Abstract
Background and Aims:
Non-invasive biomarkers are increasingly used to assess fibrosis in patients with chronic liver disease. We determined the utility of dual cut-offs for non-invasive biomarkers to exclude and confirm advanced fibrosis in HBV-HIV co-infected patients receiving combined anti-retroviral therapy (cART).
Patients and Methods:
Participants were anti-HIV/HBsAg positive adults from 8 clinical sites in the US and Canada of the Hepatitis B Research Network. Fibrosis was staged by a central pathology committee using the Ishak fibrosis score (F). Clinical, laboratory and vibration-controlled transient elastography (VCTE) data were collected at each site. Dual cut-offs for three non-invasive biomarkers (APRI, FIB-4 and liver stiffness by VCTE) with the best accuracy to exclude or confirm advanced fibrosis (F ≥3) were determined using established methodology.
Results:
Of 139 enrolled participants, 108 with a liver biopsy and having ≥1 non-invasive biomarker were included; 22% had advanced fibrosis; 54% had normal ALT. The median (IQR) of APRI (n=106), FIB-4 (n=106) and VCTE (n=63) were 0.34 (0.26-0.56), 1.35 (0.99-1.89), and 4.9 (3.8-6.8) kPa, respectively. The AUROC for advanced fibrosis was 0.69 for APRI, 0.66 for FIB-4, and 0.87 for VCTE. VCTE cut-offs of ≤5.0 kPa (to exclude) and ≥8.8 kPa (to confirm) advanced fibrosis had a sensitivity=92.3% and specificity=96.0%, respectively, and accounted for 65.1% of participants. Among the 34.9% with values between the cut-offs, 26.1% had advanced fibrosis. Considering APRI or FIB-4 jointly with VCTE did not improve the discriminatory capacity.
Conclusions:
VCTE is a better biomarker of advanced fibrosis compared to APRI or FIB-4 in HBV/HIV co-infected adults on cART. Using VCTE dual cut-offs approximately two thirds of patients could avoid biopsy to determine advanced fibrosis.
Introduction
Accurate assessment of liver fibrosis to determine both the severity and progression of liver disease and candidacy for liver cancer screening is essential in the management of those with chronic liver disease. In the past, this was accomplished by percutaneous liver biopsy. However, given the risks and low rate of acceptance by patients, there has been extensive research into non-invasive markers for hepatic fibrosis or cirrhosis. These markers include indices or algorithms based on routine laboratory tests such as the aspartate aminotransferase (AST) to platelet ratio index (APRI)1 and Fibrosis-4 index (FIB-4)2, as well as liver stiffness measured via vibration controlled transient elastography (e.g., VCTE or Fibroscan®)3-6 that have now become standard practice. Although most of these tests were developed in patients with chronic hepatitis C virus (HCV), they have also been applied to those with chronic hepatitis B virus (HBV) 7-9. However, the majority of studies in those with HBV were performed in subjects who were not on anti-HBV therapy,4-6, 10-14 making their applicability to HBV-treated patients unclear.
Due to shared routes of transmission, chronic HBV infection (or chronic hepatitis B) is common in those living with human immunodeficiency virus (HIV)15-21. The majority of such co-infected patients are on combination anti-retroviral therapy (cART) that includes medications that suppress HBV, which may impact the performance of non-invasive tests22. Although several studies have reported a single non-invasive marker of fibrosis in HBV-HIV subjects on cART23-27, few investigations have evaluated the discriminatory capacity of non-invasive markers (i.e., with comparisons to liver histology as the gold standard) in this special population28-30, and only one has compared the relative discriminatory capacity of APRI and FIB-4, the two most widely used serum-based markers, to VCTE 31.
Although most HBV-HIV co-infected subjects on cART have suppressed HBV DNA and normal liver enzymes, we have previously shown that a high proportion can have significant underlying fibrosis.32 However, neither HIV nor HBV virologic or single serologic markers (e.g. ALT) sufficiently predict advanced fibrosis in co-infected adults.32 Because liver biopsy is rarely performed in HBV-HIV co-infected patients to identify those at highest risk for liver-related morbidity and for developing hepatocellular carcinoma, there is a strong need for non-invasive fibrosis markers in this population. The aim of this report was to determine the diagnostic accuracy of APRI, FIB-4 and liver stiffness from VCTE for advanced fibrosis, as determined by liver biopsy, in a well-defined cohort of HBV-HIV co-infected adults in North America, all of whom were receiving cART. Two types of cut-offs were identified. The first was a single “optimal” cut-off for classifying participants as having or not having advanced fibrosis29. However, because no single cut-off has had sufficient accuracy to replace the need for biopsy in HBV, we also identified dual cut-offs with the best accuracy to exclude and confirm advanced fibrosis4.
Patients and Methods
This is a cross sectional diagnostic accuracy study using data from the enrollment assessment of a multi-center prospective cohort study (R01-DK94818). Adult patients with HBV/HIV were recruited from eight Hepatitis B Research Network (HBRN) sites in the US and Canada (Virginia Commonwealth University, University of California San Francisco, UT Southwestern Medical Center, Johns Hopkins University, University Health Network, Toronto, Washington University Saint Louis, Massachusetts General Hospital, National Institutes of Health, National Institute Diabetes and Digestive and Kidney Diseases, NIDDK). All included subjects were anti-HIV positive and hepatitis B surface antigen (HBsAg) positive for at least 6 months. Those with detectable hepatitis C virus (HCV) ribonucleic acid (RNA), decompensated cirrhosis, or hepatocellular carcinoma (HCC) were excluded. The institutional review board at each center approved the protocol, and participants gave written informed consent. The study is registered at ClinicalTrials.gov ( NCT01924455).
The study screened 351 potential participants. The most common reasons for not participating were refused liver biopsy/no consent (n=195), hepatic decompensation (n=9) and liver cancer (n=4). By close of enrollment 139 participants consented, 19 of whom did not undergo liver biopsy due to financial or logistical (transportation and scheduling conflicts) reasons. Of the 120 participants with histology data, 108 had at least one valid non-invasive biomarker within 24 weeks of the biopsy and were included in this report.
Standardized research assessments were conducted by study personnel (clinical research investigators and their research staff) based on a common research protocol operations manual. Data were entered by research staff or the central lab and transmitted to the HBRN Data Coordinating Center (University of Pittsburgh).
Liver Histology.
Liver biopsy was performed in the standard fashion. Total length of biopsy was recorded. Unstained slides were submitted for hematoxylin and eosin and Masson trichrome staining by a central lab. Histological findings were scored blindly with respect to clinical data, including HIV status, by the HBRN Pathology Committee (DEK, chair). While the number of portal tracts was not recorded, only biopsies determined as adequate (i.e., representative) by the HBRN Pathology committee were included. Fibrosis was staged with the Ishak fibrosis score (F), which ranges from 0-6 (F0=none, F1=early portal, F2=established portal, F3=early bridging, F4=established bridging, F5=early cirrhosis, and F6=established cirrhosis)35. Our primary outcome was advanced fibrosis or worse, defined as ≥F3.
Transient elastography.
VCTE (Fibroscan®, EchoSens, Paris, France) was used to measure liver stiffness expressed in kiloPascal’s (kPa) (range: 2-75 kPa) at six clinical sites. All scans were performed by trained study personnel certified by Echosens at each site. Participants were instructed to fast for at least 3 hours prior to assessment. At least 10 successful measurements were required to calculate the primary data point, median liver stiffness (referred to simply as liver stiffness from here forward), across measurements. Among participants with a liver biopsy who had VCTE within 24 weeks of the biopsy (n=75), VCTE results were excluded due to fasting <3 hours (n=4), inadequate data (i.e. <10 measurements) (n=4), or an interquartile range (IQR)/median liver stiffness ratio >0.3 (n=4).
Serum models.
Laboratory testing by local site included aspartate aminotransferase (AST), alanine aminotransferase (ALT) and platelets (PLT). Age was calculated based on date of birth. The following published serum models were computed.
Covariates.
Laboratory testing by local site included hematology, basic metabolic, hepatic panel and CD4/CD8. HIV stage (1-4) was defined by CD4 count at entry according to 2005 World Health Organization Guidelines (≥500, 350-499, 200-349, and <200 cells/mm3, respectively)36. Local viral testing included HBsAg, hepatitis B e antigen (HBeAg)/antibody, HBV DNA, HCV antibody, HIV RNA, and hepatitis delta antibody. Serum was collected for central testing, stored at the NIDDK biorepository and sent to the HBRN Central Laboratory (University of Washington) for quantitative HBV surface antigen (qHBsAg), quantitative HBeAg (qHBeAg), and HBV DNA (Roche, Branchburg, NJ).
Data on demographics and health behaviors were self-reported at the time of enrollment. Alcohol consumption was graded as none or minimal (<1 drink/month), low-risk (more than minimal but ≤ 4 drinks/day or 14 drinks/week in men; ≤3 drinks/ day or 7 drinks/week in women), or heavy (more than moderate or ≥5 drinks on ≥1 day in past month)37. Clinical assessment included height and weight, utilized to calculated body mass index (BMI), as well as evidence of lipodystrophy (mild, moderate or severe)38. Duration of HIV and/or HBV infection as well has current and past cART use was collected but unable to be verified in many subjects due to the fragmented care they received from different health providers from different sites from diagnosis to enrollment.
Statistical analysis.
Descriptive statistics (frequencies and percentages for categorical data, and medians and interquartile ranges (IQR) for continuous data) summarized demographic, clinical and virologic characteristics measured at study entry. Associations between each non-invasive histology marker (APRI, FIB-4 and liver stiffness by VCTE) and the Ishak fibrosis score were evaluated with Kendall’s tau-b. The distribution of non-invasive histology markers by Ishak fibrosis score were presented graphically using boxplots; F ≥3, indicating advanced fibrosis or worse, were collapsed due to low frequency.
The discriminatory capacity of each non-invasive biomarker (continuous) for the diagnosis of advanced fibrosis was calculated using receiver operating characteristics (ROC) curves. Optimal cut-off values for advanced fibrosis were defined using the highest sum of sensitivity (SE) and specificity (SP)29. In addition, dual cut-off values with the best accuracy to 1) rule in (confirm), or 2) rule out (exclude) advanced fibrosis were identified using established criteria4, 33-34. Criteria for the confirmatory cut-off were specificity >90% and positive likelihood ratio (PLR) ≥10; if more than one cut-off met this criteria, the cut-off with the highest specificity was selected; if no cut-off met these criteria, among cut-offs with >90% specificity, the cut-off with the highest PLR was selected. Criteria for the exclusionary cut-off were sensitivity >90% and negative likelihood ratios (NLR) ≤0.1; if no cut-off met these criteria, among cut-offs with >90% sensitivity, the cut-off with the lowest NLR was selected. For each cut-off value the AUROC, sensitivity, specificity, PLR, NLR, positive predictive value (PPV) and negative predictive value (NPV) are reported. The SE+SP and the diagnosis accuracy (i.e., sum of true positives and true negatives over the number of participants) is also reported for optimal cut-offs. All statistics were also reported for the optimal VCTE cut-off for advanced fibrosis suggested by a prior study of HIV/HBV co-infected adults (>7.6 kPa)29. As a sensitivity analysis, statistics were also calculated for all cut-offs as determined above, among the subset of participants whose non-invasive measures were measured within 12 weeks of the biopsy (N=97 for APRI and FIB-4; N=58 for VCTE).
Finally, we investigated whether identification of advanced fibrosis could be improved by combining VCTE with APRI or FIB-4. For this, we used a classification and regression tree (CART) analysis. Because we previously showed that after consideration of ALT or AST and platelets (i.e., components of APRI and FIB-4) no other factors (i.e., sociodemographics, health-behaviors, and HIV- and HBV-specific markers) were significantly associated with advanced fibrosis in this sample32, the current study did not consider the addition of these factors.
All analyses except CART were conducted using SAS, version 9.4 (SAS Institute Inc., Cary NC, 2000). The CART analysis was conducted using Salford Predictive Modeler, version 8.2 (Salford Systems; San Diego, CA).
Results:
Study participants:
Among the 108 participants, 106 (98%) had the required measures for calculation of APRI and FIB-4 (i.e., AST, ALT, and platelets), while 63 (58%) participants had a valid measure of liver stiffness from VCTE, 61 of whom also had APRI and FIB-4.
Patient demographics:
Demographic and clinical features of the 108 participants are reported in Table 1. The median age was 49 (IQR: 44-55) years, and 92.6% were male. Approximately half (53.8%) had normal ALT. All participants had a history of taking cART, 99% were on cART at the time of enrollment, 77.6% had complete suppression of HIV RNA, the median CD4 count was 567 (IQR: 366-718) cells/mm3, 56.5% were HBeAg positive, 82.4% had suppressed (<1000 IU/mL) HBV DNA, 5.6% were anti-HCV positive with an undetectable HCV RNA, and 3.7% had delta virus co-infection. Characteristics among the subgroups with APRI and FIB-4 (n=106) and VCTE (n=63) are reported in Supplemental table 1; characteristics of both analysis subsamples were representative of the full sample.
Table 1.
Participant characteristics for our cohort.
| Total Cohort N=108† |
|
|---|---|
| Age (years) | |
| Median (25th, 75th) | 49 (44, 55) |
| Min, Max | 28, 70 |
| Male | 100 (92.6%) |
| Race | n=107 |
| Non-Hispanic White | 34 (31.8%) |
| Non-Hispanic Black | 55 (51.4%) |
| Other | 18 (16.8%) |
| Alcohol risk | |
| None or minimal | 60 (55.6%) |
| Low-risk | 34 (31.5%) |
| Heavy | 14 (13.0%) |
| Body mass index (kg/m2) | n=104 |
| Median (25th, 75th) | 26.3 (22.4, 30.6) |
| Min, Max | 16.6, 48.5 |
| ALT (IU/L) | n=106 |
| Median (25th, 75th) | 27 (19, 39) |
| Min, Max | 8, 223 |
| ALT (by standard ULN‡) | n=106 |
| ≤1 | 57 (53.8%) |
| >1-≤2 | 35 (33.0%) |
| >2 | 14 (13.2%) |
| AST (IU/L) | n=106 |
| Median (25th, 75th) | 28 (23, 39) |
| Min, Max | 13, 202 |
| AST (by lab-specific ULN§) | n=106 |
| ≤1 | 82 (77.4%) |
| >1-≤2 | 21 (19.8%) |
| >2 | 3 (2.8%) |
| Platelets (x103/mm3) | n=106 |
| Median (25th, 75th) | 199 (174, 234) |
| Min, Max | 84, 344 |
| Anti-HCV positive | 6 (5.6%) |
| Anti-HDV positive | 4 (3.7%) |
| Sexually transmitted | n=99 |
| 94 (94.9%) | |
| Estimated duration of HIV Infection (years) | n=97 |
| Median (25th, 75th) | 20 (10, 25) |
| Min, Max | 1, 4 |
| HIV RNA copies/ml | n=98 |
| 0 - <20 | 76 (77.6%) |
| 20 - <100 | 8 (8.2%) |
| 100 - <10000 | 11 (11.2%) |
| ≥ 10000 | 3 (3.1%) |
| CD4 (cells/mm3) | n=99 |
| Median (25th, 75th) | 567 (366, 718) |
| Min, Max | 38, 1395 |
| HIV stage | n=80 |
| 1 (CD4 ≥500 cells/mm3) | 61 (76.3%) |
| 2 (CD4 350-499 cells/mm3) | 10 (12.5%) |
| 3 (CD4 200-349 cells/mm3) | 5 (6.3%) |
| 4 (CD4 <200 cells/mm3) | 4 (5.0%) |
| Estimated duration of HBV Infection (years) | n=82 |
| Median (25th, 75th) | 13.5 (8.0, 22.0) |
| Min, Max | 1.0, 52.0 |
| HBeAg Positive | 61 (56.5%) |
| Quantitative HBeAg (IU/mL), among HBeAg positive | n=60 |
| Median (25th, 75th) | 15.0 (1.9, 198.9) |
| Min, Max | 0.5, 2058.1 |
| Quantitative HBsAg (IU/mL) | n=106¶ |
| Median (25th, 75th) | 1440.5 (307.1, 7755.0) |
| Min, Max | BLD#, 647460.0 |
| HBV DNA level (IU/mL) | |
| <1000 | 89 (82.4%) |
| 1000-<20000 | 7 (6.5%) |
| ≥20000 | 12 (11.1%) |
| Currently on cART treatment | 107 (99.1%) |
| Tenofovir, alone or in combination | 92 (85.2%) |
| Emtricitabine | 80 (74.8%) |
| Lamivudine, alone or combination | 19 (17.6%) |
| Entecavir | 16 (14.8%) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; cART, combination antiretroviral therapy; CD, cluster of differentiation, DNA, deoxyribonucleic acid; HBeAg, quantitative e antigen; HBsAg, quantitative s antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HDV, hepatitis Delta virus; HIV, human immunodeficiency virus; RNA ribonucleic acid.
Unless otherwise specificied due to missing data.
Upper limit of normal (ULN) ALT ≤30 IU/L in men, ALT ≤19 U/L in women
Upper limit of normal (ULN) AST by each local laboratory
Two participants who were HBsAg positive via local laboratory did not have HBsAg measured by central laboratory.
Four participants who were HBsAg positive via local laboratory had HBsAg below level of detection by central laboratory.
Assessment of liver fibrosis:
Median liver biopsies length was 17 (IQR: 14-23.5) mm long; 92.6% were ≥ 10 mm in length. Based on liver biopsy, 22.2% (n=24) of participants had advanced fibrosis (F ≥ 3): 18.5% (n=20) with Ishak 3-4 and 3.7% (n=4) with Ishak 5-6. Liver histology by analysis sample is shown in Table 2.
Table 2.
Measures of liver fibrosis by analysis sample.
| APRI/FIB-4 Sample n=106 |
VCTE Sample n=63 |
|
|---|---|---|
| FIB-4 | n=61 | |
| Median (25th, 75th) | 1.35 (0.99, 1.89) | 1.38 (0.99, 1.87) |
| Min, Max | 0.44, 5.96 | 0.44, 5.96 |
| APRI | n=61 | |
| Median (25th, 75th) | 0.34 (0.26, 0.56) | 0.33 (0.26, 0.56) |
| Min, Max | 0.10, 3.98 | 0.10, 3.98 |
| VCTE (kPa) | n=61 | |
| Median (25th, 75th) | 4.9 (3.8, 6.6) | 4.9 (3.8, 6.8) |
| Min, Max | 2.7, 29.7 | 2.7, 29.7 |
| Histological Activity Index | ||
| Median (25th, 75th) | 3 (2, 4) | 3 (2, 4) |
| Min, Max | 0, 14 | 0, 14 |
| Fibrosis Ishak score | ||
| 0 | 28 (26.4%) | 18 (28.6%) |
| 1 | 39 (36.8%) | 26 (41.3%) |
| 2 | 15 (14.2%) | 6 (9.5%) |
| 3 | 16 (15.1%) | 8 (12.7%) |
| 4 | 4 (3.8%) | 2 (3.2%) |
| 5 | 1 (0.9%) | 1 (1.6%) |
| 6 | 3 (2.8%) | 2 (3.2%) |
| Advanced Fibrosis (F ≥ 3) | 24 (22.6%) | 13 (20.6%) |
Abbreviations: APRI, aspartate aminotransferase to platelet ratio index; Fibrosis Ishak score, F; FIB-4, fibrosis index based on four factors; VCTE, vibration controlled transient elastography; kPa, kiloPascals
Non-invasive assessment:
Measures used to calculate APRI and FIB-4 among the 106 participants with qualifying measures were determined a median of 29 (IQR: 14-50) days before or after the liver biopsy. Among the 63 qualifying VCTE measures, VCTE was performed a median of 1 (IQR: 7-29) day before or after the liver biopsy; median fasting time was 11 (IQR: 4-12) hours and median IQR/stiffness ratio was 0.1 (IQR: 0.1, 0.2). Median (IQR) of APRI was 0.34 (0.26-0.56) and FIB-4 was 1.35 (0.99-1.89); median (IQR) of liver stiffness was 4.9 kPa (3.8-6.8).
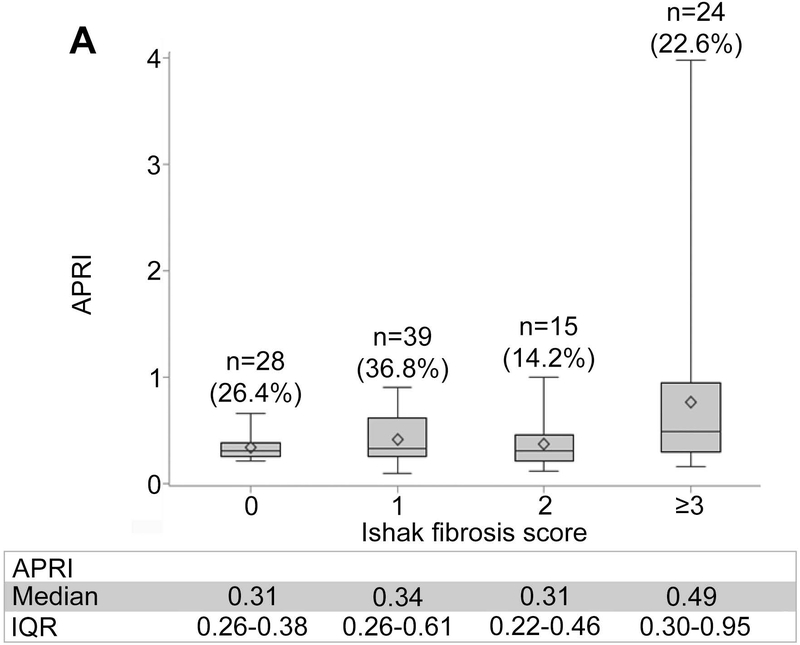
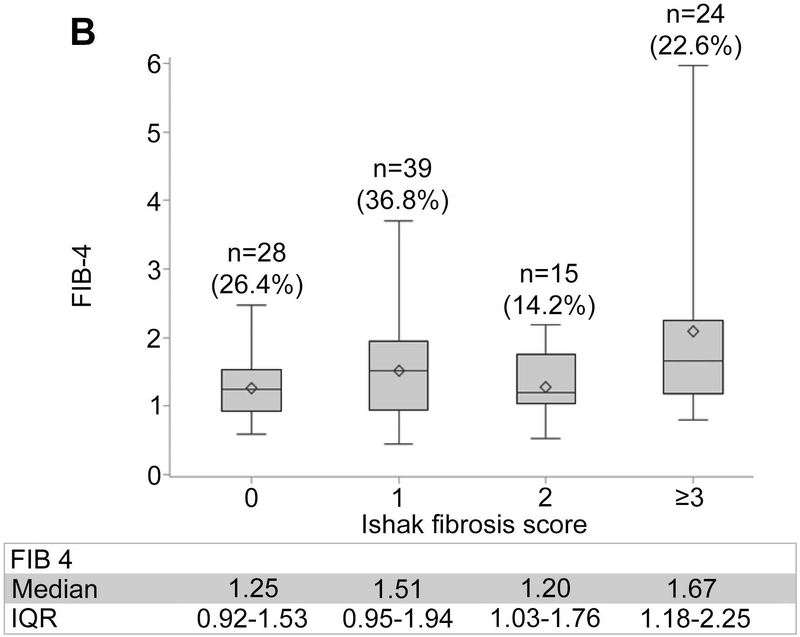
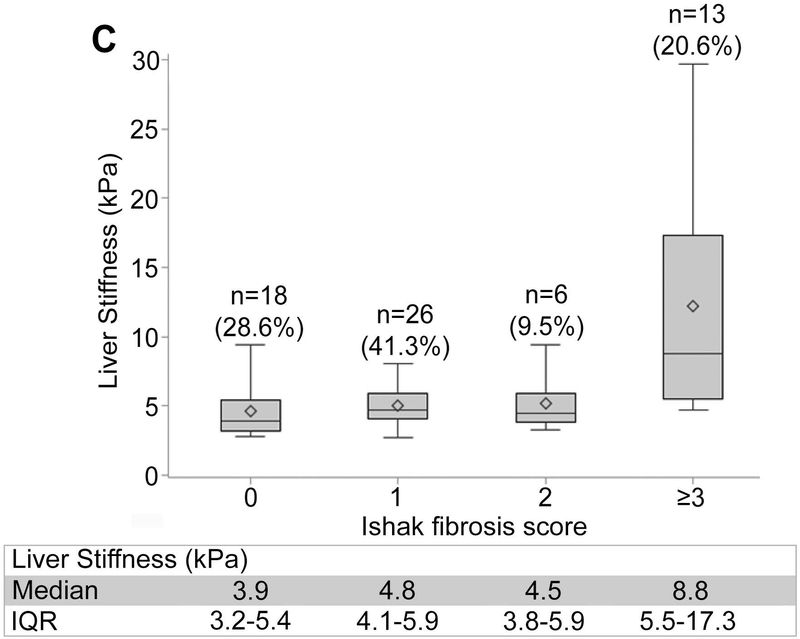
Non-invasive markers of liver fibrosis by Ishak fibrosis score are shown in Figure 1, panels A-C. The median (IQR) of liver stiffness via VCTE was 3.9 kPa (3.2–5.4) for F0, 4.8 kPa (4.0–5.9) for F1, 4.5 kPa (3.8–5.9) for F2, and 8.8 kPa (5.5–17.3) for ≥F3, demonstrating participants with advanced fibrosis had markedly higher liver stiffness. Statistically significant but weak positive correlations were observed between each of the three non-invasive markers and the Ishak fibrosis score (Kendall’s tau-b: ARPI: 0.179; p=0.014; FIB-4: 0.181 p=0.013; liver stiffness: 0.393; p<0.0001).
Figure 1.
Distribution of non-invasive markers according to ISHAK fibrosis scoring. (A) APRI. (B) FIB-4. (C) Liver stiffness.
Discriminatory capacity of non-invasive markers:
The diagnostic accuracy of each non-invasive marker for advanced fibrosis is shown in Figure 2. The area under the receiver operating characteristics curve (AUROC) for differentiation of advanced fibrosis by biomarkers was 0.615 for APRI, 0.665 for FIB-4, and 0.865 for VCTE. The sensitivity and specificity of each non-invasive marker for advanced fibrosis are shown in supplemental Figure 1, panels A-C.
Figure 2.
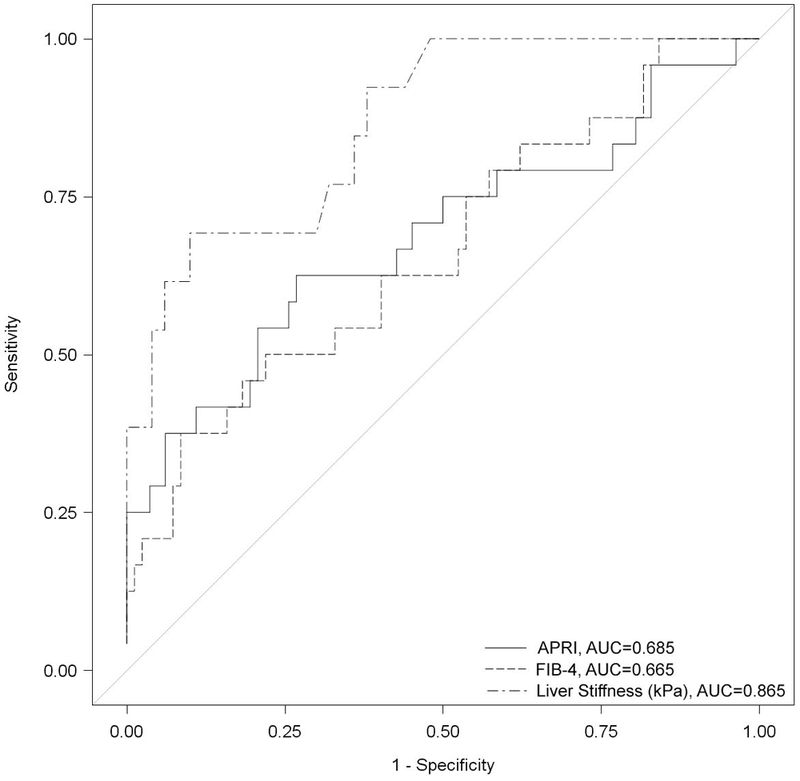
Stiffness by Ishak Score. Receiver operating characteristics curve representing the relationship between sensitivity and 1 – specificity of the three non-invasive markers for the diagnosis of advanced fibrosis.
Optimal cut-off:
The diagnostic accuracy of optimal cut-offs (i.e., producing the highest SE+SP) of non-invasive markers in identifying advanced fibrosis are reported in Table 3. The optimal VCTE cut-off of ≥7.8 kPa yielded the highest SE+SP (155.5), as well as the highest PPV (72.7%) and NPR (90.5%), compared to the optimal APRI cut-off of ≥0.42 (SE+SP=135.7, PPV=40.5%, NPV=87.0%) and the optimal FIB-4 cut-off of ≥1.76 (SE+SP=126.8, PPV=38.7%, NPV=84.0%). The optimal VCTE cut-off determined by this study was only 0.1 different from the established cut-off (>7.6 kPa)29, thus, the diagnostic accuracy for the established cut-off was similar (SE+SP=153.5, PPV=66.7%, NPV=90.2%).
Table 3.
The diagnostic accuracy of optimal cut-offs† for non-invasive markers in identifying advanced fibrosis in adults with HBV-HIV coinfection.
| Biomarker | AUROC | SE, % | SP, % | SE + SP | PLR | NLR | PPV, % | NPV, % | AC, % |
|---|---|---|---|---|---|---|---|---|---|
| APRI ≥ 0.42 (n=106) | 0.678 | 62.5 | 73.2 | 135.7 | 2.3 | 0.51 | 40.5 | 87.0 | 70.8 |
| FIB-4 ≥ 1.76 (n=106) | 0.634 | 50.0 | 76.8 | 126.8 | 2.2 | 0.65 | 38.7 | 84.0 | 35.8 |
| VCTE ≥ 7.8 kPa (n=63) | 0.778 | 61.5 | 94.0 | 155.5 | 10.3 | 0.41 | 72.7 | 90.4 | 87.3 |
| Established > 7.6 kPa | 0.768 | 61.5 | 92.0 | 153.5 | 7.69 | 0.42 | 66.7 | 90.2 | 85.7 |
Abbreviations: AC, accuracy; APRI, aspartate aminotransferase to platelet ratio index; AUROC, area under the receiver operating characteristic curve; FIB-4, fibrosis index based on four factors (i.e., age, platelet count, aspartate aminotransferase and alanine aminotransferase); VCTE, vibration controlled transient elastography; kPa, kiloPascals; NLR, negative likelihood ratio, NPV, negative predictive value; PLR, positive likelihood ratio; PPV positive predictive value; SE, sensitivity; SP, specificity.
The cut-off with the highest SE+SP; the higher number, the higher the diagnostic accuracy.
The frequency and percentage of participants 1) below vs meeting the optimal cut-off to identify advanced fibrosis for each non-invasive marker, and 2) with advanced fibrosis (as determined by biopsy) by these groups, are reported in supplemental Table 2. For APRI, 65.1% (n=69) of participants fell below the cut-off for advanced fibrosis, 13.0% (n=9) of whom had it. Likewise, 70.8% (n=75) fell below the FIB-4 cut-off, 16.0% (n=12) of whom had advanced fibrosis. In contrast, 82.5% (n=52) fell below the VCTE cut-off, only 9.6% (n=5) of whom had advanced fibrosis.
Exclusionary and confirmatory cut-offs:
The diagnostic accuracy of dual cut-offs to rule out (exclude) and rule in (confirm) advanced fibrosis are reported in Table 4. Due to the methodology of selecting cut-offs, the sensitivity of the lower exclusionary cut-offs, and the specificity of the higher confirmatory cut-offs were similar across the three non-invasive markers (i.e., sensitivity for APRI ≤ 0.22, FIB-4 ≤ 0.88 and liver stiffness ≤ 5.0 were 95.8%, 95.8% and 92.3%, respectively; specificity for APRI ≥ 1.00, FIB-4 ≥ 3.01 and liver stiffness ≥ 8.8 were 98.8%, 98.8% and 96.0%, respectively). However, the percentage of participants who were excluded, confirmed, and fell between the dual cut-offs, differed by non-invasive marker. That is, far fewer participants fell between the exclusionary and confirmatory cut-offs (indicating the need for liver biopsy) for VCTE (36.5%; n=22) vs APRI (79.3%; n=84) and FIB-4 (80.2%; n=85). Despite this difference, the percentage of participants between the exclusionary and confirmatory cut-offs with advanced fibrosis was similar for the three non-invasive markers: 20.2% (n=17) for APRI, 22.4% (n=19) for FIB-4, and 26.1% (n=5) for VCTE (supplemental Table 3).
Table 4.
The diagnostic accuracy of non-invasive markers to exclude† and confirm‡ advanced fibrosis in adults with HBV-HIV coinfection.
| Biomarker | AUROC | SE, % | SP, % | PLR | NLR | PPV, % | NPV, % |
|---|---|---|---|---|---|---|---|
| APRI (n=106) | |||||||
| Exclusionary cut-off ≤ 0.22 | 0.565 | 95.8 | 17.1 | 1.2 | 0.24 | 25.3 | 93.3 |
| Confirmatory cut-off ≥ 1.00 | 0.619 | 25.0 | 98.8 | 20.5 | 0.76 | 85.7 | 81.8 |
| FIB-4 (n=106) | |||||||
| Exclusionary cut-off ≤ 0.88 | 0.571 | 95.8 | 18.3 | 1.2 | 0.23 | 25.6 | 93.8 |
| Confirmatory cut-off ≥ 3.01 | 0.577 | 16.7 | 98.8 | 13.7 | 0.84 | 80.0 | 80.2 |
| VCTE, kPa (n=63) | |||||||
| Exclusionary cut-off ≤ 5.0 | 0.772 | 92.3 | 62.0 | 2.43 | 0.12 | 38.7 | 96.9 |
| Confirmatory cut-off ≥ 8.8 | 0.749 | 53.8 | 96.0 | 13.5 | 0.48 | 77.8 | 88.9 |
Abbreviations: AC, accuracy; APRI, aspartate aminotransferase to platelet ratio index; AUROC, area under the receiver operating characteristic curve; FIB-4, fibrosis index based on four factors (i.e., age, platelet count, aspartate aminotransferase and alanine aminotransferase); VCTE, vibration controlled transient elastography; kPa, kiloPascals; NLR, negative likelihood ratio, NPV, negative predictive value; PLR, positive likelihood ratio; PPV positive predictive value; SE, sensitivity; SP, specificity.
Criteria for the exclusionary cut-off were sensitivity >90% and negative likelihood ratios (NLR) ≤0.1; if no cut-off met these criteria, among cut-offs with >90% sensitivity, the cutoff with the lowest NLR was selected.
Criteria for the confirmatory cut-off were specificity >90% and positive likelihood ratio (PLR) ≥10; if more than one cut-off met these criteria, the cut-off with the highest specificity was selected; if no cut-off met these criteria, among cut-offs with >90% specificity, the cut-off with the highest PLR was selected.
Sensitivity analysis:
Statistics for the optimal, exclusionary and confirmatory cut-offs determined among the analysis samples (n=106 for APRI and FIB-4; n=63 for VCTE), which required non-invasive markers to be measured within 24 weeks of liver biopsy, were similar when calculated only among participants with non-invasive markers measured within 12 weeks of the liver biopsy (n=97 for APRI and FIB-4; n=58 have VCTE) (supplemental Table 4).
Combining non-invasive markers using CART:
CART analysis also identified VCTE as the primary predictor of advanced fibrosis. Addition of APRI or FIB-4 did not improve the miss-classification rates.
Discussion
Over one-fifth (22%) our cohort of HBV-HIV patients on cART with liver biopsy had advanced fibrosis (≥F3). Comparing the performance of three common non-invasive markers of liver fibrosis used in North America, we found that VCTE out-performed the serum-based non-invasive markers, APRI and FIB-4. This was true for the single optimal cut-off and the dual (i.e., exclusionary and confirmatory) cut-offs. Unlike their performance in HBV mono-infected patients not on anti-HBV therapy4-6, 10-14, the poor performance of the serum-based models in HBV-HIV patients receiving cART may be attributed to their reliance on serum ALT and AST levels, which were normal or near normal due to HBV viral suppression with therapy.
Although studies in HBV patients consistently show a significant correlation between liver stiffness and severity of liver disease, no single cut-off has had sufficient accuracy to replace the need for biopsy in HBV to determine stages of fibrosis9. We therefore asked ourselves whether the use of a dual cut-off VCTE algorithm in patients on cART concurrently examined by liver biopsy would better predict and rule out advanced fibrosis in HIV-HBV co-infected adults, versus the use of a single optimal VCTE cut-off4, 39. Based on our results we recommend a low cutoff of VCTE of ≤ 5.0 kPa to exclude advanced fibrosis and a cutoff of ≥ 8.8 kPa to confirm advanced fibrosis. Using these cut-offs we could avoid liver biopsy to determine advanced fibrosis or worse in approximately two thirds of our sample. In those with VCTE in-between 5.0 and 8.8 kPa, liver biopsy would still be required, as over a quarter had advanced fibrosis and consideration of other non-invasive measures did not differentiate this group.
Consistent with literature suggesting that cut-offs in treated versus untreated patients differ9, 29, the optimal cut-offs for all three noninvasive makers in our sample are lower than cut-offs established in untreated patients3-6, perhaps due to the lower liver enzymes in our cART treated population. Only a handful of studies of HBV-HIV patients have compared non-invasive tests with liver histology. In a multicenter French study, VCTE was performed in over 1000 patients with viral hepatitis, of which 110 had HIV and were coinfected with HCV or HBV9. The overall accuracy of VCTE (≥12.5 kPa) was higher for identifying cirrhosis (AUROC 0.90) compared to serum biomarkers (AUROC 0.77-0.86), but was not different for determining significant fibrosis (≥7.1 kPa) (AUROC 0.77-0.86), with slightly better results in those with coinfection compared to those with HCV or HBV alone. However, results are difficult to interpret as there was no differentiation between those with HCV/HIV versus HBV/HIV, and no data was provided regarding cART use. In contrast, we excluded those with HCV RNA and most (99%) of our cohort was on cART. Furthermore, the outcomes (i.e., cirrhosis and significant fibrosis) were different from our study hampering comparison.
The optimal single VCTE cut-off for advanced fibrosis in our study (≥7.8 kPa) is similar compared to the smaller study by Mialhes29 of 59 HBV-HIV patients from a single center. Using a cut-off of >7.6 kPa their diagnostic accuracy was 86%. Applying this cut-off, the diagnostic accuracy was also 86% in our sample; it was 87% with our similar optimal cut-off of ≥7.8 kPa. Applying this later cut-off, less than one-fifth (17.5%) of the sample was identified, the majority (72.7%) of whom had advanced fibrosis. However, nearly 10% (9.7%) of participants below the cutoff also had advanced fibrosis, and thus would be missed using a single cut-off to guide decision to biopsy. In contrast, only 3% of participants would be missed applying our VCTE dual cut-offs, which implicate biopsy in one third.
We did not observe any additional benefit of adding assessment of other non-invasive serum markers, namely APRI or FIB-4, to VCTE to improve the performance of non-invasive indices to identify advanced fibrosis in our HBV-HIV cohort. In contrast, in the study by Miailhes, performance was improved with the addition of the serum-based Fibrotest®29. The PPV was 100% in those who had both VCTE ≥ 7.6 and Fibrotest ≥ 0.42. Conversely, when VCTE was < 7.6 kPa and Fibrotest < 0.42, only 1 patient with advanced fibrosis was misclassified, yielding a negative predictive value of 95%29. However, 33% of participants had a discordant result. Thus, liver biopsy would still be required in approximately one third of patients, similar to our findings using VCTE alone with the dual cut-off approach.
Strengths of this study are the geographic diversity of the sample, the central reading of liver biopsies by the HBRN pathology committee, and the evaluation of three competing non-invasive markers. Conversely, limitations of our study include the self-selection of patients who were willing to undergo liver biopsy who therefore may not reflect all adults with HBV-HIV coinfection. In addition, we could not account for duration of infections or impact or duration of anti-HBV therapy alone or as part of prior cART, as many patients received care by other providers at other sites prior to study enrollment. As with any liver biopsy study, there may have been sampling error. Furthermore, not all noninvasive tests were performed on all subjects on the same day as the liver biopsy. However, the examination of liver biopsy and non-invasive markers within a short time span (<24 weeks) limited the potential confounding role of hepatitis flares that may unpredictably ensue during the course of infection and challenge the diagnostic accuracy of non-invasive markers. In addition, a sensitivity analysis excluding non-invasive tests performed >12 weeks from the biopsy yielded similar results4. Because our sample size was modest, we were not able to split our cohort into derivation and validation subsets; thus, the cut-offs identified in this study require validation in other cohorts. However, the similarity in the single optimal VCTE cut-off between our study and the Mialhes study29 provides support for the reproducibility of results. Cirrhosis was not specifically evaluated in our study due to a combination of the sample size and low frequency of this outcome (7 of 108). However, our outcome (i.e., advanced fibrosis or worse) included cirrhosis. Lastly, were not able to compare VCTE to other serum algorithms or magnetic resonance elastography40. Notwithstanding these limitations, our study is one of very few studies on non-invasive assessment compared to liver histology in this understudied population.
In conclusion, compared to APRI or FIB-4, VCTE is a better biomarker of advanced fibrosis in HBV-HIV co-infected adults on cART, perhaps due to treatment effect on liver enzymes. Given the poor performance of APRI and FIB-4, we do not recommend their use in HBV-HIV patients on cART. VCTE exclusionary and confirmatory cutoffs can be used to reduce the number of patients who need biopsy to determine advanced fibrosis by over one half in this understudied population.
Supplementary Material
Acknowledgments
This study was funded by NIDDK (R01-DK94818) as an ancillary study ( NCT01924455) of the Hepatitis B Research Network to Dr. Richard K. Sterling. Dr. Sulkowski was partially supported by K24DA034621. Dr. Khalili was partially supported by K24AA022523.
References
- 1.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:51826. [DOI] [PubMed] [Google Scholar]
- 2.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, M SS, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:131725. [DOI] [PubMed] [Google Scholar]
- 3.Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011. April;54(4):650–9. [DOI] [PubMed] [Google Scholar]
- 4.Viganò M, Paggi S, Lampertico P, Fraquelli M, Massironi S, Ronchi G, Rigamonti C, Conte D, Colombo M. Dual cut-off transient elastography to assess liver fibrosis in chronic hepatitis B: a cohort study with internal validation. Aliment Pharmacol Ther. 2011. August;34(3):353–62. [DOI] [PubMed] [Google Scholar]
- 5.Chan HL, Wong GL, Choi PC, Chan AW, Chim AM, Yiu KK, Chan FK, Sung JJ, Wong VW. Alanine aminotransferase-based algorithms of liver stiffness measurement by transient elastography (Fibroscan) for liver fibrosis in chronic hepatitis B. J Viral Hepat. 2009. January;16(1):36–44. [DOI] [PubMed] [Google Scholar]
- 6.Marcellin P, Ziol M, Bedossa P, Douvin C, Poupon R, de Lédinghen V, Beaugrand M. Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int. 2009. February;29(2):242–7. [DOI] [PubMed] [Google Scholar]
- 7.Parikh P, Ryan JD, Tsochatzis EA. Fibrosis assessment in patients with chronic hepatitis B virus (HBV) infection. Ann Transl Med. 2017. February;5(3):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Branchi F, Conti CB, Baccarin A, Lampertico P, Conte D, Fraquelli M. Non-invasive assessment of liver fibrosis in chronic hepatitis B. World J Gastroenterol. 2014. October 28;20(40):14568–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Degos F, Perez P, Roche B, Mahmoudi A, Asselineau J, Voitot H, Bedossa P. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol 2010;53:101321. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Cai Q, Zhang Y, Xie Q, Xu N, Jiang X, Li J, Li X, Zhang Z. Development of algorithms based on serum markers and transient elastography for detecting significant fibrosis and cirrhosis in chronic hepatitis B patients: Significant reduction in liver biopsy. Hepatol Res. 2016. December;46(13):1367–1379. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Chen Y, Zhao Y. The diagnostic value of the FIB-4 index for staging hepatitis B-related fibrosis: a meta-analysis. PLoS One. 2014. August 28;9(8):e105728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology. 2015. January;61(1):292–302. [DOI] [PubMed] [Google Scholar]
- 13.Liang XE, Dai L, Yang SL, Zhong CX, Peng J, Zhu YF, Chen YP, Hou JL. Combining routine markers improves the accuracy of transient elastography for hepatitis B cirrhosis detection. Dig Liver Dis. 2016. May;48(5):512–518. [DOI] [PubMed] [Google Scholar]
- 14.Lee JM, Seo YS, Kim TH, Ahn JM, Yim SY, Kim SU, Jung YK, Kim JH, An H, Yim HJ, Yeon JE, Lee HS, Byun KS, Um SH, Kim CD, Ryu HS. The LAW index as an accurate indicator of the initiation of antiviral treatment in patients with chronic hepatitis B. J Gastroenterol Hepatol. 2017. January;32(1):208–214. [DOI] [PubMed] [Google Scholar]
- 15.Sorrell MF, Belongia EA, Costa J, Gareen IF, Grem JL, Inadomi JM, Kern ER, McHugh JA, Petersen GM, Rein MF, Strader DB, Trotter HT. National Institutes of Health Consensus Development Conference Statement: management of hepatitis B. Ann Intern Med 2009;150:104–10. [DOI] [PubMed] [Google Scholar]
- 16.Benhamou Y Hepatitis B in the HIV-coinfected patient. J Acquir Immune Defic Syndr 2007;45 Suppl 2:S57–65; discussion S66-7. [DOI] [PubMed] [Google Scholar]
- 17.Soriano V, Puoti M, Peters M, Benhamou Y, Sulkowski M, Zoulim F, Mauss S, Rockstroh J. Care of HIV patients with chronic hepatitis B: updated recommendations from the HIV-Hepatitis B Virus International Panel. Aids 2008;22:1399–410. [DOI] [PubMed] [Google Scholar]
- 18.Sulkowski MS. Viral hepatitis and HIV coinfection. J Hepatol 2008;48:353–67. [DOI] [PubMed] [Google Scholar]
- 19.Ockenga J, Tillmann HL, Trautwein C, Stoll M, Manns MP, Schmidt RE. Hepatitis B and C in HIV-infected patients. Prevalence and prognostic value. J Hepatol 1997;27:18–24. [DOI] [PubMed] [Google Scholar]
- 20.Thio CL. Hepatitis B in the human immunodeficiency virus-infected patient: epidemiology, natural history, and treatment. Semin Liver Dis 2003;23:125–36. [DOI] [PubMed] [Google Scholar]
- 21.Gaglio PJ, Sterling R, Daniels E, Tedaldi E. Hepatitis B virus and HIV coinfection: results of a survey on treatment practices and recommendations for therapy. Clin Infect Dis 2007;45:618–23. [DOI] [PubMed] [Google Scholar]
- 22.Stasi C, Salomoni E, Arena U, Corti G, Montalto P, Bartalesi F, Marra F, Laffi G, Milani S, Zignego AL, Pinzani M. Non-invasive assessment of liver fibrosis in patients with HBV-related chronic liver disease undergoing antiviral treatment: A preliminary study. Eur J Pharmacol. 2017. July 5;806:105–109. [DOI] [PubMed] [Google Scholar]
- 23.Maida I, Soriano V, Castellares C, Ramos B, Sotgiu G, Martin-Carbonero L, Barreiro P, Rivas P, González-Lahoz J, Núñez M. Liver fibrosis in HIV-infected patients with chronic hepatitis B extensively exposed to antiretroviral therapy with anti-HBV activity. HIV Clin Trials. 2006. September-October;7(5):246–50. [DOI] [PubMed] [Google Scholar]
- 24.Audsley J, Robson C, Aitchison S, Matthews GV, Iser D, Sasadeusz J, Lewin SR. Liver Fibrosis Regression Measured by Transient Elastography in Human Immunodeficiency Virus (HIV)-Hepatitis B Virus (HBV)-Coinfected Individuals on Long-Term HBV-Active Combination Antiretroviral Therapy. Open Forum Infect Dis. 2016. February 12;3(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin-Carbonero L, Teixeira T, Poveda E, Plaza Z, Vispo E, Gonzalez-Lahoz J, Soriano V. Clinical and virological outcomes in HIV-infected pateints with chronic hepatitis B on long-term nucleos(t)ide analogues. AIDS 2011;25:73–79. [DOI] [PubMed] [Google Scholar]
- 26.Hawkins C, Agbaji O, Ugoagwu P, Thio CL, Auwal MM, Ani C, Okafo C, Wallender E, Murphy RL. Assessment of liver fibrosis by transient elastography in patients with HIV and hepatitis B virus coinfection in Nigeria. Clin Infect Dis. 2013. December;57(12):e189–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyd A, Lacombe K. More long-term assessment of transient VCTE is needed for HIV/Hepattiis B virus coinfected patients undergoing treatment with tenofovir. CID 2016:62:128–130. [DOI] [PubMed] [Google Scholar]
- 28.Bottero J, Lacombe K, Guéchot J, Serfaty L, Miailhes P, Bonnard P, Wendum D, Molina JM, Lascoux-Combe C, Girard PM. Performance of 11 biomarkers for liver fibrosis assessment in HIV/HBV co-infected patients. J Hepatol. 2009. June;50(6):1074–83. [DOI] [PubMed] [Google Scholar]
- 29.Miailhes P, Pradat P, Chevallier M, Lacombe K, Bailly F, Cotte L, Trabaud MA, Boibieux A, Bottero J, Trepo C, Zoulim F. Proficiency of transient elastography compared to liver biopsy for the assessment of fibrosis in HIV/HBVcoinfected patients. J Viral Hepat 2011;18:61–69. [DOI] [PubMed] [Google Scholar]
- 30.Taibi L, Boyd A, Bosselut N, Bottero J, Guéchot J, Lacombe K, Lasnier E, Baudin B, Vaubourdolle M. Diagnostic accuracy of the Coopscore© to predict liver fibrosis in human immunodeficiency virus/hepatitis B virus co-infection. Ann Clin Biochem. 2018. March;55(2):236–243. [DOI] [PubMed] [Google Scholar]
- 31.Stockdale AJ, Phillips RO, Beloukas A, Appiah LT, Chadwick D, Bhagani S, Bonnett L, Sarfo FS, Dusheiko G, Geretti AM; Hepatitis B Infection in Kumasi (HEPIK) Study Group. Liver fibrosis by transient elastography and virologic outcomes after introduction of tenofovir in lamivudine-experienced adults with HIV and hepatitis B virus coinfection in Ghana. Clin Infect Dis. 2015. September 15;61(6):883–91. [DOI] [PubMed] [Google Scholar]
- 32.Sterling RK, Wahed AS, King WC, Kleiner DE, Khalili M, Sulkowski M, Chung RT, Jain MK, Lisker-Melman M, Wong DK, Ghany MG; and the HIV-HBV Cohort Study of the Hepatitis B Research Network Spectrum of Liver Disease in Hepatitis B Virus (HBV) Patients Co-infected with Human Immunodeficiency Virus (HIV): Results of the HBV-HIV Cohort Study. Am J Gastroenterol. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lijmer JG, Mol BW, Heisterkamp S, et al. Empirical evidence of design related bias in studies of diagnostic tests. JAMA 1999; 282: 1061–6. [DOI] [PubMed] [Google Scholar]
- 34.Wong GL, Wong VW, Choi PC, et al. Evaluation of alanine transaminase and hepatitis B virus DNA to predict liver cirrhosis in hepatitis B e antigen-negative chronic hepatitis B using transient elastography. Am J Gastroenterol 2008; 103: 3071–81. [DOI] [PubMed] [Google Scholar]
- 35.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696–9. [DOI] [PubMed] [Google Scholar]
- 36.WHO. Report of the consensus meeting on WHO antiretroviral therapy guidelines for adults and adolescents 2009. Available from: http://www.who.int/hiv/topics/treatment/art_consensus_meeting_091016.pdf.
- 37.National Institute on Alcohol Abuse and Alcoholism. What is “low-risk” drinking? Available from https://www.rethinkingdrinking.niaaa.nih.gov/How-much-is-too-much/Is-Your-Drinking-Pattern-Risky/Whats-Low-Risk-Drinking.aspx Accessed 1/16/19
- 38.Carr A, Law M Objective Lipodystrophy Severity Grading Scale Derived From the Lipodystrophy Case Definition Score. JAIDS Journal of Acquired Immune Deficiency Syndromes 2003;33(5):571–6. [DOI] [PubMed] [Google Scholar]
- 39.Juan Lopez Cristina S, Martin Marta C, Sanchez Mercedes G, et al. Clinical and Molecular Hepatology 2018;24:384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao H, Shi M, Xie Y, Chi X. Comparison of diagnostic accuracy of magnetic resonance elastography and Fibroscan for detecting liver fibrosis in chronic hepatitis B patients: A systematic review and meta-analysis. PLOS One November 6, 2017: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.