Abstract
Background:
Ex vivo confocal microscopy (CM) works under two modes, fluorescence and reflectance, allowing the visualization of different structures. Fluorescence CM (FCM) requires a contrast agent and has been used for the analysis of basal cell carcinomas (BCC) during Mohs surgery. Conversely, reflectance CM (RCM) is mostly used for in vivo diagnosis of equivocal skin tumours. Recently, a new, faster ex vivo confocal microscope has been developed which simultaneously uses both lasers (fusion mode).
Objectives:
To describe the BCC features identified on reflectance, fluorescence and fusion modes using this novel device. To determine the best mode to identify characteristic BCC features. To develop a new staining protocol to improve the visualization of BCC under the different modes.
Methods:
From September 2016 to June 2017, we prospectively included consecutive BCCs which were excised using Mohs surgery in our department. The lesions were evaluated using ex vivo CM after routine Mohs surgery. The specimens were first stained with acridine orange and then stained using both acetic acid and acridine orange.
Results:
We included 78 BCCs (35 infiltrative, 25 nodular, 12 micronodular, 6 superficial). Most features were better visualized with the fusion mode using the double staining. We also identified new CM ex vivo features, dendritic and plump cells, which have not been previously reported.
Conclusions:
Our results suggest that nuclei characteristics are better visualized in FCM but cytoplasm and surrounding stroma are better visualized in RCM. Thus, the simultaneous evaluation of reflectance and fluorescence seems to be beneficial due to its complementary effect.
Keywords: reflectance confocal microscopy, fluorescence confocal microscopy, fusion confocal microscopy, ex vivo confocal microscopy, Mohs surgery, basal cell carcinoma, skin cancer
Introduction
Paraffin-embedded blocks have been used in conventional histopathology for a century.1 Imaging technologies such as confocal microscopy (CM) had made definitive changes in dermatological and histopathological diagnosis in the last decades. Reflectance CM (RCM) has shown to provide high diagnostic accuracy in diagnosing skin cancers.2, 3, 4 In addition, CM using fluorescence has also been used to evaluate excised specimens (ex vivo FCM) showing correct identification of residual tumour after Mohs surgery.5 In this setting, high resolution ex vivo CM allows for fresh tissue analysis immediately after excision.6 In order to improve the detection of residual tumour, specimen staining protocols have been described. The first stain was 5% acetic acid (AA), which induced whitening of the epithelium and brightening of the nuclei by chromatin compaction.7 Acridine orange (AO) was also used, resulting in an increase of the nuclei brightness by selectively highlighting nucleic acid (green fluorescence) and RNA (red fluorescence) in fluorescence mode.8 Other stains have been tested such as Nile blue, methylene blue or patent blue, but results have not improved on those obtained with AO in ex vivo CM.9
Ex vivo CM saves at least 1/3 of the time while processing BCC Mohs samples, providing a sensitivity of 88% and a specificity of 99%, whilst not affecting posterior staining of permanent sections.10, 5, 7
The first ex vivo confocal microscope, the VivaScope 2500® (Lucid Inc, Henrietta, NY), had two different diode lasers: one for RCM with a wavelength of 830 nm, and another for FCM with a laser wavelength of 488 nm. However, the simultaneous acquisition in both modes was not possible and it was relatively slow, taking ~30 minutes. Recently, a novel confocal microscope (VivaScope 2500 4th Gen®, MAVIG GmbH, Munich) has been developed which allows the visualization with reflectance and fluorescence simultaneously, providing high resolution images in both modes separately and in combination (fusion mode).
Herein, we present the first results using this new device and we describe the features encountered in a series of consecutive BCC. Moreover, we describe a new staining protocol which combines AO and AA to enhance the visualisation of structures in both reflectance and fluorescence, thus allowing a better definition of both stroma, and tumour characteristics.
MATERIAL AND METHODS
After approval by the Ethics Committee at Hospital Clínic de Barcelona, we conducted a prospective, descriptive and exploratory study in order to describe different staining and laser modes of ex vivo confocal microscopy. The main objective was to describe the criteria visualized with each protocol and compare between different protocols (combination of staining and mode). From September 2016 to June 2017, we included consecutive BCCs, which were excised using Mohs surgery in our centre. Lesions included in the study were at least 1 cm in their maximum diameter and complied with the indications for Mohs surgery. We excluded basosquamous BCCs, and BCCs which were removed using conventional excision.
Patients who agreed to participate signed an informed consent. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Tissue Processing
The tissue used was obtained after Mohs surgery, both from the debulking and the lateral and deep margins. The tissue used in the study was obtained from the specimen leftover after frozen sections were obtained for standard-of-care Mohs surgery. Initially, the tissue was processed for visualisation using CM after Mohs surgery, and later processed for routine histopathology analysis (vertical sections for the debulking and the lateral margins, and horizontal sections for the deep margins) (Supplementary figure 1).
In order to compare both staining effects, two separate fusion images were obtained for each specimen, the first image was taken after AO staining and the second after AO+AA staining. The samples were first immersed for 20 seconds in 1 mmol/L AO and subsequently washed with saline solution for 20 seconds as described in the literature.10 They were then scanned using the ex-vivo confocal microscopy in fusion mode with both fluorescence and reflectance lasers. Then, the same sample was stained again in AO at the same concentration for 10 seconds to avoid the bleaching effect, and after washing in saline solution for 20 seconds, it was immersed in acetic acid 50% (AA) for 20 seconds, washed again in saline solution for 20 seconds, and finally re-scanned in the CM with the same setting as the first imaging.11
In order to analyse 100% of the lesion using CM, the specimen was placed on a slide, two small pieces of reusable adhesive putty (Blu Tack, Bostik Inc, Paris) were placed on each side of the specimen, and an additional slide was placed on top in order to flatten the specimen. Later, the slides were placed in the 4th Gen VivaScope 2500 (MAVIG GmbH, Munich) to capture images of RCM and FCM ex vivo. This device uses two laser sources with a resolution image of 1024×1024 pixels, and an objective lens of 38x with a numerical aperture of 0.85. Mosaics of individual horizontal images and stacks of vertical images were obtained to render a 3D approximation of the sample. The field of view of single images is 550 × 550 μm and the maximum mosaic area is 25 × 25mm.
After CM acquisition, the specimens were placed in the same exact position in a processing cassette for paraffin section and H&E staining to demonstrate the viability of the samples for routine histopathological analysis after all the manipulations for ex-vivo CM.
After optimisation of the technique, we developed an optimised standard operating procedure which can be used either for fresh tissue or after frozen tissue has been thawed (supplementary figure 1, supplementary document 1).
Data management and analysis
The images were stored in a secure drive within our centre. The patient’s age and sex, tumour location, initial and final histopathologic diagnoses were recorded. All CM images were evaluated by consensus by two Mohs surgeons, one an expert in ex vivo CM, and one an expert in in vivo CM. Histopathology slides from frozen sections and H&E slides were evaluated by consensus by two dermatopathologists.
For the evaluation of BCC using RCM, we used the previously described criteria for in vivo RCM:11, 12, 13, 14 bright tumour islands, dark silhouettes, palisading, peritumoural clefting, peritumoural stromal reaction, thickened/distorted collagen bundles, horizontal blood vessels, dendritic cells and plump cells. For the evaluation using fluorescence CM we evaluated: delimitation of the tumour, crowding, nuclear pleomorphism, increased nucleus/cytoplasm ratio, and presence of fluorescence in the cell nuclei. Later, we compared the findings in fluorescence, reflectance and fusion obtained with AO as well as AA + AO.
Statistical analysis
The study variables were formally tabulated into descriptive variables and treated as paired binomial variables for each one of the experiments and stain protocols. The exact p-values of the McNemar test, which is especially suited for paired nominal data, was used to assess the statistical differences between the results before and after staining the samples with AA. All p-values have also been adjusted for multiple comparisons using the Benjamini-Hochberg15 procedure to control the false discovery rate. Finally, the exact confidence intervals of the binomial variables were computed and plotted and adjusted for multiple comparisons (97.7 % CI).16 All tests were performed with a significance level of p= 0.05 with the PASW statistical software (SPSS Corp, Chicago, USA). Each of the structures analyzed in this manuscript are well described and used for diagnosis using confocal microscopy. This paper pretends to help guide technicians to decide when to use a given staining technique to visualize each one of the particular structures of interest.
RESULTS
Clinical features
A total of 78 BCCs from 78 patients (41 males; mean age 63 years [SD: 38–93]) were imaged. The face was the most frequent location (80%), followed by the limbs (10.5 %) and the trunk (9.5 %). Fifty-seven cases were primary and the remaining 21 had received prior treatment. Regarding BCC subtype, 35 were infiltrative, 25 were nodular, 12 were micronodular and 6 were superficial.
Confocal microscopy results
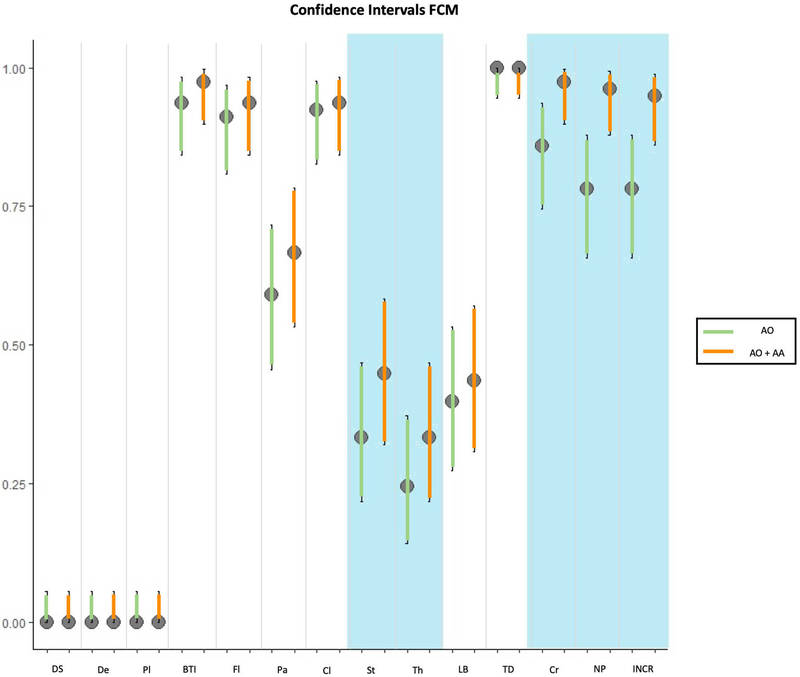
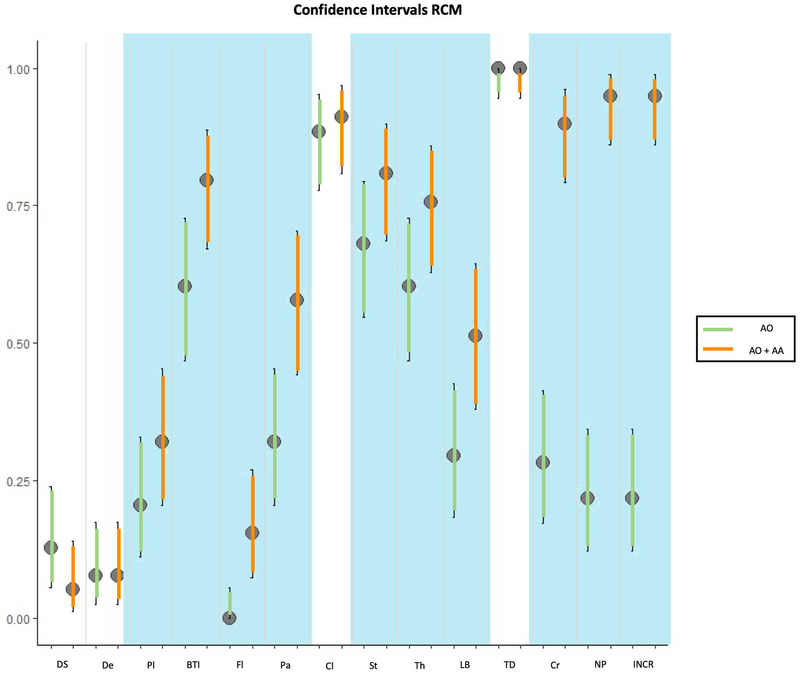
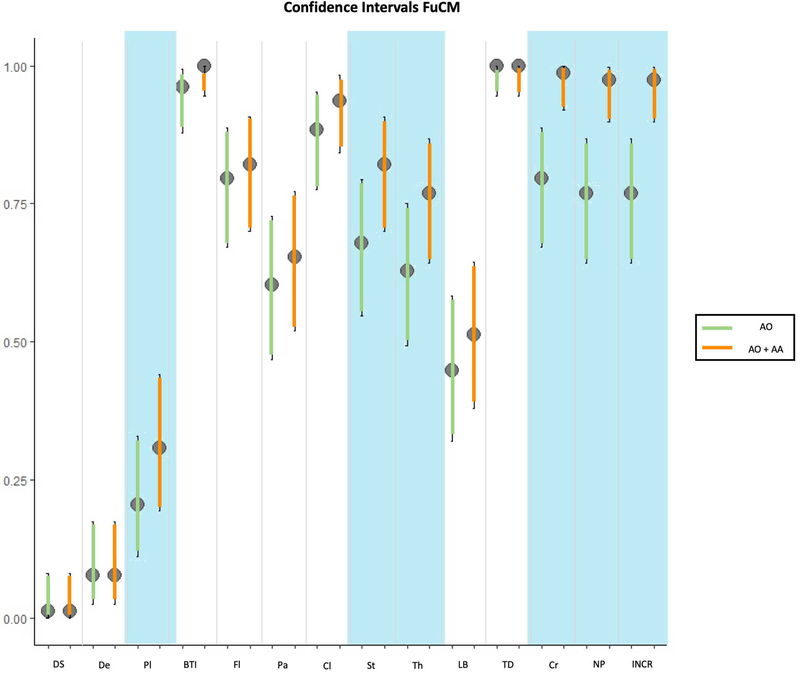
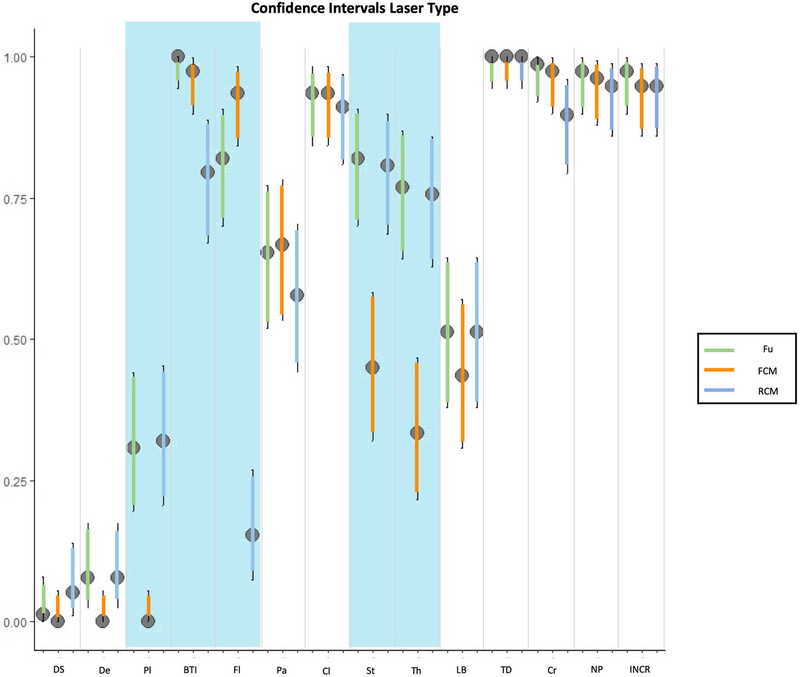
The results of the confocal findings comparing AO with AO+AA in fluorescence, reflectance or fusion are summarized in Figure 1A, 1B, 1C respectively and supplementary Table 1. The results of the different laser modes for the best stain for feature visualization are summarized in Figure 1D and supplementary Table 2. Seven minutes was the mean time required to scan each skin sample, including the stain protocol, with a 3 to 15-minute range depending on the size of the specimen. In addition, our final histology evaluation confirmed that the specimens were not damaged after the staining or CM scanning processes. The most relevant results are summarised therein.
Figure 1:
Confidence intervals between different laser and staining procedures.
1A: comparison of staining in fluorescent confocal microscopy (FCM); 1B: comparison of staining in reflectance confocal microscopy (RCM); 1C: comparison of staining in fusion mode of confocal microscopy (FuCM); 1D comparison between different laser modes with the best staining (AO+AA).
Grey colour for significant comparisons for p-McNemar.
Results in fluorescence mode
The double stain increased the visualisation of most features. Statistical differences were found between the results before and after staining with AA, especially changes were found in the visualization of nuclear pleomorphism (61 vs. 75, p<0.001), increased nuclear/cytoplasm visualization ratio (61 vs. 75, p<0.001), and crowding (67 vs. 76, p=0.01). There were also statistical differences in the visualization of stroma (26 vs. 35, p=0.01) and thickened collagen bundles (19 vs. 26, p=0.04). The visualisation of palisading, bright tumour islands, fluorescence, clefting, and linear blood vessels also showed differences with the double stain. However, no statistically significant differences were found between AO vs. AA+AO for the aforementioned features. Tumour demarcation was identified in all cases using both stain protocols, while dark silhouettes, dendritic cells and plump cells were not identified in the fluorescence mode with any stain (Figures 1A, 2, 3 and supplementary table 1).
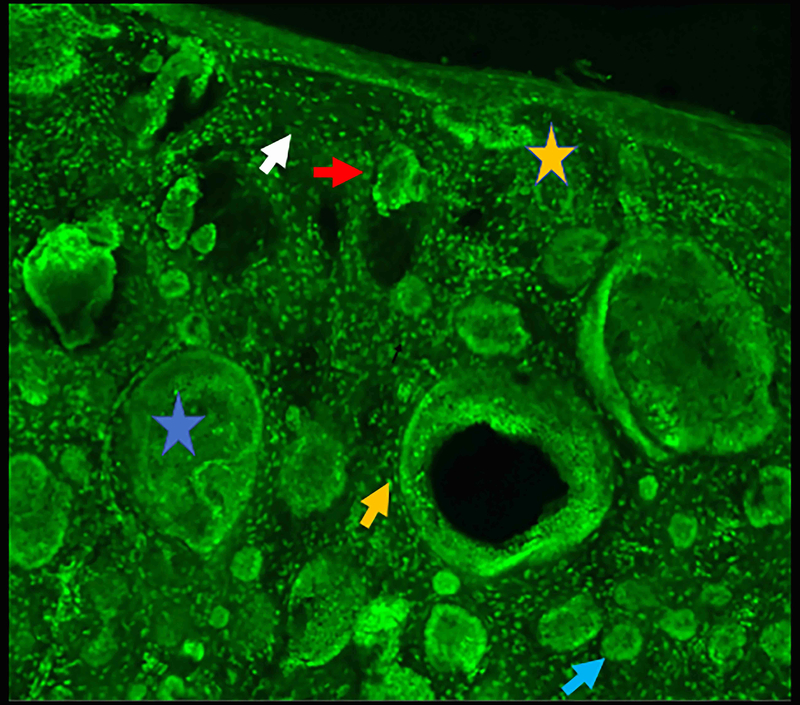
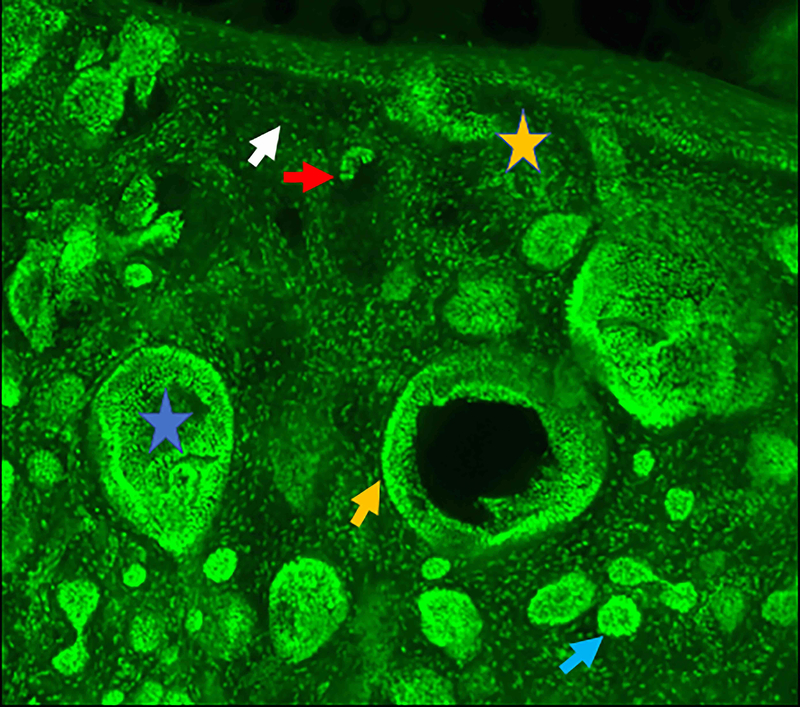
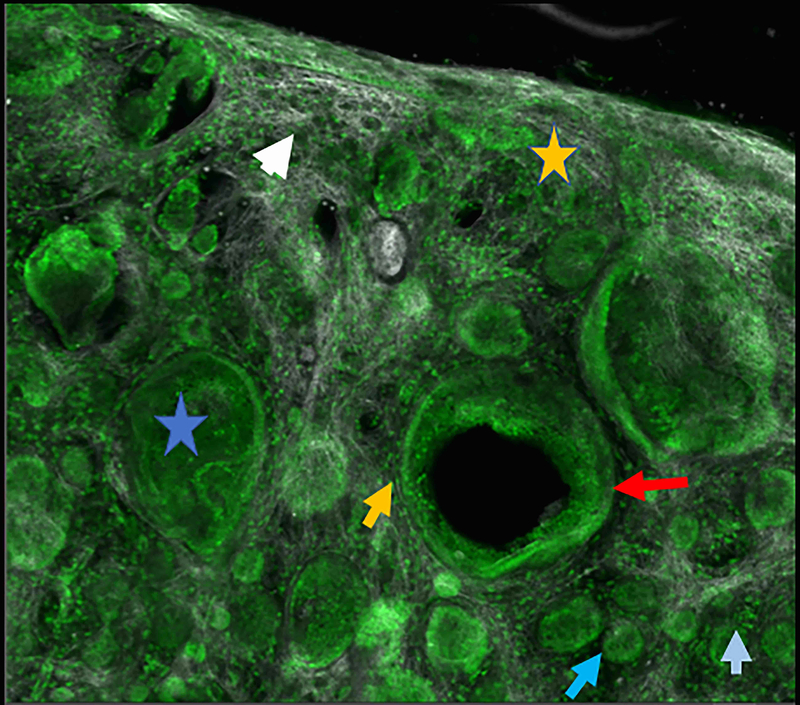
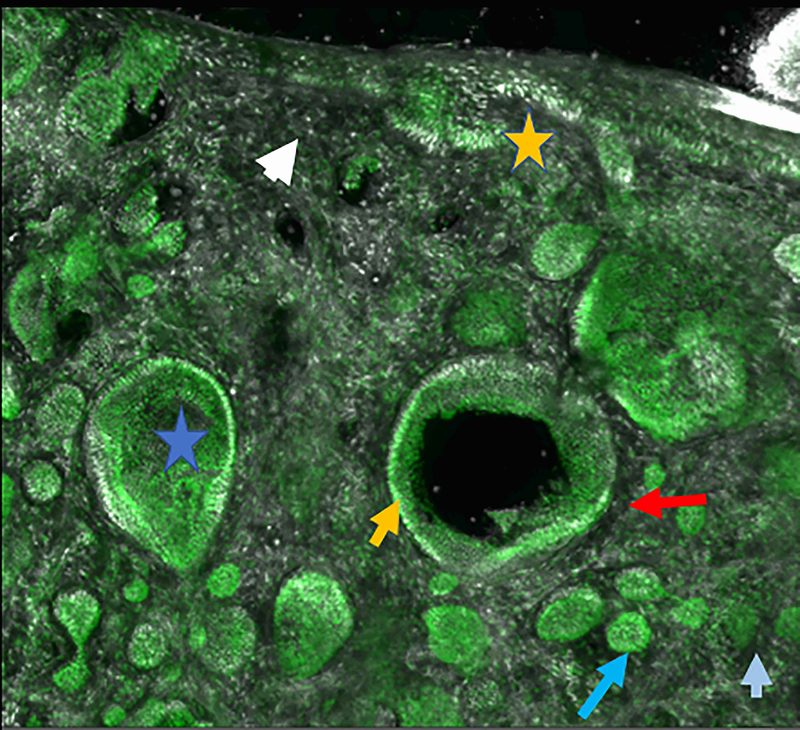
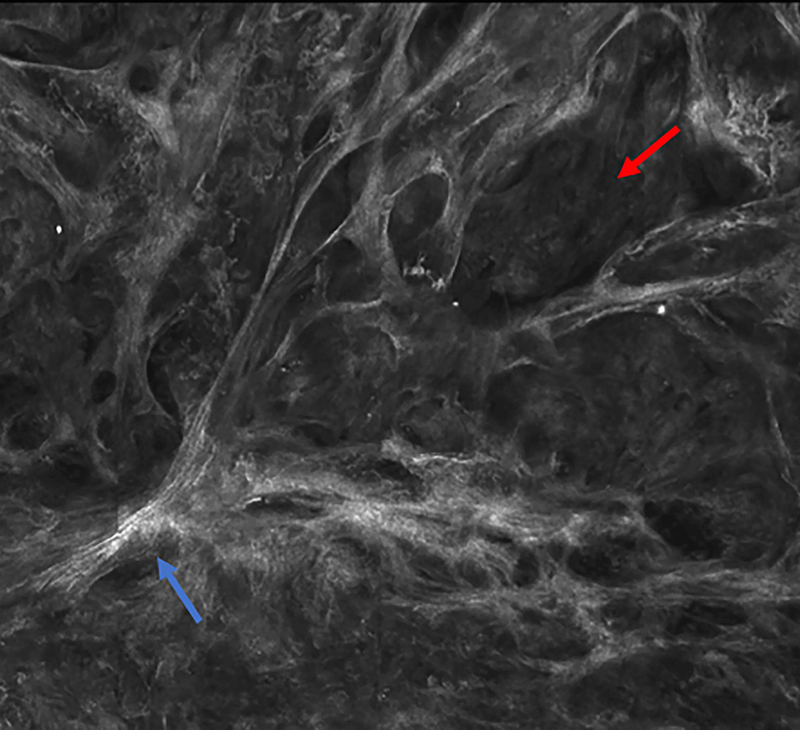
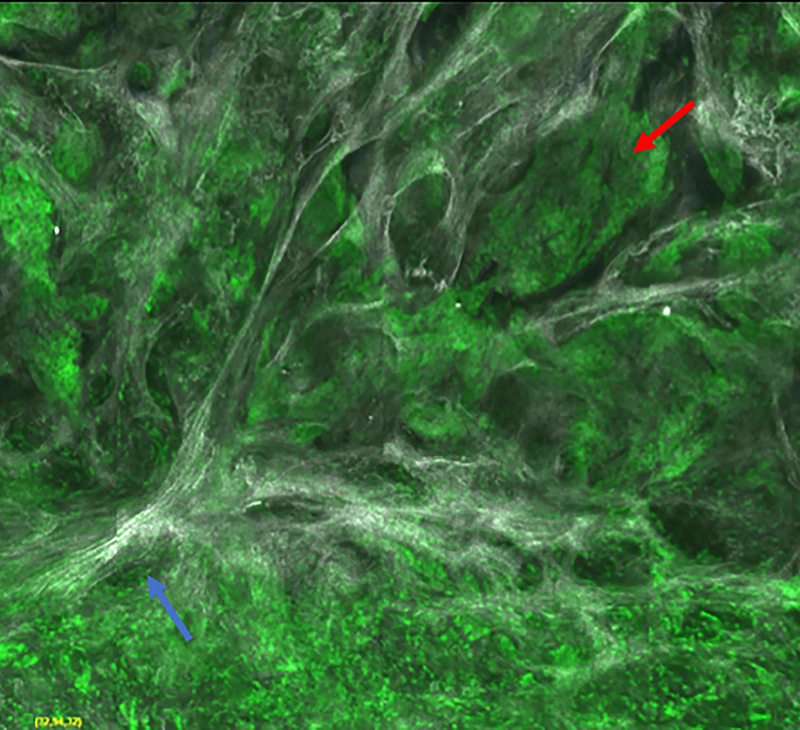
Figure 2.
Micronodular basal cell carcinoma. Feature differences in distinct lasers and stain protocols. Right column Acridine orange + acetic acid. Left column: Acridine orange. A-A1: Fluorescence confocal microscopy. B-B1: Reflectance confocal microscopy. C-C1: Fusion confocal microscopy. Differences between stroma reactions can be observed (white arrow); clefting (red arrow); palisading (yellow arrow); thickened collagen bundles (sky blue arrow); well delineated and bright tumour islands (blue arrow); dark silhouettes (pink arrows); presence of fluorescence (yellow star); nuclear features: crowding, nuclear pleomorphism, increased nucleus/cytoplasm ratio (blue star).
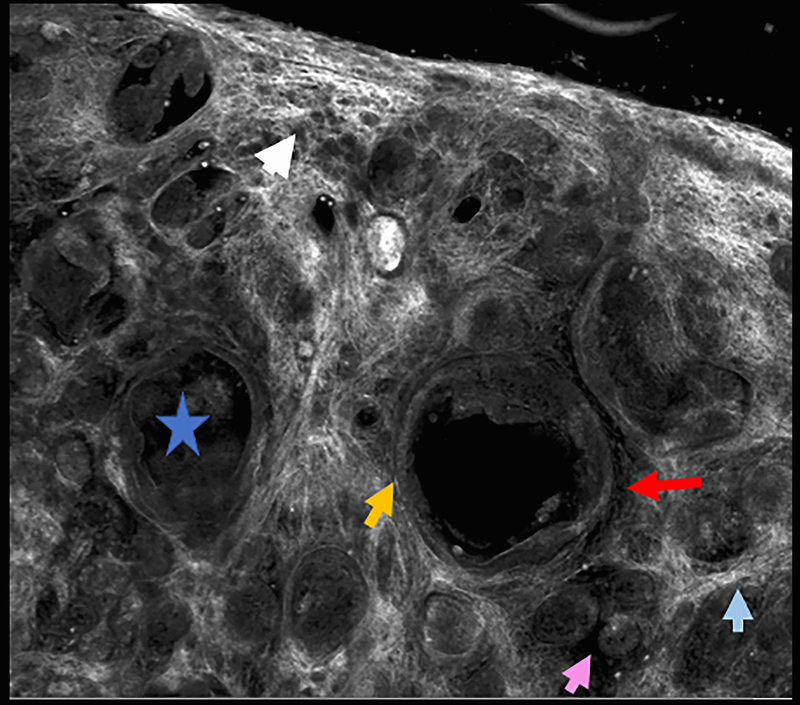
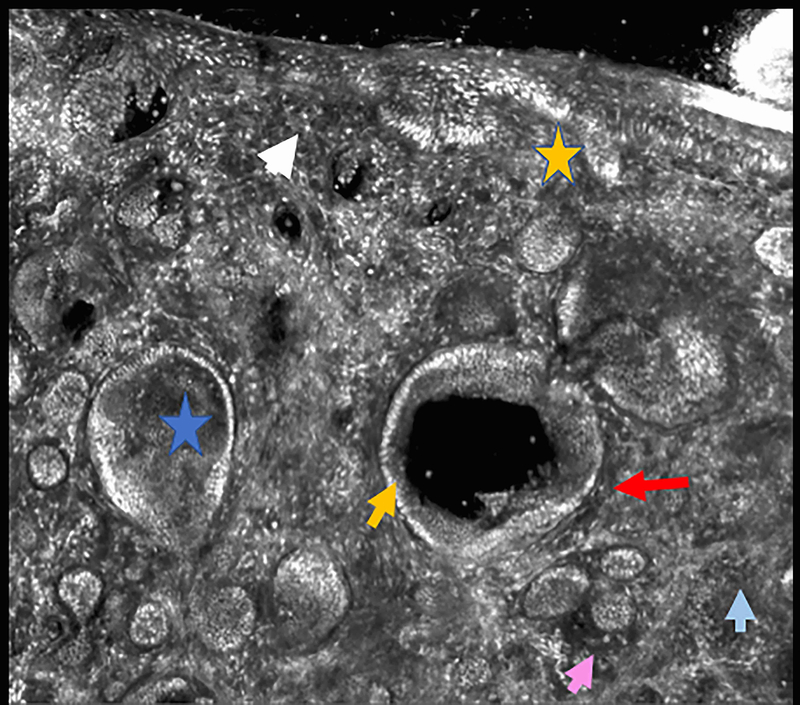
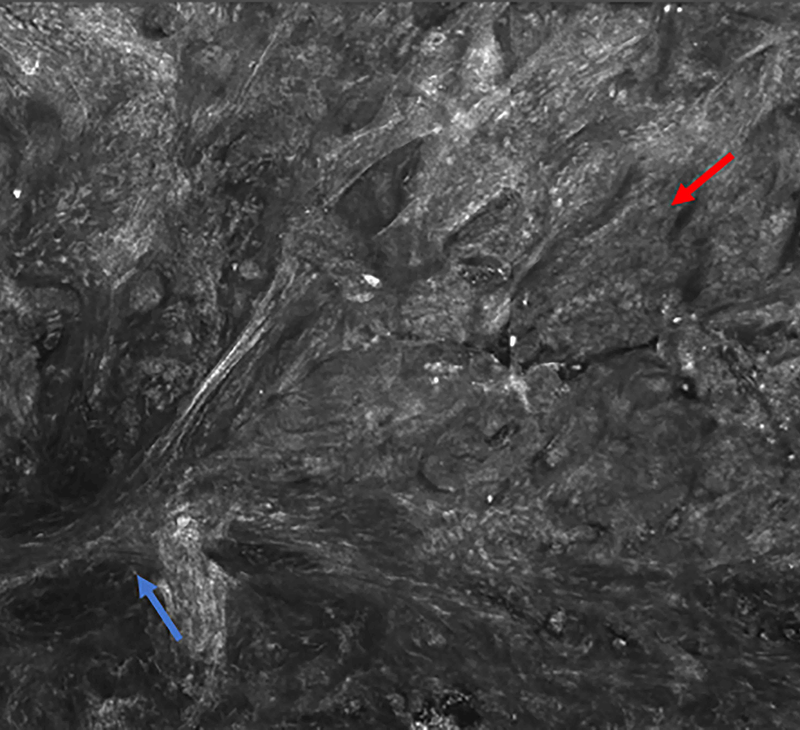
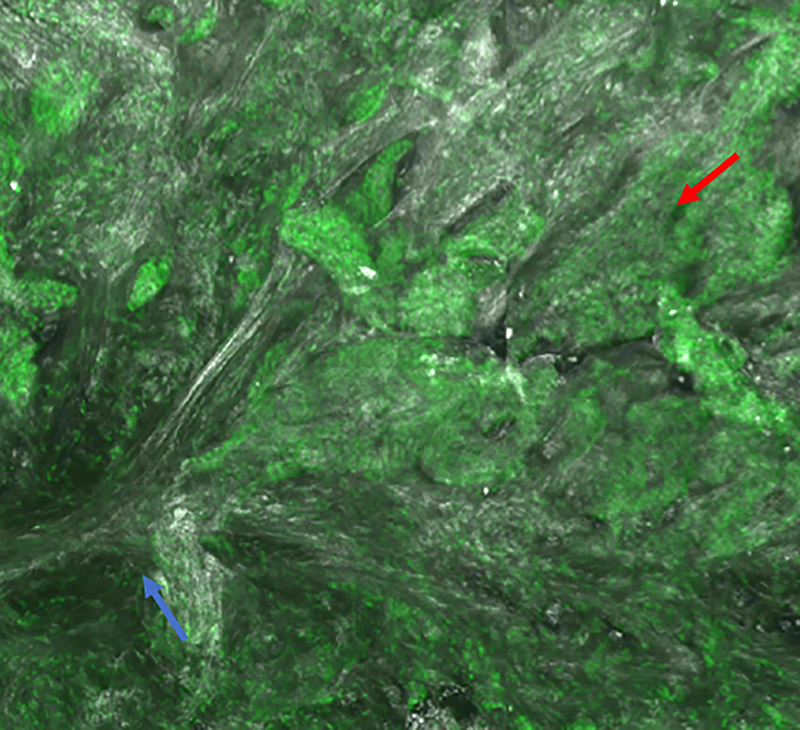
Figure 3.
Infiltrating basal cell carcinoma. Feature differences: distinct lasers and stain protocols. Right column Acridine orange + acetic acid. Left column: Acridine orange. A-A1: Fluorescence confocal microscopy. B-B1: Reflectance confocal microscopy. C-C1: Fusion confocal microscopy. Dark silhouettes are better observed without the nuclear enhancement of the acetic acid. They are poorly visualized in fluorescence mode and better observed in reflectance mode. When the acetic acid is applied, bright and well delineated tumour islands can be observed instead of them (red arrow). Collagen bundles are poorly observed in fluorescence mode (blue arrow). Clefting is not always observed in infiltrating tumours.
Results in reflectance mode
The double stain significantly showed differences and improved the visualisation of nuclear pleomorphism (17 vs. 74, p<0.001), nuclear/cytoplasm ratio (17 vs. 74, p<0.001) and crowding (22 vs. 73 p<0.001). The same stain also significantly differentiated the visualisation of plump cells (16 vs. 25, p=0.01), bright tumour islands (47 vs 62 p<0.001), fluorescence (0 vs 12, p<0.002), palisading (25 vs. 45, p<0.001), stroma (53 vs. 63, p=0.02), thickened collagen (47 vs. 59, p=0.006) and linear blood vessels (23 vs. 40, p<0.001). Clefting visualization did not change significantly with the addition of acetic acid (69 vs.71, p=0.91). Conversely, the visualisation of dark silhouettes in RCM decreased with the double stain (10 vs 4, p=0.14) (Figures 1B, 3, and supplementary figure 2). Dendritic cells and tumour demarcation were observed independently of the stain protocol (p>0.99).
Results in fusion mode
Similar to reflectance and fluorescence, the double stain showed differences and improved the visualisation of nuclear pleomorphism (60 vs. 76, p<0.001), nuclear/cytoplasm ratio (60 vs. 76, p<0.001) and crowding (62 vs. 77, p<0.001) in the fusion mode. It also showed significant differences (p<0.05) in the visualization of stroma (p=0.004), thickened collagen bundles (p=0.004) and plump cells (p<0.02). The visualization of dark silhouettes, dendritic cells, bright tumour islands, fluorescence, palisading, clefting, linear blood vessels and tumour demarcation did not significantly change with the double stain protocol (Figures 1C and 2).
Overall laser results using acetic acid plus acridine orange
When comparing fluorescence and reflectance modes using the double stain, statistical differences were found in the visualisation of plump cells (0 vs 25, p<0.001), bright tumour islands (76 vs 62, p=0.005), fluorescence (73 vs 12, p<0.001), stroma reaction (35 vs 63, p<0.001) and thickened collagen bundles (26 vs 59, p<0.001). It slightly improved the observation of the linear blood vessels (34 vs 40, p= 0.07). No differences between FCM and RCM were noted when evaluating palisading (52 vs. 45, p=0.14), clefting (73 vs. 71, p=0.74), tumour demarcation (78 vs 78, p>0.99), crowding (76 vs 73, p 0.63), nuclear pleomorphism (75 vs. 74, p>0.99), and increased nuclear/cytoplasm ratio (p>0.99). Conversely, in FCM, dark silhouettes (0 vs 4, p=0.23) and dendritic cells (0 vs 6, p=0.07) could not be observed.
Overall, FCM improved the visualisation of bright tumour islands and fluorescence and RCM was better for identifying dark silhouettes, dendritic and plump cells (Figure 1D, 2, 3), whereas Fusion CM was also good compared with the other lasers for identifying the remaining features observed in supplementary Table 2. (Figures 1–4) (Supplementary figure 2).
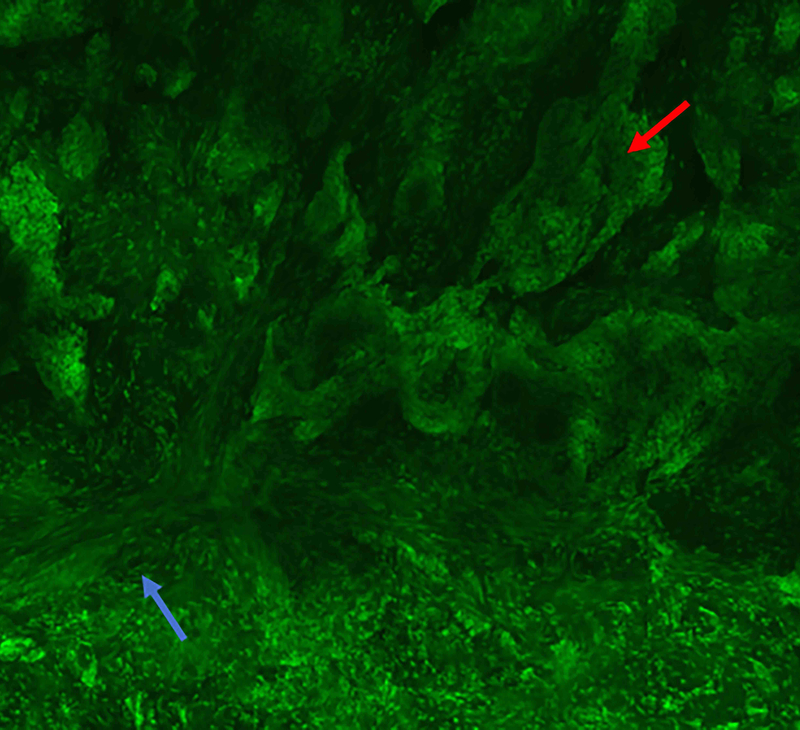
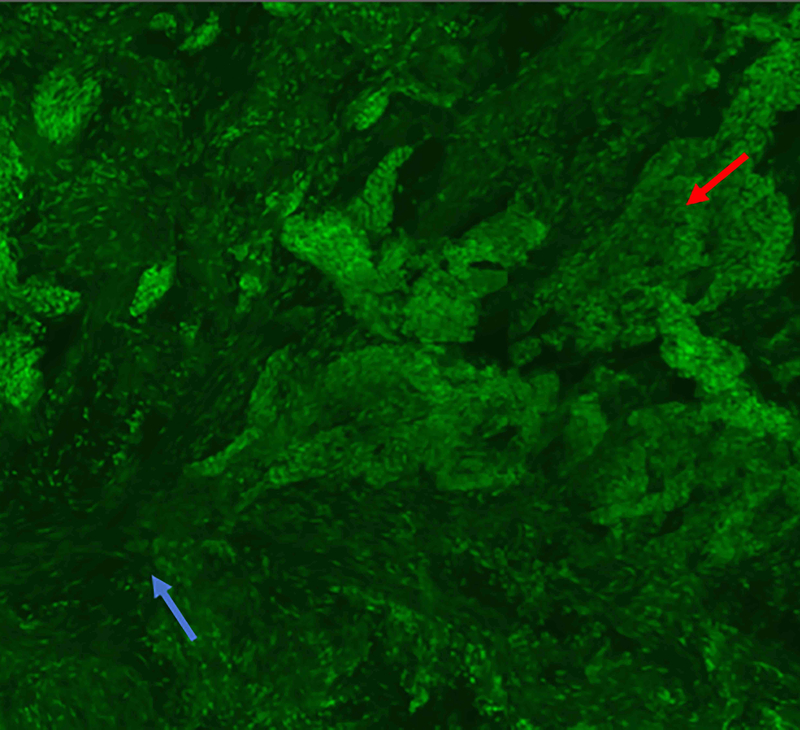
Figure 4.
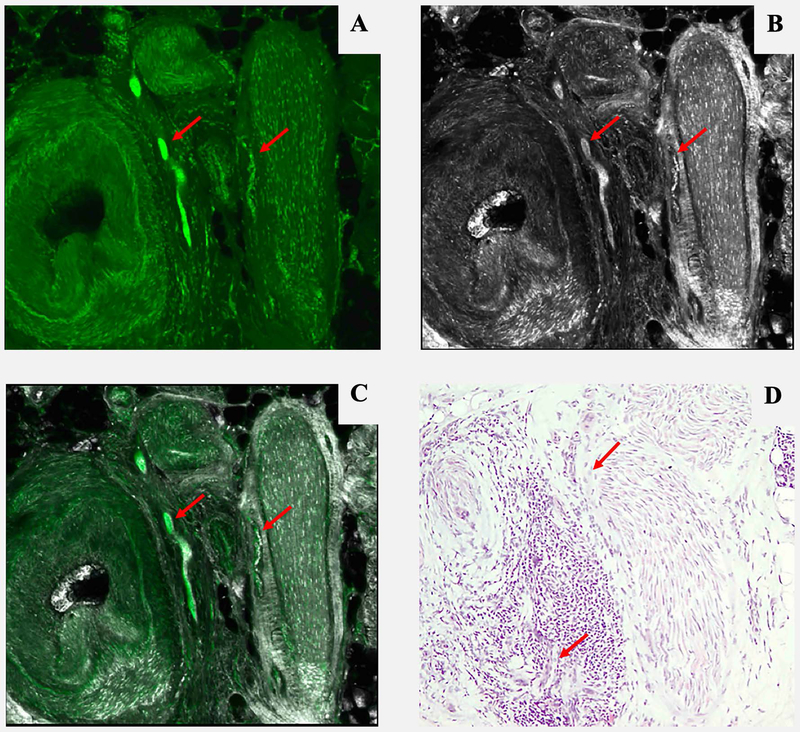
Three-dimensional peri vascular and peri neural infiltrating basal cell carcinoma (red arrow). A: Fluorescence mode. B: Reflectance mode. C: Fusion mode. D: Hematoxylin and Eosin. 10x. Even small amounts of tumour can be detected by this method, but not always clearly by conventional pathology.
DISCUSSION
In this study we present the first results in skin using a novel confocal microscope which combines reflectance and fluorescence modes simultaneously. To date, most studies using ex vivo CM to diagnose BCC described features use the fluorescence mode and only a few criteria have been described for ex vivo in RCM. Some authors have described the use of in vivo CM for intraoperative margin control.5, 10, 17, 18, 19
Both RCM and FCM have advantages and disadvantages when evaluating BCCs. Reflectance improves the visualisation of the stromal and cytoplasmic features while fluorescence also enhances the visualization of the nuclei.20,21 Therefore, a combined approach may improve the visualisation of nuclear, cytoplasmic and stromal details. Our results support this statement, since fusion CM resulted in an improvement of the visualisation of most BCC features such as bright tumour islands, crowding, nuclear pleomorphism, or stromal reaction (supplementary Table 2). However, for select features such as fluorescence or dark silhouettes, FCM or RCM individually seem to perform better than fusion CM. This may be due to a sharper visualisation of such features when assessed using only one laser. Nevertheless, we do not believe fusion should be used alone, but we advocate for the parallel use of RCM, FCM and fusion when performing ex vivo CM since they allow a more comprehensive evaluation of the different cellular and stromal features. A similar scenario occurs in dermoscopy, where controversy has arisen when assessing whether polarised or non-polarised dermoscopy is better. Indeed, many advocate for the integration of both light sources in the same device in order to toggle from polarised to non-polarised to identify certain structures not visible with both modes (blink sign).22,23 Hence, this could be the same scenario in ex vivo CM, where one could toggle from fluorescence to reflectance and vice versa, and get the complete image in fusion in order to help identify residual tumour areas that may facilitate the surgical procedure.
In addition, since multiple staining methods exist to improve tumour visualisation in both fluorescence and reflectance, we have developed a staining method which combines AA and AO to improve the visualization of the tumour (nucleus and cytoplasm) and the stroma at the same moment (supplementary figure 1, supplementary document 1).24,25,20 Our results suggest that, overall, the majority of BCC features are better visualised in both reflectance and fluorescence when using the double stain with AA+AO compared to AO alone. However, the visualisation of dark silhouettes in RCM decreased with the double stain. This seems logical since AA compacts the chromatin and improves the visualisation of nuclear details.14 It is also the case in our study where, in the cases where dark silhouettes were poorly visualised with the double stain, they enhanced the identification of bright tumour islands, crowding and other nuclear details. Therefore, the combination of AA and AO gives an efficient contrast with clear and sharper differentiation between cellular structures and the surrounding stroma. This may facilitate the interpretation of confocal images potentially leading to a more widespread use of the technology. However, further studies comparing novices vs. experts are needed, as well as reviewing the published criteria which occasionally may have little clinical applicability (i.e. fluorescence feature in fluorescence mode).
Another advantage of our approach is that the entire process is faster than previous ones. Since the new generation of ex vivo CM is faster and the different staining phases are performed sequentially, the entire process of staining and scanning in both lasers requires seven minutes on average, compared to 15–30 minutes using previous methods.10 Therefore, fast margin assessment allows almost simultaneous reconstruction in case of negative margins. The main advantage of the commercial model of this device is the possibility of the visualization of the fusion mode with a digital stain similar to haematoxylin and eosin which needs to be the main focus of another study (supplementary figure 3).21,26
However, our approach also has limitations. As reported previously, factors that could interfere in staining samples in ex vivo CM might be the life time of the fluorescence.7, 27 We also presume that not only the thickness of the tumour, but also the compaction of its cells in solid tumours does not allow for sufficient stain penetration in some BCC. In these cases, 20 seconds of AO stain is probably not enough to stain the tissue and more time could possibly be required. The complete flattening of the tissue and reaching the correct depth before scanning are also main interfering factors for perfect imaging. In addition, in the current manuscript we did not intend to assess the diagnostic accuracy of this novel CM device. Finally, we also acknowledge the fact that this novel CM device may be only present in select centres and, therefore, the results of our current study may not be generalizable to previous iterations of the device.
CONCLUSION:
To sum up, we have described BCC features using a novel ex vivo CM which uses fluorescence and reflectance simultaneously. Our results suggest that nuclei characteristics are better visualized in FCM but cytoplasm and surrounding stroma are better visualized in RCM. Thus, the simultaneous evaluation of reflectance and fluorescence seems to be beneficial due to its complementary effect. The adjunct use of multiple stains such as AA and AO also seemed beneficial to highlight the different tumour characteristics and seems to be promising to help identify residual tumour.
Supplementary Material
Bulleted statements.
What is already known on this topic
Ex vivo fluorescent confocal microscopy (FCM) is an imaging technique that allows histopathologic analysis of fresh tissue.
FCM is faster - at least 1/3 of the time - than conventional methods.
FCM has a sensitivity of 88% and a specificity of 99% in detecting basal cell carcinomas
What does this study adds
Reflectance and fluorescence modes can be used simultaneously in a new ex vivo confocal microscopy device.
Each mode complements the other, resulting in an increase in the detection of basal cell carcinoma (BCC) features in a fusion mode.
A combined staining using acetic acid and acridine orange enhances the visualization of tumour and stroma without damaging the tissue for further histopathological analysis.
ACKNOWLEDGEMENTS:
We want to thank the members of Surgery Unit and Dermatopathology Department of the Hospital Clinic Barcelona, for providing technical support to this project. We also want to thank our patients and their families who are the main reason for our studies, and the nurses from the Melanoma Unit of Hospital Clínic de Barcelona, Daniel Gabriel, Pablo Iglesias and Maria E Moliner for helping collect patient data. We also thank Helena Kruyer for her help with text edition.
Funding statement: The research at the Melanoma Unit in Barcelona is partially funded by Spanish Fondo de Investigaciones Sanitarias grants PI15/00716 and PI15/00956; CIBER de Enfermedades Raras of the Instituto de Salud Carlos III, Spain, co-financed by European Development Regional Fund “A way to achieve Europe” ERDF; AGAUR 2014_SGR_603 of the Catalan Government, Spain; European Commission under the 6th Framework Programme, Contract No. LSHC-CT-2006–018702 (GenoMEL) and by the European Commission under the 7th Framework Programme, Diagnoptics; The National Cancer Institute (NCI) of the US National Institute of Health (NIH) (CA83115); a grant from “Fundació La Marató de TV3” 201331–30, Catalonia, Spain; a grant from “Fundación Científica de la Asociación Española Contra el Cáncer” GCB15152978SOEN, Spain, and CERCA Programme / Generalitat de Catalunya. Part of the work was carried out at the Esther Koplowitz Center, Barcelona.
The whole exome sequencing analysis was in part supported by the intramural research program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics.
Abbreviations:
- DS
dark silhouettes
- De
dendritic cells
- Pl
plump cells
- BTI
bright tumour islands
- Fl
fluorescence
- Pa
palisading
- Cl
clefting
- St
stroma
- Th
thickened collagen bundles
- TD
tumour demarcation
- Cr
crowding
- NP
nuclear pleomorphism
- INCR
increased nucleus/cytoplasm ratio
- AO
Acridine Orange
- AO+AA
Acridine Orange plus Acetic acid
- FCM
fluorescence confocal microscopy
- RCM
reflectance confocal microscopy
- s
seconds
Footnotes
Disclosures: The authors have no disclosures or conflicts of interest to report.
Bibliography
- 1.Forkner C A method for supravital staining of animals with neutral red and its preservation in paraffin sections. J Exp Med 1930; 52:379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alarcon I, Carrera C, Palou J, et al. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br J Dermatol 2014; 170:802–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pellacani G, Pepe P, Casari A, Longo C. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: A longitudinal prospective study. Br J Dermatol 2014; 171. doi: 10.1111/bjd.13148. [DOI] [PubMed] [Google Scholar]
- 4.Stevenson AD, Mickan S, Mallett S, Ayya M. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept 2013; 3:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennàssar A, Vilata A, Puig S, Malvehy J. Ex vivo fluorescence confocal microscopy for fast evaluation of tumour margins during Mohs surgery. Br J Dermatol 2014; 170:360–5. [DOI] [PubMed] [Google Scholar]
- 6.Longo C, Ragazzi M, Rajadhyaksha M, et al. In Vivo and Ex Vivo Confocal Microscopy for Dermatologic and Mohs Surgeons . Dermatol Clin: 2016; 34:497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajadhyaksha M, Menaker G, Flotte T, et al. Confocal examination of nonmelanoma cancers in thick skin excisions to potentially guide mohs micrographic surgery without frozen histopathology. J Invest Dermatol 2001; 117:1137–43. [DOI] [PubMed] [Google Scholar]
- 8.Karen JK, Gareau DS, Dusza, MPH, Tudisco M RM. Detection of basal cell carcinomas in Mohs excisions with fluorescence confocal misaiming microscopy. Br J Dermatol 2009 June ; 160(6) 1242–1250 2009; 160:1242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welzel J, Kästle R, Sattler EC. Fluorescence (Multiwave) Confocal Microscopy . Dermatol Clin: 2016; 34:527–33. [DOI] [PubMed] [Google Scholar]
- 10.Bennàssar A, Carrera C, Puig S, et al. Fast Evaluation of 69 Basal Cell Carcinomas With Ex Vivo Fluorescence Confocal Microscopy: Criteria Description, Histopathological Correlation, and Interobserver Agreement. JAMA Dermatol 2013; 149:1–8. [DOI] [PubMed] [Google Scholar]
- 11.Nori S, Rius-Díaz F, Cuevas J, et al. Sensitivity and specificity of reflectance-mode confocal microscopy for in vivo diagnosis of basal cell carcinoma: A multicenter study. J Am Acad Dermatol 2004; 51:923–30. [DOI] [PubMed] [Google Scholar]
- 12.Segura S, Puig S, Carrera C, et al. Dendritic cells in pigmented basal cell carcinoma: a relevant finding by reflectance-mode confocal microscopy. Arch Dermatol 2007; 143:883–6. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez S, Tannous Z. Real-time, in vivo confocal reflectance microscopy of basal cell carcinoma. J Am Acad Dermatol 2002; 47:869–74. [DOI] [PubMed] [Google Scholar]
- 14.Tannous Z, Torres A, Gonzalez S. In Vivo Real-Time Confocal Reflectance Microscopy:A Noninvasive Guide for Mohs Micrographic SurgeryFacilitated by Aluminum Chloride, an Excellent Contrast Enhancer. Dermatologic Surg 2003; 29:839–46. [DOI] [PubMed] [Google Scholar]
- 15.And YB, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc 1995; 57:289–300. [Google Scholar]
- 16.Benjamini Y, Yekutieli D, Edwards D, et al. False discovery rate-adjusted multiple confidence intervals for selected parameters. J Am Stat Assoc 2005; 100:71–93. [Google Scholar]
- 17.Alejandra V-M, Bennàssar A, Salvador G, et al. Application of in vivo reflectance confocal microscopy (RCM) and ex vivo fluorescence confocal microscopy (FCM) in most common subtypes of Basal Cell Carcinoma and correlation with histopathology. Br J Dermatol 2018; 6. doi: 10.1111/bjd.16421. [DOI] [PubMed] [Google Scholar]
- 18.Sierra H, Damanpour S, Hibler B, et al. Confocal imaging of carbon dioxide laser-ablated basal cell carcinomas: An ex-vivo study on the uptake of contrast agent and ablation parameters. Lasers Surg Med 2016; 48:133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sierra H, Larson B a, Chen C-SJ, Rajadhyaksha M. Confocal microscopy to guide erbium:yttrium aluminum garnet laser ablation of basal cell carcinoma: an ex vivo feasibility study. J Biomed Opt 2013; 18:095001.1–095001.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pérez-Anker J, Puig S, Malvehy J. July 2016 1. 2016. [Google Scholar]
- 21.Pérez-Anker J, Malvehy J, Moreno-Ramírez D. Microscopia confocal ex vivo con método de fusión y tinción digital: cambiando paradigmas en el diagnóstico histológico. Actas Dermosifiliogr. [DOI] [PubMed] [Google Scholar]
- 22.Benvenuto-Andrade C, Dusza SW, Agero ALC, et al. Differences between polarized light dermoscopy and immersion contact dermoscopy for the evaluation of skin lesions. Arch Dermatol 2007; 143:329–338vanc. [DOI] [PubMed] [Google Scholar]
- 23.Liebman TN, Jaimes-Lopez N, Balagula Y, et al. Dermoscopic features of basal cell carcinomas: Differences in appearance under non-polarized and polarized light. Dermatologic Surg 2012; 38:392–9. [DOI] [PubMed] [Google Scholar]
- 24.Karen JK, Gareau DS, Dusza SW, et al. Detection of basal cell carcinomas in Mohs excisions with fluorescence confocal mosaicing microscopy. Br J Dermatol 2009; 160:1242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bini J, Spain J, Nehal K, et al. Confocal mosaicing microscopy of human skin ex vivo: spectral analysis for digital staining to simulate histology-like appearance. J Biomed Opt 2011; 16:076008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yélamos O, Pérez-Anker J. Avances en el manejo del cáncer cutáneo : videomosaicos y microscopía confocal de fusión. Rev Chil dermatología 2018; 34:6–8. [Google Scholar]
- 27.Galletly NP, McGinty J, Dunsby C, et al. Fluorescence lifetime imaging distinguishes basal cell carcinoma from surrounding uninvolved skin. Br J Dermatol 2008; 159:152–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.