (Age 12 was chosen as the average age of menarche for girls, as well as an age when participants were likely to be able to understand the basic idea of the study topic and thus give informed assent.) There were an estimated 1622 daughters in the relevant age range. For this feasibility study, we proposed recruiting 240 of those daughters. The sample size was chosen based a sample size calculation that this would provide power to find plausible if fairly large effect sizes as well as the pilot funding available (supplementary material).
Table 1.
Events and study measures across three generations, the Bogalusa Heart Study
| 1970s | 1980s | 1990s | 2000s | 2010–2015* | 2015-* | |
|---|---|---|---|---|---|---|
| data collection phase | Parent BHS | Parent BHS | Parent BHS | Parent BHS | Bogalusa Babies | Bogalusa Daughters |
| mother events | CV measures | CV measures pregnancies | CV measures pregnancies | CV measures pregnancies | CV measures pregnancies | CV measures |
| daughter events | births | births | births | CV measures | ||
| grandchild events | births | births |
Data collection continued and is ongoing for other studies of generation 1, not mentioned in this paper CV, cardiovascular
Study procedure varied slightly depending on the age and geographic location of the participants. For the Bogalusa Daughters protocol, Bogalusa Babies participants were first contacted by mail and phone, and contact information for their daughters obtained. The daughter was then contacted by telephone, email and/or text message. If she was able to attend a clinic visit, blood pressure, height, and weight were measured and a fasting blood draw taken. An interview was conducted about her reproductive history, and medical records were requested about her pregnancies. If the daughter was aged 12–17, a modified version of this protocol was implemented, with fewer questions about pregnancy. Daughters who lived too far away to visit the clinic, or who did not wish to, were interviewed by telephone. If they were willing to provide biological measures but did not live near Bogalusa, they were invited to visit a local LabCorp for lipid, glucose, and blood pressure measurements. Mothers were provided with $10 for providing contact information for a daughter, and daughters received $20 for the interview, $20 for the blood draw, and $20 for clinical measurements (height, weight, blood pressure). All payments were in the form of gift cards.
Cardiovascular risk factors
Anthropometric measurements were conducted on individuals in light clothing without shoes using a standard protocol. Determinations of height (to nearest 0.1 cm) and weight (to nearest 0.1 kg) were performed in duplicate. Blood pressure was measured using the HEM 907 ZL Non-Invasive Blood Pressure Monitor, by Omron HealthCare CO, LTD, Kyoto Japan. A total of four readings were taken and averaged for analyses. Plasma glucose was measured using the hexokinase method. Serum total cholesterol and triglycerides were measured by enzymatic procedures.
Reproductive outcomes
Adult women were interviewed about their age at menarche, gravidity, parity, fertility issues, birthweight and gestational age of their children, and pregnancy complications such as gestational diabetes (GDM), pregnancy-induced hypertension (PIH), and pre-eclampsia, using a standardized form. These questions have been used in the Bogalusa Babies study and self-report for pregnancy history is generally reliable.27–29 Other related data was also collected: indicators of infertility (time to pregnancy, fertility treatments, symptoms and diagnosis of polycystic ovarian syndrome) and duration of breastfeeding. Adolescents (<18) were asked about age at menarche, and any children they had; if they reported having children, information about those pregnancies was gathered.
Covariates
Additional information was collected on age, race, marital status, smoking, and educational level. For comparisons between participants and non-participants, covariate information was taken from the mother’s report. Race of daughter was assumed to be the same as the mother’s. As part of a Babies data collection on social factors, mothers were asked for their oldest child’s highest level of education and whether their oldest child lived in Bogalusa.
Analysis
First, we examined the numbers and proportions of women who were recruited, the channels of recruitment, the response rate, and the type of visit (home or clinic). Characteristics of the recruited sample and their mothers were then compared to the population eligible for the study, as defined by the mothers’ report during Bogalusa Babies of the year of birth and sex of her children. Study-related variables included whether the woman was a participant as well as a daughter, number of visits by the mother, and year of earliest and most recent study visit. Demographic factors compared included age, race, county of residence, financial situation, and educational level. Cardiovascular risk factors included BMI, history of hypertension and diabetes, systolic blood pressure, and total cholesterol. These were compared across the relevant study populations using t-tests and ANOVA for continuous variables, and chi-square tests for categorical and dichotomous variables. We also created a predictive logistic model incorporating all variables that were significant at 0.15 or less, to determine which were the strongest predictors. Participating and non-participating daughters were compared on race, age, number of biological siblings, education, and whether they lived in Bogalusa. The last two, because of the type of data collected from the mother, were limited to daughters who were the oldest child in their family.
Correlations across generations were examined using Pearson and Spearman correlations. Generalized estimating equations were used to examine the strength of the association with adjustment for clustering within family, as well as with adjustment for maternal and daughter’s age.
All participants 18+ provided written informed consent, and parental consent and daughters’ assent was obtained for those <18. The project was approved by the Tulane Institutional Review Board.
Results
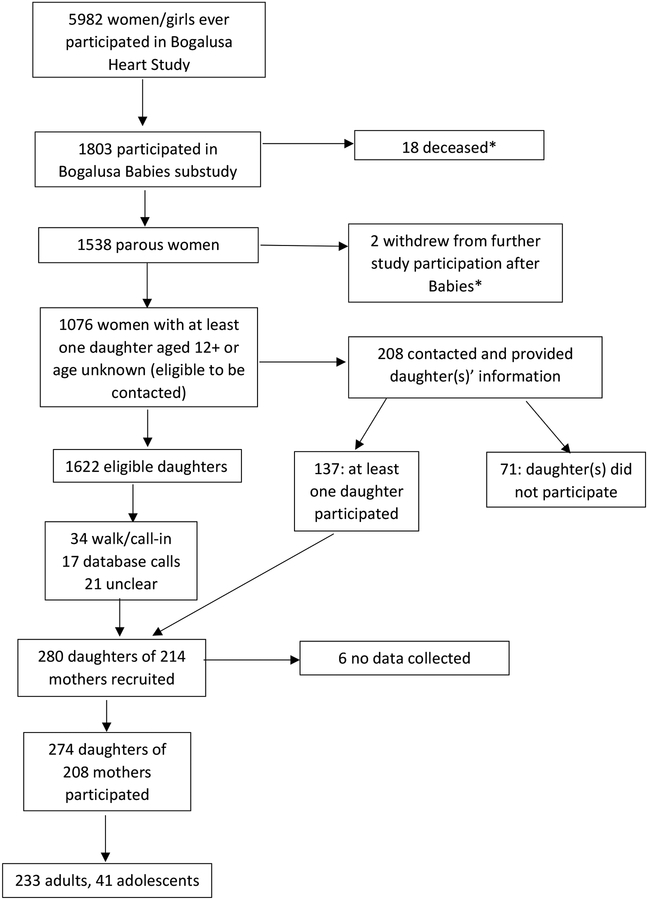
Of the 208 mothers who were contacted and provided their daughter’s information, 137 had at least one daughter participate. Some of those women had multiple daughters participate, for a total of 208 daughters (Figure 1). Other participants were recruited via telephone calls, call- or walk-ins (who usually learned about the study from friends, family members, or other word of mouth), letter, or word of mouth. We did not use social media to recruit because the eligibility requirements were quite different from standard BHS follow-ups and it was thought to be potentially confusing, especially for a pilot study. Generally, at least two calls were necessary, one as an introduction, leaving a voicemail message, and a second for the actual recruitment/interview. 223 (81%) of Daughters participants attended a clinic visit and 51 (19%) did a phone interview. 41 completed a phone interview and agreed to attend a LabCorp near them for basic measures; 15 followed through on this. Ninety-seven daughters had previously participated in BHS (because the first decades of the study conducted cross-sectional studies of the range of school age in the community, it was possible for someone recruited as, say, a 16-year-old in 1978 to have a daughter participate as a 6-year-old in 1988). Forty-one (15%) of the participants were adolescents and the remainder adults (Figure 1).
Figure 1.
Study population
*Daughters of deceased participants or participants who withdrew were eligible to participate if they learned about the study through other means
Maternal predictors of daughters’ participation included black race, lower education, older age at oldest visit, living and being born in the Bogalusa area, less good financial situation, and recency of study visits (Table 2). In a multivariable model, the strongest predictors were race, current residence in the Bogalusa area, and recency of study visits (data not shown). From a selection perspective (do those who participated differ in their cardiovascular risk), participants were somewhat more likely to have a history of diabetes and a higher BMI; history of hypertension was highest in those who gave information on their daughters but whose daughters did not participate. Mean cholesterol and systolic blood pressure did not differ across the groups. Daughters who participated were slightly older than the average of the eligible population (Table 3), consistent with our recruiters’ impression that is was harder to recruit adolescents than adults. Living in Bogalusa was a strong predictor of participating among daughters, while education and number of siblings were not.
Table 2.
Maternal factors predicting daughters’ participation
| daughter participated (n=208 mothers) | mother provided information, daughter did not participate (n=71) | other eligible Babies participants (n=797) | p for difference | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| number of visits | <0.01 | ||||||
| 1 | 21 | 10.6 | 4 | 5.6 | 84 | 11.1 | |
| 2 | 15 | 7.5 | 3 | 4.2 | 86 | 11.4 | |
| 3–4 | 27 | 13.6 | 4 | 5.6 | 160 | 21.1 | |
| 5–6 | 33 | 16.6 | 15 | 21.1 | 160 | 21.1 | |
| 7 or more | 103 | 51.8 | 45 | 63.4 | 268 | 35.4 | |
| race | <0.01 | ||||||
| black | 105 | 50.5 | 41 | 57.8 | 303 | 38.2 | |
| white | 103 | 49.5 | 30 | 42.3 | 490 | 61.8 | |
| education | <0.01 | ||||||
| high school or less | 119 | 57.2 | 35 | 49.3 | 292 | 36.6 | |
| associates/some college | 55 | 26.4 | 18 | 25.4 | 278 | 34.9 | |
| college or more | 34 | 16.4 | 18 | 25.4 | 227 | 28.5 | |
| mean | SD | mean | SD | mean | SD | ||
| age at youngest visit | 10.7 | 6.4 | 9.6 | 3.4 | 10.3 | 7.2 | 0.47 |
| age at oldest visit | 33.0 | 12.7 | 34.1 | 11.9 | 27.3 | 12.6 | <0.01 |
| age in 2016 | 50.4 | 7.0 | 50.8 | 6.2 | 49.3 | 7.7 | 0.06 |
| year at first visit* | 1974 | 1974–1981 | 1974 | 1973–1978 | 1976 | 1974–1982 | <0.01 |
| year at most recent visit* | 2004 | 1994–2008 | 2008 | 1993–2008 | 1996 | 1989–2004 | <0.01 |
| Current residence | <0.01 | ||||||
| Washington Parish** | 172 | 84.7 | 56 | 78.9 | 482 | 62.1 | |
| neighboring parish/county | 12 | 5.9 | 8 | 11.3 | 86 | 11.1 | |
| Other Louisiana/Mississippi | 15 | 7.4 | 4 | 5.6 | 111 | 14.3 | |
| Other | 4 | 2.0 | 3 | 4.2 | 97 | 12.5 | |
| Born in Bogalusa | 0.03 | ||||||
| Yes | 172 | 84.3 | 62 | 88.6 | 620 | 78.5 | |
| No | 32 | 15.7 | 8 | 11.4 | 170 | 21.5 | |
| Current financial situation | <0.01 | ||||||
| less than enough/poor | 26 | 12.6 | 12 | 16.9 | 99 | 12.4 | |
| enough to get by/neither poor nor well-off | 125 | 24.8 | 42 | 59.2 | 338 | 42.5 | |
| more than enough/well-off | 55 | 26.7 | 17 | 23.9 | 359 | 45.1 | |
| ever smoker | 95 | 51.4 | 32 | 48.5 | 379 | 55.7 | 0.22 |
| ever drank alcohol regularly | 68 | 11.2 | 10 | 16.7 | 24 | 14.0 | 0.23 |
| history of hypertension | 50 | 24.0 | 25 | 35.2 | 153 | 19.2 | 0.04 |
| history of diabetes | 17 | 8.2 | 1 | 1.4 | 36 | 4.5 | 0.06 |
| child BMI | 17.7 | 3.5 | 17.0 | 2.7 | 17.3 | 3.0 | 0.25 |
| adolescent BMI | 21.2 | 4.0 | 21.4 | 4.6 | 21.4 | 4.4 | 0.91 |
| adult BMI | 28.0 | 6.7 | 26.9 | 7.1 | 26.5 | 6.6 | 0.05 |
| child cholesterol | 181.2 | 27.8 | 184.5 | 25.9 | 166.2 | 26.9 | 0.15 |
| adolescent cholesterol | 161.6 | 26.1 | 161.0 | 24.5 | 160.4 | 26.5 | 0.88 |
| adult cholesterol | 181.2 | 27.8 | 184.5 | 25.9 | 183.0 | 34.3 | 0.75 |
| child systolic blood pressure | 98.0 | 8.8 | 96.7 | 7.8 | 97.0 | 8.2 | 0.42 |
| adolescent systolic blood pressure | 107.2 | 7.8 | 107.6 | 7.0 | 107.8 | 8.1 | 0.75 |
| adult systolic blood pressure | 120.6 | 18.0 | 122.6 | 17.8 | 121.5 | 18.3 | 0.80 |
median, IQR, Wilcoxon test
Counties are called parishes in Louisiana. Washington Parish is the location of Bogalusa, and it borders Mississippi.
Table 3.
Daughter factors predicting daughters’ participation
| Daughters (n=274) | other eligible daughters (n=1282) | ||||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Race* | <0.01 | ||||
| black | 124 | 45.3 | 524 | 41.0 | |
| white | 150 | 54.7 | 754 | 59.0 | |
| Daughter’s age in 2016 | 25.9 | 8.4 | 24.7 | 8.6 | 0.03 |
| Number of biological siblings | 0.18 | ||||
| 0 | 21 | 8.1 | 103 | 8.1 | |
| 1 | 84 | 32.2 | 461 | 36.3 | |
| 2–3 | 130 | 49.8 | 543 | 42.8 | |
| 4+ | 26 | 10.0 | 163 | 12.8 | |
| Education** | 0.21 | ||||
| High school or less | 69 | 44.2 | 323 | 50.2 | |
| associates/some college | 57 | 36.5 | 189 | 29.4 | |
| college or more | 30 | 19.2 | 131 | 20.4 | |
| Lives in Bogalusa** | 91 | 70.0 | 288 | 51.0 | <0.01 |
For non-participants, assumes daughter of same race as mother
Limited to oldest children. Self-report for participating daughters, maternal report for non-participants. As part of a Babies data collection on social factors, mothers were asked for their oldest child’s highest level of education and whether their oldest child lived in Bogalusa
The completeness of clinical data varied by whether the participant could attend a clinic visit (for the daughters) and whether the woman had participated in BHS as an adult (for the mothers). Some measures were also not consistently measured in BHS, such as age at menarche, leading to missing data from the earlier visits. BMI and glucose levels were higher in the daughters than mothers or the overall study population, while age at menarche and birthweight at first pregnancy were similar. Lipid levels were not significantly different for those who attended a LabCorp versus those who had a clinic visit (data not shown).
Simple correlations across generations were between r=0.19 (systolic blood pressure) and r=0.39 (BMI, LDL; Table 4). When adjusted for clustering within family and maternal age, the same general patterns held, with the exception that glucose was no longer significantly correlated (although the size of the beta coefficient was similar to other factors).
Table 4.
Correlations for cardiovascular health between mother and daughters
| daughters (total N=274) | mothers (total N=208) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | mean | median | SD | min | max | N | mean | median | SD | min | max | |
| current age | 274 | 27.0 | 27.0 | 8.4 | 12 | 53.2 | 198 | 46.9 | 47.0 | 6.8 | 28.0 | 61.0 |
| Age at 1st pregnancy | 137 | 20.7 | 20.0 | 4.3 | 13.0 | 32.7 | 198 | 20.5 | 19.8 | 4.2 | 14.3 | 33.7 |
| BMI* | 227 | 31.9 | 30.4 | 9.2 | 14.1 | 71.1 | 161 | 28.0 | 26.2 | 6.7 | 17.9 | 46.8 |
| systolic blood pressure | 227 | 118.5 | 116.0 | 13.5 | 84.0 | 175.0 | 161 | 110.9 | 109.7 | 10.1 | 92.7 | 147.1 |
| diastolic blood pressure | 225 | 75.5 | 75.0 | 11.1 | 51.0 | 112.0 | 161 | 73.6 | 72.9 | 6.8 | 56.3 | 96.9 |
| HDL | 219 | 51.6 | 50.0 | 13.7 | 21.0 | 99.0 | 161 | 52.9 | 53.0 | 11.4 | 29.0 | 99.0 |
| LDL | 218 | 100.0 | 98.0 | 29.1 | 37.0 | 228.0 | 161 | 114.1 | 14.8 | 25.3 | 60.9 | 187.8 |
| total cholesterol | 219 | 170.1 | 168.0 | 34.4 | 18.0 | 289.0 | 161 | 181.3 | 182.4 | 27.8 | 126.5 | 260.0 |
| triglycerides | 218 | 92.8 | 77.0 | 49.9 | 30.0 | 376.0 | 161 | 97.6 | 89.0 | 55.6 | 33.0 | 548.3 |
| glucose | 209 | 97.4 | 93.0 | 26.5 | 66.0 | 323.0 | 160 | 84.7 | 82.0 | 13.6 | 65.0 | 171.3 |
| age at menarche | 271 | 12.6 | 12.0 | 1.8 | 8.0 | 20.0 | 98 | 12.6 | 13.0 | 1.6 | 9.0 | 18.0 |
| birthweight of first baby | 132 | 3160 | 3175 | 527 | 907 | 4323 | 196 | 3184 | 3175 | 646 | 907 | 5613 |
| GEE, clustering within family | GEE, clustering within family+ adjustment for age | ||||||
|---|---|---|---|---|---|---|---|
| r* | beta | SE | p | beta | SE | p | |
| current age | |||||||
| Age at 1st pregnancy | |||||||
| BMI* | 0.39 | 0.55 | 0.12 | <0.01 | 0.54 | 0.12 | <0.01 |
| systolic blood pressure | 0.19 | 0.26 | 0.10 | 0.01 | 0.22 | 0.1 | 0.03 |
| diastolic blood pressure | 0.24 | 0.41 | 0.13 | <0.01 | 0.39 | 0.11 | <0.01 |
| HDL | 0.31 | 0.35 | 0.07 | <0.01 | 0.34 | 0.07 | <0.01 |
| LDL | 0.34 | 0.36 | 0.07 | <0.01 | 0.34 | 0.08 | <0.01 |
| total cholesterol | 0.33 | 0.37 | 0.08 | <0.01 | 0.36 | 0.08 | <0.01 |
| triglycerides | 0.32 | 0.24 | 0.06 | <0.01 | 0.23 | 0.05 | <0.01 |
| glucose | 0.19 | 0.39 | 0.28 | 0.17 | 0.26 | 0.28 | 0.35 |
| age at menarche | |||||||
| birthweight of first baby | 0.20 | 0.17 | 0.06 | <0.01 | 0.15 | 0.05 | <0.01 |
all p<0.05
GEE, generalized estimating equation; BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein
Discussion
Our feasibility study suggests that children of participants in a community-based, long-term study can be recruited via the original study participants. Participants were generally willing to provide contact information for their children, although not all children responded when contacted. Black women, those with less education, and those who lived in the study area were over-represented in the study group compared to the eligible population. Completeness of data was in some cases limited by practical factors, including the extent and timing of participation of the mother previously and the data that had been collected as part of the parent study. As expected, maternal and daughter cardiovascular risk factors were correlated (with the possible exception of glucose). These correlations were statistically strong though weak to moderate in magnitude.
Transgenerational research has become of increasing interest, as developmental origins of disease and epigenetic research have expanded, and the ‘missing heritability’ paradox has left family correlations unexplained.30, 31 However, few transgenerational studies have been conducted in humans.32–37,38 The relatively small number of studies reflects the difficulty of assembling such cohorts, requiring as it does long-term record availability or follow-up of an existing cohort. Even when long-term records are available, routinely collected data such as medical records may not provide the level of detail that researchers require to investigate developmental origins. Overall, previous studies recruiting multiple generations emphasize the importance of personal connections and family buy-in. A study in Japan found that grandparents most often participated if both a pregnant woman and her partner participated, instead of just the pregnant woman.39 Complex eligibility criteria limit recruitment, while recommendations from community contacts or other participants are most effective.40 Theory-informed recruitment of children and grandchildren of Jackson Heart Study participants found the employment as recruiters of individuals with multiple contact networks, communication skills, and personalities that influence others to be an effective strategy.41 These studies’ experiences are consistent with ours, in which recent and frequent participation in the study by the mother was one of the strongest predictors, a sizable proportion of daughters were also primary BHS participants, and sisters sometimes learned of the study from each other. The recruiters’ impressions were that a mother’s continued participation in the BHS was a major plus in recruitment of daughters. If the mother was a willing and invested participant, the odds of enrolling the daughters were greater. A large proportion of our study population lived in or near Bogalusa, suggesting that other methods may be necessary for transgenerational research in populations that are more mobile. The somewhat narrow eligibility criteria (and budget) also limited our ability to extend to recruitment avenues such as social media or community outreach.
Transgenerational studies will be important to understand the development of disease in populations. Traditional genetic mechanisms explain only a small proportion of the familial clustering of phenotypes, suggesting other underlying mechanisms need to be explored. e10. While environment and behavior are also strongly correlated within families,42–44 recent works in epigenetics suggests possible routes of multigenerational effects as well45, 46
Strengths of our study include the community-based original study sample, the racial diversity, and the rigorous measures of cardiovascular health. Limitations include the inevitable loss to follow-up and possible associated selection bias, as well as the variability in length of time before pregnancy of measurements in generation 1. In addition, we consider only the daughters of female participants; future work may expand to sons and offspring of male participants. With this study, we lay the groundwork for future cross-generational study in this population.
Supplementary Material
Acknowledgements
Financial support
The Bogalusa Heart Study is supported by grants R01HD069587, R01HL016592, R01AG041200, P50HL015103, R01HD032194.
Bogalusa Daughters is supported in part by U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support provided by the Tulane Bridge Fund and the Bernick Faculty Research Grants.
Footnotes
None of the authors have a conflict of interest.
References
- 1.Duijts L, Reiss IK, Brusselle G, de Jongste JC. Early origins of chronic obstructive lung diseases across the life course. Eur J Epidemiol. 2014;29(12), 871–885. [DOI] [PubMed] [Google Scholar]
- 2.Koletzko B, Decsi T, Molnár D, Hunty A. Early nutrition programming and health outcomes in later life: obesity and beyond, 2009. Springer Science & Business Media. [Google Scholar]
- 3.Barker DJ. Fetal origins of coronary heart disease. BMJ (Clinical research ed). 1995;311(6998), 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31(6), 1235. [DOI] [PubMed] [Google Scholar]
- 5.Sly PD. The early origins of asthma: who is really at risk? Curr Opin Allergy Clin Immunol. 2011;11(1), 24–28. [DOI] [PubMed] [Google Scholar]
- 6.Galley JD, Bailey M, Kamp Dush C, Schoppe-Sullivan S, Christian LM. Maternal obesity is associated with alterations in the gut microbiome in toddlers. PLoS One. 2014;9(11), e113026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529(7585), 212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei Y, Schatten H, Sun QY. Environmental epigenetic inheritance through gametes and implications for human reproduction. Hum Reprod Update. 2015;21(2), 194–208. [DOI] [PubMed] [Google Scholar]
- 9.Stegemann R, Buchner DA. Transgenerational inheritance of metabolic disease. Semin Cell Dev Biol. 2015;43, 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manolio TA, Collins FS, Cox NJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461(7265), 747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shasa DR, Odhiambo JF, Long NM, Tuersunjiang N, Nathanielsz PW, Ford SP. Multigenerational impact of maternal overnutrition/obesity in the sheep on the neonatal leptin surge in granddaughters. Int J Obes (Lond). 2015;39(4), 695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Bulnes A, Astiz S, Ovilo C, et al. Early-postnatal changes in adiposity and lipids profile by transgenerational developmental programming in swine with obesity/leptin resistance. J Endocrinol. 2014;223(1), M17–29. [DOI] [PubMed] [Google Scholar]
- 13.Plautz SC, Guest T, Funkhouser MA, Salice CJ. Transgenerational cross-tolerance to stress: parental exposure to predators increases offspring contaminant tolerance. Ecotoxicology. 2013;22(5), 854–861. [DOI] [PubMed] [Google Scholar]
- 14.Kimberly DA, Salice CJ. If you could turn back time: understanding transgenerational latent effects of developmental exposure to contaminants. Environ Pollut. 2014;184, 419–425. [DOI] [PubMed] [Google Scholar]
- 15.Taki FA, Pan X, Lee MH, Zhang B. Nicotine exposure and transgenerational impact: a prospective study on small regulatory microRNAs. Sci Rep. 2014;4, 7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thamotharan M, Garg M, Oak S, et al. Transgenerational inheritance of the insulin-resistant phenotype in embryo-transferred intrauterine growth-restricted adult female rat offspring. Am J Physiol Endocrinol Metab. 2007;292(5), E1270–1279. [DOI] [PubMed] [Google Scholar]
- 17.Pinheiro AR, Salvucci ID, Aguila MB, Mandarim-de-Lacerda CA. Protein restriction during gestation and/or lactation causes adverse transgenerational effects on biometry and glucose metabolism in F1 and F2 progenies of rats. Clin Sci (Lond). 2008;114(5), 381–392. [DOI] [PubMed] [Google Scholar]
- 18.Tran M, Gallo LA, Jefferies AJ, Moritz KM, Wlodek ME. Transgenerational metabolic outcomes associated with uteroplacental insufficiency. J Endocrinol. 2013;217(1), 105–118. [DOI] [PubMed] [Google Scholar]
- 19.Hanafi MY, Saleh MM, Saad MI, Abdelkhalek TM, Kamel MA. Transgenerational effects of obesity and malnourishment on diabetes risk in F2 generation. Mol Cell Biochem. 2016;412(1–2), 269–280. [DOI] [PubMed] [Google Scholar]
- 20.Ding GL, Wang FF, Shu J, et al. Transgenerational glucose intolerance with Igf2/H19 epigenetic alterations in mouse islet induced by intrauterine hyperglycemia. Diabetes. 2012;61(5), 1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gea-Horta T, Silva Rde C, Fiaccone RL, Barreto ML, Velasquez-Melendez G. Factors associated with nutritional outcomes in the mother-child dyad: a population-based cross-sectional study. Public Health Nutr. 2016;19(15), 2725–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Chen HJ, Liang L, Wang Y. Parent-child resemblance in weight status and its correlates in the United States. PLoS One. 2013;8(6), e65361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dearth-Wesley T, Gordon-Larsen P, Adair LS, Zhang B, Popkin BM. Longitudinal, cross-cohort comparison of physical activity patterns in Chinese mothers and children. Int J Behav Nutr Phys Act. 2012;9, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.An Investigation of Coronary Heart Disease in Families: The Framingham Offspring Study. Am J Epidemiol. 2017;185(11), 1093–1102. [DOI] [PubMed] [Google Scholar]
- 25.Cruickshanks KJ, Nondahl DM, Johnson LJ, et al. Generational Differences in the 5-Year Incidence of Age-Related Macular Degeneration. JAMA Ophthalmol. 2017;135(12), 1417–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dougan MM, Willett WC, Michels KB. Prenatal vitamin intake during pregnancy and offspring obesity. Int J Obes (Lond). 2015;39(1), 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellison GT, de Wet T, Matshidze KP, Cooper P. The reliability and validity of self-reported reproductive history and obstetric morbidity amongst Birth to Ten mothers in Soweto. Curationis. 2000;23(4), 76–80. [DOI] [PubMed] [Google Scholar]
- 28.Rice F, Lewis A, Harold G, et al. Agreement between maternal report and antenatal records for a range of pre and peri-natal factors: the influence of maternal and child characteristics. Early Hum Dev. 2007;83(8), 497–504. [DOI] [PubMed] [Google Scholar]
- 29.Troude P, L’Helias LF, Raison-Boulley AM, et al. Perinatal factors reported by mothers: do they agree with medical records? Eur J Epidemiol. 2008;23(8), 557–564. [DOI] [PubMed] [Google Scholar]
- 30.Marian AJ. Elements of ‘missing heritability’. Curr Opin Cardiol. 2012;27(3), 197–201. [DOI] [PubMed] [Google Scholar]
- 31.Knuiman MW, Divitini ML, Welborn TA, Bartholomew HC. Familial correlations, cohabitation effects, and heritability for cardiovascular risk factors. Ann Epidemiol. 1996;6(3), 188–194. [DOI] [PubMed] [Google Scholar]
- 32.Harville EW, Jacobs MB, Qi L, Chen W, Bazzano LA. Multigenerational Cardiometabolic Risk as a Predictor of Birth Outcomes: The Bogalusa Heart Study. J Pediatr. 2017;181, 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naess O, Stoltenberg C, Hoff DA, et al. Cardiovascular mortality in relation to birth weight of children and grandchildren in 500,000 Norwegian families. Eur Heart J. 2013;34(44), 3427–3436. [DOI] [PubMed] [Google Scholar]
- 34.Miller LL, Pembrey M, Davey Smith G, Northstone K, Golding J. Is the growth of the fetus of a non-smoking mother influenced by the smoking of either grandmother while pregnant? PLoS One. 2014;9(2), e86781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Golding J, Northstone K, Gregory S, Miller LL, Pembrey M. The anthropometry of children and adolescents may be influenced by the prenatal smoking habits of their grandmothers: a longitudinal cohort study. Am J Hum Biol. 2014;26(6), 731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agius R, Savona-Ventura C, Vassallo J. Transgenerational metabolic determinants of fetal birth weight. Exp Clin Endocrinol Diabetes. 2013;121(7), 431–435. [DOI] [PubMed] [Google Scholar]
- 37.Bygren LO, Tinghog P, Carstensen J, et al. Change in paternal grandmothers’ early food supply influenced cardiovascular mortality of the female grandchildren. BMC Genet. 2014;15, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis MM, McGonagle K, Schoeni RF, Stafford F. Grandparental and parental obesity influences on childhood overweight: implications for primary care practice. J Am Board Fam Med. 2008;21(6), 549–554. [DOI] [PubMed] [Google Scholar]
- 39.Ishikuro M, Obara T, Osanai T, et al. Strategic Methods for Recruiting Grandparents: The Tohoku Medical Megabank Birth and Three-Generation Cohort Study. Tohoku J Exp Med. 2018;246(2), 97–105. [DOI] [PubMed] [Google Scholar]
- 40.Hughes D, Hutchinson A, Prichard I, Chapman J, Wilson C. Challenges associated with recruiting multigenerational, multicultural families into a randomised controlled trial: Balancing feasibility with validity. Contemp Clin Trials. 2015;43, 185–193. [DOI] [PubMed] [Google Scholar]
- 41.Beech BM, Bruce MA, Crump ME, Hamilton GE. The Jackson Heart KIDS Pilot Study: Theory-Informed Recruitment in an African American Population. J Racial Ethn Health Disparities. 2017;4(2), 288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taouk L, Schulkin J. Transgenerational transmission of pregestational and prenatal experience: maternal adversity, enrichment, and underlying epigenetic and environmental mechanisms. J Dev Orig Health Dis. 2016;7(6), 588–601. [DOI] [PubMed] [Google Scholar]
- 43.Vassoler FM, Sadri-Vakili G. Mechanisms of transgenerational inheritance of addictive-like behaviors. Neuroscience. 2014;264, 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karatsoreos IN, Thaler JP, Borgland SL, Champagne FA, Hurd YL, Hill MN. Food for thought: hormonal, experiential, and neural influences on feeding and obesity. J Neurosci. 2013;33(45), 17610–17616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Youngson NA, Whitelaw E. Transgenerational epigenetic effects. Annu Rev Genomics Hum Genet. 2008;9, 233–257. [DOI] [PubMed] [Google Scholar]
- 46.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8(4), 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.