Abstract
Chronic postoperative osteomyelitis represents an important health problem due to its significant morbidity and low mortality rate. This pathology is challenging because of difficulties in understanding the pathogenesis and the decision-making involving the treatment. The present article had the goal of reviewing the definition, pathogenesis, clinical aspects, diagnosis, and treatment of chronic postoperative osteomyelitis, and of gathering this information in a single Brazilian updated publication.
The PubMed, LILACS, and the Cochrane Library medical databases were analyzed using pertinent keywords. Current and relevant articles were selected.
The present article gathered the established information, as well as innovations related to chronic osteomyelitis and its treatment, to offer updated data to assist the professionals involved in the management of chronic osteomyelitis.
Keywords: osteomyelitis, postoperative complications, bacterial infections
Introduction
As a medical term, osteomyelitis has been present in the specialized literature since its description by Nelaton, in 1844, as an inflammatory process of infectious origin in the bones. However, the clinical manifestation as a secretory wound after injury has been mentioned throughout history since carved plates in Sumer. The treatment was based on keeping the wound open for the elimination of purulent discharge and the local application of ointments and other substances.
The advent of anesthesia and the expansion of surgical procedures, as well as the discovery of antibiotics, resulted in significant changes in the clinical and surgical treatments of osteomyelitis. 1
Chronic osteomyelitis is defined as an infectious disease sustained for more than a month. Its causes include an incorrectly-treated acute infectious process, and contiguous bone infection from chronic adjacent soft tissue infection.
Postoperative chronic osteomyelitis represents a major health problem due to its significant morbidity and low mortality rate. 2 3 This infection occurs in approximately 5 to 50% of open fractures and in less than 1% of osteosynthetic closed fractures; in addition, 5% result from an acute hematogenous spread. 3 The main problem associated with chronic bone infection is the ability of the organisms to stay in necrotic bone tissues with increased survival.
In short, this subject has been continuously revised and updated regarding the understanding of its pathogenesis, classifications and treatment options with the advent of new surgical techniques and drug innovations.
The present paper aims to review the definition, pathogenesis, clinical aspects, diagnosis, and treatment of chronic postoperative osteomyelitis, gathering all of this information in a single Brazilian updated publication.
Materials and Methods
In order to prepare this literature update article, data were collected by querying scientific papers in the PubMed, BVS-LILACS and Cochrane Library databases. Three keywords (MeSH terms) that were relevant to the proposed subject were selected: osteomyelitis , chronic and long bones .
In the PubMed database, the terms were searched in isolation and in combination ( osteomyelitis AND chronic ; osteomyelitis AND long bones ). The papers filtered for inclusion were mainly overviews and clinical practice guidelines. Studies on vertebral or pelvic chronic osteomyelitis related to joint prosthesis and hematogenous-only infection, or those involving only the pediatric population, were excluded.
In the BVS-LILACS database, the three terms were concurrently applied to the same search, and the same exclusion criteria were applied.
In the Cochrane Library, the term chronic osteomyelitis was used to retrieve systematic reviews.
Results
The PubMed database search found 75 papers with the first term combination ( osteomyelitis AND long bones ) and 587 papers with the second combination ( osteomyelitis AND chronic ) when applying the aforementioned inclusion and exclusion criteria. The BVS-LILACS database search resulted in 142 papers. The information used to prepare this review came from the retrieved material, in addition to their cited bibliographic sources through a subsequent direct search. In the Cochrane Library, only two systematic reviews of osteomyelitis were found.
Current papers, in addition to those considered most relevant and of high-quality, were selected to prepare this update.
Discussion
The medical knowledge gathered through the literature search on chronic osteomyelitis in the long bones can be didactically organized in the following topics: definition (as previously explained), classification, pathogenesis and aspects related to disease development, clinical manifestations, clinical diagnosis, armed propaedeutic and treatment.
Osteomyelitis classification
The evolution of the medical understanding on osteomyelitis has resulted in the proposition over time of several classification systems.
Historically, there are the etiological classifications of Kelly, Weiland and May; however, these systems are currently little used and disseminated. The two most widely employed classifications in the medical literature are those of Lew and Waldvogel 3 and of Cierny and Mader and Cierny et al. 5
Lew and Waldvogel 3 classify osteomyelitis as having three potential etiologies: hematogenous, contiguous and chronic.
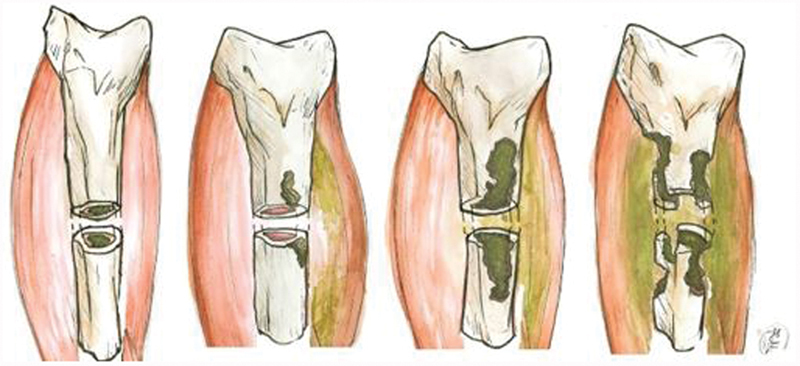
Cierny and Mader 4 and Cierny et al 5 considered the bone involvement pattern according to the etiology (types 1 to 4) ( Fig. 1 ) and the conditions of the host (types A, systemic B, local B, systemic and local B, C). This classification has the aim of guiding treatment decisions.
Fig. 1.
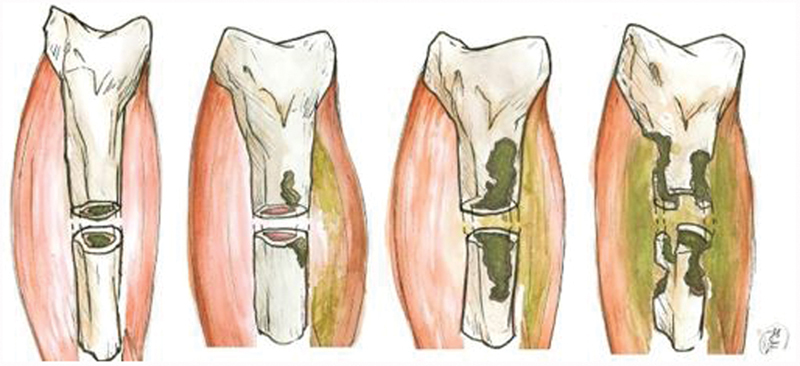
Cierny and Mader classification for osteomyelitis according to the pattern of bone involvement. Type 1, spinal cord; type 2, superficial; type 3, stable permeative; type 4, unstable permeative osteomyelitis.
Type 1–intramedullary lesion, usually due to intramedullary pinning.
Type 2–superficial lesion, usually due to pressure ulcer contiguity.
Type 3–stable permeative lesion, in which the infection penetrates the cortical layer and gains access to the medulla, but the bone remains biomechanically stable (that is, it supports load). This type is usually observed in the postoperative period in cases of infection after plaque osteosynthesis.
Type 4–unstable permeative lesion, in which the infection is extensive, affects the cortical and medullary layers, and the bone is biomechanically unstable. It may occur after aggressive infection or extensive debridement.
Host A – healthy patient and limbs.
Host B, systemic type – history of diabetes mellitus, senility, alcohol or drug use, immunodeficiencies.
Host B, local B type – previous local burn, scar, cellulitis, previous surgery, local vascular disease.
Host B, both systemic and local types – combined systemic and local involvement.
Host C – multiple comorbidities make the patient unable to tolerate the treatment. 4 5 6
Pathogeny and biofilm
The pathogenic understanding has also changed with the knowledge on bacteria behavior in biofilm formation. This knowledge enabled us to understand the phenomena of infection recurrence, antibiotic resistance, and the impact of surgical implantation on the infected site. 1 7
There are two forms of biofilm-forming bacteria: planktonic and fixed. In the planktonic (free) form, the bacteria are free outside the extracellular matrix, being isolated and vulnerable to host defense mechanisms. However, in high volumes, planktonic bacteria can migrate into the bloodstream, resulting in septicemia. Planktonic bacteria may adhere to a surface, such as necrotic tissue or foreign matter (surgical implant), becoming fixed. In the fixed (sessile) form, sessile bacteria usually form a polysaccharide biofilm on the tissue surfaces or implants.
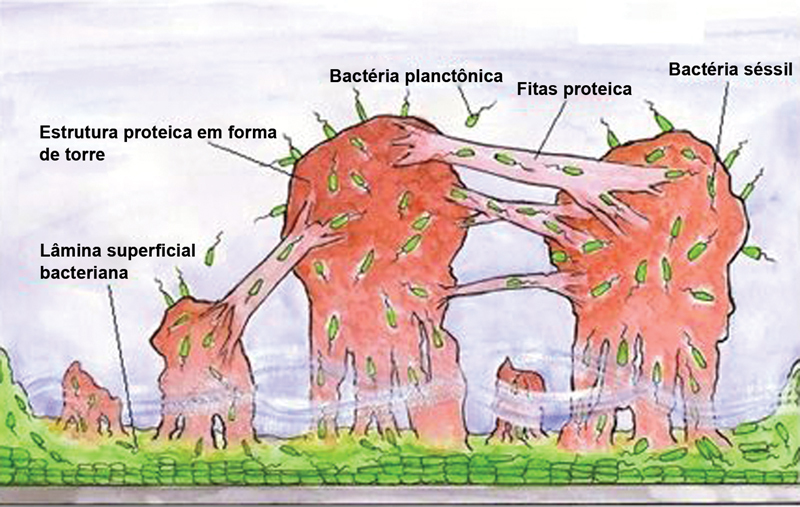
After colonization and biofilm formation, bacteria may remain inert or cause an infection. An infection in the presence of biofilm is more resistant to antibiotics. This is due to the fact that the antibiotic agent is not able to successfully pass through the glycocalyx (outer layer) of the biofilm (that is, there is a low-concentration gradient in the region occupied by bacteria). At the core of the colony, bacteria are in a low metabolic state, hindering the action of certain antibiotics. This may explain the greater antibiotic resistance in chronic infections (with more latent bacteria) compared to acute infections 7 8 ( Fig. 2 ).
Fig. 2.
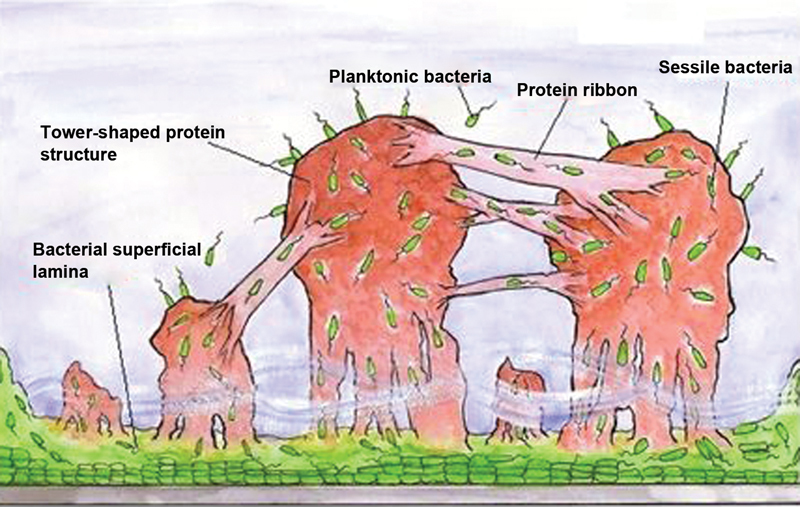
Structural model of bacterial biofilm. Note the presence of bacterial superficial lamina adhered to the metallic material, the protein tower structure filled with bacteria in transit, the fluid at the base of the towers, and protein strips enabling bacterial transit. Planktonic bacteria are present around the biofilm.
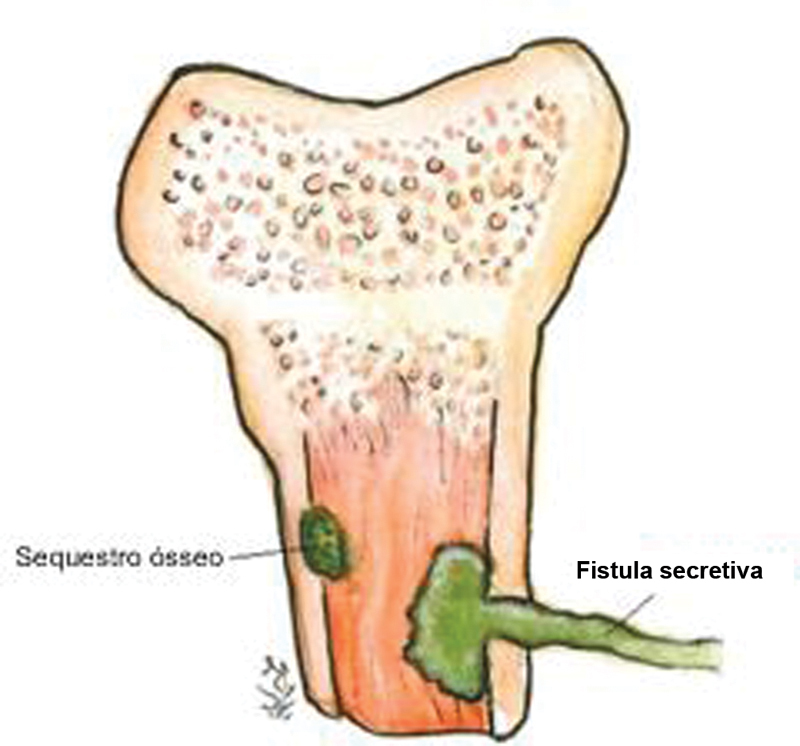
The interaction between the colony, the host tissue and the immunological response may lead to the formation of encapsulated necrotic bone, which may also be colonized, resulting in bone sequestration. 7 This collection can protrude, forming a sinus tract up to the skin, leading to a fistula 9 ( Fig. 3 ).
Fig. 3.
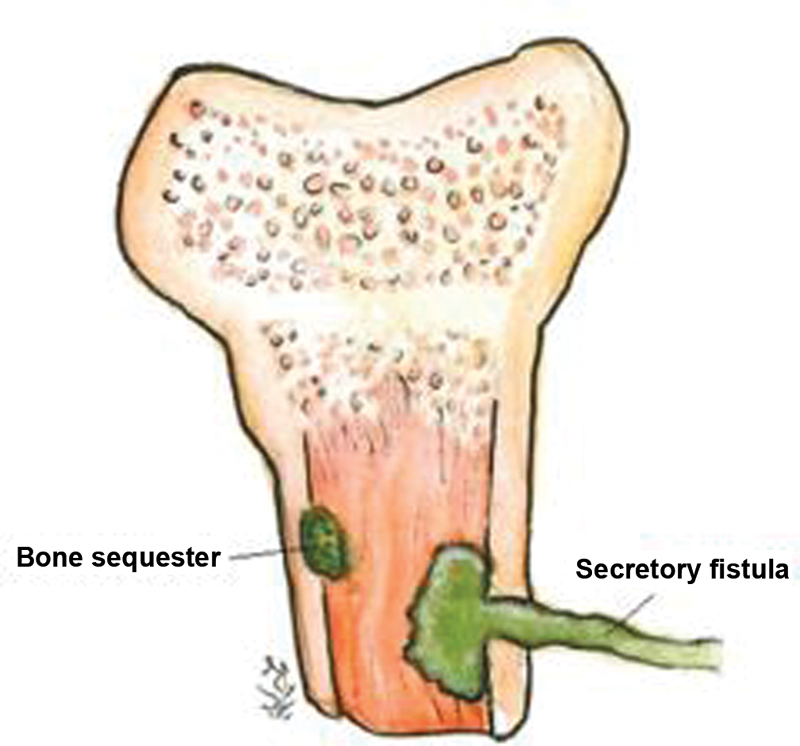
Interaction phenomena between the infectious tissue and the host result in bone sequestration and fistula formation.
Fractures and infection
Fracture-related osteomyelitis usually occurs in cases of bone exposure or after surgical treatment (with or without implant placement). In open fractures, contamination is certain. The determinants associated with the evolution from contamination to infection include the immune response of the host, the ability of the mechanical cleanliness to lower the local bacterial concentration, and debridement to leave healthy and viable wound tissue less susceptible to bacterial adherence.
Microbiology
The bacteria usually identified in acute exposed wounds are not the ones that will cause chronic infection. The most aggressive hospital pathogens gain importance in these open fracture cases, as they can contaminate and cause infection within days of the accident, even after the proper initial treatment with cleaning and debridement. 3
Chronic osteomyelitis, usually due to an incorrectly-treated acute infection or a postsurgery infection, may be caused by a pathogen from the typical hospital flora ( Pseudomonas aeruginosa , other Gram-negative organisms, Staphylococcus aureus ), or have a polybacterial origin. 8
Fungal osteomyelitis is more common in immunocompromised or diabetic patients, or those with indwelling catheters. Dissemination can be hematogenous or contiguous, and the most common agent is Candida sp . 1 3
Evaluation and diagnosis
Patient assessment begins with a detailed anamnesis for the evaluation of the clinical history. Information such as previous focal or systemic infections may raise suspicion of infectious spread to a particular site, whether it is a recent or remote event. History of previous trauma leading to local skin or soft tissue complication is also relevant. The occurrence of fracture and its characteristics, such as degree of exposure and performed treatment (surgery for local cleaning, fracture fixation, debridement, presence of an implant or foreign body) are fundamental during the initial approach.
Specific manifestations may include deep local (bone) pain, heat sensation, edema and skin rash, as well as general symptoms, such as inappetence and fever. Purulent secretory surgical wounds or formation of cutaneous fistulas are also very suggestive findings at inspection. 7
The relevant laboratory tests include complete blood counts (CBCs), since leukocytosis is the main suggestive marker in acute infections. In chronic osteomyelitis, however, white blood cell (WBC) counts may be normal. Inflammatory markers, such as erythrocyte sedimentation rate (ESR) and serum C-reactive protein (CRP), increase in the acute phase of the infection and after surgical manipulation; CRP and ESR levels peak on the third and fifth days after manipulation respectively. C-reactive protein levels return to baseline within three weeks, while ESR normalization takes longer. A new peak in CRP three days after surgical manipulation or the beginning of the antibiotic therapy is suggestive of reinfection or treatment failure. Normal values on both tests are excellent predictors of absence of osteomyelitis. These tests are useful during the follow-up of osteomyelitis through serial analysis. The CRP is the first marker to return to normal values in response to a successful treatment. 7 10 11
Blood culture is not very useful because its results are negative even in the presence of osteomyelitis, especially when there is no septicemia. 1
The radiological findings are usually normal in the acute phase of the disease, especially during the first two weeks of hematogenous osteomyelitis. The presence of fracture, bone callus or surgical implant may mask specific infectious findings. In the late phase of chronic osteomyelitis, the findings may be atypically well-localized local bone rarefaction or lytic lesions (arising after the destruction of 50 to 75% of the bone matrix). Other abnormalities include formation of visible bone sequestration, bone sclerosis, neoformation and cortical thickening, and periosteal reaction. 10 12
Bone scintigraphy with technetium-99 or indium-labeled red blood cells or gallium-67-labeled WBCs or with bone marrow activity markers have been a useful resource for osteomyelitis screening and early diagnosis. These bone scans highlight areas of inflammatory activity; however, there is no consensus on which marker would be the most sensitive for the early detection of osteomyelitis (screening and increased sensitivity). 10 13 14
Gallium-67 scintigraphy highlights areas of WBC concentration, usually infection sites, but also tumors. The gallium-67 examination is best suited for osteomyelitis complications, except for the spine. 13 15 Bone marrow activity can be assessed indirectly by local labeling with technetium-99m colloid in a three-phase examination.
Other markers have been proposed to offer more specific tests for chronic osteomyelitis diagnosis and follow-up, including in biotin for vertebral infections. Radio-labelled ubiquicidin fragments show promise in enhancing bacteria-infected tissues. 14
Computed tomography (CT) may help establish the extension of the bone fragments to the soft tissues, and provide a better characterization of bone sequestration, besides offering detailed images of the cortical bone layer, enabling the evaluation of axial stability.
Magnetic resonance imaging (MRI) enables a detailed study of the extent of the infection, including the soft tissues. The affected areas are often highlighted in T2-weighted hypersignal, such as muscle or subcutaneous tissue secretory collection and bone edema, which characterize an increased inflammatory activity at this site. The MRI enables the earlier detection of acute changes when simple segmental radiographs are normal. In chronic infections, periosteal reactions can be more accurately verified by visualizing lamellar thickening (“onion skin” formation) or, if this process is stopped, it leads to the formation of a “Codman triangle”. 10 The test may suffer interference from surgical manipulation and present artifacts due to the presence of metallic implants. 1 10
The positron emission tomography (PET) scan with the 2-[18F]-fluoro-2-deoxy-D-glucose (FDG) marker is another promising imaging modality, with high sensitivity (approximately 95%) and specificity (75-99%). This method is limited by its low availability and high cost. 15
In many cases, culture from wound secretion, open fracture and fistula material do not agree with the etiological agent of the osteomyelitis. There is no consensus in the literature if this is a relevant diagnostic method. Treatment based on “wrong” bacteria (identified through culture) can harm the patient, resulting in ineffective antibiotic use, which stimulates the development of resistance. 9 10
Affected bone biopsy is the preferred method, especially in chronic osteomyelitis, in which pathogen isolation in the blood is very unlikely. 10 Another diagnostic method is the sonication of surgical implants (including prostheses) removed from infected sites. The material is submitted to preparation and application of ultrasonic waves that structurally break the bacterial biofilm, enabling the molecular identification of the infectious agent. 16 17 Other microbiology techniques, such as polymerase chain reaction, are also used, but mainly restricted to the academic and research settings. 10
Treatment
Chronic osteomyelitis treatment must be multiphasic, involving three combined strategies: 1) clinical stabilization; 2) antibiotic therapy; and 3) surgical approach.
-
Clinical stabilization
The first step after diagnosis is the improvement of the clinical conditions of the patient, aiming at the control of systemic diseases such as diabetes, malnutrition, immunosuppression and vascular disease.
Antibiotic therapy
The antibiotic agent to be used must be inexpensive, convenient in terms of administration and dosage, and offer high serum and bone tissue concentration. 2 8
Antibiotics can be used in three ways: systemic, either as prophylaxis or treatment; in an irrigation solution for surgical cleaning; and in a device introduced during the surgical procedure.
Systemic antibiotic therapy
Since systemic antibiotic therapy in open fractures is based on empirical recommendations, studies supporting specific drug classes and treatment duration are required. The literature supports the use of cephalosporin in low contamination fractures and its association with aminoglycosides in more contaminated lesions presenting soft-tissue injury and higher energy trauma. The duration of the treatment is controversial: antibiotics are usually used for one to three days, and extended use is reserved for cases with signs of infection at wound inspection. 10
In a systematic review of the literature, there is no consensus on the use of systemic antibiotics to treat a chronic infection regarding therapy duration and medication choice. 18 Two- to six-week regimen schedules are recommended to enable local wound improvement and revascularization. However, according to other authors, there are guidelines to extend the treatment time to several months. 1 8
There are many options of drugs, but there are recommendations based on clinical observations, efficacy studies, accumulated clinical experience, and outcome analyses. Some options have already been established among experts, and are guided by protocols that are widespread in the scientific environment, such as the one developed by Lima and Zumiotti, 2 in Brazil, and the 2014 Korean Society for Chemotherapy antibiotic therapy protocol for bone and joint infections 19 ( Table 1 ).
Table 1. Main bacterial etiological agents in osteomyelitis and respective antibiotics for treatment.
| Organism | First-line antibiotic agent | Optional antibiotic agents |
|---|---|---|
| Staphylococcus aureus or methicillin-sensitive, coagulase-negative staphylococci | Oxacillin or cefazolin. | Vancomycin or clindamycin or ampicillin/sulbactam. |
| Methicillin-resistant S. aureus (MRSA) or methicillin-resistant, coagulase-negative staphylococci | Vancomycin (associated or not with rifampicin) or teicoplanin. | Linezolid or sulfamethoxazole/trimethoprim (associated or not with rifampicin) or daptomycin (associated or not with rifampicin) or tigecycline or clindamycin or fluoroquinolone (associated or not with rifampicin). |
| Streptococcus spp. | Penicillin or ceftriaxone or cefazolin or vancomycin. | Clindamycin or vancomycin or fluoroquinolone. |
| Enterococcus spp. | Penicillin or ampicillin + gentamycin (association). | Ampicillin/sulbactam or linezolid or daptomycin or tigecycline associated with rifampicin. |
| Pseudomonas spp. | Cefepime or meropenem or imipenem. | Fluoroquinolone. |
| Extended-spectrum beta-lactamase (ESBL) producing enterobacteria | Ertapenem or imipenem or meropenem. | Ceftriaxone. |
| Non-ESBL enterobacteria | Ceftriaxone or fluoroquinolone. | Ceftriaxone. |
| Anaerobic agents | Amoxicillin/Clavulanate or ampicillin/sulbactam or piperacillin/tazobactam. | Metronidazole or clindamycin or meropenem or imipenem. |
| Aerobic and anaerobic polymicrobial infection | Amoxicillin/Clavulanate. | Ertapenem. |
In cases of chronic infection with no sepsis, systemic or limb involvement, the use of antibiotics can be discontinued one week before surgical cleaning and the collection of culture material. An empirical medication is initiated immediately after surgery, and replaced by a specific therapy following the culture and sensitivity results.
In septic patients, antibiotic therapy should be initiated during anesthetic induction for the surgical procedure to be performed (surgical cleaning, which will be discussed later in the present paper), aiming to reduce the risk of bacteremia and its complications, without compromising the results of the bacterial culture from the collected material. 2 8
Final considerations
Osteomyelitis has been the subject of new updates in the medical literature and knowledge accumulation, especially regarding a better understanding of the pathogenic phenomena and the development of postoperative chronic infections, as well as new techniques and options for surgical treatment.
The definition, the historical and the most recently used classifications are well-established and described in the present paper, as well as the pathogenic theories.
The suggestive clinical diagnosis is already well-documented. However, there are new developments regarding the use of laboratory, imaging and microbiological tests for diagnostic confirmation, disease follow-up and collection of important information to guide the treatment. The treatment of chronic osteomyelitis in the long bones presents divergences in clinical and drug management, mainly because many recommendations are not yet based on solid scientific evidence. However, protocols and isolated studies show successful treatment combinations. The surgical treatment has evolved significantly in recent years, with the introduction of new techniques for infectious tissue cleaning, the use of bone substitutes for dead space management and stability maintenance, new fixation implants, and the local use of associated antibiotics.
The knowledge gathered allows us to establish promising combined clinical and surgical treatment strategies that have satisfactory results in various settings. As a result, the mastery of this theme by experts in orthopedics and in infectious diseases enables a better management of patients with chronic osteomyelitis in the long bones.
The present work has gathered the classic information and innovations related to chronic osteomyelitis and its treatment. It offers updated material to assist professionals involved with the treatment of chronic osteomyelitis during the decision-making process.
Conflitos de Interesse Os autores declaram não haver conflitos de interesse.
Originalmente Publicado por Elsevier Editora Ltda.
Originally Published by Elsevier.
Referências
- 1.Mast N H, Horwitz D. Osteomyelitis: a review of current literature and concepts. Oper Tech Orthop. 2002;12(04):232–241. [Google Scholar]
- 2.Lima A LLM, Zumiotti A V. Aspectos atuais do diagnóstico e tratamento das osteomielites. Acta Ortop Bras. 1999;7(03):135–142. [Google Scholar]
- 3.Lew D P, Waldvogel F A.Osteomyelitis Lancet 2004364(9431):369–379. [DOI] [PubMed] [Google Scholar]
- 4.Cierny G, Mader J T. Thorofare, NJ: Slack; 1989. Adult chronic osteomyelitis. A review; pp. 31–48. [Google Scholar]
- 5.Cierny G, III, Mader J T, Penninck J J.A clinical staging system for adult osteomyelitis Clin Orthop Relat Res 2003; (4147–24. [DOI] [PubMed] [Google Scholar]
- 6.Mader J T, Shirtliff M, Calhoun J H. Staging and staging application in osteomyelitis. Clin Infect Dis. 1997;25(06):1303–1309. doi: 10.1086/516149. [DOI] [PubMed] [Google Scholar]
- 7.Hake M E, Oh J K, Kim J W et al. Difficulties and challenges to diagnose and treat post-traumatic long bone osteomyelitis. Eur J Orthop Surg Traumatol. 2015;25(01):1–3. doi: 10.1007/s00590-014-1576-z. [DOI] [PubMed] [Google Scholar]
- 8.Jorge L S, Chueire A G, Rossit A RB. Osteomyelitis: a current challenge. Braz J Infect Dis. 2010;14(03):310–315. [PubMed] [Google Scholar]
- 9.Mackowiak P A, Jones S R, Smith J W. Diagnostic value of sinus-tract cultures in chronic osteomyelitis. JAMA. 1978;239(26):2772–2775. doi: 10.1001/jama.239.26.2772. [DOI] [PubMed] [Google Scholar]
- 10.Lazzarini L, Mader J T, Calhoun J H. Osteomyelitis in long bones. J Bone Joint Surg Am. 2004;86(10):2305–2318. doi: 10.2106/00004623-200410000-00028. [DOI] [PubMed] [Google Scholar]
- 11.Alrashidi Y, Galhoum A E, Wiewiorski M et al. How to diagnose and treat infection in totalankle arthroplasty. Foot Ankle Clin. 2017;22(02):405–423. doi: 10.1016/j.fcl.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Butt W P. The radiology of infection. Clin Orthop Relat Res. 1973;96(96):20–30. [PubMed] [Google Scholar]
- 13.Sapienza M T, Hironaka F, Lima A LLM et al. Avaliação de atividade inflamatória na osteomielite crônica. Contribuição da cintilografia com anticorpos policlonais. Rev Assoc Med Bras (1992) 2000;46(02):106–112. doi: 10.1590/s0104-42302000000200004. [DOI] [PubMed] [Google Scholar]
- 14.Love C, Palestro C J. Nuclear medicine imaging of bone infections. Clin Radiol. 2016;71(07):632–646. doi: 10.1016/j.crad.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Hogan A, Heppert V G, Suda A J. Osteomyelitis. Arch Orthop Trauma Surg. 2013;133(09):1183–1196. doi: 10.1007/s00402-013-1785-7. [DOI] [PubMed] [Google Scholar]
- 16.Trampuz A, Piper K E, Jacobson M J et al. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357(07):654–663. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 17.Piper K E, Jacobson M J, Cofield R H et al. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J Clin Microbiol. 2009;47(06):1878–1884. doi: 10.1128/JCM.01686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conterno L O, Turchi M D. Antibiotics for treating chronic osteomyelitis in adults. Cochrane Database Syst Rev. 2013;(09):CD004439. doi: 10.1002/14651858.CD004439.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korean Society for Chemotherapy.Korean Society ofInfectious Diseases; Korean Orthopaedic Association. Clinicalguidelines for the antimicrobial treatment of bone and jointinfections in Korea Infect Chemother 20144602125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bharti A, Saroj U K, Kumar V, Kumar S, Omar B J. A simple method for fashioning an antibiotic impregnated cemented rod for intramedullary placement in infected non-union of long bones. J Clin Orthop Trauma. 2016;7 02:171–176. doi: 10.1016/j.jcot.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo S, Jiang T, Yang Y, Yang X, Zhao J. Combination therapy with vancomycin-loaded calcium sulfate and vancomycin-loaded PMMA in the treatment of chronic osteomyelitis. BMC Musculoskelet Disord. 2016;17(01):502. doi: 10.1186/s12891-016-1352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKee M D, Li-Bland E A, Wild L M, Schemitsch E H. A prospective, randomized clinical trial comparing an antibiotic-impregnated bioabsorbable bone substitute with standard antibiotic-impregnated cement beads in the treatment of chronic osteomyelitis and infected nonunion. J Orthop Trauma. 2010;24(08):483–490. doi: 10.1097/BOT.0b013e3181df91d9. [DOI] [PubMed] [Google Scholar]
- 23.Nandi S K, Bandyopadhyay S, Das P et al. Understanding osteomyelitis and its treatment through local drug delivery system. Biotechnol Adv. 2016;34(08):1305–1317. doi: 10.1016/j.biotechadv.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Gálvez-López R, Peña-Monje A, Antelo-Lorenzo R et al. Elution kinetics, antimicrobial activity, and mechanical properties of 11 different antibiotic loaded acrylic bone cement. Diagn Microbiol Infect Dis. 2014;78(01):70–74. doi: 10.1016/j.diagmicrobio.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Calzia E, Oter S, Muth C M, Radermacher P. Evolving career of hyperbaric oxygen in sepsis: From augmentation of oxygen delivery to the modulation of the immune response. Crit Care Med. 2006;34(10):2693–2695. doi: 10.1097/01.CCM.0000240782.44414.3A. [DOI] [PubMed] [Google Scholar]
- 26.Marx R E, Ehler W J, Tayapongsak P, Pierce L W. Relationship of oxygen dose to angiogenesis induction in irradiated tissue. Am J Surg. 1990;160(05):519–524. doi: 10.1016/s0002-9610(05)81019-0. [DOI] [PubMed] [Google Scholar]
- 27.Kanakaris N, Gudipati S, Tosounidis T, Harwood P, Britten S, Giannoudis P V. The treatment of intramedullary osteomyelitis of the femur and tibia using the Reamer-Irrigator-Aspirator system and antibiotic cement rods. Bone Joint J. 2014;96-B(06):783–788. doi: 10.1302/0301-620X.96B6.32244. [DOI] [PubMed] [Google Scholar]
- 28.Hashmi M A, Norman P, Saleh M. The management of chronic osteomyelitis using the Lautenbach method. J Bone Joint Surg Br. 2004;86(02):269–275. doi: 10.1302/0301-620x.86b2.14011. [DOI] [PubMed] [Google Scholar]
- 29.Fry D E. Pressure Irrigation of Surgical Incisions and Traumatic Wounds. Surg Infect (Larchmt) 2017;18(04):424–430. doi: 10.1089/sur.2016.252. [DOI] [PubMed] [Google Scholar]
- 30.Bhandari M, Thompson K, Adili A, Shaughnessy S G. High and low pressure irrigation in contaminated wounds with exposed bone. Int J Surg Investig. 2000;2(03):179–182. [PubMed] [Google Scholar]
- 31.Campbell R, Berry M G, Deva A, Harris I A, Harris I A. Aggressive management of tibial osteomyelitis shows good functional outcomes. Eplasty. 2011;11:e3. [PMC free article] [PubMed] [Google Scholar]
- 32.Gülabi D, Erdem M, Ceçen G S. Treatment of chronic osteomyelitis of the femur with combined technique. Eklem Hastalik Cerrahisi. 2014;25(03):173–178. doi: 10.5606/ehc.2014.37. [DOI] [PubMed] [Google Scholar]
- 33.Giannoudis P V, Harwood P J, Tosounidis T, Kanakaris N K. Restoration of long bone defects treated with the induced membrane technique: protocol and outcomes. Injury. 2016;47(47) 06:S53–S61. doi: 10.1016/S0020-1383(16)30840-3. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Wang Z, Fu J, Huang K, Xie Z. Induced membrane technique for the treatment of chronic hematogenous tibia osteomyelitis. BMC Musculoskelet Disord. 2017;18(01):33. doi: 10.1186/s12891-017-1395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masquelet A C, Fitoussi F, Begue T, Muller G P. [Reconstruction of the long bones by the induced membrane and spongy autograft] Ann Chir Plast Esthet. 2000;45(03):346–353. [PubMed] [Google Scholar]
- 36.Marais L C, Ferreira N. Bone transport through an induced membrane in the management of tibial bone defects resulting from chronic osteomyelitis. Strateg Trauma Limb Reconstr. 2015;10(01):27–33. doi: 10.1007/s11751-015-0221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papineau L J. L'excision-greffe avec fermeture retardée délibérée dans l'ostéomyélite chronique. Nouv Presse Med. 1973;2(41):2753–2755. [PubMed] [Google Scholar]
- 38.Archdeacon M T, Messerschmitt P. Modern papineau technique with vacuum-assisted closure. J Orthop Trauma. 2006;20(02):134–137. doi: 10.1097/01.bot.0000184147.82824.7c. [DOI] [PubMed] [Google Scholar]
- 39.Drago L, Romanò D, De Vecchi E et al. Bioactive glass BAG-S53P4 for the adjunctive treatment of chronic osteomyelitis of the long bones: an in vitro and prospective clinical study. BMC Infect Dis. 2013;13:584. doi: 10.1186/1471-2334-13-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu H, Shen J, Yu X et al. Two stage management of Cierny-Mader type IV chronic osteomyelitis of the long bones. Injury. 2017;48(02):511–518. doi: 10.1016/j.injury.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Kojima T, Kohno T, Ito T. Muscle flap with simultaneous mesh skin graft for skin defects of the lower leg. J Trauma. 1979;19(10):724–729. doi: 10.1097/00005373-197910000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Lowenberg D W, Buntic R F, Buncke G M, Parrett B M. Long-term results and costs of muscle flap coverage with Ilizarov bone transport in lower limb salvage. J Orthop Trauma. 2013;27(10):576–581. doi: 10.1097/BOT.0b013e31828afde4. [DOI] [PubMed] [Google Scholar]
- 43.Malizos K N, Zalavras C G, Soucacos P N, Beris A E, Urbaniak J R. Free vascularized fibular grafts for reconstruction of skeletal defects. J Am Acad Orthop Surg. 2004;12(05):360–369. doi: 10.5435/00124635-200409000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Zalavras C G, Femino D, Triche R, Zionts L, Stevanovic M. Reconstruction of large skeletal defects due to osteomyelitis with the vascularized fibular graft in children. J Bone Joint Surg Am. 2007;89(10):2233–2240. doi: 10.2106/JBJS.E.01319. [DOI] [PubMed] [Google Scholar]