Abstract
Lassa fever is a zoonotic disease endemic in some West African countries. It is exported to countries in America, Asia, and Europe. Antivirals against Lassa fever are important to provide a cure in patients with the disease and provide protection against it. In addition, due to the potential utilization of Lassa virus as a bioterrorism agent, vaccines against the disease can be utilized as a counterterrorism measure. Developing antiviral compounds and vaccines against the disease requires understanding of the pathogenesis of Lassa fever and its disease course, including the signs, symptoms, complications, and sequelae. An important sequela of Lassa fever is ataxia. A few cases of postviral ataxia following Lassa fever have been described in the literature. This review focuses on highlighting these cases, the gaps in scientific knowledge where further research is needed, and possible ways of diagnosing postviral ataxia after Lassa fever in resource-limited settings.
Keywords: Lassa fever, ataxia, sequelae of Lassa fever, neurodegenerative disorder, postviral, ataxia
Lassa fever (LF) is an acute human viral disease [1]. Its etiologic agent is Lassa virus (LASV), an enveloped, single-stranded, bisegmented negative-strand RNA arenavirus belonging to the family Arenaviridae [2, 3]. It has a lipid-containing envelope and is spherical, with a diameter of 70–150 µm [4]. Its natural reservoir is commonly Mastomys natalensis, a rodent species indigenous to West Africa [5, 6]. A study involving wild rodents trapped in Nigeria from 2011–2015 by Olayemi et al. demonstrated a higher frequency of LASV or arenavirus infection present in the Natal multimammate mouse—Mastomys natalensis—and the Guinea multimammate mouse—Mastomys erythroleucus—than in Praomys daltoni, Mus boaulei, Rattus rattus, Crocidura spp., Mus minutoides, and Praomys misonnei [7]. The first known human case of LF was reported in 1969, when a missionary nurse stationed in the Nigerian village of Lassa in the state of Borno became ill. She was transferred to the Evangel Hospital (now Bingham University Teaching Hospital) in Jos Plateau State, Nigeria, where other health care workers became infected, leading to the first recorded nosocomial spread of the disease [8]. LF can also be laboratory-acquired [9] and can spread from person to person in community settings. The virus is isolated from serum, pleural fluid, urine, throat wash [1, 4] and cerebrospinal fluid (CSF) [10]. LF is endemic in the West African countries of Sierra Leone, Liberia, Guinea, and Nigeria and is reported in Mali, Ghana, Cote d’Ivoire, Burkina Faso, Togo, Benin, and Central African Republic. Cases have been exported to Sweden, United Kingdom, Canada, United States, Germany, Netherlands, Japan, and Israel. Though reports estimate that ~500 000 LF cases occur annually, with ~5000 fatalities, outbreaks of the disease reported between 1969 and 1972 resulted in a total of 101 cases [5]. Over 4 decades later, in 2018, the most significant LF outbreak in Nigeria, described as unprecedented, occurred in 633 laboratory reverse transcription quantitative polymerase chain reaction–confirmed cases out of 3498 suspected cases, with 171 deaths from January 1, 2018, to December 31, 2018 [11]. LF is an important public health concern because of its incidence, virulence, the consequences of uncontrolled disease spread, and its potential as a bioterrorism agent. It is therefore critically important to understand the clinical manifestations (including the signs, complications, and sequelae) and viral pathogenesis of the disease in order to develop effective drugs for treatment of cases and vaccines for the prevention and control of the disease. This review focuses on ataxia as a sequela of LF.
ATAXIA
Ataxia is a degenerative manifestation marked by abnormalities in movement, affecting gait, coordination, and fine motor skills. It results in tremors, involuntary eye movements (nystagmus), dizziness, blurred vision, slurred speech (dysarthria), and difficulty swallowing (dysphagia) [12]. Some patients with ataxia demonstrate a lack of proprioception. Ataxia can be identified as a nervous system degenerative disorder (nonhereditary), or it can be inherited or acquired due to damage to the cerebellum—the brain region that controls muscle coordination—or cerebellar pathways. Damage to the cerebellum can result from chronic alcoholism, stroke, tumors, malaria, typhoid fever, other infectious diseases, hypothyroidism, multiple sclerosis, head trauma, bleeding in the cerebellum, exposure to toxins, and deficiency of vitamin B-1, B-12, or E [13]. Moreover, cerebellar damage is also reported to be immune-mediated by antibodies in patients with tumors, including lung, breast, ovarian, and gynecological cancers, digestive tract adenocarcinomas, malignant thymoma, and Hodgkin’s lymphoma [12, 13]. Similarly, patients with insulin-dependent diabetes mellitus may develop antibody-mediated ataxia [12–14]. Some types of immune-mediated ataxia are associated with long-term sequelae and poor prognoses [12].
Acute cerebellar ataxia (ACA) that accounts for ~40% of ataxias in children [14, 15]. ACA in children has been extensively reported, with the most common cause being varicella-zoster virus (VZV) [13]. Children are reported to make a full recovery following ACA in most cases [14]. Although postinfectious ataxia in adults is not reported as frequently as in children, it does occur [12]. The most common cause of postviral ataxia in adults is infection with Epstein-Barr virus (EBV) [13]. Some other viral agents linked to ataxia include herpes simplex virus (HSV), enterovirus, influenza A and B viruses, mumps virus, measles virus, hepatitis A virus, coxsackievirus, West Nile Virus (WNV), and cytomegalovirus [12, 15]. Postinfectious ataxia is described after a number of bacterial infections, such as tabetic neurosyphilis, Lyme borreliosis, and Whipple’s disease [13]. Postinfectious ataxia in association with HIV infection is thought to be a result of opportunistic infections and not to be caused by direct damage to the cerebellum by HIV in most cases [13].
Regarding postviral ataxia, a distinction is made between cerebellar ataxia caused by acute viral infection, which has a more favorable prognosis, and cerebellar ataxia caused by chronic viral infection, which can lead to progressive ataxia with a direr prognosis [13].
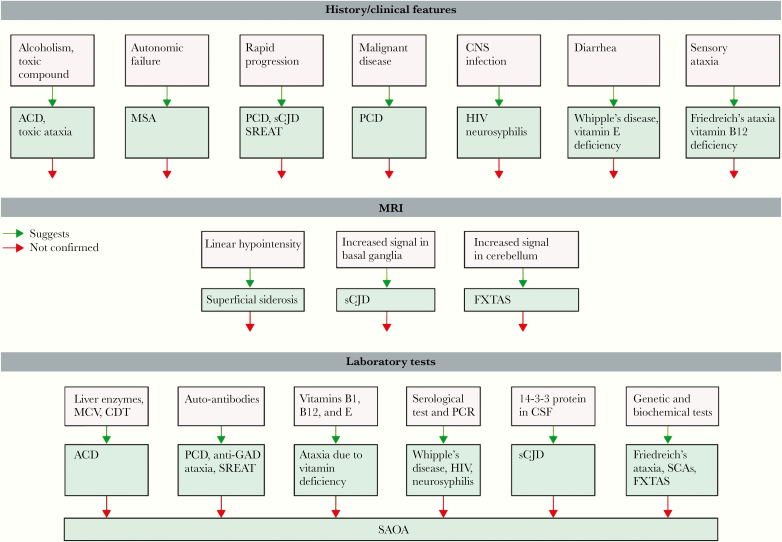
Ataxia is evaluated by physical examination, review of the medical history, computerized tomography (CT), magnetic resonance imaging (MRI), biochemical or molecular tests, or genetic testing (in cases of hereditary ataxia). Though the diagnosis of ataxia can be challenging, Klockgether’s chart can be used as a guide to accurately diagnose and classify ataxia (Figure 1). Further diagnostic guidelines have been detailed by Klockgether [13].
Figure 1.
Diagnostic approach to sporadic adult-onset ataxia3. History and 3clinical features3 are often highly suggestive of a specific diagnosis. Similarly, typical 3MRI3 features might suggest a particular diagnosis. If the suspected diagnosis is not confirmed by appropriate 3laboratory tests3, wider laboratory screening is required, which will eventually lead to the final specific diagnosis. If all tests are negative, then a diagnosis of SAOA is made by exclusion. Abbreviations: ACD, alcoholic 3cerebellar degeneration3; CDT, carbonyl-deficient 3transferrin3; FXTAS, fragile-X-associated tremor/ataxia syndrome; GAD, glutamic acid decarboxylase; MCV, mean corpuscular volume; MSA, multiple system atrophy; PCD, paraneoplastic cerebellar degeneration; SAOA, sporadic adult-onset 3ataxia3 of unknown etiology; SCA, spinocerebellar ataxia; sCJD, sporadic Creutzfeldt-Jakob disease; SREAT, steroid-responsive 3encephalopathy3 associated with 3autoimmune thyroiditis3 [13].
ATAXIA IN LASSA FEVER
Multiorgan involvement is reported in LF, leading to complications including gastrointestinal symptoms, pleuritic chest pain, bleeding, acute kidney injury, convulsion, shock, and coma [10, 16–18]. In addition, central nervous system (CNS) involvement is well documented in LF, with complications including seizures, labile hypotension, encephalitis, encephalopathy, aseptic meningitis, vertigo, unilateral or bilateral sensorineural hearing loss, and ataxia (less frequently) [10, 16, 17, 19–21]. Furthermore, LASV was isolated from CSF in a patient whose serum had no circulating detectable LASV [10]. This implies that LASV has the ability to infect cells of the CNS and replicate even in the absence of detected viremia.
The ability of viral agents to cross the blood–brain barrier (BBB) and the blood–cerebrospinal fluid barrier (BCSFB) and cause damage to the CNS is well documented [22, 23], and viruses utilize a variety of mechanisms. One mechanism includes invading BBB endothelial cells and astrocytic glial cells by direct infection (either through receptor-mediated mechanisms or endocytosis/colloidal transport) [24, 25]. In addition to direct invasion of brain endothelial cells, viral proteins can trigger host cell signaling pathways, thereby altering the permeability of the BBB by weakening the tight junctions that hold the endothelial cells together [24, 26] or they can enter through infecting leukocytes that pass through the BBB (also known as the Trojan horse mechanism) [22, 24, 26, 27]. Lymphocytic choriomeningitis virus (LCMV), WNV, herpes simplex virus–1 (HSV), coxsackievirus B3 (CVB3), measles virus, and poliovirus are reported to use this route of entry [23, 24].
Another mechanism involves exploiting the vulnerabilities of the CNS to evade the BBB and/or BCSFB to establish neuroinvasion [22, 27]. A commonly exploited area of vulnerability in the CNS is the nerves and ganglia in the peripheral nervous system that connect the CNS to peripheral tissue, including cranial nerves [27]. Viruses such as rabies virus, HSV, reovirus, enterovirus 71, Theiler’s murine encephalomyelitis virus, adenovirus, and Bornavirus utilize this pathway to travel from peripheral tissue to the CNS, circumventing the BBB and BCSFB by axoplasmic transport [23]. LASV causes damage to the vestibulocochlear nerve, leading to hearing loss. In an in vivo experiment performed by Yun et al., LASV was detected in the spiral ganglion in both signal transducer– and activator of transcription 1 (STAT1)–deficient mice and interferon alpha/beta/gamma receptor (IFN α/β/γR)–deficient mice. LASV infection of the auditory nerve resulted in damage to the spiral ganglion in the STAT1-deficient mice but not in the IFN α/β/γR–deficient mice [28]. This may indicate cranial nerve involvement in LASV neuroinvasion.
The BBB is made up of brain capillary endothelial cells, mural cells, the tight and adherens junctions that hold the endothelial cells together, and the foot processes of astrocytic glial cells [22, 27]. These form a barrier that regulates and protects the brain parenchyma from infiltrating substances and microbes. The BCSFB is made up of epithelial–endothelial cells and tight junctions [29, 30]. The BCSFB–choroid plexus interface is prone to neuroinvasion by microorganisms because the endothelial cells are fenestrated, unlike the endothelial cells in the BBB that lack fenestration. Moreover, the tight junctions in the BCSFB possess a lower electrical resistance than in the BBB that makes them more vulnerable to invasion than the BBB [27, 29]. LCMV and mumps virus invade the cerebrospinal fluid through the BCSFB [30]. Though the arenavirus LCMV has been shown to invade the cerebrospinal fluid through the BCSFB, it is unclear what mechanism LASV utilizes to invade the CSF.
Furthermore, viral factors (strain, receptor affinity, mutations) in conjunction with host factors (age, gender, genetic conditions, immune status) affect the neuroinvasiveness and neurovirulence of different viruses [25, 31].
The mechanisms of LASV neuroinvasion remain unclear, but Cassady and Whitley propose that LASV affects the function of the endothelium and propose the choroid plexus as a CNS region that promotes viral neurotropism [25]. In the arenavirus prototype LCMV, a CD8+ T-cell-dependent mechanism of BBB disruption is proposed [24, 32].
After viral neuroinvasion, damage to CNS structures could result from direct viral infection, immune-mediated injury, or a combination of both [22, 28]. Though there are several possible mechanisms of damage to the CNS, the mechanism and pathogenesis of postviral ataxia, which can occur after viral infection, are not completely understood [33]. Further studies are needed to elucidate the mechanisms of neuroinvasion utilized by LASV.
Clinical cases of ataxia linked to LF have been documented. Fisher-Hoch et al. reported a case of a surgical nurse and a student nurse who washed blood-soaked materials (clothes, instruments, and gloves) from a patient who died after developing a febrile illness during an LF outbreak driven by nosocomial spread in a hospital in Imo State, Nigeria. Both the nurse and student developed febrile illnesses within 10 days of the surgery. Their sera were positive for LASV-specific IgG and IgM antibodies. About 3 weeks after onset of febrile illness, the surgical nurse had developed deafness and was severely ataxic [34]. No further follow-up of this patient was reported regarding the persistence of the ataxia or her survival.
In a study involving 32 patients with LF by Solbrig et al. in Sierra Leone, 2 patients developed convalescent-phase ataxia. A 9-year-old febrile male patient, whose exposure to LASV was confirmed by serological methods, was placed on antiviral ribavirin for 10 days. On day 8 of hospitalization, he developed sudden-onset ataxia marked by difficulty sitting, truncal ataxia, poor tandem walk, and vertical nystagmus [35]. The second patient, a 20-year-old female whose exposure to LASV was confirmed by serological methods, developed convalescent-phase ataxia while she was afebrile and hospitalized. Ataxia in this patient was of sudden onset and was characterized by truncal and right-sided ataxia. Her CSF contained anti–Lassa virus IgG, demonstrated by indirect immunofluorescence antibody assay. She still had ataxia at the time of her discharge [35]. No further follow-up was reported to determine whether ataxia persisted in this patient.
Macher et al. reported a case of an aid worker who was infected with Lassa virus in Sierra Leone in 1975. Neurological examinations conducted in a US hospital in 1976 showed a right-sided Babinski reflex, indicating possible damage to the corticospinal tract that transmits motor information from the motor cortex to the spinal cord. The patient also had vertigo, hypotension, and left-sided facial weakness. Thirty years after the onset of neurological manifestations, the patient still suffered headache, dizziness, and staggering movements, symptoms and signs of ataxia [20].
Though ataxia can be diagnosed by simple neurologic examination, postviral ataxia resulting from LF might be underreported as a result of the poor health care–seeking behavior of patients in endemic countries in West Africa, inadequate follow-up of patients discharged from hospitals, and prohibitive cost of health care when it is available.
DIAGNOSTIC CHALLENGES OF POSTVIRAL ATAXIA IN RESOURCE-LIMITED SETTINGS
Diagnosis of postviral ataxia in endemic West African countries such as Nigeria is met with multifactorial challenges that affect the delivery of accessible and affordable health care services [36]. Some of these challenges include the absence of a system to keep track of a patient’s medical history, creating disconnections in a patient’s medical story. These challenges also include limited availability of diagnostic equipment and expensive cost of delivery for relevant health care services. The absence of effective social support systems further limits patients’ ability to access and utilize already inadequate health care services [37, 38]. The health care system is further weakened by limited operation of a national health insurance scheme [37, 39] with a burdensome effect on patients in the form of out-of-pocket payments for health care services [40] that are prohibitive.
CONCLUSIONS
Postviral ataxia has been described in the literature following LF. To better identify and manage patients with postviral ataxia related to LF, hospitals in endemic countries might consider adopting or modifying the diagnostic and classification recommendations proposed by Klockgether (Figure 1). In addition, hospitals might consider creating and maintaining registries for patients who recover from LF as well as regular follow-ups with them in order to document any disease conditions that arise following LF, including ataxia. Further research is needed to understand the mechanism, prevalence, and incidence of postviral ataxia in patients who recover from LF in endemic regions in West Africa.
Acknowledgments
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.s
References
- 1. Johnson KM, McCormick JB, Webb PA, et al. Clinical virology of Lassa fever in hospitalized patients. J Infect Dis 1987; 155:456–64. [DOI] [PubMed] [Google Scholar]
- 2. Speir RW, Wood O, Liebhaber H, Buckley SM. Lassa fever, a new virus disease of man from West Africa. IV. Electron microscopy of Vero cell cultures infected with Lassa virus. Am J Trop Med Hyg 1970; 19:692–4. [DOI] [PubMed] [Google Scholar]
- 3. Rowe WP, Murphy FA, Bergold GH, et al. Arenoviruses: proposed name for a newly defined virus group. J Virol 1970; 5:651–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buckley SM, Casals J. Lassa fever, a new virus disease of man from West Africa. 3. Isolation and characterization of the virus. Am J Trop Med Hyg 1970; 19:680–91. [DOI] [PubMed] [Google Scholar]
- 5. Monath TP, Newhouse VF, Kemp GE, et al. Lassa virus isolation from Mastomys natalensis rodents during an epidemic in Sierra Leone. Science 1974; 185:263–5. [DOI] [PubMed] [Google Scholar]
- 6. Murphy F, Walker D. Arenaviruses: persistent infection and viral survival in reservoir hosts. In: Kurstak E, Maramoroch K, eds. Viruses and environment. New York: Academic Press; 1978:155–80. [Google Scholar]
- 7. Olayemi A, Oyeyiola A, Obadare A, et al. et al. Widespread arenavirus occurrence and seroprevalence in small mammals, Nigeria. Parasit Vectors 2018; 11:416–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frame JD, Baldwin JM Jr, Gocke DJ, Troup JM. Lassa fever, a new virus disease of man from West Africa. I. Clinical description and pathological findings. Am J Trop Med Hyg 1970; 19:670–6. [DOI] [PubMed] [Google Scholar]
- 9. Leifer E, Gocke DJ, Bourne H. Lassa fever, a new virus disease of man from West Africa. II. Report of a laboratory-acquired infection treated with plasma from a person recently recovered from the disease. Am J Trop Med Hyg 1970; 19:677–9. [DOI] [PubMed] [Google Scholar]
- 10. Günther S, Weisner B, Roth A, et al. Lassa fever encephalopathy: Lassa virus in cerebrospinal fluid but not in serum. J Infect Dis 2001; 184:345–9. [DOI] [PubMed] [Google Scholar]
- 11. NCDC. 2018 Lassa fever outbreak in Nigeria. Situation report. 2018. Available at: 3Uhttps://www.ncdc.gov.ng/diseases/sitreps/?cat=5&name=An%20update%20of%20Lassa%20fever%20outbreak%20in%20NigeriaU3. Accessed 9 August 2019. [Google Scholar]
- 12. Joubert B, Rostásy K, Honnorat J. Immune-mediated ataxias. Handb Clin Neurol 2018; 155:313–32. [DOI] [PubMed] [Google Scholar]
- 13. Klockgether T. Sporadic ataxia with adult onset: classification and diagnostic criteria. Lancet Neurol 2010; 9:94–104. [DOI] [PubMed] [Google Scholar]
- 14. Ryan MM, Engle EC. Acute ataxia in childhood. J Child Neurol 2003; 18:309–16. [DOI] [PubMed] [Google Scholar]
- 15. van der Maas NA, Bondt PE, de Melker H, Kemmeren JM. Acute cerebellar ataxia in the Netherlands: a study on the association with vaccinations and varicella zoster infection. Vaccine 2009; 27:1970–3. [DOI] [PubMed] [Google Scholar]
- 16. Grahn A, Bråve A, Lagging M, et al. Imported case of Lassa fever in Sweden with encephalopathy and sensorineural hearing deficit. Open Forum Infect Dis 2016; 3(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Okokhere PO, Bankole IA, Iruolagbe CO, et al. Aseptic meningitis caused by Lassa virus: case series report. Case Rep Neurol Med 2016; 2016:684–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okokhere P, Colubri A, Azubike C, et al. Clinical and laboratory predictors of Lassa fever outcome in a dedicated treatment facility in Nigeria: a retrospective, observational cohort study. Lancet Infect Dis 2018; 18:684–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cummins D, Bennett D, Fisher-Hoch SP, et al. Lassa fever encephalopathy: clinical and laboratory findings. J Trop Med Hyg 1992; 95:197–201. [PubMed] [Google Scholar]
- 20. Macher AM, Wolfe MS. Historical Lassa fever reports and 30-year clinical update. Emerg Infect Dis 2006; 12:835–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Solbrig M. Lassa virus and central nervous system diseases. In: SMS, ed. The Arenaviridae. Boston, MA: Springer; 1993:325–9. [Google Scholar]
- 22. McGavern DB, Kang SS. Illuminating viral infections in the nervous system. Nat Rev Immunol 2011; 11:318–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salinas S, Schiavo G, Kremer EJ. A hitchhiker’s guide to the nervous system: the complex journey of viruses and toxins. Nat Rev Microbiol 2010; 8:645–55. [DOI] [PubMed] [Google Scholar]
- 24. Spindler KR, Hsu TH. Viral disruption of the blood-brain barrier. Trends Microbiol 2012; 20:282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cassady K, Whitley R. Pathogenesis and pathophysiology of viral infections of the central nervous system. In: Scheld M, Whitley R, Marra C, eds. Infections of the central nervous system. Philadelphia, PA: Lippincott Williams & Wilkins; 2014; 49–64. [Google Scholar]
- 26. Dahm T, Rudolph H, Schwerk C, et al. Neuroinvasion and inflammation in viral central nervous system infections. Mediators Inflamm 2016; 2016:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dando SJ, Mackay-Sim A, Norton R, et al. Pathogens penetrating the central nervous system: infection pathways and the cellular and molecular mechanisms of invasion. Clin Microbiol Rev 2014; 27:691–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yun NE, Ronca S, Tamura A, et al. Animal model of sensorineural hearing loss associated with Lassa virus infection. J Virol 2015; 90:2920–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wolburg H, Paulus W. Choroid plexus: biology and pathology. Acta Neuropathol 2010; 119:75–88. [DOI] [PubMed] [Google Scholar]
- 30. Strazielle N, Ghersi-Egea JF. Choroid plexus in the central nervous system: biology and physiopathology. J Neuropathol Exp Neurol 2000; 59:561–74. [DOI] [PubMed] [Google Scholar]
- 31. David S. Neuroinflammation: New Insights Into Beneficial and Detrimental Functions. Hoboken, NJ: John Wiley & Sons Inc.; 2014. [Google Scholar]
- 32. Kang SS, McGavern DB. Microbial induction of vascular pathology in the CNS. J Neuroimmune Pharmacol 2010; 5:370–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abele M, Bürk K, Schöls L, et al. The aetiology of sporadic adult-onset ataxia. Brain 2002; 125:961–8. [DOI] [PubMed] [Google Scholar]
- 34. Fisher-Hoch SP, Tomori O, Nasidi A, et al. Review of cases of nosocomial Lassa fever in Nigeria: the high price of poor medical practice. BMJ 1995; 311:857–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Solbrig M, McCormick J. Lassa fever: central nervous system manifestations. J Trop Geogr Neurol 1991; 1:23–30. [Google Scholar]
- 36. Singh P, Sachs JD. 1 million community health workers in sub-Saharan Africa by 2015. Lancet 2013; 382:363–5. [DOI] [PubMed] [Google Scholar]
- 37. Fatusi O, Akinpelu O, Amusa Y. Challenges of managing nasopharyngeal carcinoma in a developing country. J Natl Med Assoc 2006; 98:758–64. [PMC free article] [PubMed] [Google Scholar]
- 38. Adeloye D, David RA, Olaogun AA, et al. Health workforce and governance: the crisis in Nigeria. Hum Resour Health 2017; 15:32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Adegboyega O, Abioye K. Effects of health-care services and commodities cost on the patients at the primary health facilities in Zaria Metropolis, North Western Nigeria. Niger J Clin Pract 2017; 20:1027–35. [DOI] [PubMed] [Google Scholar]
- 40. Aregbeshola BS, Khan SM. Out-of-pocket payments, catastrophic health expenditure and poverty among households in Nigeria 2010. Int J Health Policy Manag 2018; 7:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]