Abstract
Intravesical BCG is a highly effective treatment for high-grade non-muscle invasive bladder cancer and carcinoma in situ (CIS); however, for patients who are either resistant or become unresponsive to BCG therapy there is a need for alternative treatment approaches. This study examined the safety and feasibility of intravesically administered recombinant fowlpox virus encoding GM-CSF (Arm A) or TRICOM (Arm B); and the local and systemic immunologic responses generated to the vector(s). Twenty bladder cancer patients scheduled for cystectomy as their standard of care received pre-operatively 4 weekly doses of intravesical recombinant fowlpox. Treatment was well tolerated, however, 3 patients experienced transient elevations of liver transaminases, with 1 rising to the level of a DLT. Cystectomy derived tumor and normal bladder mucosa demonstrated mRNA for the virally encoded LacZ gene supporting effective infection/transfection. Detected serum antibody to the LacZ encoding β-galactosidase indicated successful expression of vector-encoding gene products and the ability to immunize via the bladder site. H&E and IHC using a panel of immune cell specific antigens demonstrated immune cell infiltration of the bladder wall. These findings demonstrate good safety profile, successful infection/transfection, ability to generate systemic immune response and local recruitment of immune cell populations with intravesical administration of fowlpox-based constructs encoding for GM-CSF(rF-GM-CSF) or TRICOM (rF-TRICOM), and support further evaluation of this treatment modality for bladder cancer.
Introduction
Bladder cancer is the most common cancer of the urinary tract, with a total of 81,190 new cases estimated in 2018 and constituting approximately 54% of all projected urinary cancers (1). Approximately 70-80% of these cases are classified as non-muscle invasive bladder cancer (NMIBC), in which the cancer is limited to the superficial mucosa and submucosa of the bladder (2). Management of NMIBC includes a combination of surgical and medical therapeutic interventions, involving transurethral resection (TURBT) and intravesical drug(s) therapy. The mainstay first line intravesical drug treatment is with the bacillus Calmette-Guerin (BCG), an immunologic agent, which induces a BCG-specific immune response with resultant antitumor activity via exposure to the generated inflammatory milieu (3, 4). However, 20-40% of patients will not respond to BCG therapy and up to 20% will progress to muscle invasive disease (5, 6). Surgical intervention with radical cystectomy is the next most common therapeutic approach, but it is associated with significant perioperative complications and a decreased in quality of life (7, 8). Hence, there is a need for the development of alternative organ-sparing intravesical treatment options in this high-risk group of patients.
Given responsiveness of the NMIBC to the BCG-mediated immune modulation, immune-based intravesical therapy options for this disease appear particularly attractive. The use of recombinant viral vectors allows one to specifically engineer bioactive vectors to induce cytokine and/or immune stimulatory molecules chosen based on identified immune regulatory mechanisms known to be associated with the generation of an optimal immunologic response (9, 10). In addition, peritumoral administration of these agents is thought to provide immune-modulation directly at the site of the tumor antigens that are specific for the disease in a given individual. Consequently, our group has extensively studied the use of local, peritumoral administration of the poxviral vectors as a mean to alter the tumor microenvironment and enhance systemic antitumor immunity. We have demonstrated in preclinical and clinical studies that replicative vaccinia virus has broad tropism and high efficiency of infection/transfection, making it an excellent candidate as a vector for gene delivery (11). Our in vitro studies with murine bladder tumors MBT2 and MB49 and in vivo studies in C57BL/6 mice bearing intravesical MB49 tumor demonstrated effective infection/transfection of tumor despite preexisting immunity to the vaccinia vector which would be analogous to patients who had developed systemic immunity due to initial vaccinia exposure via the smallpox vaccine (9).
As a prelude to the described here study, we carried out a phase I trial of intravesical vaccinia vector in patients with muscle-invasive bladder tumor prior to cystectomy to assess the safety profile and whether vaccinia can transfect human bladder tumor (11). We found that intravesical vaccinia virus can be administered safely, with dysuria as the most common adverse event. Obtained cystectomy samples showed tissue immune infiltrates in patients receiving higher doses of vaccinia, consistent with vaccinia infecting/transfecting cells in the tumor microenvironment (11). Though this study showed no significant toxicity, vaccinia is a fully replicating virus, and as such has the potential to produce significant systemic toxicities in patients with compromised immunity. As a result, we selected related non-replicating fowlpox vector for our current phase I study, based on the advantage that this non-replicating virus could be less likely to cause systemic toxicities and has increased length of transgenic expression (12).
Major advances in cancer immunology have led to an increased understanding of the molecular events needed to induce an effective anti-tumor immune response, among them, importance of costimulatory molecules and specific cytokine(s) production. Extensive pre-clinical data supports the use of the combination of multiple co-stimulatory molecules together with the tumor antigen in order to induce a greater antigen specific T-cell response (13, 14). Also, the addition of GM-CSF, a cytokine involved in the recruitment and activation of antigen presenting dendritic cells (DC) was demonstrated to provide an important signal for enhanced antigen presentation in the tumor microenvironment and consequent stronger anti-tumor immune response (10, 15).
Based on the above studies by us and others, discussed here phase I trial assessed safety and feasibility of intravesical administration of recombinant fowlpox virus constructs encoding GM-CSF (rFGM-CSF) or three immune costimulatory molecules: B7.1, ICAM-1, and LFA-3 (rF-TRICOM) in patients with bladder carcinoma scheduled for cystectomy as a standard of care for their disease. Our secondary objectives were to determine the efficiency of viral infection and gene function as well as host response to the intravesical recombinant fowlpox vectors. Our underlying hypothesis for the described study is that local and/or systemic anti-tumor effects induced by neoadjuvant recombinant fowlpox constructs have the potential to increase the chance of tumor control following radical cystectomy. With this approach, the patient has the benefit of receiving standard surgical treatment for their bladder disease and the possible benefit from the generation of a systemic and/or local anti-tumor-specific immune response elicited by intravesical rF-GM-CSF and/or rF-TRICOM.
Patients and Methods
Treatment Plan:
The described study was an open label phase I dose escalation trial ( NCT00072137). The primary objective was to determine the maximum tolerated dose (MTD) of intravesically administered recombinant fowlpox virus encoding GM-CSF or TRICOM (Therion Biologics Corporation). A standard 3+3 dose escalation schema was utilized in this study with two treatment arms. Patients treated on Arm A received rF-GM-CSF, and those on Arm B received rF-TRICOM. The starting dose on Arm A was rF-GM-CSF 7.02 × 107 PFU and Arm B was rF-TRICOM 5.67 × 107 PFU with dose escalation according to Table 1. There was no intra-patient dose escalation. Patients received 4 weekly doses (Days 1, 8, 15, 22) prior to undergoing cystectomy with the last dose given 4–6 days prior to surgery as seen in Figure 1. Recombinant fowlpox construct was suspended in 50 mL of buffered saline suitable for intravesical instillation (Normosol-R pH 7.4, Abbot Laboratories) and deposited in the bladder via urethral catheter. After a dwell time of 2 hrs, male patients were allowed to void their bladders and in female patients, the virus containing solution was removed by the catheter.
Table 1: Modified Dose Escalation Schema for Arms A & B:
Dose escalation began at dose level 1 for both Arms. The dose for Arm B was de-escalated to dose levels 0 and 0.5 due to elevated liver transaminases.
| Dose level | Arm A: rF-GM-CSF | Arm B: rF-TRICOM |
|---|---|---|
| 0 | N/A | 5.67 × 106 PFU |
| 0.5 | N/A | 2.84 × 107 PFU |
| 1 | 7.02 × 107 PFU | 5.67 × 107 PFU |
| 2 | 7.02 × 108 PFU | 5.67 × 108 PFU |
| 3 | 1.4 × 109 PFU | 1.13 × 109 PFU |
Figure 1. Treatment Schema:
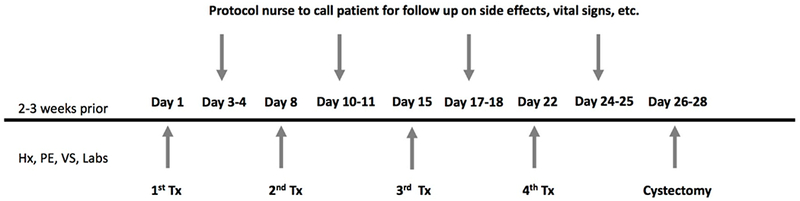
Planned treatment administration and schema was identical for both arms of the trial.
Patients:
Eligible patients had histologically confirmed bladder cancer (transitional cell carcinoma, adenocarcinoma, or squamous cell carcinoma including carcinoma in situ) scheduled for cystectomy as a standard therapy for their tumor stage. Patients were required to have an ECOG performance status of 0 or 1, an estimated life expectancy of at least 6 months, and adequate renal (serum creatinine < 1.5 mg/dl or a creatinine clearance > 60 mL/min), hepatic (bilirubin <2.0 mg/dl, AST and ALT < 2× normal range) and bone marrow function (ANC> 1,500 mm3 and platelets > 75,000/mm3). Patients with known immunodeficiency disorders, altered immune competence, and/or current or past autoimmune disease were excluded from this trial. Additionally, patients who had received neoadjuvant chemotherapy prior to planned cystectomy or bladder radiation therapy were excluded. Prior intravesical BCG and/or intravesical chemotherapy was allowed. Prior systemic chemotherapy, radiation therapy, immunotherapy or systemic doses of steroids were permitted if given at least 4 weeks prior to the start of study vaccination and patients had recovered from all acute toxicities of previous treatments. The study protocol was reviewed and approved by the Cancer Institute of New Jersey Scientific Review Board and Institutional Review Board and written informed consent was obtained from all patients. Entry assessments included history and physical examinations, vital signs, complete blood counts, chemistries (liver and renal function), serum for anti-fowlpox and anti-β-galactosidase antibody titers, and urine for urinalysis was obtained at baseline, weekly prior to therapy administration, and during the 1year follow-up period. Toxicity assessments were obtained for all patients who received at least one dose of protocol therapy at baseline, weekly while on treatment, in between weekly therapy (48-72 hours post vaccination), and on follow up visits. Toxicities were graded using the NCI Common Toxicity Criteria Version 2.0 and were deemed unrelated, unlikely, possibly, probably, or definitely related to protocol therapy by the Principle Investigator.
Correlative Studies:
Tissue retrieval:
Bladder tissue was obtained from patients at the time of cystectomy for immunohistochemical staining (IHC) and reverse transcription polymerase chain reaction (RT-PCR) by the Rutgers Cancer Institute of New Jersey Biospecimen Repository Shared Resource. Samples were fixed in formalin and sections prepared for IHC. For identification of viral transfection, tissue was fast frozen in liquid N2. Blood was collected and serum frozen for determination of systemic immunity to vector and encoded lac-Z gene product β -galactosidase at screening, weekly during treatment and following cystectomy.
Immunohistochemical (IHC) staining for cell subsets:
Immune cell infiltrates were assessed by IHC staining using cell/lineage specific antibodies. Formalin-fixed, paraffin embedded sections from the cystectomy specimen were stained by the Cancer Institute of New Jersey Immunohistochemistry Shared Resource to elucidate infiltrating cell subsets to include CD3, CD4, CD8, CD45 RO, CD1a, CD30, and Factor XIIIa (dendritic cells) as we have previously reported (16, 17). Primary Abs for CD3 (clone PS1, immuno- globulin G2a (IgG2a), Novocastra, Newcastle on Tyne, UK), CD4 (clone 1F6, IgG1; Novocastra); CD8 (clone CB/144B, IgG1; Dako, Carpinteria, CA), CD45RO (clone UCHL1, IgG2a; Dako), CD30 (clone Ber-H2, IgG1; Dako), CD1a (clone MTB1, IgG1; Dako), Factor XIIIa (clone AC-1A1, IgG1, Ventana Med. Sys., Tucson, AZ) were used to identify T cells, helper/inducer T cells, cytotoxic T cells, activated T cells, and antigen presenting populations, and tissue macrophages, respectively. IHC stained samples were read in a blinded fashion and scored on a scale of 0 to 3 based on positivity.
RT-PCR:
Bladder tissue was examined by RT-PCR to assess viral gene function at the time of cystectomy using primers for the vector encoding LacZ gene product (β-galactosidase). Total RNA was isolated from snap-frozen bladder biopsies and was quantitated spectrophotometrically before the RT-PCR step. cDNA synthesis was performed using 10 ug of total RNA, random primer (Life Technologies, Gaithersburg, Md), and RT buffer in diethyl pyrocarbonate-thromboxane water. The reaction mixture was incubated at 65°C for 10 minutes and subsequently maintained at 4°C. To the mixture, 10 mM dithiothreitol (Life Technologies); 0.5 mM each of deoxyadenosine triphosphate, deoxycytidine triphosphate, deoxyguanosine triphosphate, and thymidine triphosphate (Life Technologies); and 500 U Moloney murine leukemia virus RT (Life Technologies) were added to achieve a final reaction volume of 50 uL. Samples were incubated at 37°C for 1 hour and then heated to 95°C for 5 minutes. For amplification by PCR, 5 uL of each cDNA was then added to MicroAmp reaction tubes (Perkin Elmer, Norwalk, Conn) containing PCR buffer; 0.2 mM each of deoxyadenosine triphosphate, deoxycytidine triphosphate, deoxyguanosine triphosphate, and thymidine triphosphate; 1.25 U AmpliTaq DNA Polymerase (Perkin Elmer); MgCl2 concentrations determined to be optimal in our laboratory for each primer pair (final concentrations of 1.5–4.0 mM); and 0.5 uM each of the appropriate primer pairs in a final volume of 50 uL. The two primer pairs used in this study included beta-actin (+) (5’-tga-cgg-ggt-cac-cca-cac-tgt-gcc-cat-cta-3’), beta-actin (–) (5’-cta-gaa-gca-ttg-cgg-tgg-acg-atg-gag-gg-3’) and Laczp (5’-gtc aat ccg ccg ttt gtt cc-3’), Laczm (5’-tgc acc atc gtc tgc tca tc-3’).
PCR samples were amplified using a GeneAmp System 9600 thermocycler (Perkin Elmer). PCR products and size markers (100-base pair ladder; Promega, Madison, Wis) were separated in a 2% SeaKem GTG agarose gel (FMC BioProducts, Rockland, Me). The gel was stained with ethidium bromide and visualized and photographed under ultraviolet illumination. Electrophoresis of PCR products revealed a band corresponding to the predicted fragment size for each set of primers (16).
Humoral immunity to fowlpox and β-galactosidase:
To measure serum titers of anti-Fowlpox antibody, 96-well Maxisorb plates (Nunc, Rochester, NY) were first coated overnight with a 20fold dilution of fowlpox used for therapy and then blocked with fetal calf serum in phosphate-buffered saline. A dilution series of patient sera before and after treatment was then added to the wells. Following a four hour incubation, the patient sera were removen and the plates were washed. A peroxidase-labeled anti-human IgG heavy and light chain second antibody (Caltag, South San Francisco, Calif) was added to bind to any anti-Fowlpox antibodies in the wells; the second antibody was visualized using o-phenylenediamine as substrate. Titers were read as the reciprocal serum dilution yielding 50% maximum absorbance in the assay. To measure antibodies to β-galactosidase, wells were coated with a solution of β-galactosidase (10 ug/mL; Sigma, St. Louis, Mo) in place of the fowlpox described previously, and the assays were performed similarly (10, 11, 16).
Results
Patients and Toxicities:
Of the total 25 patients consented, 21 patients were eligible to participate in this clinical trial. All enrolled participants had high-grade non-metastatic bladder cancer scheduled for a cystectomy. One patient on Arm A did not receive protocol therapy and was replaced in the dose cohort. Twenty patients were included in the analysis (Figure 2). Two patients received the full course of study treatment but did not receive a cystectomy due to cardiac ischemia/infarction in one patient and development of a second primary tumor (mixed neuroendocrine and adenocarcinoma lung lesion) in the other. Patient demographics are listed in Table 2. Nineteen patients (95%) had urothelial carcinoma and 1 patient had mixed urothelial and adenocarcinoma histology determined by a TURBT. Eleven patients (55%) presented with pathologically determined muscle invasive disease and 6 patients presented with NMIBC. Seven of the treated patients had previous intravesical therapy. Five patients were considered BCG refractory (25%).
Figure 2. Patient Disposition:
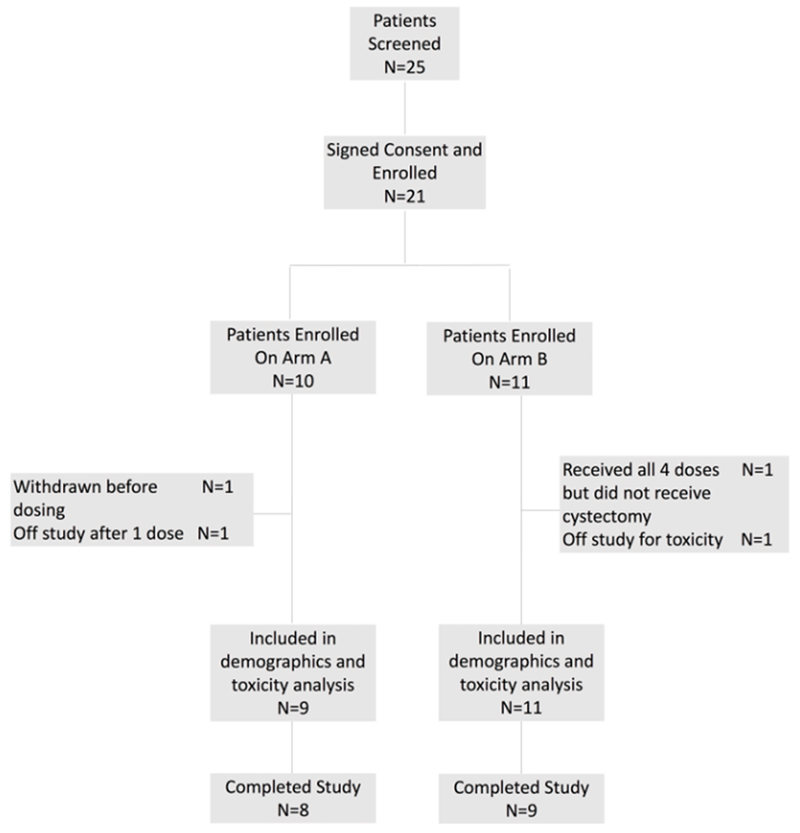
Twenty patients (Arm A: 9; Arm B: 11) were included in the analysis.
Table 2:
| Baseline demographics of treated patients | Patients(N=20) |
|---|---|
| Gender, n (%) | |
| Male | 17 (85) |
| Female | 3 (15) |
| Race, n (%) | |
| White | 19 (95) |
| Black or African American | 1 (5) |
| Ethnicity, n (%) | |
| Hispanic | 0 (10) |
| Non-Hispanic | 20 (100) |
| Age, years | |
| Median (range) | 65 (45-76) |
| ECOG Performance Status1 | |
| 0 | 15 (75) |
| 1 | 5 (25) |
| Pre-Treatment Disease Stage, n | |
| I | 7 (35) |
| II | 12 (60) |
| III | 1 (5) |
| Grade, n | |
| High Grade | 20 (100) |
| Histology, n | |
| Urothelial | 19 (95) |
| Mixed Urothelial and Adenocarcinoma | 1 (5) |
| Prior Lines of lines of intravesical Therapy, n | |
| 0 | 13 (70) |
| 1 | 6* (25) |
| 2 | 0 |
| 3 | 1 (5) |
| Prior Intravesical Therapy, n3 | |
| BCG Immunotherapy | 6* (25) |
| Mitomycin C | 2 (10) |
| Interferon | 1 (5) |
| No Prior Intravesical Therapy | 13 (70) |
Abbreviations:
ECOG = Eastern Cooperative Oncology Group;
BCG = Bacille Calmette-Guerin
Patients can fit into more than one group
1 patient received 2 cycles of BCG therapy
Ten patients were enrolled in Arm A across three dose cohorts. One patient in dose cohort 1 was unable to be catheterized and was removed from the study. Dose escalation through Dose level 3 in Arm A occurred without any DLTs and the MTD was not reached. Eleven patients were enrolled in Arm B. The first 3 patients enrolled on dose level 1 of Arm B experienced increased AST/ALT. One of these patients experienced a DLT (grade 3 ALT and grade 3 AST), did not receive the last dose of therapy, and went off-study. A second patient experienced Grade 3 AST elevation after cystectomy and it was not considered to be a DLT. The third patient experienced grade 1 ALT and grade 2 AST elevations. There were no grade 4 or 5 adverse events attributable to protocol therapy.
After observing AST elevation in a second study participant (though, per protocol, this was not considered to be a DLT) the trial was halted and the liver transaminases levels were reviewed. Subsequently, all was consulted with the CTEP and the trial was reopened to accrual with modifications in dosing levels. Upon reopening, 3 patients were enrolled on Dose Level 0 of Arm B, which was a ten-fold lower dose from the initial dose level 1, as determined by the investigators and the sponsor. There were no DLTs observed at the Dose Level 0. Dose escalation continued to Dose Level 0.5 without further dose limiting toxicities. Two additional patients were then enrolled at Dose Level 1 (with no toxicity) for a total of 5. The MTD, as defined in the protocol, was not reached in either study Arm A or Arm B. With regards to toxicity, a total of 6 patients (30%) experienced treatment related adverse events, 2 patients in Arm A and 4 patients in Arm B (Table 3).
Table 3. Treatment Related Adverse Events (AEs) by Arm and Dose Level:
Treatment related adverse events are broken down by Arm and Dose Level. A total of 9 patients were treated on Arm A on Dose Levels 1,2, and 3 (3 per dose level) and 2 patients total experienced AEs. A total of 11 patients were treated on Arm B, on Dose Levels 0, 0.5, and 1. AST/ALT elevations were observed in 3 patients on Dose Level 1 of Arm B. Treatment related adverse events in Arm B occurred in 4 of 11 patients.
| ARM A: Fowlpox GM-CSF | ||||||
|---|---|---|---|---|---|---|
| Adverse Event | Dose Level 0 (N=0) |
Dose Level 0.5 (N=0) |
Dose Level 1 (N=3) |
Dose Level 2 (N=3) |
Dose Level 3 (N=3) |
Grade ≥ 3 (N=9) |
| Patients with AE | 1 | - | 1 | - | ||
| Dysuria | N/A | N/A | - | - | 1 | - |
| Urinary Frequency | 1 | - | - | - | ||
| ARM B: Fowlpox-TRICOM | ||||||
| Adverse Event | Dose Level 0 (N=3) |
Dose Level 0.5 (N=3) |
Dose Level 1 (N=5) |
Dose Level 2 (N=0) |
Dose Level 3 (N=0) |
Grade ≥ 3 (N=11) |
| Patients with AE | - | - | 4 | 2 | ||
| ALT Increase1 | - | - | 3 | 1 | ||
| AST Increase2 | - | - | 3 | 2 | ||
| Constipation | - | - | 1 | - | ||
| Decreased Appetite | - | - | 1 | - | ||
| Decreased Hemoglobin | - | - | 1 | N/A | N/A | - |
| Erythema | - | - | 1 | - | ||
| Fever without Neutropenia | - | - | 1 | - | ||
| Malaise | - | - | 1 | - | ||
| Nausea | - | - | 1 | - | ||
ALT = Alanine aminotransferase;
AST = Aspartate aminotransferase
Correlative Studies:
Patients in the first cohort of Arms A & B were assessed for biomarkers associated with effective infection/transfection of the bladder microenvironment using IHC for immune cell infiltration, RT-PCR identifying transcription of the Lac-Z gene product β-galactosidase, and the generated humoral immunity to both the fowlpox vectors and to the encoded β-galactosidase via serum antibody ELISA.
Immunohistochemistry:
Following completion of the first dose level cohort (DL1, 3 rFGM-CSF, 3 rF-TRICOM), the 6 patients were studied for immune cell infiltration via IHC. Figure 3 (patient #3, rF-GM-CSF) represents an example of the IHC staining. IHC results from the patient samples from the first cohort of patients treated with rF-GM-CSF and rF-TRICOM are shown in Table 4. Given that the bladder cancer staging used for study participation was done using small TURBT-based biopsies, insufficient for IHC, we lacked pretreatment IHC analysis for comparison with post-treatment IHC analysis of cystectomy specimens from treated patients. For this reason, we identified a group of comparable cystectomy specimens from untreated patients for use as controls. Supplemental Table 1 provides detailed IHC results and pathology from treated specimens and Supplemental Table 2 detailed IHC results and pathology from control specimens. While the limited numbers of treated (Arm A, GM-CSF and Arm B TRICOM) and untreated patients make statistical significance impossible to determine, data presented in Supplemental Table 1 (untreated cystectomy) and Supplemental Table 2 (treated patients) provided results that when analyzed suggest the following trends. Overall, there were no consistent differences throughout between tumor specimens and adjacent non tumor specimens from individual patients. We therefore combined the data from tumor and adjacent non-tumor to increase numbers of specimens evaluated. When comparing IHC scores, the following trends were identified. Independent of which treatment arm the patients were on, treated patients had clearly enhanced levels of inflammation and T cell infiltration (CD3, CD4, CD8) over the untreated controls however there was no consistent difference between treatment arms in inflammation and T cell infiltration. This finding would be consistent with the conclusion that productive infection with the viral vector (See Fig. 4), independent of Arm enhanced the levels of inflammation and T cell infiltration. Regarding differences between Arms A and B, while inflammation and T cell markers were similar, the levels of Factor XIIIa expressing cells were somewhat higher in Arm A (GM-CSF) than Arm B (TRICOM) in tumor. While this would be consistent with the vector induced GM-CSF enhancing DC, the small number of data points would preclude a significant conclusion.
Figure 3. Immunohistochemical assessment of the tumor from patient #003 treated with the fowlpox vector expressing GM-CSF following cystectomy:
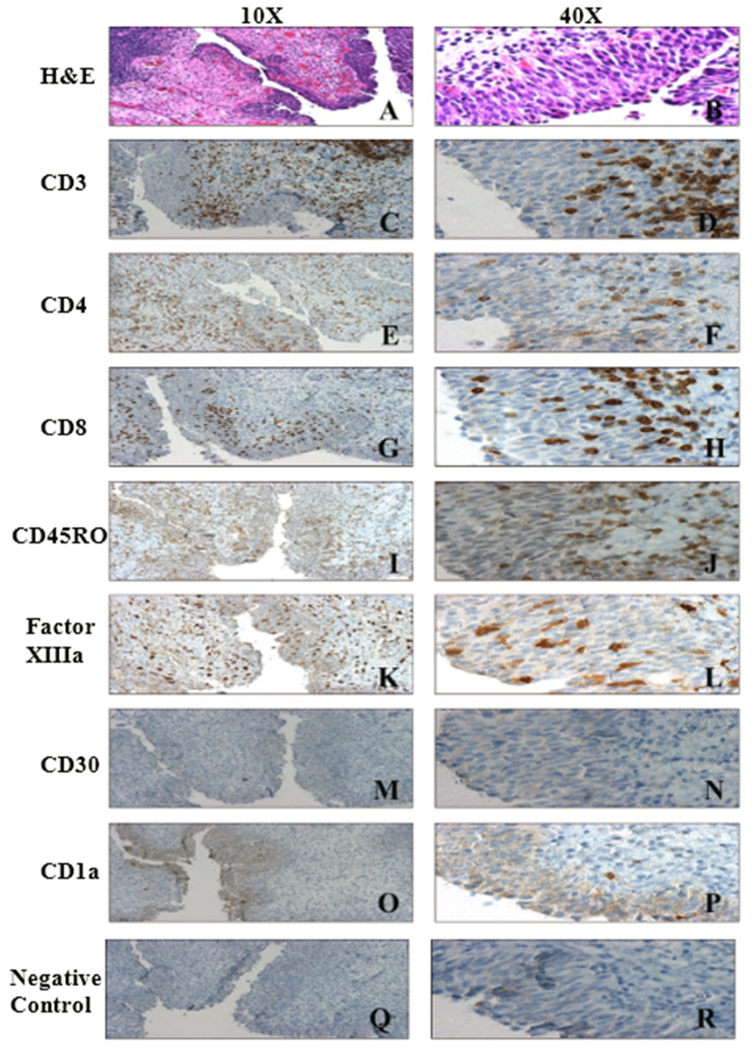
Patient #003 received a weekly intravesical dose of 7.02 × 107 PFU rFP-GM-CSF with the fourth dose instilled 2 days prior to cystectomy. A and B H&E stain, C and D CD3, E and F CD4, G and H CD8, I and J CD45RO, K and L factor XIIIa (Dendritic cells), M and N CD30, O and P CD1a, Q and R Immunostaining negative control.
Table 4. Immunohistochemical assessment of the tumor from patients treated with FP-GM-CSF and FP-TRICOM following cystectomy:
IHC staining of cystectomy specimens from treated patients using immune subset antibodies including CD3, CD4, CD8, CD45RO, CD1a. Factor XIIIa and the negative control CD30. IHC staining: 0, no infiltration; .3, less than 5 cells / high power field (hpf); 1, 5-20 cells/hpf; 2, 20-50 cells / hpf; 3, >50 cells /hpf; ne, nonevaluable.
| Marker | Patient#1 GM-CSF | Patient#3 GM-CSF | Patient#5 GM-CSF | Patient#4 TRICOM | Patient#6 TRICOM | Patient#7 TRICOM |
|---|---|---|---|---|---|---|
| CD3 | ++ | ++++ | +++ | +++ | ++++ | + |
| CD4 | + | ++++ | +++ | +++ | +++ | + |
| CD8 | + | +++ | ++ | ++ | ++ | + |
| CD45RO | ++ | ++++ | +++ | +++ | +++ | ++ |
| CD1a | − | − | − | − | − | − |
| CD30 | − | Few | − | − | − | − |
| Factor XIIIa | +++ | +++ | ++ | ++ | ++ | Few |
Figure 4. Effective transfection/transcription of the recombinant Lac-Z gene:
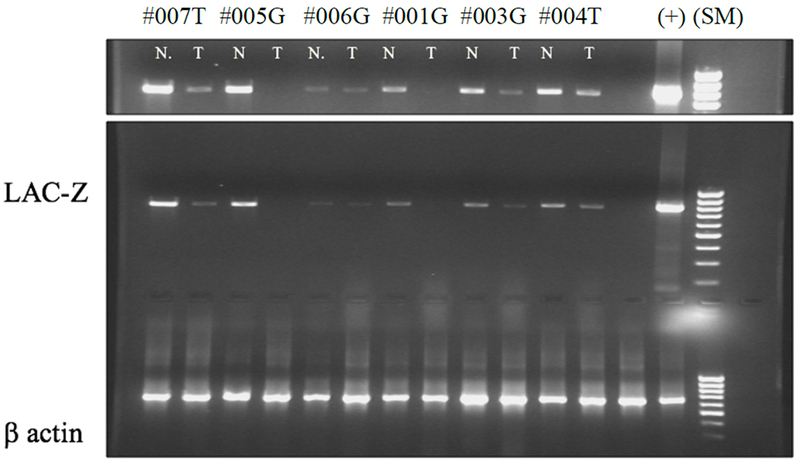
Expression of Lac-Z and b actin mRNA in the tumor (T) and normal adjacent tissue (N), post cystectomy, by RT-PCR in the dose level 1 patient cohorts treated with G (rF-GM-CSF) and T (rF-TRICOM). (+ positive control, and (s)- size markers). (Top panel was the gel in the lower panel with extended exposure time).
RT-PCR:
As noted above for IHC, patients from the first cohort of each treatment arm (3 rF-GM-CSF, 3 rF-TRICOM) were examined for effective infection/transfection of the vector(s) by using RT-PCR for the lacZ gene in both tumor and normal-adjacent tissue. These RT-PCR analyses of the lacZ gene in both tissue types indicate that rF-GM-CSF and rF-TRICOM productively infected/transfected bladder mucosa by inducing local expression of lac-Z mRNA in either or both tumor and normal adjacent mucosa (Figure 4).
Systemic Immune Response to Virus:
All patients in the first cohort demonstrated serum antibodies to both the fowlpox vector and the encoded lac-Z product β-galactosidase (Table 5). The presence of serum antibodies to β-galactosidase demonstrate further that the vectors were successful in infecting/transfecting the bladder and, more importantly, that the patients were effectively immunized via the bladder compartment following this therapeutic strategy.
Table 5: Anti-Fowlox and Anti-LacZ Antibody Titers in Post Immune Patients’ Sera Determined by ELISA:
Post immune titer is the reciprocal of the concentration corresponding to the mean OD+ 3× STDEV equivalent to the pre-immune baseline set for each patient. The baseline was set as the mean OD of the pre-immune serum sample at a 1:80 dilution + 3 times the standard deviation. Baseline titer is set to 80 for Antib-galactosidase and to 50 for Anti-Fowlpox. The immune sera were tested in 2-fold dilution increments from an initial of 1:10 and a curve was prepared.
| Patient ID | Arm | Anti-LacZ | Anti-Fowlpox |
|---|---|---|---|
| 1 | rF-GM-CSF | 81.9 | 45.3 |
| 3 | rF-GM-CSF | 131.9 | 30.5 |
| 4 | rF-TRICOM | 106.7 | 67.2 |
| 5 | rF-GM-CSF | 102.5 | 294 |
| 6 | rF-TRICOM | 90.1 | 64.2 |
| 7 | rF-TRICOM | 82.3 | 60.3 |
Discussion
Despite clear recognition that the anti-tumor effect of intravesical BCG is due to the generated strong inflammatory response the exact mechanism of its anti-tumor effect is poorly understood. Although BCG remains the agent of choice for intravesical treatment of NMIBC, there is still sizable number of patients who either don’t respond to BCG or develop subsequent resistance with recurrent and/or progressive disease treated in second line less effective intravesical agents or radical cystectomy creating an unmet need for more effective bladder-spearing treatment strategies.(11, 18). Based on our previous clinical studies with vaccinia demonstrating the ability of the virus to induce systemic immunity, we developed a phase I trial of recombinant intravesical fowlpox in patients with bladder cancer (11, 16, 19). We report here the findings of the first completed phase I trial of recombinant intravesical pox-vector.
Our results provideed three important findings: (1) individual intravesical administration of both rFGM-CSF and rF-TRICOM induces systemic immunity as shown by the anti-fowlpox and anti-β-galactosidase antibody titers in post-treatment patients’ sera; (2) both recombinant fowlpox vectors have the ability to transfect normal bladder mucosa and bladder tumor; and (3) both recombinant fowlpox vectors encoding GMCSF or TRICOM can be safely administered with minimal side effects at 1.4 × 109 PFU and 5.67 × 107 PFU, respectively.
The ability of the fowlpox vector to induce a systemic immune response is consistent with our previous clinical studies utilizing intravesical vaccinia in bladder cancer patients and intralesional recombinant vaccinia encoding GM-CSF in patients with melanoma respectively (11, 16). We hypothesized that the mechanism of intravesical rF-GM-CSF would be similar to that of our prior experience with vaccinia-GMCSF in melanoma in enhancing a systemic immune response via recruiting and activating dendritic cells (Factor XIIIa, Figure 3K–L) (16, 20).
With the increasing costs of the diagnosis and treatment of bladder cancer and the significant morbidity of radical cystectomy, there is a need for more focus on research efforts to develop alternative treatment options for patients with BCG unresponsive disease (21). In these patients, chemotherapeutic agents such as intravesical gemcitabine has been shown to be superior to treatment with mitomycin C, retreatment with BCG, or placebo in reducing disease recurrence based on clinical trials data (22–25). The use of BCG plus intravesical interferon showed 45% of BCG failure patients still remaining disease free after 24 months of follow-up (26). In a phase II trial, patients with high grade (HG) NMIBC were treated with intravesical recombinant adenovirus encoding interferon alfa with Syn3 (rAd-IFN∞/Syn3). After 12 months, 35% of patients remained free of HG recurrence (90% CI: 22.6% to 49.2%) (27). Valrubicin is the only intravesical agent approved by the FDA for BCG unresponsive carcinoma in situ (CIS); however, it demonstrated a rather modest response with an 18% complete response rate in two clinical trials (28, 29). The efficacy of intravesical docetaxel has been investigated in a phase I trial of 18 patients, with a response rate of 59% and a 1year recurrence free survival of 40% and 5-year overall survival rate of 71% (30). Other intravesical therapies such as Ty21a ( NCT03421236), Nanoxel®M ( NCT02982395), BCG with and without PANVAC ( NCT02015104), and Alt-803 with BCG ( NCT02138734) are currently under investigation and recruiting patients with NMIBC.
We and others are currently studying the effects of immune checkpoint inhibitors, such as those targeting programmed cell death protein-1 (PD-1) and programmed death ligand-1 (PD-L1) in patients with NMIBC. Currently, there are several phase I/II studies investigating the use of systemic PD-1/PD-L1 inhibitors such as Atezolizumab ( NCT02844816), Durvalumab ( NCT02901548), Pembrolizumab ( NCT02625961), and Nivolumab ( NCT03106610) in BCG unresponsive NMIBC.
We recognize that our study has several important limitations including relatively small sample size and the lack of pre-treatment tissue for comparison with post-treatment effects in correlative studies. However, it provides prove of principle for ability to successfully vaccinate at the bladder site using recombinant fowlpox-based viral constructs. Observed toxicity profile was also acceptable though the etiology of transient elevations in liver transaminases in the 2 patients in Arm B proved difficult to characterize.
In summary, our described study of intravesical delivery of rF-GM-CSF and rF-TRICOM in patients with urothelial cancer demonstrated good tolerance and encouraging preliminary correlative findings that support its further study in this disease.
Supplementary Material
Acknowledgements:
Studies made use of the Rutgers Cancer Institute of New Jersey Biospecimen and Immunohistochemistry and Research Pharmacy, Shared Resources.
Support: This trial was supported by the Cancer Therapy Evaluation Program (CTEP), a division of the National Cancer Institute (NCI/CTEP #5585, U01CA132194), R21CA121589, and a grant from the National Cancer Institute (P30CA072720). The Fowlpox vaccine, rF-GM-CSF (NSC 707299) and rF-TRICOM (NSC 710658) was manufactured by Therion Biologics Corporation and supplied by the Pharmaceutical Management Branch, CTEP, DCTD, NCI.
Dr. Singer receives research funding from Astellas/Medivation. The remaining authors declared no potential conflicts.
Footnotes
Conflicts: Dr. Lattime is an inventor of the patented recombinant VacciniaGMCSF that has been licensed to Sillajen and is being studied as JX-594 (Pexa-Vec). As such, he derives royalties and licensing fees from the Thomas Jefferson University where the patent is held.
References:
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Alfred Witjes J, Lebret T, Comperat EM, Cowan NC, De Santis M, Bruins HM, et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur Urol 2017;71(3):462–75. [DOI] [PubMed] [Google Scholar]
- 3.Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Comperat E, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol 2013;64(4):639–53. [DOI] [PubMed] [Google Scholar]
- 4.Freyne B, Marchant A, Curtis N. BCG-associated heterologous immunity, a historical perspective: experimental models and immunological mechanisms. Trans R Soc Trop Med Hyg 2015;109(1):46–51. [DOI] [PubMed] [Google Scholar]
- 5.Cookson MS, Herr HW, Zhang ZF, Soloway S, Sogani PC, Fair WR. The treated natural history of high risk superficial bladder cancer: 15-year outcome. J Urol 1997;158(1):62–7. [DOI] [PubMed] [Google Scholar]
- 6.Ahn JJ, Ghandour RA, McKiernan JM. New agents for bacillus Calmette-Guerin-refractory nonmuscle invasive bladder cancer. Curr Opin Urol 2014;24(5):540–5. [DOI] [PubMed] [Google Scholar]
- 7.Herr HW, Milan TN, Dalbagni G. BCG-refractory vs. BCG-relapsing non-muscle-invasive bladder cancer: a prospective cohort outcomes study. Urol Oncol 2015;33(3):108 e1–4. [DOI] [PubMed] [Google Scholar]
- 8.Tyson MD 2nd, Barocas DA. Quality of Life After Radical Cystectomy. Urol Clin North Am 2018;45(2):249–56. [DOI] [PubMed] [Google Scholar]
- 9.Lee SS, Eisenlohr LC, McCue PA, Mastrangelo MJ, Lattime EC. Intravesical gene therapy: in vivo gene transfer using recombinant vaccinia virus vectors. Cancer Res 1994;54(13):3325–8. [PubMed] [Google Scholar]
- 10.Mastrangelo MJ, Eisenlohr LC, Gomella L, Lattime EC. Poxvirus vectors: orphaned and underappreciated. J Clin Invest 2000;105(8):1031–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomella LG, Mastrangelo MJ, McCue PA, Maguire HJ, Mulholland SG, Lattime EC. Phase i study of intravesical vaccinia virus as a vector for gene therapy of bladder cancer. J Urol 2001;166(4):1291–5. [PubMed] [Google Scholar]
- 12.Schlom J, Hodge JW. The diversity of T-cell co-stimulation in the induction of antitumor immunity. Immunol Rev 1999;170:73–84. [DOI] [PubMed] [Google Scholar]
- 13.Grosenbach DW, Barrientos JC, Schlom J, Hodge JW. Synergy of vaccine strategies to amplify antigen-specific immune responses and antitumor effects. Cancer Res 2001;61(11):4497–505. [PubMed] [Google Scholar]
- 14.Hodge JW, McLaughlin JP, Abrams SI, Shupert WL, Schlom J, Kantor JA. Admixture of a recombinant vaccinia virus containing the gene for the costimulatory molecule B7 and a recombinant vaccinia virus containing a tumor-associated antigen gene results in enhanced specific T-cell responses and antitumor immunity. Cancer Res 1995;55(16):3598–603. [PubMed] [Google Scholar]
- 15.Kass E, Panicali DL, Mazzara G, Schlom J, Greiner JW. Granulocyte/macrophage-colony stimulating factor produced by recombinant avian poxviruses enriches the regional lymph nodes with antigen-presenting cells and acts as an immunoadjuvant. Cancer Res 2001;61(1):206–14. [PubMed] [Google Scholar]
- 16.Mastrangelo MJ, Maguire HC Jr., Eisenlohr LC, Laughlin CE, Monken CE, McCue PA, et al. Intratumoral recombinant GM-CSF-encoding virus as gene therapy in patients with cutaneous melanoma. Cancer Gene Ther 1999;6(5):409–22. [DOI] [PubMed] [Google Scholar]
- 17.Deguchi M, Aiba S, Ohtani H, Nagura H, Tagami H. Comparison of the distribution and numbers of antigen-presenting cells among T-lymphocyte-mediated dermatoses: CD1a+, factor XIIIa+, and CD68+ cells in eczematous dermatitis, psoriasis, lichen planus and graft-versus-host disease. Arch Dermatol Res 2002;294(7):297–302. [DOI] [PubMed] [Google Scholar]
- 18.Anastasiadis A, de Reijke TM. Best practice in the treatment of nonmuscle invasive bladder cancer. Ther Adv Urol 2012;4(1):13–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mastrangelo MJ, Maguire HC Jr., McCue PA, Lee SS, A. A, L.N. N, et al. A Pilot Study Demonstrating the Feasibility of Using Intratumoral Vaccinia Injections as a Vector for Gene Transfer. Vaccine Research. 1995; 4(2). [Google Scholar]
- 20.Conry RM, Westbrook B, McKee S, Norwood TG. Talimogene laherparepvec: First in class oncolytic virotherapy. Hum Vaccin Immunother 2018;14(4):839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noyes K, Singer EA, Messing EM. Healthcare economics of bladder cancer: cost-enhancing and cost-reducing factors. Curr Opin Urol 2008;18(5):533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Lorenzo G, Perdona S, Damiano R, Faiella A, Cantiello F, Pignata S, et al. Gemcitabine versus bacille Calmette-Guerin after initial bacille Calmette-Guerin failure in non-muscle-invasive bladder cancer: a multicenter prospective randomized trial. Cancer. 2010;116(8):1893–900. [DOI] [PubMed] [Google Scholar]
- 23.Skinner EC, Goldman B, Sakr WA, Petrylak DP, Lenz HJ, Lee CT, et al. SWOG S0353: Phase II trial of intravesical gemcitabine in patients with nonmuscle invasive bladder cancer and recurrence after 2 prior courses of intravesical bacillus Calmette-Guerin. J Urol 2013;190(4):1200–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Addeo R, Caraglia M, Bellini S, Abbruzzese A, Vincenzi B, Montella L, et al. Randomized phase III trial on gemcitabine versus mytomicin in recurrent superficial bladder cancer: evaluation of efficacy and tolerance. J Clin Oncol 2010;28(4):543–8. [DOI] [PubMed] [Google Scholar]
- 25.Messing EM, Tangen CM, Lerner SP, Sahasrabudhe DM, Koppie TM, Wood DP Jr., et al. Effect of Intravesical Instillation of Gemcitabine vs Saline Immediately Following Resection of Suspected Low-Grade Non-Muscle-Invasive Bladder Cancer on Tumor Recurrence: SWOG S0337 Randomized Clinical Trial. JAMA. 2018;319(18):1880–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joudi FN, Smith BJ, O’Donnell MA, National BCGIPIG. Final results from a national multicenter phase II trial of combination bacillus Calmette-Guerin plus interferon alpha-2B for reducing recurrence of superficial bladder cancer. Urol Oncol. 2006;24(4):344–8. [DOI] [PubMed] [Google Scholar]
- 27.Shore ND, Boorjian SA, Canter DJ, Ogan K, Karsh LI, Downs TM, et al. Intravesical rAd-IFNalpha/Syn3 for Patients With High-Grade, Bacillus Calmette-Guerin-Refractory or Relapsed Non-Muscle-Invasive Bladder Cancer: A Phase II Randomized Study. J Clin Oncol 2017;35(30):3410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinney CP, Greenberg RE, Steinberg GD. Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette-Guerin. Urol Oncol 2013;31(8):1635–42. [DOI] [PubMed] [Google Scholar]
- 29.Steinberg G, Bahnson R, Brosman S, Middleton R, Wajsman Z, Wehle M. Efficacy and safety of valrubicin for the treatment of Bacillus Calmette-Guerin refractory carcinoma in situ of the bladder. The Valrubicin Study Group. J Urol 2000;163(3):761–7. [PubMed] [Google Scholar]
- 30.Barlow LJ, McKiernan JM, Benson MC. Long-term survival outcomes with intravesical docetaxel for recurrent nonmuscle invasive bladder cancer after previous bacillus Calmette-Guerin therapy. J Urol 2013;189(3):834–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


