Abstract
Cancer stem cells (CSC) are highly associated with poor prognosis in cancer patients. Our previous studies report that isorhapontigenin (ISO) down-regulates SOX2-mediated cyclin D1 induction and stem-like cell properties in glioma stem-like cells. The present study revealed that ISO could inhibit stem cell-like phenotypes and invasivity of human bladder cancer (BC) by specific attenuation of expression of CD44 but not SOX-2, at both the protein transcription and degradation levels. On one hand, ISO inhibited cd44 mRNA expression through decreases in Sp1 direct binding to its promoter region-binding site, resulting in attenuation of its transcription. On the other hand, ISO also down-regulated USP28 expression, which in turn reduced CD44 protein stability. Further studies showed that ISO treatment induced miR-4295, which specific bound to 3′-UTR activity of usp28 mRNA and inhibited its translation and expression, while miR-4295 induction was mediated by increased Dicer protein to enhance miR-4295 maturation upon ISO treatment. Our results provide the first evidence that ISO has a profound inhibitory effect on human BC stem cell-like phenotypes and invasivity through the mechanisms distinct from those previously noted in glioma stem-like cells.
Electronic supplementary material
The online version of this article (10.1007/s00018-019-03185-3) contains supplementary material, which is available to authorized users.
Keywords: Isorhapontigenin, Bladder cancer, Stem cell-like properties, CD44, USP28
Introduction
Bladder cancer (BC) originates from the urothelial epithelium and at least 50% of patients with muscle-invasive bladder cancer (MIBC) die from metastases within 2 years, and the 5-year survival rate for metastatic BC is only 6% [1]. In 2019, about 80,470 new cases of BC will have been diagnosed in the US [2]. Although the mortality rate from bladder cancer is not as high as that of lung and breast cancer, its high recurrence rate is an urgent problem that needs to be addressed. Based on clinic pathology, BC is mainly divided into two phenotypes: muscle-invasive bladder cancer (MIBC) and non-muscle-invasive bladder cancer (NMIBC). Approximately 90% patients with NMIBC are treated by surgery alone or in combination with other treatments; however, about 50–70% of patients will have a recurrence and 5–20% of relapsed patients even will progress to MIBC. Compared with NMIBC, patients with MIBC have a higher risk of mortality [3–5]. Increasing evidence has proven that cancer stem cells (CSC), which have some traits of normal stem cells including self-renewal ability, play a significant role in tumor recurrence and metastasis and drug resistance [5–7]. For these reasons, evaluating potential therapeutic approaches, for example, finding new special compounds for CSC-targeted cancer therapies, is of extreme importance for improving the clinical outcomes of BC patients. Along these lines, a natural saponin ginsenoside-Rb1 has already been shown to effectively suppress ovarian cancer CSC self-renewal without regrowth [8].
Isorhapontigenin (ISO), a highly functionalized derivative stilbene, was original isolated from the Chinese herb Gnetum cleistostachyum [9, 10] and is now commercially available. Our earlier in vitro and in vivo studies demonstrated that ISO possesses a lot of anti-cancer activities. Those studies showed that ISO treatment of T24T human-invasive BC cells induced apoptosis through down-regulation of the anti-apoptotic protein XIAP [10]. Recently, our studies also found that ISO inhibited cell invasion in both UMUC3 and T24T cells through a targeting of the STAT1–FOXO1–MMP2 axis [11]. Moreover, our work showed that ISO exhibited clear inhibitory effects on BC cell growth that was mediated by the miR-137/Sp1/cyclin D1 pathway, both in vitro and in vivo [12]. These results provided substantial support for defining ISO as a promising therapeutic natural compound worthy of further exploration of its potentially powerful anti-cancer utility. Even so, to date, nothing is known about if ISO can inhibit bladder stem cell-like cancer cells. Thus, in the current studies reported here, inhibitory effects of ISO on human BC cell sphere formation properties and potential underlying molecular mechanisms for these effects were investigated.
CD44, an important surface receptor for hyaluronate, has been identified as a major cancer stem cell marker for certain kinds of epithelial tumors, including bladder cancer [5, 13]. CD44 possesses an extracellular, transmembrane, and intracellular domain [14, 15] that contribute to interactions with various ligands. These interactions are essential for its many cellular functions, such as modulation of migration/invasion processes during cancer progression [16], angiogenesis [17], as well as bone metastasis [18]. Moreover, CD44, as a transcription factor, enables it to control cell signaling via binding to promoter response elements [19, 20]. Indeed, data from the recent study found that knockdown of ATG7 accelerates CD44 protein degradation, which subsequently had an inhibitory effect on cell sphere formation and invasivity by human BC cells [21].
To our knowledge, the study reported here is the first to show that ISO inhibition of CD44 protein expression both at protein degradation and transcription level, consequently, impacted on BC cell stem-like properties and invasivity.
Materials and methods
Plasmids, antibodies, and reagents
Short Palindromic Repeats (CRISPR)/Cas9 system-specific targeting FOXO1 were bought from Applied Biological Materials (British Columbia, Canada). The plasmid containing luciferase reporter under control of a human CD44 gene promoter was constructed in a PGL3-BASIC vector using the primers (F) 5′-CCGCTCGAGACGTATGGGTGGATGAGAG-3′ and (R) 5′-CCCAAGCTTATGAGGGCCTGAAGT. A CD44 promoter-driven luciferase reporter with a mutation in the SP1-binding site was constructed using a CD44 promoter-driven luciferase reporter with primers (F) 5′-CTCTTTCCACTTGGAAGATTCACCA-3′ and (R) 5′-TGGATATCCTGGGAGAGGAGCT-3′. A plasmid containing a luciferase reporter under control of human WT usp28 mRNA 3′UTR was constructed in a pMIR-report vector using primers (F) 5′-CCCAAGCTTACATAAGATTCCGATCAGAC-3′ and (R) 5′-CCGCTCGAGTTATTGCACATTTATAAAATCTG-3′. The primers for cloning the mutant of usp28 mRNA 3′UTR luciferase reporter were (F) 5′-ATTGAAAATAAAATTCGTGTTTTTAGGTACAAAA-3′ and (R) 5′-TTTTGTACCTAAAAACACGAATTTTATTTTCAAT-3′. The miR-4295 inhibitor plasmid (HmiR-AN1540-AM02) was purchased from GeneCopoeia (Rockville, MD, USA).
Antibody-specific against human CD44 (#5640S), Oct4 (#2840S), Sox2 (#23064S), Nanog (#4903P), Skp1 (#2156), Skp2 (#4358), ITCH (#12117S), USP4 (#2651S), LC3A, LC3B (#4445S, autophagy kit), p62 (#88588), ELK1 (#9182), GAPDH (#5174), GFP (#2956S), FOXO1 (#9454S), STAT5 (#9363S), NFAT1 (#5861), and Dicer (#5362S) were all purchased from Cell Signaling (Beverly, MA, USA). Antibodies specific for c-MYC (sc-40), Sp1 (sc-14027), E2F1 (sc-193), ACTB (sc-58673), and TUBA (sc-53646) were bought from Santa Cruz (Santa Cruz, CA, USA). Antibodies specific for FBW7 (QC43532-42348) were purchased from Aviva Systems Biology (San Diego, CA, USA). Antibodies specific for USP8 (#SAB1300068) was purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA). Alkaline phosphatase (AP)-conjugated secondary antibodies were purchased from Cell Signaling (Beverly, MA, USA).
Actinomycin D (ActD; sc-200906), CHX (sc-3508), MG132 (sc-201270), and Bafilomycin (sc-201550) were purchased from Santa Cruz (Santa Cruz, CA, USA). The Dual Luciferase Assay kit was purchased from Promega (E1960). PolyJetTM DNA in Vitro Transfection Reagent was purchased from SignaGen Laboratories (SignaGen Laboratories, Gaithersburg, MD). TRIzol (#15596026) reagent and a SuperScript™ First-Strand Synthesis system (#18080051) were bought from Invitrogen (Invitrogen, USA). Isorhapontigenin (ISO; purity > 99%) was purchased from Roche Pharma (New York, USA). ISO was dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich Corporation, USA, 67-68-5) to make a 20 mM stock solution; the same concentration of DMSO was used as a vehicle control in all the experiments.
Culture medium DMEM mixture Ham’s F-12 was purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA), and penicillin and streptomycin were bought from Corning (Manassas, USA). 0.5% Trypsin–EDTA (10×) was purchased from Gibco (Maryland, USA). Fetal bovine serum (FBS) was purchased from JRH Biosciences (Lenexa, Kansas, USA).
Western blot analysis
Western blotting was performed as previously reported [22, 23]. In brief, cells were seeded into 6-well plates and cultured in normal FBS medium until a 70–80% confluence. The cells were then cultured in 0.1% FBS-containing medium for 12 h, followed by treatment with different doses of ISO for the indicated times. Whole cell extracts were then prepared using cell lysis buffer, and proteins subsequently resolved by SDS-PAGE before electrotransfer to polyvinylidene fluoride membranes (Bio-Rad, Hercules, CA, USA). Each membrane was probed with a specific primary antibody that was ultimately detected by incubation of the membrane with the AP-secondary antibody and treatment with ECL Western blot substrate. All images of the stained membranes were acquired by scanning with a Typhoon FLA 7000 Phosphorimager (General Electric, Chicago, IL, USA).
Cell culture and transfection
The human BC cell line T24T was used as in the previous studies [24, 25]. These cells were cultured in DMEM/Ham’s F-12 (1:1 volume) mixed medium-containing 5% FBS, 1% penicillin/streptomycin, and 2 mM l-glutamine and maintained at 37 °C in a 5% CO2 incubator. All cell lines were subjected to DNA tests and authenticated before/after utilization for research by Genetica DNA Laboratories (PowerPlex 16 HS System). Stable transfections were performed using PolyJet™ DNA In Vitro Transfection Reagent according to the manufacturer’s protocol. To isolate stable transfectants, cells were selected using G418, puromycin, or hygromycin during incubation for 4–6 weeks; surviving cells after selection were pooled as stable mass transfectants as described previously [26, 27].
RT-PCR and quantitative real-time PCR
Total RNA was extracted using TRIzol as described in the instructions accompanying the miRNeasy Mini Kit (#217004) (Qiagen, Valencia, CA, USA). cDNA was synthesized with a SuperScript™ First-Strand Synthesis system. Analyses of mRNA expression were carried out using a Fast SYBR Green Master Mix Kit (#4385614) and a 7900HT Fast Real-Time PCR System (both Applied Biosystems, Pittsburg, PA, USA). Data were analyzed as described previously [28]. The primers used were: pre-miR-4295 ([F] 5′-AATGGT-TTCCTTGCCTGTG-3′; [R] 5′-AGTCTAGTTTCTTAGCCTTG), human USP28 ([F] 5′-AAA-ATTGTCGAAGTCATAC-3′; [R] 5′-GGATACTGGCCGAAGGTCTCA-3′), human GAPDH ([F] 5′-GACTCATGACCACAGTCCATGC-3′; [R] 5′-CAGGTCAGGTCCACCACTGA-3′), human CD44 ([F] 5′-CACAATCCAGGCAACTCCTA-3′; [R] 5′-TACTCTGCTGCGTTGTCATT-3′); and human Dicer ([F] 5′-GAGTGTTTGAGGGATAG-3′; [R] 5′-CTGAGGTATGGGTTTGG-3′).
Luciferase reporter assays
A miR-4295 promoter-driven luciferase reporter, CD44 promoter-driven luciferase reporter, usp28 mRNA 3′UTR luciferase reporter, and pRL-TK, were each stably transfected into T24T cells. The stable transfectants were seeded into dedicated 96-well plates (104/well) and treated with ISO for the indicated time periods. The cells were then extracted with lysis buffer (25 mM Tris–phosphate [pH 7.8] 2 mmol/L EDTA, 1% Triton X-100, and 10% glycerol), and the luciferase activity in the extract was then evaluated in a MicroplateLuminometerLB96 V microplate lumino-meter (Berthold GmbH & Co).
Chromatin immunoprecipitation (ChIP) Assay
A ChIP assay was performed using an EZ CHIP kit (Millipore Technologies) according to the manufacturer’s instructions. In brief, genomic DNA and total cell proteins were isolated as in a previous study [29]. Amplification of the region-containing putative responsive elements of the human CD44 promoter was done using PCR and the primers (F) 5′-CTCTTTCCACTTGGAAGATTCACCA-3′ and (R) 5′-TGGATATCCTGGGAGAGGAGCT-3′. Resulting PCR products were separated over 2% agarose gels that were in turn stained with ethidium bromide. Final images were then generated using a standard UV light Alpha Innotech SP Image system (Alpha Innotech Corporation, San Leandron, CA, USA).
Sphere formation assay
To determine any impact of ISO on BC sphere formation, T24T cells were sorted from cultures by selection of sphere-formed cells. The isolated cells were then plated into 6-well ultra-low attachment culture plates (Corning, Kennebunk, ME, USA) and grown for 5–7 days as described in our previous studies [21]. The images were acquired using an Olympus DP71 microscope (Olympus 654 America Inc. Center Valley, PA, USA). At least six fields per well were counted. Data were expressed as relative sphere formation.
Actinomycin D chase experiment
Actinomycin D (ActD) chase experiments were used to test mRNA stability after ISO exposure. In brief, T24T cells were seeded into 6-well plates and cultured in 5% DMEM/Ham’s F-12 (1:1 volume) medium until 60–70% confluent. Thereafter, the cells were cultured in 0.1% FBS-containing medium for 12 h, and subsequently treated with 20 μM ISO or 0.1% DMSO in the presence or absence of 20 μg/ml Act D for the indicated time periods. At the end of each period, total RNA was extracted and subjected to RT-PCR for evaluation of miR-4295 expression.
Bioinformatic analysis
The CD44 promoter region was analyzed for potential transcription factor binding sites using ALGGEN-PROMO Software. The usp28 mRNA 3′UTR region was analyzed for potential miRNA-binding sites using TargetScanHuman 7.1 Software.
Statistical analysis
All data were plotted as mean ± SD of triplicate assays. A Student’s t test was employed to determine the significance of any differences between the various groups. A p value < 0.05 was considered significant. All analyses were conducted using Prism 5.0 Software (GraphPad).
Results
Down-regulation of CD44 contributed to ISO inhibition of BC stem cell-like properties and invasivity
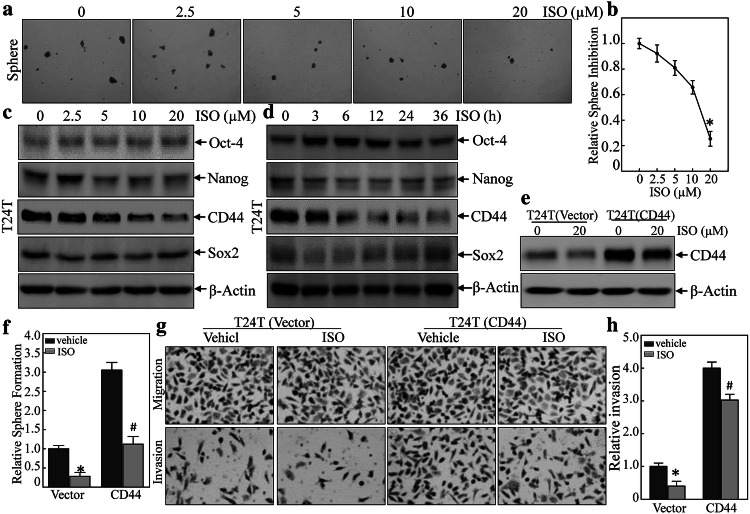
Prior studies noted that ISO could significantly inhibit mouse invasive BC development following BBN treatment in vivo and also inhibit BC cell invasivity in vitro [30]. Our recent work has also demonstrated that ATG7 promoted BC cell stem cell-like properties through an up-regulation in CD44 s’ expression [21]. To explore whether ISO also impacted BC cell stem cell-like properties, sphere formation was assessed. The results (Fig. 1a, b) show that treatment of the T24T cells with ISO significantly reduced the numbers and sizes of tumor spheres that formed in a dose-dependent manner. To study how ISO influenced this property, Western blotting analyses of potential ISO downstream targets were performed. The data showed that ISO dramatically inhibited CD44 protein expression in a dose- and time-dependent manner in the T24T cells (Fig. 1c, d). In contrast, there was no consistent change observed in expression of other stem markers such as Oct4, Sox2, and Nanog.
Fig. 1.
ISO inhibition of BC stem-like properties through inhibiting CD44 was also important for changes in invasivity. a, b T24T cells were treated with ISO at indicated concentrations for 7 days to determine impact on sphere formation ability. The number of spheroid-forming cells in each image was counted as described in “Materials and methods”. Bars shown are mean ± SD from three independent experiments. *Significant difference relative to controls (p < 0.05). c, d T24T cells were treated with ISO at indicated concentrations or for indicated times, and then evaluated for stemness marker protein expression using Western blots. e Over-expression of CD44 constructs were stably transfected into T24T cells; the transfectants were then treated with 20 μM ISO for 12 h, and then underwent Western Blot analyses to determine CD44 expression levels. f Over-expression of CD44 could not reverse ISO inhibition of sphere formation. *Significant difference between indicted cells treated with or without 20 μM ISO (p < 0.05). g, h Invasivity of T24T(vector) and T24T(CD44) cells treated with or without 20 µM ISO for 24 h was evaluated using a BD BioCoat TM Matrigel TM Invasion Chamber. After incubation for 24 h, cells were fixed and stained as described in “Materials and methods”. *Significant difference (p < 0.05)
To further test what role a CD44 protein inhibition might have, CD44 over-expression in T24T cells was first established, and then, the transfectants were treated with ISO to determine how ectopic expression of CD44 might regulate this cell stem cell-like property. It was found that ectopic expression of CD44 (Fig. 1e) remarkably increased sphere formation ability, and partially reversed the ISO suppression of sphere formation (Figs. 1f and S1) and invasion (Fig. 1g, h). Consistently, ISO also had partially inhibitory effect on over-expressed CD44 (Fig. 1e). Taken together, these results demonstrated that ISO inhibited BC stem cell-like properties and invasion through down-regulation of CD44 expression.
ISO attenuated CD44 protein stability by down-regulating USP28 in human BC cells
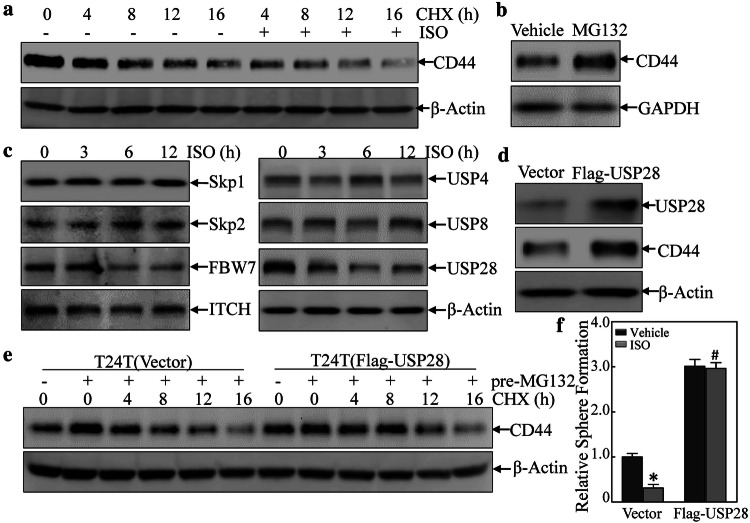
Because ISO treatment not only inhibited endogenous CD44 expression but also reduced ectopic CD44 protein expression in T24T cell, the study next examined if down-regulation of CD44 expression due to ISO was a result of changes in degradation of CD44 protein. In T24T cells treated with CHX alone or in combination with ISO for varying lengths of time, CD44 protein degradation rates were markedly increased in cells incubated with ISO + CHX as compared to in cells incubated with CHX alone (Fig. 2a). Based on this, it appeared that ISO exerted a down-regulating effect on CD44 protein expression via promotion of its degradation. Since protein degradation by either proteasomes or lysosomes plays an indispensable role in cells [31], the study next tested if autophagy could induce CD44 protein degradation. As seen in Fig. S2A, there were no obvious differences in CD44 degradation rates between cells treated with ISO + BafA1 vs. those that received only ISO. On the other hand, MG132 could lead to accumulation of the CD44 protein (Fig. 2b), suggesting that ISO treatment regulated CD44 protein degradation via proteasomal degradation.
Fig. 2.
ISO proceeding CD44 degradation through attenuating USP28 expression. a Cycloheximide (CHX) (50 µg/ml) in combination with/without ISO (20 μM) was added into the medium and the cells were incubated for the indicated times. Cells were then extracted for determination of CD44 protein expression using Western blots. b CD44 protein accumulation due to MG132 (10 μM). c T24T cells were treated with 20 μM ISO for the indicted times and then extracted; extracts were subjected to Western blot analyses to determine the protein expressions indicated. d T24T cells were transfected with Flag-USP28 expression vector or control vector; thereafter, the transfectants were extracted and subjected to Western blot to determine the protein expressions indicated. e T24T(vector) and T24T(Flag-USP28) cells were pre-treated with or without MG132 in the presence or absence of CHX for the indicated time periods before cell extracts were prepared and analyzed by Western blot for determination of CD44 status. f Cell sphere formation abilities of T24T(vector) and T24T(Flag-USP28) cells treated with or without 20 µM ISO were determined. *Significant difference (p < 0.05)
To further explore the above findings, the study here also examined proteins (such as ITCH, USPs, FBW7, etc.) that are associated with proteasomal-dependent protein degradation. Of these, USP28 protein expression was dramatically down-regulated by ISO treatment (Fig. 2c), suggesting USP28 might be involved in the modulation of CD44 protein degradation. To test this, a Flag-USP28 expression construct was transfected into T24T cells; as expected, USP28 over-expression led to increased CD44 protein expression (Fig. 2d). Furthermore, ectopic expression of USP28 led to increased CD44 protein stability (Fig. 2e). Moreover, USP28 expression attenuated the ISO-induced inhibition of BC cell sphere formation (Figs. 2f and S2B). These outcomes were consistent with the findings of Zhu et al who reported that USP28 was a key de-ubiquitination enzyme that binds to CD44 protein and removes the ubiquitin group from CD44 protein, resulting in enhanced CD44 protein stability [21]. Taken together, these results showed that ISO promoted CD44 protein degradation via decreasing USP28 protein expression.
ISO inhibited CD44 transcription specifically mediated by attenuating Sp1 in BC cells
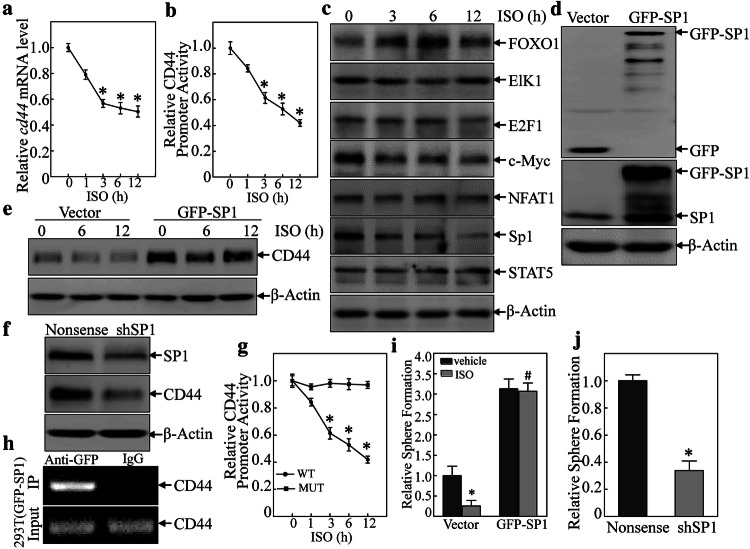
Since the inhibition of ISO on endogenous CD44 was more than on exogenous ectopic expressed CD44, we anticipated that ISO might also have impact on cd44 mRNA in BC cells. To test this notion, T24T cells were treated by ISO to determine effects on cd44 mRNA expression. As shown in Fig. 3a, ISO treatment resulted in significant time-dependent decreases in the standard form of cd44 (cd44s) mRNA. Using a luciferase reporter assay to determine CD44 promoter activity, it was seen here that ISO also inhibited CD44 promoter activity in a time-dependent manner (Fig. 3b). These results indicated ISO inhibited cd44 mRNA at the transcriptional level.
Fig. 3.
Sp1 down-regulation mediated ISO inhibition of CD44 transcription. a Total RNA isolated from T24T cells treated with 20 μM ISO for indicated time periods was subjected to RT-PCR to determine cd44 mRNA expression. b Both CD44 luciferase reporter and pRL-TK were co-transfected into T24T cells, and the transfectants were treated with 20 μM ISO for indicated times. Luciferase activity was evaluated as described in “Materials and methods”. *Significant difference (p < 0.05). c Indicated transcription factors in T24T cells were determined by Western Blot after cells were treated with 20 μM ISO for indicated times. d GFP-Sp1 plasmid or vector was transfected into T24T cells; the transfectants were extracted and subjected to Western blot to determine the protein expressions indicated. e, f Sp1 was responsible for ISO inhibition of CD44 transcription. g T24T cells were stably transfected with CD44 promoter-driven luciferase or the Sp1-binding site mutant, and the stable transfectants were treated with 20 μM ISO. Luciferase activity was evaluated as described in “Materials and methods”. h ChIP assay was carried out with anti-GFP antibody. DNA was extracted from GFP agarose or control IgG. Total sonicated nuclei were used as Input control. Specific Sp1 binding DNA regions of the CD44 promoter were then amplified by PCR. PCR products were separated over 2% agarose gels that were then stained with ethidium bromide and examined under UV light. i T24T(vector) and T24T(GFP-Sp1) cells were treated with/without ISO (20 μM) to determine sphere formation ability. *Significant difference (p < 0.05). j T24T(nonsense) and T24T(shSp1) cells were used in sphere formation assay as described in “Materials and methods”. *Significant difference (p < 0.05)
To build on these findings, bioinformatics was used to analyze potential transcription factor-binding sites in the CD44 promoter region. Though there are many potential transcription factor-binding sites in the CD44 promoter region (including for FOXO1, Elk1, E2F1, c-MYC, NFAT1, Sp1, and STAT5; Fig. S3A), to identify the specific transcription factor(s) participating in the modulation of CD44 transcription, related transcription factor protein abundance was determined after ISO treatment. As shown in Fig. 3c, treatment of T24T cells with ISO resulted in significant dose-dependent induction of FOXO1 and inhibition of c-MYC and Sp1 expression. There were no observable effects on expression of Elk1, E2F1, NFAT1, and STAT5. These results suggested that FOXO1, Sp1, and c-MYC were CD44 promoter transcription factor candidates. To test this, the effects of FOXO1 knockdown, c-MYC over-expression, and GFP-Sp1 ectopic expression on CD44 expression in T24T cells were next evaluated. As shown in Figs. S3B & S3C, FOXO1 knockout and c-MYC over-expression had no impact on CD44 expression. These results excluded both of FOXO1 and c-MYC in ISO inhibition of CD44 transcription. Western blotting confirmed the efficiency of Sp1 over-expression (Fig. 3d). Ectopic expression of GFP-Sp1 increased CD44 expression, and ISO treatment only a partially inhibition of ectopic expressed GFP-Sp1-induced CD44 expression (Fig. 3e). These results indicated that Sp1 is a key transcription factor-mediating inhibition of CD44 transcription after ISO treatment. Consistent with GFP-Sp1 up-regulation of CD44 expression, knockdown of Sp1 resulted in attenuation of CD44 expression (Fig. 3f). To verify if Sp1 is able to specific binding to the CD44 promoter, a point mutation in the Sp1-binding sites in a CD44 promoter luciferase reporter eliminated ISO-induced inhibition of CD44 promoter luciferase activity (Fig. 3g). Furthermore, a chromatin immunoprecipitation (ChIP) assay—followed by PCR with primers specifically targeting the Sp1 binding region in the human CD44 promoter in T24T cells was carried out. As shown in Fig. 3h, Sp1 did directly bind to putative Sp1-binding sites in the CD44 promoter.
Finally, to show the importance of Sp1 in the BC cell phenotype, cell sphere formation was again assayed. The results indicated that ectopic expression of GFP-Sp1 attenuated ISO inhibition of BC cell sphere formation (Figs. 3i and S3D). Furthermore, knockdown of Sp1 inhibited BC cell sphere formation (Figs. 3j and S3E). Collectively, these findings revealed Sp1 played an important role in ISO-induced inhibition of CD44 transcription and protein expression in human BC cells, which in turn also impacted on cell phenotype/functionality.
ISO-induced miR-4295 inhibited USP28 translation by affecting mRNA 3′-UTR activation
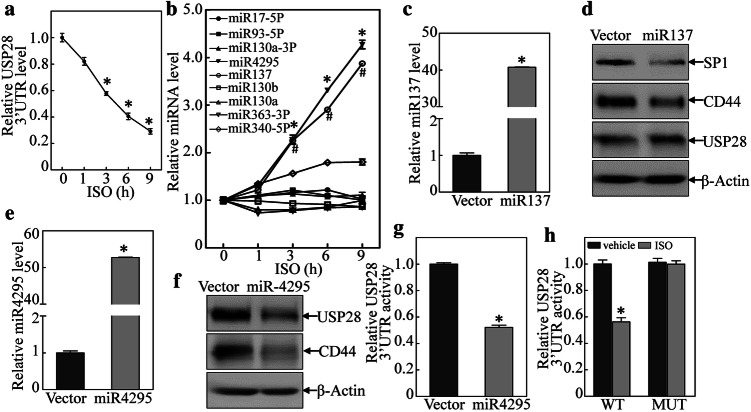
It was shown above that ISO inhibited USP28 expression that, in turn, weakened CD44 protein stability and ultimately led to inhibition of BC cell sphere formation. To clarify the mechanisms leading to the repressed USP28 protein expression, usp28 mRNA expression was evaluated. The results indicated that ISO treatment did not significant influence usp28 mRNA expression (Fig. S4A), thereby precluding the possibility of effects at the transcriptional/post-transcriptional level. To ascertain if ISO affected USP28 protein stability in the T24T cells, cells treated with cycloheximide (CHX) or CHX + ISO were analyzed. The data indicated that USP28 protein expression was not overtly impacted (Fig. S4B), indicating protein degradation was not affected by ISO. As this suggested to us that ISO regulated USP28 expression at the translation level, and it is well recognized that the 3′-UTR of a transcript can influence protein translation, usp28 mRNA 3′-UTR luciferase reporters were, therefore, transfected into T24T cells, and the cells were then treated with ISO. As shown in Fig. 4a, ISO treatment resulted in significant time-dependent reductions in usp28 mRNA 3′-UTR activity, suggesting that ISO inhibited USP28 protein translation via regulation of usp28 mRNA 3′-UTR activity.
Fig. 4.
Induction of miR-4295 by ISO bound to USP28 3′UTR and inhibited USP28 protein translation in human BC cells. a USP28 3′-UTR luciferase reporters were co-transfected with pRL-TK into T24T cells; stable transfectants were exposed to 20 μM ISO for the indicated time periods to assess the ISO inhibition of USP28 3′UTR luciferase activity. *Significant difference (p < 0.05). b Expression levels of predicted miRNA were evaluated using quantitative real-time PCR. T24T cells were treated with medium-containing either vehicle or 20 μM ISO. c, e Over-expression of miR-137 and miR-4295 in T24T cells. *Significant difference (p < 0.05). d, f miR-4295, but not miR-137, inhibited USP28 and CD44 protein expression. g Inhibition of USP28 3′UTR activity by ectopic expression of miR-4295 in T24T cells. *Significant difference (p < 0.05). h miR-4295-binding site was crucial for ISO inhibition of USP28 3′UTR luciferase activity. *Significant difference (p < 0.05)
Given that miRNAs are known to regulate mRNA 3′-UTR [32], a TargetScan software was employed to screen potential miRNA-binding sites in the 3590-4840 bp region of the usp28 mRNA 3′-UTR (Fig. S4C). To identify which of the nine miRNAs was responsible for regulation of USP28 protein translation, real-time PCR was used to assess expression of each miRNA in the T24T cells treated with ISO. The results showed that only miR-137 and miRNA-4295 were markedly induced by ISO treatment (in time-dependent manner), while miR-340-5P was slight affected and the other miRNA were unaffected (Fig. 4b). To identify whether miR-137 and/or miRNA-4295 acted as a regulator for inhibition of USP28 protein translation, miR-137 and miRNA-4295 were stably transfected into T24T cells (Fig. 4c, e). Over-expression of miRNA-4295 impaired USP28 and CD44 protein expression, while over-expression of miRNA-137 did not show any inhibitory effect on USP28 protein expression, but did partially inhibit expression of CD44 protein and Sp1 (Fig. 4d, f). This suggested that miR-4295, and not miR-137, played a major role in inhibition of USP28 translation.
Consistent with the inhibition of USP28 and CD44 by miR-4295, ectopic expression of miR-4295 was found to block USP28 3′-UTR luciferase reporter activity in a dual-luciferase reporter assay (Fig. 4g). To assess whether miR-4295 directly bound to the USP28 3′-UTR and then inhibited USP28 translation, an miR-4295-targeted site-mutated USP28 3′-UTR luciferase reporter was constructed (Fig. S4D) and transfected into T24T cells. Upon treatment with ISO, compared with outcomes in WT USP28 3′-UTR luciferase reporter-transfected cells, cells with a point mutation in the miR-4295 binding site in the USP28 3′-UTR-luciferase reporter displayed a loss of ISO inhibitory effects on USP28 3′UTR activity (Fig. 4h). Together, the data revealed how miR-4295 induced by ISO inhibited USP28 translation by binding directly to the usp28 mRNA 3′-UTR region.
miR-4295 induction was crucial for ISO inhibitory effects on USP28 and CD44 expression, and BC sphere formation and invasivity
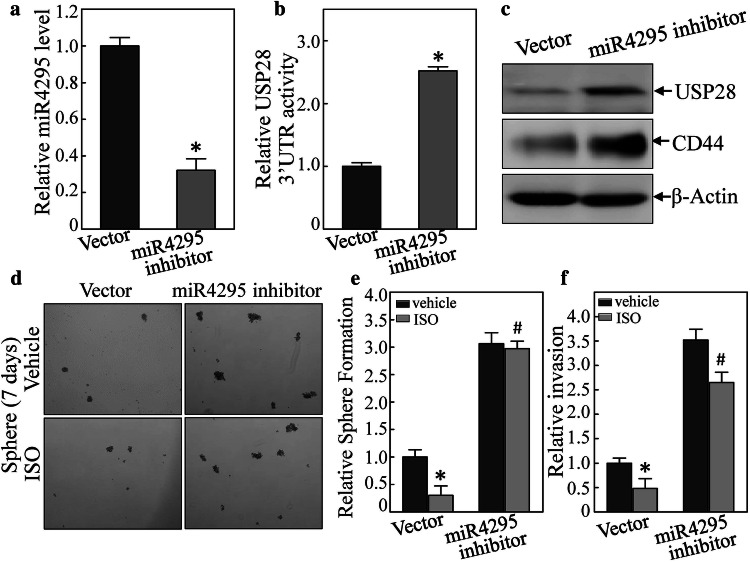
Both miR-4295 inhibitor plasmid and its vector were transfected into T24T cells; once the stable cell lines were established, miR-4295 expression level was evaluated. As expected, miR-4295 expression was decreased (Fig. 5a). In addition, this inhibition of miR-4295 expression not only increased USP28 3′-UTR activity but also promoted USP28 and CD44 protein expression (Fig. 5b, c). To determine if miR-4295 was involved in the effect of ISO on inhibiting cell sphere formation and cell invasivity, a cell sphere formation assay was also done. The results indicated that ISO inhibited this T24T(vector) cells stem cell-like property, and that this was reversed by the presence of the miR-4295 inhibitor (Fig. 5d, e). Inhibition of miR-4295 expression also blocked ISO inhibition of cell invasion in the BC cells (Figs. 5f and S5). These results revealed that miR-4295 induction is crucial for ISO inhibition of expression of USP28 and CD44, and BC sphere formation and invasion.
Fig. 5.
miR-4295 inhibitor reversed ISO inhibition of USP28 and CD44 expression, USP28 3′UTR activity, and cell sphere formation, as well as cell invasion in human BC cells. a miR-4295 inhibitor constructs were stably transfected into T24T cells. The inhibition efficiency of miR-4295 was assessed by quantitative real-time PCR. *Significant difference (p < 0.05). b, c miR-4295 inhibitor could not block USP28 3′UTR luciferase activity and inhibit USP28 and CD44 expression (assessed via Western blot). d, e miR-4295 inhibitor reversed ISO inhibition of cell sphere formation. *Significant difference in comparison to vehicle control (p < 0.05). f miR-4295 inhibitor also reversed ISO inhibition of cell invasion. *Significant difference in comparison to vehicle control (p < 0.05)
ISO promoted miR-4295 expression through up-regulation of Dicer protein expression
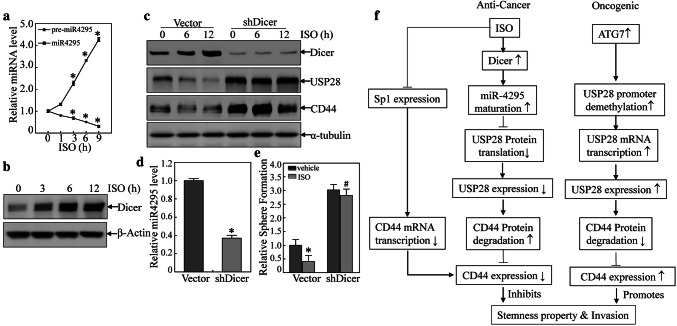
In the investigation of potential underlying mechanisms leading to increase miR-4295 expression, it was seen that ISO treatment only showed a slight effect on miR-4295 promoter-driven luciferase activity (Fig. S6A). As a result, it is possible to exclude an ISO impact at the miR-4295 transcription level. To see whether ISO influenced miR-4295 expression via changes in miR-4295 degradation, actinomycin D (ActD) was used to block new miRNA transcription in T24T cells treated with ISO or vehicle control (DMSO) and miR-4295 degradation kinetics was assessed. As shown in Fig. S6B, there was no significant difference in rates of miR-4295 degradation due to ISO treatment. As degradation was not overtly affected, this suggested that ISO might regulate miR-4295 maturation. To test this, pre-miR-4295 and miR-4295 expression in T24T cells treated with ISO for varying time periods was evaluated. As expected, miR-4295 expression was dramatically up-regulated by ISO; in contrast, pre-miR-4295 was down-regulated upon ISO treatment (Fig. 6a), suggesting that ISO promotes pre-miR-4295 maturation. The ISO treatment also resulted in significant time-dependent up-regulation of Dicer protein expression in the cells (Fig. 6b). Given that Dicer is an important RNase III expressed in eukaryotes and is essential for miRNA maturation [33, 34], these results revealed that ISO promoted miR-4295 maturation via up-regulation of Dicer.
Fig. 6.
ISO accelerated miR-4295 maturation by induction of Dicer. a miR-4295 and pre-miR-4295 assessed (via qRT-PCR) in T24T cells treated with 20 μM ISO for the time periods indicated. *Significant difference (p < 0.05). b Dicer expression induced by ISO (20 μM) was evaluated using Western blot. c T24T(vector) and T24T(shDicer) cells were treated with 20 μM ISO for the time periods indicated, and the presence of indicated proteins evaluated using Western blot. d Knockdown Dicer reduced miR-4295 expression using qRT-PCR. *Significant difference in comparison to vector (p < 0.05). e Cell sphere formation by cells transfected with shDicer or nonsense vector. Transfectants were exposed to 20 μM ISO for 7 days before analyses using microscopy. *Significant difference between indicated transfectants and between vehicle- and ISO-treated groups (p < 0.05). f The schematic summary of molecular mechanisms underlying inhibition of human bladder cancer stem cell-like phenotypes and invasivity upon ISO treatment
To determine its functional role in the observed outcomes with ISO, analyses using knockdown of Dicer showed that this protein could reverse the inhibitory effects of ISO upon USP28, CD44 expression, and also blocked miR-4295 expression (Fig. 6c, d). Knocked down of Dicer also attenuated ISO effects on expression of BC stem cell-like phenotypes (Figs. 6E and S6C). Collectively, these results demonstrated that ISO-induced Dicer expression could promote a miR-4295 maturation that subsequently inhibited USP28 translation by targeting usp28 mRNA 3′UTR and ultimately a decrease in CD44 protein stability as diagram in Fig. 6f.
Discussion
A presence of cancer stem cells (CSC) is closely associated with potential tumor metastasis and resistance to anti-cancer therapeutics [35, 36]. As recurrence and drug resistance are two important characteristics of bladder cancer (BC) [37], it is urgent to find novel therapeutics to inhibit human BC stem-like cells. Given the results of our previous studies assessed the various biological effects of ISO both in vitro and in vivo, we believed that ISO could be a novel cancer therapeutic for BC patients. In the current study, we demonstrated that ISO imparted an inhibitory effect on expression of BC stem-like properties and invasivity, in part, by causing a down-regulation of CD44 expression. It was also demonstrated that the down-regulation of CD44 by ISO was not only caused by an inhibition of the expression of/interactions of deubiquitinating enzyme USP28 with CD44 leading to enhance CD44 protein degradation but was also a result of a reduction in the expression/binding activity of transcription factor Sp1 that binds to CD44 promoter sites and resulted in inhibited CD44 transcription.
Accumulating evidence has demonstrated that CD44 expression is highly correlated to cancer stem cell phenotype, as well as overall tumor invasivity, metastatic potential, and chemo-resistance. CD44 has been shown to be a cancer stem cell marker in several cancers, including lung [38], breast [39], liver [40], bladder [5], and colorectal cancers [41]. Wu et al. [42] reported that higher CD44 expression levels could increase spheroid colony formation by BC cells. Our previous study also showed that CD44 is a crucial ATG7 downstream mediator critical for invasivity and metastatic potentials of human BC cells [21]. Thus, CD44 might be a potential target for treatments of human high invasive BC.
The current novel findings of the ISO impact on BC stem cell-like properties indicate that these arose via distinctly different means from those through which ISO suppressed the growth of patient-derived glioblastoma spheres (i.e., via inhibition of SOX2 expression) [43]. The current study revealed that ISO decreased both Sp1-mediated cd44 mRNA transcription and USP28 direct binding-mediated stabilization of CD44 protein. Further investigation of mechanisms underlying the relationship between CD44 expression and cancer stem cell phenotypes are essential.
Dicer is an RNase involved in the biogenesis of microRNA (miRNA) as well as other small non-coding RNA (ncRNA) [44]. In recent years, studies have been performed to define factors regulating Dicer gene expression. Given the complexity of Dicer regulation, it is not surprising that a large amount of contradictory data has been generated. SOX4, a well-known transcription factor, positively regulates Dicer transcription by directly binding to its promoter; in regards to cancer, this effect leads to subsequent suppression of melanoma cell invasivity [45]. It is of note that let-7 repressed Dicer expression by directly binding the dicer 3′-UTR region; this down-regulation leads to reduced expression of mature let-7, indicating a possible novel negative feedback loop existed [46]. Another report showed that expression of human Dicer could be regulated by modification of phosphorylation or SUMOylation [47, 48]. The present study found that ISO treatment led to time-dependent increases in Dicer protein abundance, which, in turn, subsequently promoted miR-4295 maturation that resulted in down-regulation of USP28, acceleration of CD44 protein degradation, and ultimately brought about a significant reduction in stem-like properties of the human BC cells. Since precise regulation of Dicer expression is important in various physiological processes, future studies will analyze underlying mechanisms leading to Dicer accumulation due to ISO treatment.
Accumulating evidence has demonstrated that miR-4295, an essential miRNA, is involved in various biological processes. Over-expression miR-4295 is known to promote cell proliferation by a direct targeting of BTG1 in BC cells [49]. Our previous studies also found that miR-4295 could inhibit p63α protein expression, an event that promoted malignant transformation of human bladder epithelial UROtsa cells [50]. Based on the literature, we anticipate that miR-4295 can act as an oncogenic miRNA in several cancers. On the other hand, by targeting deubiquitinating enzyme USP28, miR-4295 may also function as a tumor suppressor in BCs, may also in some cancer cells [51]. The current study showed that due to ISO treatment, increasing miR-4295 maturation through changes in Dicer expression, and increased direct binding of miR-4295 to usp28 mRNA 3′UTR and inhibited USP28 protein translation, resulting in enhanced CD44 degradation. These changes, in turn, suppressed expression of BC stem-like properties. It is interesting that miR-4295 appears to display dual functions in BC cells in different phases of BC development. Of even greater note, according to Kaplan–Meier Plotter database analyses, miR-4295 expression is positive correlated with survival time of BC patients (see Supplementary Fig. S7A), which supports our hypothesis that induction of miR-4295 by ISO resulted in the inhibition of BC stem-like properties and invasion.
That ISO treatment impacted on USP28, a member of the ubiquitin-specific protease family that plays an important role in regulation of oncogene and tumor suppressor expression [52, 53], represents an important finding. Deubiquitinating enzymes (DUB) have long been thought of as targets in many cancer treatments [54, 55]. USP28 expression in BC is known to be positively associated with decreased survival of BC patients (Kaplan–Meier Plotter database; Supplementary Fig. S7B). This is consistent with several studies, reporting that USP28 over-expression was involved in the progression of certain cancers, including gliomas [56] and BC [57]. The current study found that ISO inhibited USP28 expression, thereby promoting CD44 protein degradation and suppressing human BC sphere formation. Consistently, our previous study found that ATG7 regulates CD44 protein expression, through USP28 binding to CD44 and promotes the debiquitination of CD44, consequently, increasing its protein stabilization [21]. Importantly, we further demonstrated here that USP28 could bind CD44 by immunoprecipitation [21]. Based on our results here, we identify that USP28, a ubiquitin-specific protease, plays a significant role in inhibition of human BC stem-like properties by ISO treatment.
In summary, the current study showed that ISO imparted critical inhibitory effects on human BC cell stem-like properties through down-regulation of expression of the well-known stem marker CD44. Mechanistically, on one hand, ISO-induced reduction of CD44 expression appeared to be mediated by inhibition of transcription factor Sp1 expression. On the other hand, Dicer induction by ISO promoted miR-4295 maturation, which, in turn, inhibited USP28 protein translation by targeting its 3′UTR region and then led to a subsequent speeding up of CD44 protein degradation. These findings not only revealed that ISO could impart inhibitory effects on human BC cell stem-like properties, but also provided a basis for the possible clinical use of ISO as a novel mechanism-based cancer therapeutic against human bladder cancer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig S1. T24T(vector) and T24T(CD44) cells were treated with ISO or DMSO at indicated concentrations for 7 days to determine impact on sphere formation ability (JPEG 650 kb)
Fig S2. (A) T24T cells were treated with Bafomycin A1 (5 nM) or ISO (20 μM) alone or Bafomycin A1+ ISO, which tested whether CD44 protein accumulated by Bafomycin A1. (B) Cell sphere formation by cells transfected with Flag-USP28 or vector. Transfectants were exposed to 20 μM ISO for 7 days before analyses using microscopy (JPEG 778 kb)
Fig S3. (A) Schematic representation of transcription factor binding sites in human CD44 promoter region from -1133 to -1. (B & C) FOXO1 or c-MYC did not affect CD44 protein expression. (D) T24T(vector) and T24T(GFP-Sp1) cells were treated with/without ISO (20 μM) to determine sphere formation ability. (E) T24T(nonsense) and T24T(shSp1) cells were used in sphere formation assay as described in Methods (JPEG 900 kb)
Fig S4. (A) usp28 mRNA expression levels in T24T cells were determined by RT-PCR after cells were treated with 20 μM ISO for indicated time periods. (B) T24T cells were treated with/without 20 μM ISO in the presence of CHX for indicated time periods, then underwent extraction; extracts were then analyzed by Western blot to assess USP28 protein expression. (C) Schematic representation of potential miRNA-binding sites in USP28 3’UTR were analyzed with TargetScan software. (D) Schematic sequence of intact miR-4295-binding site in wide-type USP28 3’UTR and its mutation of USP28 3’UTR luciferase reporter (JPEG 912 kb)
Fig S5. Invasivity of T24T(vector) and T24T(miR-4295 inhibitor) cells treated with or without 20 µM ISO for 24 hours was evaluated using a BD BioCoat TM Matrigel TM Invasion Chamber. After incubation for 24 hours, cells were fixed and stained and took pictures as described in Methods (JPEG 838 kb)
Fig S6. (A) T24T(miR-4295 promoter) stable transfectants were treated with 20 μM ISO for indicated time periods; thereafter, miR-4295 promoter luciferase activity was measured (via Dual-Luciferase Reporter Assay System). (B) T24T cells treated with 20 μM ISO or vehicle control - both along with ActD - for the time periods indicated. Total RNA was isolated and subjected to qRT-PCR evaluation of miR-4295 expression levels. (C) Cell sphere formation abilities of T24T(vector) and T24T(shDicer) cells treated with or without 20 µM ISO were determined as described in Methods (JPEG 811 kb)
Fig S7. (A) Kaplan-Meier estimation about has-mir-4295 of overall survival (OS) in bladder cancer (BC) patients from the kmplot.com database. Overall survival (OS) curves showing that patients with high miR-4295 expression (n=564) was related with better OS, compared with lower expression of miR-4295 (n=186). (B) Kaplan-Meier estimation about USP28 of overall survival (OS) in bladder cancer (BC) patients from the kmplot.com database. Overall survival (OS) curves showing that patients with high USP28 expression (n=294) were positively associated with decreased survival of BC patients, lower expression of miR-4295 (n=191) was associated with longer survival (JPEG 833 kb)
Abbreviations
- ActD
Actinomycin D
- AP
Alkaline phosphatase
- BAF
Bafilomycin A1
- BC
Bladder cancer
- ChIP
Chromatin immunoprecipitation
- CHX
Cycloheximide
- CSC
Cancer stem cells
- DMSO
Dimethyl sulfoxide
- DUB
Deubiquitinating enzymes
- FBS
Fetal bovine serum
- IgG
Immunoglobulin G
- ISO
Isorhapontigenin
- MIBC
Muscle-invasive bladder cancer
- miR-4295
Hsa-microRNA-4295
- ncRNA
Non-coding RNA
- NMIBC
Non-muscle-invasive bladder cancer
- RT-PCR
Reverse transcription-polymerase chain reaction
- WT
Wide type
- 3′UTR
3′-Untranslated regions
Funding
This work was partially supported by Grants from NIH/NCI CA177665, CA217923, CA229234, CA165980, and NIH/NIEHS ES000260.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yisi Luo and Zhongxian Tian contributed equally to this work.
References
- 1.Choi W, Czerniak B, Ochoa A, Su X, Siefker-Radtke A, Dinney C, et al. Intrinsic basal and luminal subtypes of muscle-invasive bladder cancer (Review) Nat Rev Urol. 2014;11(7):400–410. doi: 10.1038/nrurol.2014.129. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society . Cancer facts & figures 2019. Atlanta: American Cancer Society; 2019. [Google Scholar]
- 3.Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21(3):283–296. doi: 10.1016/j.ccr.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khandelwal P, Abraham SN, Apodaca G. Cell biology and physiology of the uroepithelium. Am J Physiol Renal Physiol. 2009;297(6):F1477–F1501. doi: 10.1152/ajprenal.00327.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan KS, Espinosa I, Chao M, Wong D, Ailles L, Diehn M, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci. 2009;106(33):14016–14021. doi: 10.1073/pnas.0906549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Britton KM, Kirby JA, Lennard TW, Meeson AP. Cancer stem cells and side population cells in breast cancer and metastasis. Cancers. 2011;3(2):2106–2130. doi: 10.3390/cancers3022106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dou J, Gu N. Emerging strategies for the identification and targeting of cancer stem cells. Tumor Biol. 2010;31(4):243–253. doi: 10.1007/s13277-010-0023-y. [DOI] [PubMed] [Google Scholar]
- 8.Deng S, Wong CKC, Lai H-C, Wong AST. Ginsenoside-Rb1 targets chemotherapy-resistant ovarian cancer stem cells via simultaneous inhibition of Wnt/β-catenin signaling and epithelial-to-mesenchymal transition. Oncotarget. 2017;8(16):25897. doi: 10.18632/oncotarget.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang K-S, Wang Y-H, Li R-L, Lin M. Stilbene dimers from the lianas of Gnetum hainanense. Phytochemistry. 2000;54(8):875–881. doi: 10.1016/s0031-9422(00)00151-5. [DOI] [PubMed] [Google Scholar]
- 10.Fang Y, Yu Y, Hou Q, Zheng X, Zhang M, Zhang D, et al. The Chinese herb isolate isorhapontigenin induces apoptosis in human cancer cells by down-regulating overexpression of antiapoptotic protein XIAP. J Biol Chem. 2012;287(42):35234–35243. doi: 10.1074/jbc.M112.389494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang G, Wu AD, Huang C, Gu J, Zhang L, Huang H, et al. Isorhapontigenin (ISO) inhibits invasive bladder cancer (BC) formation in vivo and human BC invasion in vitro by targeting STAT1/FOXO1 Axis. Cancer Prevent Res. 2016 doi: 10.1158/1940-6207.capr-15-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng X, Xu Z, Gu J, Huang H, Gao G, Zhang X, et al. Induction of miR-137 by isorhapontigenin (ISO) directly targets Sp1 protein translation and mediates its anticancer activity both in vitro and in vivo. Mol Cancer Therapeut. 2016;15:512–522. doi: 10.1158/1535-7163.MCT-15-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura Y, Goi T, Nakazawa T, Hirono Y, Katayama K, Urano T, et al. CD44variant exon 9 plays an important role in colon cancer initiating cells. Oncotarget. 2013;4(5):785. doi: 10.18632/oncotarget.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Culty M, Nguyen HA, Underhill CB. The hyaluronan receptor (CD44) participates in the uptake and degradation of hyaluronan. J Cell Biol. 1992;116(4):1055–1062. doi: 10.1083/jcb.116.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iczkowski KA. Cell adhesion molecule CD44: its functional roles in prostate cancer. Am J Transl Res. 2010;3(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Lokeshwar B, Lokeshwar V, Block N. Expression of CD44 in prostate cancer cells: association with cell proliferation and invasive potential. Anticancer Res. 1995;15(4):1191–1198. [PubMed] [Google Scholar]
- 17.Savani RC, Cao G, Pooler PM, Zaman A, Zhou Z, DeLisser HM. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J Biol Chem. 2001;276(39):36770–36778. doi: 10.1074/jbc.M102273200. [DOI] [PubMed] [Google Scholar]
- 18.McFarlane S, Coulter JA, Tibbits P, O’Grady A, McFarlane C, Montgomery N, et al. CD44 increases the efficiency of distant metastasis of breast cancer. Oncotarget. 2015;6(13):11465. doi: 10.18632/oncotarget.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okamoto I, Kawano Y, Murakami D, Sasayama T, Araki N, Miki T, et al. Proteolytic release of CD44 intracellular domain and its role in the CD44 signaling pathway. J Cell Biol. 2001;155(5):755–762. doi: 10.1083/jcb.200108159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorne RF, Legg JW, Isacke CM. The role of the CD44 transmembrane and cytoplasmic domains in co-ordinating adhesive and signalling events. J Cell Sci. 2004;117(3):373–380. doi: 10.1242/jcs.00954. [DOI] [PubMed] [Google Scholar]
- 21.Zhu J, Huang G, Hua X, Li Y, Yan H, Che X, et al. CD44s is a crucial ATG7 downstream regulator for stem-like property, invasion, and lung metastasis of human bladder cancer (BC) cells. Oncogene. 2019 doi: 10.1038/s41388-018-0664-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding J, Li J, Xue C, Wu K, Ouyang W, Zhang D, et al. Cyclooxygenase-2 induction by arsenite is through a nuclear factor of activated T-cell-dependent pathway and plays an antiapoptotic role in Beas-2B cells. J Biol Chem. 2006;281(34):24405–24413. doi: 10.1074/jbc.M600751200. [DOI] [PubMed] [Google Scholar]
- 23.Zhang D, Li J, Zhang M, Gao G, Zuo Z, Yu Y, et al. The requirement of c-Jun N-terminal kinase2 in regulation of hypoxia inducing factor-1α mRNA stability. J Biol Chem JBC. 2012;M112:365882. doi: 10.1074/jbc.M112.365882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin H, Yu Y, Hu Y, Lu C, Li J, Gu J, et al. Divergent behaviors and underlying mechanisms of cell migration and invasion in non-metastatic T24 and its metastatic derivative T24T bladder cancer cell lines. Oncotarget. 2015;6(1):522. doi: 10.18632/oncotarget.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gildea JJ, Golden WL, Harding MA, Theodorescu D. Genetic and phenotypic changes associated with the acquisition of tumorigenicity in human bladder cancer. Genes Chromosom Cancer. 2000;27(3):252–263. doi: 10.1002/(sici)1098-2264(200003)27:3<252::aid-gcc5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 26.Song L, Li J, Zhang D, Liu Z-G, Ye J, Zhan Q, et al. IKKβ programs to turn on the GADD45α–MKK4–JNK apoptotic cascade specifically via p50 NF-κB in arsenite response. J Cell Biol. 2006;175(4):607–617. doi: 10.1083/jcb.200602149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song L, Li J, Ye J, Yu G, Ding J, Zhang D, et al. p85α acts as a novel signal transducer for mediation of cellular apoptotic response to UV radiation. Mol Cell Biol. 2007;27(7):2713–2731. doi: 10.1128/MCB.00657-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang C, Zeng X, Jiang G, Liao X, Liu C, Li J, et al. XIAP BIR domain suppresses miR-200a expression and subsequently promotes EGFR protein translation and anchorage-independent growth of bladder cancer cell. J Hematol Oncol. 2017;10(1):6. doi: 10.1186/s13045-016-0376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song L, Gao M, Dong W, Hu M, Li J, Shi X, et al. p85α mediates p53 K370 acetylation by p300 and regulates its promoter-specific transactivity in the cellular UVB response. Oncogene. 2011;30(11):1360. doi: 10.1038/onc.2010.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang G, Wu AD, Huang C, Gu J, Zhang L, Huang H, et al. Isorhapontigenin (ISO) inhibits invasive bladder cancer (BC) formation in vivo and human BC invasion in vitro by targeting STAT1/FOXO1 Axis. Cancer Prevent Res Canprevres. 2016;0338:2015. doi: 10.1158/1940-6207.CAPR-15-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Robbins J. Proteasomal and lysosomal protein degradation and heart disease. J Mol Cell Cardiol. 2014;71:16–24. doi: 10.1016/j.yjmcc.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim DH, Sætrom P, Snøve O, Rossi JJ. MicroRNA-directed transcriptional gene silencing in mammalian cells. Proc Natl Acad Sci. 2008;105:16230–16235. doi: 10.1073/pnas.0808830105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11(3):228. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 34.Ha M, Kim VN. Regulation of microRNA biogenesis (Review Article) Nat Rev Mol Cell Biol. 2014;15:509. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 35.Enderling H, Hlatky L, Hahnfeldt P. Cancer stem cells: a minor cancer subpopulation that redefines global cancer features. Front Oncol. 2013;3:76. doi: 10.3389/fonc.2013.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kondo T. Stem cell-like cancer cells in cancer cell lines. Cancer Biomark. 2007;3(4–5):245–250. doi: 10.3233/cbm-2007-34-508. [DOI] [PubMed] [Google Scholar]
- 37.Ohishi T, Koga F, Migita T. Bladder cancer stem-like cells: their origin and therapeutic perspectives. Int J Mol Sci. 2015;17(1):43. doi: 10.3390/ijms17010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang P, Gao Q, Suo Z, Munthe E, Solberg S, Ma L, et al. Identification and characterization of cells with cancer stem cell properties in human primary lung cancer cell lines. PLoS One. 2013;8(3):e57020–e57020. doi: 10.1371/journal.pone.0057020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13(2):153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Dalerba P, Dylla SJ, Park I-K, Liu R, Wang X, Cho RW, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci. 2007;104(24):10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu K, Ning Z, Zeng J, Fan J, Zhou J, Zhang T, et al. Silibinin inhibits β-catenin/ZEB1 signaling and suppresses bladder cancer metastasis via dual-blocking epithelial–mesenchymal transition and stemness. Cell Signal. 2013;25(12):2625–2633. doi: 10.1016/j.cellsig.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 43.Xu Z, Zeng X, Xu J, Xu D, Li J, Jin H, et al. Isorhapontigenin suppresses growth of patient-derived glioblastoma spheres through regulating miR-145/SOX2/cyclin D1 axis. Neuro-oncology. 2015;18(6):830–839. doi: 10.1093/neuonc/nov298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hannon GJ. RNA interference. Nature. 2002;418(6894):244. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 45.Jafarnejad SM, Ardekani GS, Ghaffari M, Martinka M, Li G. Sox4-mediated Dicer expression is critical for suppression of melanoma cell invasion. Oncogene. 2013;32(17):2131. doi: 10.1038/onc.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tokumaru S, Suzuki M, Yamada H, Nagino M, Takahashi T. let-7 regulates Dicer expression and constitutes a negative feedback loop. Carcinogenesis. 2008;29(11):2073–2077. doi: 10.1093/carcin/bgn187. [DOI] [PubMed] [Google Scholar]
- 47.Rigbolt KT, Prokhorova TA, Akimov V, Henningsen J, Johansen PT, Kratchmarova I, et al. System-wide temporal characterization of the proteome and phosphoproteome of human embryonic stem cell differentiation. Sci Signal. 2011;4(164):rs3. doi: 10.1126/scisignal.2001570. [DOI] [PubMed] [Google Scholar]
- 48.Gross TJ, Powers LS, Boudreau RL, Brink B, Reisetter A, Goel K, et al. A microRNA processing defect in smokers’ macrophages is linked to SUMOylation of the endonuclease DICER. J Biol Chem. 2014;289(18):12823–12834. doi: 10.1074/jbc.M114.565473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nan Y-H, Wang J, Wang Y, Sun P-H, Han Y-P, Fan L, et al. MiR-4295 promotes cell growth in bladder cancer by targeting BTG1. Am J Transl Res. 2016;8(11):4892. [PMC free article] [PubMed] [Google Scholar]
- 50.Jin H, Xu J, Guo X, Huang H, Li J, Peng M, et al. XIAP RING domain mediates miR-4295 expression and subsequently inhibiting p63α protein translation and promoting transformation of bladder epithelial cells. Oncotarget. 2016;7(35):56540. doi: 10.18632/oncotarget.10645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang L, Xu B, Qiang Y, Huang H, Wang C, Li D, et al. Overexpression of deubiquitinating enzyme USP 28 promoted non-small cell lung cancer growth. J Cell Mol Med. 2015;19(4):799–805. doi: 10.1111/jcmm.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhen Y, Knobel PA, Stracker TH, Reverter D. Regulation of USP28 de-ubiquitinating activity by SUMO conjugation. J Biol Chem JBC. 2014;M114:601849. doi: 10.1074/jbc.M114.601849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valero R, Bayés M, Francisca Sánchez-Font M, González-Angulo O, Gonzàlez-Duarte R, Marfany G. Characterization of alternatively spliced products and tissue-specific isoforms of USP28 and USP25. Genome Biol. 2001;2(10):research0043.1. doi: 10.1186/gb-2001-2-10-research0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lim K-H, Baek K-H. Deubiquitinating enzymes as therapeutic targets in cancer. Curr Pharm Des. 2013;19(22):4039–4052. doi: 10.2174/1381612811319220013. [DOI] [PubMed] [Google Scholar]
- 55.Nicholson B, Marblestone JG, Butt TR, Mattern MR. Deubiquitinating enzymes as novel anticancer targets. Fut Oncol (Lond Engl) 2007;3(2):191–199. doi: 10.2217/14796694.3.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Z, Song Q, Xue J, Zhao Y, Qin S. Ubiquitin-specific protease 28 is overexpressed in human glioblastomas and contributes to glioma tumorigenicity by regulating MYC expression. Exper Biol Med. 2016;241(3):255–264. doi: 10.1177/1535370215595468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo G, Xu Y, Gong M, Cao Y, An R. USP28 is a potential prognostic marker for bladder cancer. Tumor Biol. 2014;35(5):4017–4022. doi: 10.1007/s13277-013-1525-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. T24T(vector) and T24T(CD44) cells were treated with ISO or DMSO at indicated concentrations for 7 days to determine impact on sphere formation ability (JPEG 650 kb)
Fig S2. (A) T24T cells were treated with Bafomycin A1 (5 nM) or ISO (20 μM) alone or Bafomycin A1+ ISO, which tested whether CD44 protein accumulated by Bafomycin A1. (B) Cell sphere formation by cells transfected with Flag-USP28 or vector. Transfectants were exposed to 20 μM ISO for 7 days before analyses using microscopy (JPEG 778 kb)
Fig S3. (A) Schematic representation of transcription factor binding sites in human CD44 promoter region from -1133 to -1. (B & C) FOXO1 or c-MYC did not affect CD44 protein expression. (D) T24T(vector) and T24T(GFP-Sp1) cells were treated with/without ISO (20 μM) to determine sphere formation ability. (E) T24T(nonsense) and T24T(shSp1) cells were used in sphere formation assay as described in Methods (JPEG 900 kb)
Fig S4. (A) usp28 mRNA expression levels in T24T cells were determined by RT-PCR after cells were treated with 20 μM ISO for indicated time periods. (B) T24T cells were treated with/without 20 μM ISO in the presence of CHX for indicated time periods, then underwent extraction; extracts were then analyzed by Western blot to assess USP28 protein expression. (C) Schematic representation of potential miRNA-binding sites in USP28 3’UTR were analyzed with TargetScan software. (D) Schematic sequence of intact miR-4295-binding site in wide-type USP28 3’UTR and its mutation of USP28 3’UTR luciferase reporter (JPEG 912 kb)
Fig S5. Invasivity of T24T(vector) and T24T(miR-4295 inhibitor) cells treated with or without 20 µM ISO for 24 hours was evaluated using a BD BioCoat TM Matrigel TM Invasion Chamber. After incubation for 24 hours, cells were fixed and stained and took pictures as described in Methods (JPEG 838 kb)
Fig S6. (A) T24T(miR-4295 promoter) stable transfectants were treated with 20 μM ISO for indicated time periods; thereafter, miR-4295 promoter luciferase activity was measured (via Dual-Luciferase Reporter Assay System). (B) T24T cells treated with 20 μM ISO or vehicle control - both along with ActD - for the time periods indicated. Total RNA was isolated and subjected to qRT-PCR evaluation of miR-4295 expression levels. (C) Cell sphere formation abilities of T24T(vector) and T24T(shDicer) cells treated with or without 20 µM ISO were determined as described in Methods (JPEG 811 kb)
Fig S7. (A) Kaplan-Meier estimation about has-mir-4295 of overall survival (OS) in bladder cancer (BC) patients from the kmplot.com database. Overall survival (OS) curves showing that patients with high miR-4295 expression (n=564) was related with better OS, compared with lower expression of miR-4295 (n=186). (B) Kaplan-Meier estimation about USP28 of overall survival (OS) in bladder cancer (BC) patients from the kmplot.com database. Overall survival (OS) curves showing that patients with high USP28 expression (n=294) were positively associated with decreased survival of BC patients, lower expression of miR-4295 (n=191) was associated with longer survival (JPEG 833 kb)