Abstract
Aims
Cardiovascular magnetic resonance (CMR) imaging is an important tool in the assessment of paediatric cardiac disease. Reported reference values of ventricular volumes and masses in the paediatric population are based on small cohorts and several methodologic differences between studies exist. We sought to create steady-state free precession (SSFP) CMR reference values for biventricular volumes and mass by combining data of previously published studies and re-analysing these data in a standardized manner.
Methods and results
A total of 141 healthy children (68 boys) from three European centres underwent cine-SSFP CMR imaging. Cardiac structures were manually contoured for end-diastolic and end-systolic phases in the short-axis orientation according to current standardized CMR post-processing guidelines. Volumes and masses were derived from these contours. Age-related reference curves were constructed using the lambda mu sigma method. Median age was 12.7 years (range 0.6–18.5). We report biventricular volumes and masses, unindexed and indexed for body surface area, stratified by age groups. In general, boys had approximately 15% higher biventricular volumes and masses compared with girls. Only in children aged <6 years old no gender differences could be observed. Left ventricle ejection fraction was slightly higher in boys in this study population (median 67% vs. 65%, P = 0.016). Age-related reference curves showed non-linear relations between age and cardiac parameters.
Conclusion
We report volumetric SSFP CMR imaging reference values for children aged 0–18 years old in a relatively large multi-centre cohort. These references can be used in the follow-up of paediatric cardiac disease and for research purposes.
Keywords: CMR imaging, paediatrics, reference values, MRI, congenital heart disease
Introduction
Assessment of global ventricular size and function is an important parameter in the follow-up of patients with cardiac disease. Cardiovascular magnetic resonance (CMR) imaging is an important non-invasive tool for the assessment of both right and left ventricular dimensions and function. CMR imaging has been considered the reference standard for the assessment of these parameters due to the accuracy and reproducibility of the method.1,2. CMR often contributes to decision-making for (re)-interventions in patients with cardiac disease.3 For optimal use of CMR, reference data on ventricular volumes, function, and mass are required. Normal values in the adult population for both left ventricle (LV) and right ventricle (RV) have been published with up to 804 healthy adults.4,5 In general there is good agreement of average biventricular size and ejection fraction among these adults reference sets.6
Available reference data series for children7–9 have been criticized for the limitation of being relatively small in number or encompassing a too limited age range. The number of subjects in these studies for the commonly applied short-axis orientation ranged from 29 to 60, with ages ranging from 0 to 20 years old. However, in children under 8 years old reference data are scarce. Only the study by Buechel et al., which included 50 children aged 0–18 years, reported few data in this age range. Furthermore, several methodological differences exist across these studies.
We sought to create larger normal reference values for the paediatric population by pooling subjects included in these previous studies and to assess normal volumes and masses in a standardized manner.7–9
Materials and methods
Subjects
This study pooled subjects previously recruited in three paediatric centres, Bad Oeynhausen, Germany, Zurich, Switzerland and Rotterdam, the Netherlands.7–9 Also, seven subjects enrolled following publication of the original articles were included (age range 0.8–15.4 years). Before CMR studies were obtained, the health status of the children was assessed by questionnaire and physical examination. Scans were performed only for study purposes, except for Zurich, where CMR was obtained in addition to imaging performed for the evaluation of unrelated, mostly orthopaedic, disorders.
Inclusion criteria were children aged between 0 and 18 years with no evidence or history of cardiovascular disease. Subjects with disease potentially affecting the chest or the cardiovascular system, acute infections, arterial hypertension, arrhythmia, anaemia, neoplasm and subjects using any medication were excluded. Subjects who had developed disorders affecting the cardiovascular system since publication of the original articles (n = 5) and subjects performing vigorous physical exercise over 6 h per week were also excluded from analysis.
This study complied with the Declaration of Helsinki. All subjects or their legal guards gave written informed consent for participation in the original studies according to local legislation. The study protocols were approved by the ethics boards of the contributing institutions.
At the same day of the CMR examination, demographics such as weight and height were collected for each subject. Body mass index (BMI) was calculated as body weight (kg) per height (m)2 and body surface area (BSA) was determined by the Du Bois & Du Bois formula: 0.007184 × height (cm)0.725 × weight (kg)0.425.10
CMR studies
CMR imaging was performed using a Signa 1.5T whole-body MR imaging system (General Electric, Milwaukee, WI) in Rotterdam and Zurich. Bad Oeynhausen used a 1.5-T whole-body MR scanner (Philips Medical Systems, Intera, R11). All images were obtained using a steady-state free precession (SSFP) pulse sequence. Imaging parameters of the individual publications are summarized in Table 1. Images were obtained while holding breath whenever possible. Young children unable to properly be instructed were sedated and images were obtained while breathing freely. Two excitation signals were averaged in these children. No children were intubated for imaging. All images were reconstructed in the short-axis plane. Studies obtained in other orientations were excluded for analysis in this study.
Table 1.
Population descriptions and CMR parameters of original publications
| Robbers-Visser et al.8 | Sarikouch et al.9 | Valsangiacomo-Buechel et al.7 | Current study | |
|---|---|---|---|---|
| N (short axis) | 60 | 29 | 50 | 141 |
| Male (%) | 50 | 48 | 46 | 48 |
| Age (year) | Range 8–17 | 12.1 ± 3.8 | Median 11 (range 0.7–18) | Median 12.7 IQR 9.5–14.8 Range 0.6–18.5 |
| MR scanner | 1.5T whole-body (General Electric, Milwaukee, USA) | 1.5T whole-body (Philips Medical Systems, Eindhoven, the Netherlands) | 1.5T whole-body (General Electric, Milwaukee, USA) | |
| Breath-holds | End-expiratory | End-expiratory | End-expiratory (N = 36) or free-breathing (N = 14) | |
| Slice thickness (mm) | 7–10 | 5–6 | 5–8 | |
| Interslice gap (mm) | 0 | 0 | 0–2 | |
| Spatial resolution (mm) | 1.8–2.3 × 2.2–2.9 | 2.0–2.5 × 1.5–1.8 | 1.1–1.6 × 1.1–1.6 | |
| TR (ms) | 31.5 | 14–36 | 21–45 |
Seven subjects were included and five were excluded following publication of the original articles.
IQR, inter-quartile range; TR, true temporal resolution (temporal resolution times k-space segments per segment).
CMR post-processing
The CMR studies were analysed on a commercially available Windows workstation using MR Analytical Software System (Medis Medical Imaging Systems, Leiden, The Netherlands). Prior to analysis, a consensus between participating researchers was reached regarding guidelines for contouring. These guidelines mostly follow the 2013 Society for Cardiovascular Magnetic Resonance consensus.11 A noteworthy deviation is the contouring of right ventricular trabeculations to report right ventricular mass. An example of segmentation is shown in Figure 1.
Figure 1.
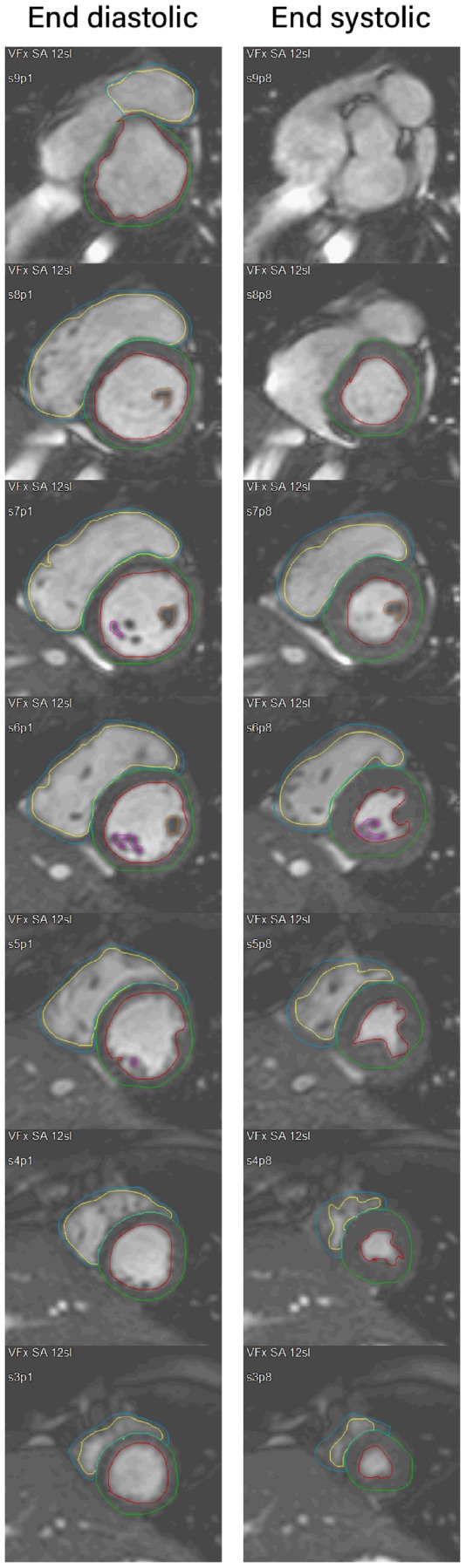
Example of CMR segmentation. Contiguous slices are shown in end-systole and end-diastole for the same subject. The most apical slice in end diastole (slice 2) is not shown.
Epicardial and myocardial borders were manually contoured in the end-diastolic and end-systolic phases in all relevant slices. End-diastole and end-systole were visually defined on multiple mid-ventricular slices. When there was any doubt on the exact phase, multiple adjacent phases were used for border detection and the calculated largest and smallest volumes were chosen as the end-diastolic phase and end-systolic phase, respectively. End-diastolic and end-systolic phases were the same for both ventricles. All datasets were analysed by one of two experienced observers (J.P.G.v.d.V. and Z.S.) and reviewed by a single experienced observer (W.A.H.). The following parameters were calculated: biventricular end-diastolic volume (EDV), end-systolic volume (ESV), stroke volume (SV), ejection fraction (EF), and ventricular wall volume. Ventricular wall volume was converted to ventricular wall mass using the specific density of myocardium, 1.05 g/mL. Myocardial masses are reported in the end-diastolic phase for the LV and end-systolic phase for the RV, as limited evidence shows these measurements are most reproducible.12–14
LV contours in the most basal slices were included in the ventricular volume if over 50% of ventricle wall was visible in a given slice, or along the aortic and mitral valve where visible. The left ventricular outflow tract was considered part of the LV and was included in the ventricular volume up to the aortic valve. Contours were traced along major trabeculae and the papillary muscles, excluding these structures from the blood pool. Papillary muscles inside the endocardial border were outlined separately and included in the myocardial mass. Trabeculae and papillary muscles were traced only if they were visible and could be contoured in ≥2 consecutive slices. The interventricular septum was included in the left ventricular mass.
In the RV contours were drawn along major trabeculae if they were connected to the ventricular wall and if there were visible in ≥2 consecutive slices. Trabecular islands (trabecula not connected to RV wall in a particular slice) were included in the blood pool and not traced because of software limitations. Slices were considered to be in the RV, rather than atrium or pulmonary artery if myocardium with trabeculations was visible.
In case of doubt, motions assessed by cine-loops of the entire cardiac cycle were used to distinguish between cardiac structures. The two-chamber and/or four-chamber reference was also used for additional information on the localization of the slice.
Ventricular volume was calculated as the sum of the ventricular cavity areas multiplied by the slice thickness.
For inter- and intra-observer variability, six studies from each centre, 18 in total, were randomly selected. After reviewing subject data to confirm the sample adequately represented the study population, this sample was used to determine inter- and intra-observer variability. For intra-observer variability, studies were re-analysed at least 6 months later by the same reviewer. An experienced reviewer, previously not involved in contouring, analysed the random sample to determine inter-observer variability.
Reference curves
Percentile curves were constructed using the lambda mu sigma (LMS) method, as described by Cole et al.15 Three curves, L, M, and S are constructed, giving rise to the name of this method. The variable of interest is transformed to a normal distribution using a Box-Cox transformation. The power of the Box-Cox transformation, lambda, is used to produce the L (lambda) curve. The M (mu) curve produces a smooth curve estimating the mean of the parameter of interest for each value of the independent parameter (in this case, age). Similarly, the S (sigma) curve estimates the coefficient of variation. Curves are smoothed using cubic splines with one smoothing parameters for each curve L, M, and S. From these L, M, and S curves percentile curves are derived. Estimated degrees of freedom for each curve need to be chosen empirically, which was done on a case-by-case basis, and the resulting curves L, M, and S were individually evaluated until researches were confident no curves were under- or over-fitted.
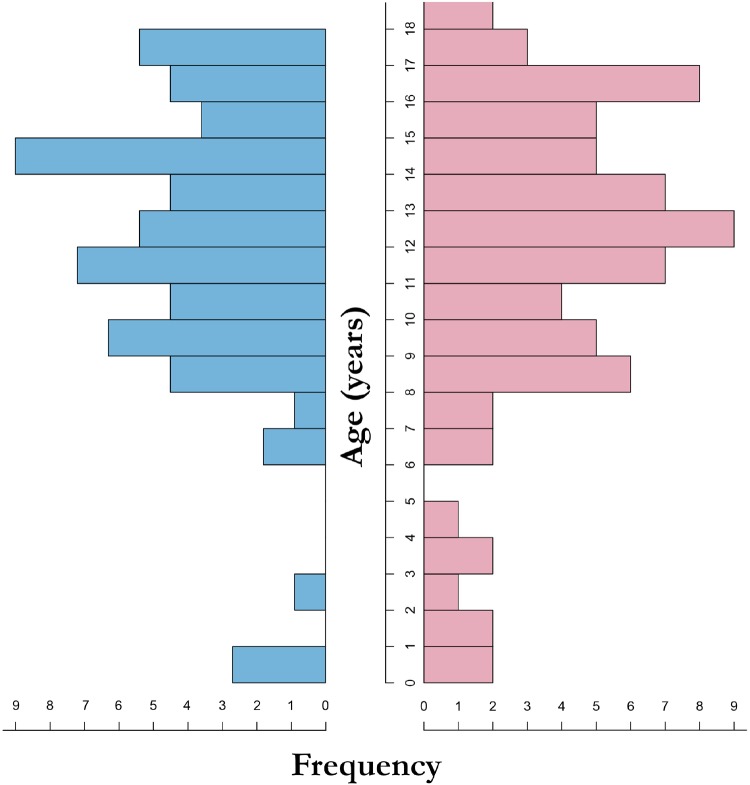
Due to small numbers in the youngest age range, we decided to include subjects of the opposite sex under 6 years old in the construction of the LMS curves, as no differences between genders were found in this age range. No differences in cardiac dimensions or function between genders are to be expected in this age range, and no gender effect has been demonstrated in large echocardiographic studies in this age range.16–18 Including subjects <6 years had a minimal effect on the course of reference curves in older age ranges, as demonstrated in Figure 2.
Figure 2.
Sex and age distribution. Left (blue) shows the distribution of the boys and right (pink) shows the distribution of the girls.
Outliers were identified on scatterplots and subject data and CMR contours for these subjects were re-evaluated and assessed for human error. No data were modified based on outlier analysis.
Statistical analysis
All continuous parameters are presented as median (inter-quartile range) and nominal data are presented as percentages, unless otherwise specified. Differences between continuous variables are tested by the non-parametric Mann–Whitney–Wilcoxon test. P-values ≤0.05 were considered to be statistically significant. Data were analysed using RStudio (Free software foundation; 2017) and LMSchartmaker Light software (Pan H, Cole T; 2011).
Inter- and intra-observer variability was assessed using Bland–Altman analysis, and expressed as the mean of the differences of the inter- or intra-observer measurements and original measurements. The upper and lower limit of agreement was defined as the mean ± 2 standard deviations of these differences. Furthermore, the intra-class correlation coefficient (ICC) and coefficient of variation (COV), defined as mean difference between observations expressed as a percentage of the mean of observations, is reported.
Allometric relationships between cardiac volumes and masses and somatic size metrics are explored by linear regression analysis. Box-cox transformation was applied to create allometric regression models.
Results
Demographics
A total of 141 healthy children (68 boys) were included in this study. All subjects were Caucasian. No subjects were excluded for insufficient image quality. About 14 subjects were unable to be instructed for breath-holds and were imaged while freely breathing, 13 of which were also sedated. Subject characteristics are shown in Table 2. Median age was 12.7 years (range 0.6–18.5). Not stratified for age, no significant difference was found for body size, height, or weight between genders.
Table 2.
Subject characteristics
| Age, years | Weight, kg | Height, cm | BMI, kg/m2 | Heart rate, bpm | BSA, m2 | |
|---|---|---|---|---|---|---|
| Total | ||||||
| Girls (n = 73) | 12.5 [9.1–14.9] | 46.0 [30.0–55.0] | 158 [137–166] | 17.5 [16.2–20.3] | 80 [71–89] | 1.41 [1.05–1.60] |
| Boys (n = 68) | 12.8 [9.8–14.7] | 43.5 [33.3–62.2] | 158 [140–174] | 18.2 [16.5–20.4] | 81 [73–92] | 1.40 [1.16–1.73] |
| 0 to 6y | ||||||
| Girls (n = 8) | 1.8 [1.2–3.3] | 13.2 [9.1–15.5] | 86 [74–97] | 16.1 [15.8–16.4] | 113 [95–134] | 0.43 [0.40–0.48] |
| Boys (n = 4) | 0.9 [0.8–1.3] | 9.1 [8.6–10.4] | 77 [73–81] | 18.0 [16.4–18.1] | 110 [106–121] | 0.55 [0.41–0.64] |
| 6 to 12y | ||||||
| Girls (n = 25) | 9.6 [8.5–11.3] | 33.0 [28.2–39.0] | 142 [132–146] | 16.4 [15.1–17.8] | 85 [77–90] | 1.13 [1.02–1.24] |
| Boys (n = 28) | 9.9 [8.9–11.1] | 33.8 [28.2–36.1] | 142 [134–149] | 16.2 [15.4–17.2] | 84 [75–94] | 1.17 [1.04–1.25] |
| 12 to 18y | ||||||
| Girls (n = 40) | 14.8 [13.3–16.4] | 53.0 [48.8–58.3] | 165 [162–170] | 19.8 [18.1–21.6] | 73 [69–81] | 1.59 [1.51–1.67] |
| Boys (n = 36) | 14.7 [13.4–16.5] | 62.0 [52.0–68.6] | 173 [166–180] | 20.1 [18.8–22.0] | 77 [67–86] | 1.73 [1.58–1.87] |
Age distribution between genders was similar and the number of children <6 years of age was limited, as shown in the demographic pyramid graph (Figure 2).
Volumes and masses
Volumetric parameters, with and without normalization for BSA, are described in Tables 3and4, respectively. Boys generally had larger ventricular volumes and masses compared with girls. In the youngest age group, from 0 to 6 years old, no differences in volumes or masses were observed between genders. In children aged 6–12, all parameters were higher in boys, except for LV and RV ESVs and masses. In the oldest age group, all volumes and masses were higher for boys, both unindexed and indexed for BSA.
Table 3.
Absolute volumes and masses of the LV and RV
| Variables | Total population |
0-6 year |
6–12 years |
12–18 years |
||||
|---|---|---|---|---|---|---|---|---|
| Girls | Boys | Girls | Boys | Girls | Boys | Girls | Boys | |
| (n = 73) | (n = 68) | (n = 8) | (n = 4) | (n = 25) | (n = 28) | (n = 40) | (n = 36) | |
| LV EDV (mL) | 92 [68–113] | 99 [85–142]* | 25 [20–30] | 22 [21–24] | 77 [61–89] | 86 [76–94]* | 112 [102–124] | 140 [120–161]*** |
| LV ESV (mL) | 35 [23–39] | 31 [26–51] | 9 [7–10] | 6 [5–7] | 27 [21–32] | 27 [21–31] | 38 [35–46] | 51 [34–59]* |
| LV SV (mL) | 61 [41–74] | 70 [57–90]** | 16 [12–22] | 17 [15–17] | 49 [40–56] | 57 [54–63]** | 74 [65–81] | 89 [72–107]*** |
| LV EF (%) | 65 [61–69] | 67 [63–72]* | 63 [61–68] | 73 [71–74] | 65 [60–68] | 69 [65–73]* | 66 [62–69] | 66 [63–71] |
| LV mass(g) | 69 [49–88] | 82 [58–120]* | 21 [16–26] | 18 [15–21] | 58 [43–65] | 58 [49–72] | 87 [77–96] | 122 [101–137]*** |
| RV EDV (mL) | 98 [72–120] | 110 [90–155]** | 23 [18–31] | 23 [21–26] | 82 [63–88] | 94 [82–104]** | 119 [108–134] | 155 [132–183]*** |
| RV ESV (mL) | 38 [26–46] | 43 [32–69]* | 8 [6–10] | 6 [5–8] | 30 [22–34] | 35 [28–38] | 44 [40–54] | 68 [49–76]*** |
| RV SV (mL) | 63 [41–75] | 68 [56–89]** | 16 [13–23] | 18 [16–20] | 47 [38–54] | 58 [51–64]** | 74 [64–85] | 89 [72–104]** |
| RV EF (%) | 63 [58–67] | 61 [57–67] | 68 [63–71] | 71 [69–75] | 61 [56–66] | 64 [60–68] | 62 [57–65] | 59 [56–62] |
| RV mass (g) | 25 [13–29] | 28 [17–44]* | 6 [4–9] | 5 [4–6] | 17 [12–25] | 21 [14–26] | 29 [25–34] | 42 [30–52]*** |
EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; LV, left ventricle; RV, right ventricle; SV, stroke volume.
P < 0.05; **P < 0.01; **P < 0.001 for differences between genders.
Table 4.
Volumes and masses of the LV and RV, indexed for BSA
| Variables | Total population |
0–6 year |
6–12 years |
12–18 years |
||||
|---|---|---|---|---|---|---|---|---|
| Girls | Boys | Girls | Boys | Girls | Boys | Girls | Boys | |
| (n = 73) | (n = 68) | (n = 8) | (n = 4) | (n = 25) | (n = 28) | (n = 40) | (n = 36) | |
| LV EDV(mL/m2) | 68 [59–73] | 76 [68–83]* | 48 [44–51] | 51 [50–52] | 65 [60–70] | 75 [67–77]* | 71 [68–77] | 82 [73–89]*** |
| LV ESV(mL/m2) | 23 [20–27] | 24 [21–29] | 17 [16–22] | 13 [ 13–15] | 22 [20–27] | 23 [21–25] | 24 [22–28] | 27 [22–33]* |
| LV SV(mL/m2) | 44 [40–48] | 51 [45–57]** | 32 [28–35] | 36 [34–38] | 43 [40–45] | 50 [45–56]** | 47 [43–51] | 52 [47–58]*** |
| LV mass(g/m2) | 49 [42–58] | 60 [49–69]** | 40 [38–42] | 38 [35–40] | 46 [42–54] | 53 [44–58] | 53 [46–61] | 67 [61–75]*** |
| RV EDV(mL/m2) | 69 [63–79] | 82 [74–92]** | 47 [41–53] | 54 [52–55] | 68 [62–72] | 79 [74–84]** | 78 [69–84] | 90 [81–96]*** |
| RV ESV(mL/m2) | 26 [22–31] | 31 [27–38]* | 16 [14–18] | 15 [13–16] | 25 [23–30] | 29 [26–32] | 29 [25–34] | 37 [30–40]*** |
| RV SV(mL/m2) | 43 [37–49] | 51 [44–55]** | 32 [28–37] | 38 [36–40] | 42 [37–46] | 51 [45–54]** | 49 [41–52] | 51 [46–56]** |
| RV mass(g/m2) | 17 [13–20] | 20 [15–25]* | 12 [9–14] | 11 [ 10–11] | 14 [12–19] | 17 [14–21] | 18 [16–22] | 23 [19–28]*** |
EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; LV, left ventricle; RV, right ventricle; SV, stroke volume.
P < 0.05; **P < 0.01; ***P < 0.001 for differences between genders.
Left ventricular ejection fraction was higher in males (median 67% vs. 65%, P = 0.016). When stratified by age groups, this difference was only observed in the age group of 6–12 years. Ejection fraction of the RV did not differ between genders.
Right ventricular EF was lower in the oldest age group compared with the youngest age group (median 61% vs. 69%, P < 0.001). Ejection fraction of the LV did not differ among age groups (P = 0.56 for comparison between the youngest and oldest age group).
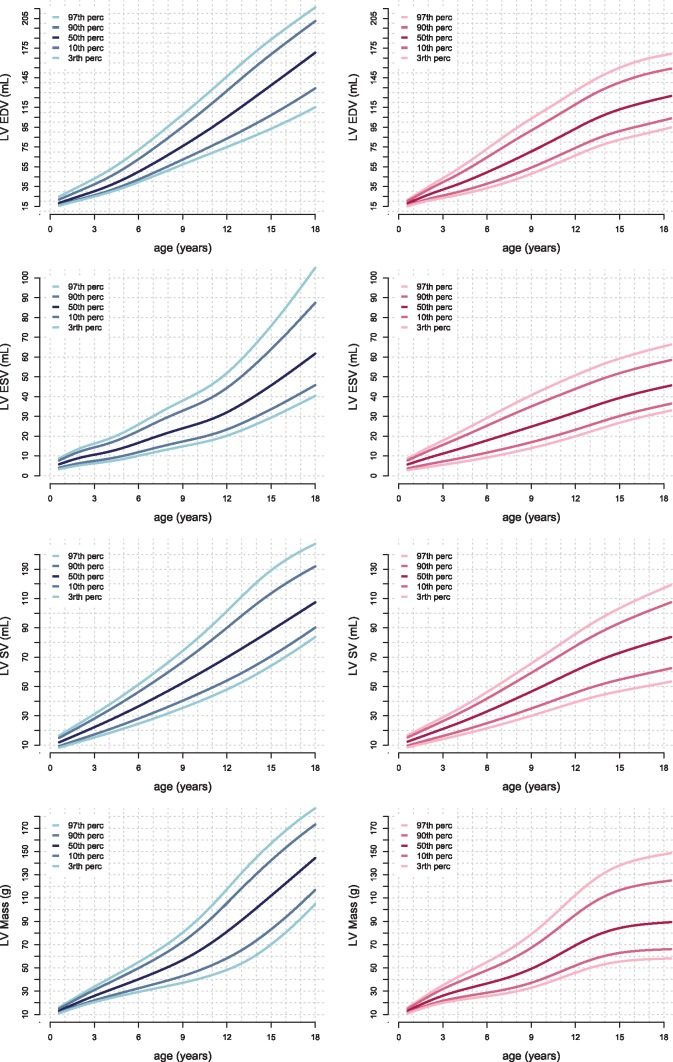
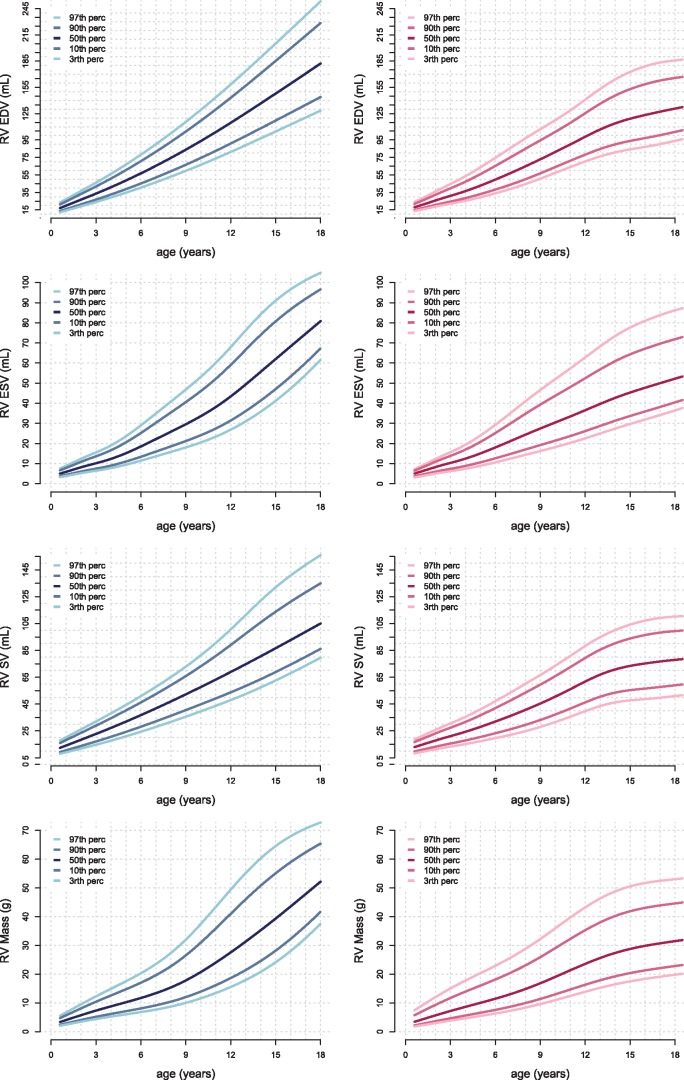
Figures 3and4 show age-related percentile curves of volumes and masses of the LV and RV. These percentile curves show non-linear, complex, relations between age and parameters. Percentile curves for parameters indexed for BSA are shown in Supplementary data online, S1 and S2.
Figure 3.
Reference curves for the volumes and masses of the LV. Boys are displayed in the left column in blue and girls in the right column in pink. Left ventricle end diastolic (LVED), end systolic (LVES), and stroke volume (LVSV) and myocardial mass are presented. Reference lines show the 3rd, 10th, 50th, 90th, and 97th percentile.
Figure 4.
Reference curves for the volumes and masses of the RV. Boys are displayed in the left column in blue and girls in the right column in pink. Right ventricle end diastolic (RVED), end systolic (RVES), and stroke volume (RVSV) and myocardial mass are presented. Reference lines show the 3rd, 10th, 50th, 90th, and 97th percentile.
Several linear and allometric sex-specific models were assessed and are summarized in Supplementary data online, S3. Volumes and masses were best explained by allometric models of BSA (R2 0.78–0.92) and length (R2 0.78–0.93). Regression formulas of volumes and masses for BSA are shown in Supplementary data online, S4.
Reproducibility
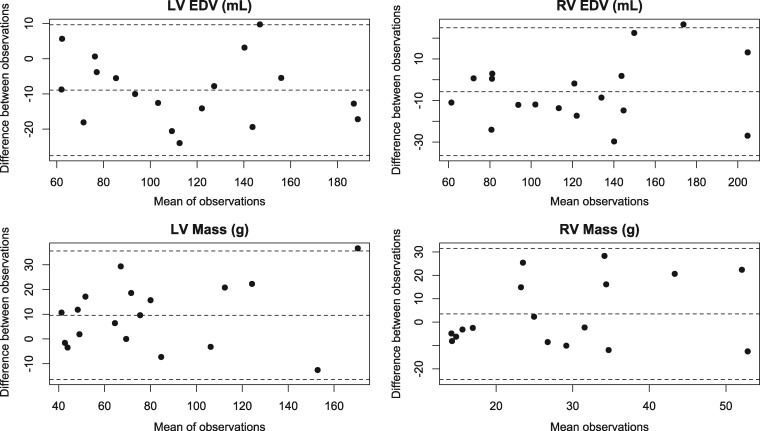
Inter- and intra-observer variability is presented in Table 5. Intra-observer variability was generally lower than inter-observer variability. Inter-observer variability was excellent (ICC > 0.90) for most variables; good for RV SV (ICC 0.88, COV 3.3%); and moderate for RV mass (ICC 0.50, COV 5.9%) and biventricular ejection fraction (ICC 0.51–0.59).19 Intra-observer variability, similarly, was excellent for all variables except for biventricular EF (ICC 0.67–0.70) and RV mass (ICC 0.82, COV 0.3%).19 Bland–Altman plots show no trend in variability with increasing mean volume or mass (Figure 5).
Table 5.
Inter-observer and intra-observer variability
| Mean difference | Limits of agreement | COV (%) | ICC | |
|---|---|---|---|---|
| Inter-observer variability | ||||
| LV EDV (mL) | 8.4 | −27.7 to 11 | 8.2 | 0.97 |
| LV ESV (mL) | 8.8 | −17.9 to 0.4 | 21.1 | 0.97 |
| LV SV (mL) | 0.2 | −18.9 to 18.5 | 1.4 | 0.94 |
| LV EF (%) | 4.5 | −5.1 to 14.1 | 8.2 | 0.59 |
| LV mass (g) | 9.7 | −16.1 to 35.4 | 12.1 | 0.94 |
| RV EDV (mL) | 4.1 | −37.4 to 29.3 | 6.0 | 0.93 |
| RV ESV (mL) | 4.7 | −19.2 to 9.8 | 9.9 | 0.94 |
| RV SV (mL) | 1.1 | −26.5 to 24.3 | 3.3 | 0.88 |
| RV EF (%) | 1.6 | −10.6 to 13.8 | 6.0 | 0.51 |
| RV mass (g) | 3.5 | −52.4 to 34.9 | 5.9 | 0.50 |
| Intra-observer variability | ||||
| LV EDV (mL) | 1.0 | −19.8 to 17.9 | 1.0 | 0.97 |
| LV ESV (mL) | 1.2 | −11.0 to 13.4 | 7.3 | 0.95 |
| LV SV (mL) | 2.2 | −22.9 to 18.6 | 4.6 | 0.92 |
| LV EF (%) | 2.5 | −12.5 to 7.5 | 1.0 | 0.70 |
| LV mass (g) | 2.4 | −28.3 to 23.5 | 0.1 | 0.96 |
| RV EDV (mL) | 5.0 | −33.4 to 23.3 | 4.2 | 0.95 |
| RV ESV (mL) | 3.3 | −19.1 to 12.6 | 4.5 | 0.94 |
| RV SV (mL) | 1.8 | −25.6 to 22.0 | 3.7 | 0.90 |
| RV EF (%) | 0.2 | −10.4 to 10.8 | 4.2 | 0.67 |
| RV mass (g) | 1.2 | −20.0 to 22.4 | 0.3 | 0.82 |
COV, coefficient of variation; EDV, end-diastolic volume; ESV, end-systolic volume; ICC, intra-class correlation coefficient; LV, left ventricle; RV, right ventricle; SV, stroke volume.
Figure 5.
Bland-Altman plots of inter-observer variability. Means and 95% confidence intervals are shown in dotted lines. These plots show no trend in variability with increasing mean volume or mass.LV, left ventricle; RV, right ventricle; EDV, end-diastolic volume.
Discussion
We report reference values for SSFP CMR-derived biventricular volumes and ejection fraction in a relatively large paediatric population. This study has reassessed and pooled the data from previously published studies from three European centres and included some new subjects.7–9 All images have been completely re-analysed to ensure uniformity in principles of segmentation. The ventricular volume parameters we obtained show non-linear growth patterns. A significant difference between girls and boys develops during adolescence.
Currently, the optimal use of CMR imaging in the paediatric population is hampered by limited reference values. Several methodological differences between previous paediatric CMR reference studies exist. The largest study analysed images obtained in both the short-axis and axial orientations.9 Buechel et al.7 included right ventricular trabeculae, the moderator band and papillary muscles in the blood pool, rather than in the myocardial mass. In contrast, Robbers-Visser et al. included these structures in the myocardial mass. Sarikouch et al.9 used automated contour detection for both the RV and LV, rather than manual segmentation. The availability of multiple CMR reference sets has caused uncertainty and a degree of arbitrariness in the choice of the adequate reference values. At a given volume or ejection fraction, the position on the reference curves may vary depending on the reference group used. Given these limitations we have re-analysed the data using contemporary segmentation guidelines to provide a larger and more generally applicable set of reference data.
For measurements in the short-axis orientation, previous references are based on 29–60 healthy children.7–9 Our study expanded the sample size to 141 children. Only Buechel et al.7 have reported data of children under 8 years old. Sarikouch et al.9 did include children under 8 years, but only used these data to estimate initial slopes for the reference curves. CMR imaging of healthy young children that are unable to properly be instructed poses an ethical problem as sedation might be required. In the present study, 14 young children where imaged without breath-holding. Respiration-related variation in CMR-derived transmitral and transtricuspid flow are approximately 16% and 24%, respectively.20 To minimize the effect of respiratory variation, images were averaged over multiple heart beats for these children. Our present analysis used all available data of children younger than 8 years of age, so that we can present a slightly larger sample size for this age range.
Our data show good agreement with previously published CMR reference values in adolescents and adults (e.g. RV EDV 87 ± 12 mL/m2 in adults under 60 years vs. 90 [81–96] in this study).6–9,21 Compared with paediatric 3D echocardiography references, our study found higher LV and RV volumes, a known inter-modality difference.22–24 Differences in cardiac dimensions between genders are well-described. In the adult population, males have up to 43% larger volumes and masses.21 We have observed that similar differences develop during adolescence. Our study found a higher LVEF only in males aged 6–12 years and no difference in RVEF between genders. In the adult population, females have significantly higher left (61% ± 5%) and right ventricular EF (58% ± 6%) compared with males (58% ± 5% and 54% ± 6%, respectively, P < 0.001 for both parameters).6 These findings implicate a change in ventricular functions in the growing and developed heart.
There is no general agreement on the optimal image orientation for volumetric evaluation, particularly in congenital heart disease. In this study, we have chosen images obtained in the short-axis orientation as this orientation is most commonly used in practice. Reproducibility of SSFP CMR measurements of the RV in the short-axis orientation was found to be non-inferior compared with axial orientations in most studies.25–27 Right ventricular mass is not routinely reported when assessing volumes and masses by SSFP CMR. Some argue that the spatial resolution of SSFP CMR is not sufficient to adequately assess the thin right ventricular wall.28 However, we found moderate to good inter-observer and intra-observer reproducibility even for RV mass, as it was reported by some other groups.29,30 Furthermore, an adequate correlation between CMR measurements of RV mass and pathology measurements have been demonstrated.31
Paediatric CMR references obtained from other techniques than SSFP cannot be used as a reference for SSFP CMR studies, as different sequences result in different measurements of volumes and masses.25,28
Construction of reference curves using the LMS method
Somatic growth in the developing child follows a complex pattern that is not reducible to a simple linear or exponential relationship with age. Growth patterns of the heart are often described in relation to parameters of somatic growth (e.g. BSA). However, the exact relationship between cardiac and somatic sizes is not well understood and probably differs across ages.32–37
We sought to report our reference values in a way that takes these complex relationships into account but can be easily used in practice. A linear or exponential regression model assumes a fixed relationship between the assessed parameters and constant variance across the model. Since these assumptions are not correct, these models probably do not adequately represent cardiac development from infancy to adolescence. The reference centile curves provided in this study have been constructed using the LMS method, as described by Cole et al.15 This method provides centile curves without any assumption of the relationship between the target and the independent variable. It also allows for non-constant variance. Age-related reference curves are commonly used in paediatrics. Multiple data points can be added to a patient-specific chart to easily assess cardiac growth with increasing age. The biggest advantage of the LMS method, however, is the well-known efficiency using small datasets. In this study, we were able to increase the data pool by more than 100% compared with the earlier published single-centre cohorts, thereby enabling more robust chart calculation. This is of importance especially in the youngest age group, where the small increase in data points is optimally utilized by the LMS method, compared with linear or exponential regression models.
Study limitations
We have divided the subjects of our study population into age groups representing developmental stages: these stages were decided arbitrarily. The correlation between volumetric measurement and large vessel flow measurement has been shown to be excellent in children.9 However, as this was not available for all datasets we did not validate volumetric measurements against large vessel flow. A limited number of subjects younger than 6 years are present in our cohort due to practical and ethical reasons. For example only four boys could be included, and their age ranged from 0 to 3 years (Figure 2). This demographic limitation has the potential of skewing the course of the reference curves. As we did not observe any significant differences between results according to gender in young children, and no significant gender differences are to be expected in this age group,16–18 we decided to pool all subjects of both genders younger than 6 years to minimize these effects.
The inter-observer variability of biventricular EF is somewhat larger than others previously reported.7–9 Previous studies performed inter-observer analysis with a second observer from the same institution. Even if we have defined rules of segmentation in common agreement, interinstitutional differences may have the increased inter-observer variance in our study.26,38 We have reassessed adherence to the CMR segmentation rules in selected cases, which was found to be good. The most frequently observed source of variance was a different endocardial border in the most basal slices, which is a well-known source of error. This uncertainty, inherent to manual segmentation of CMR images, should always be taken into account when interpreting CMR-derived data.
Future directions
The reported references values in this study were derived from a Caucasian cohort. Racial differences in cardiac volumes and masses are described in the adult population and future efforts could focus on elucidating these differences in the paediatric population.39,40
Paediatric CMR reference studies from larger healthy cohorts, such as Generation R,41 are currently being developed; these will result in much larger datasets of healthy populations, including older children. However, these studies have not yet included large numbers of children across the entire paediatric age range. Therefore reference values for children before school age remains an important target for additional future work.
Conclusion
This study provides normal reference ranges for biventricular volumes and masses from the largest published cohort of healthy Caucasian children in the most commonly used short-axis orientation covering infancy to adolescence. A significant difference in biventricular volumes and cardiac mass after the age of 6 years between girls and boys was confirmed. These paediatric CMR reference values may serve as a reference for the follow-up of children with cardiac disease and for further clinical studies.
Ethics approval and consent to participate
All subjects or their legal guards gave written informed consent for participation in the original studies according to local legislation. The study protocol was approved by the ethics boards of the contributing institutions.
Funding
This study was supported by the Magnetic Resonance Imaging Project of the Competence Network for Congenital Heart Defects funded by the German Federal Ministry of Education and Research [BMBF, FKZ 01G10210, 01GI0601].
Conflict of interest: none declared.
Supplementary Material
References
- 1. Pennell DJ, Sechtem UP, Higgins CB, Manning WJ, Pohost GM, Rademakers FE. et al. Clinical indications for cardiovascular magnetic resonance (CMR): consensus panel report. J Cardiovasc Magn Reson 2004;6:727–65. [DOI] [PubMed] [Google Scholar]
- 2. Helbing WA, Rebergen SA, Maliepaard C, Hansen B, Ottenkamp J, Reiber JH. et al. Quantification of right ventricular function with magnetic resonance imaging in children with normal hearts and with congenital heart disease. Am Heart J 1995;130:828–37. [DOI] [PubMed] [Google Scholar]
- 3. Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU. et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol 2002;90:29–34. [DOI] [PubMed] [Google Scholar]
- 4. Maceira AM, Prasad SK, Khan M, Pennell DJ.. Reference right ventricular systolic and diastolic function normalized to age, gender and body surface area from steady-state free precession cardiovascular magnetic resonance. Eur Heart J 2006;27:2879–88. [DOI] [PubMed] [Google Scholar]
- 5. Maceira AM, Prasad SK, Khan M, Pennell DJ.. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2006;8:417–26. [DOI] [PubMed] [Google Scholar]
- 6. Petersen SE, Aung N, Sanghvi MM, Zemrak F, Fung K, Paiva JM. et al. Reference ranges for cardiac structure and function using cardiovascular magnetic resonance (CMR) in Caucasians from the UK Biobank population cohort. J Cardiovasc Magn Reson 2017;19:18.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buechel EV, Kaiser T, Jackson C, Schmitz A, Kellenberger CJ.. Normal right- and left ventricular volumes and myocardial mass in children measured by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2009;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Robbers-Visser D, Boersma E, Helbing WA.. Normal biventricular function, volumes, and mass in children aged 8 to 17 years. J Magn Reson Imaging 2009;29:552–9. [DOI] [PubMed] [Google Scholar]
- 9. Sarikouch S, Peters B, Gutberlet M, Leismann B, Kelter-Kloepping A, Koerperich H. et al. Sex-specific pediatric percentiles for ventricular size and mass as reference values for cardiac MRI: assessment by steady-state free-precession and phase-contrast MRI flow. Circ Cardiovasc Imaging 2010;3:65–76. [DOI] [PubMed] [Google Scholar]
- 10. Du Bois D, Du Bois EF.. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989;5:303–11; discussion 312-3. [PubMed] [Google Scholar]
- 11. Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG. et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson 2013;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pennell DJ. Ventricular volume and mass by CMR. J Cardiovasc Magn Reson 2002;4:507–13. [DOI] [PubMed] [Google Scholar]
- 13. Altmayer SP, Teeuwen LA, Gorman RC, Han Y.. RV mass measurement at end-systole: improved accuracy, Reproducibility, and reduced segmentation time. J Magn Reson Imaging 2015;42:1291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blalock SE, Banka P, Geva T, Powell AJ, Zhou J, Prakash A.. Interstudy variability in cardiac magnetic resonance imaging measurements of ventricular volume, mass, and ejection fraction in repaired tetralogy of Fallot: a prospective observational study. J Magn Reson Imaging 2013;38:829–35. [DOI] [PubMed] [Google Scholar]
- 15. Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr 1990;44:45–60. [PubMed] [Google Scholar]
- 16. Poutanen T, Jokinen E, Sairanen H, Tikanoja T.. Left atrial and left ventricular function in healthy children and young adults assessed by three dimensional echocardiography. Heart 2003;89:544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Poutanen T, Jokinen E.. Left ventricular mass in 169 healthy children and young adults assessed by three-dimensional echocardiography. Pediatr Cardiol 2007;28:201–7. [DOI] [PubMed] [Google Scholar]
- 18. Cantinotti M, Scalese M, Murzi B, Assanta N, Spadoni I, Festa P. et al. Echocardiographic nomograms for ventricular, valvular and arterial dimensions in Caucasian children with a special focus on neonates, infants and toddlers. J Am Soc Echocardiogr 2014;27:179–91.e2. [DOI] [PubMed] [Google Scholar]
- 19. Koo TK, Li MY.. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016;15:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thavendiranathan P, Verhaert D, Walls MC, Bender JA, Rajagopalan S, Chung YC. et al. Simultaneous right and left heart real-time, free-breathing CMR flow quantification identifies constrictive physiology. JACC Cardiovasc Imaging 2012;5:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, Vogel-Claussen J, Turkbey EB, Williams R. et al. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson 2015;17:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wood PW, Choy JB, Nanda NC, Becher H.. Left ventricular ejection fraction and volumes: it depends on the imaging method. Echocardiography 2014;31:87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buccheri S, Costanzo L, Tamburino C, Monte I.. Reference values for real time three-dimensional echocardiography-derived left ventricular volumes and ejection fraction: review and meta-analysis of currently available studies. Echocardiography 2015;32:1841–50. [DOI] [PubMed] [Google Scholar]
- 24. Shimada YJ, Shiota M, Siegel RJ, Shiota T.. Accuracy of right ventricular volumes and function determined by three-dimensional echocardiography in comparison with magnetic resonance imaging: a meta-analysis study. J Am Soc Echocardiogr 2010;23:943–53. [DOI] [PubMed] [Google Scholar]
- 25. Alfakih K, Plein S, Bloomer T, Jones T, Ridgway J, Sivananthan M.. Comparison of right ventricular volume measurements between axial and short axis orientation using steady-state free precession magnetic resonance imaging. J Magn Reson Imaging 2003;18:25–32. [DOI] [PubMed] [Google Scholar]
- 26. Beerbaum P, Barth P, Kropf S, Sarikouch S, Kelter-Kloepping A, Franke D. et al. Cardiac function by MRI in congenital heart disease: impact of consensus training on interinstitutional variance. J Magn Reson Imaging 2009;30:956–66. [DOI] [PubMed] [Google Scholar]
- 27. James SH, Wald R, Wintersperger BJ, Jimenez-Juan L, Deva D, Crean AM. et al. Accuracy of right and left ventricular functional assessment by short-axis vs axial cine steady-state free-precession magnetic resonance imaging: intrapatient correlation with main pulmonary artery and ascending aorta phase-contrast flow measurements. Can Assoc Radiol J 2013;64:213–9. [DOI] [PubMed] [Google Scholar]
- 28. Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU.. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging 2003;17:323–9. [DOI] [PubMed] [Google Scholar]
- 29. Grothues F, Moon JC, Bellenger NG, Smith GS, Klein HU, Pennell DJ.. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J 2004;147:218–23. [DOI] [PubMed] [Google Scholar]
- 30. Catalano O, Antonaci S, Opasich C, Moro G, Mussida M, Perotti M. et al. Intra-observer and interobserver reproducibility of right ventricle volumes, function and mass by cardiac magnetic resonance. J Cardiovasc Med (Hagerstown) 2007;8:807–14. [DOI] [PubMed] [Google Scholar]
- 31. Farber NJ, Reddy ST, Doyle M, Rayarao G, Thompson DV, Olson P. et al. Ex vivo cardiovascular magnetic resonance measurements of right and left ventricular mass compared with direct mass measurement in excised hearts after transplantation: a first human SSFP comparison. J Cardiovasc Magn Reson 2014;16:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dewey FE, Rosenthal D, Murphy DJ Jr., Froelicher VF, Ashley EA.. Does size matter? Clinical applications of scaling cardiac size and function for body size. Circulation 2008;117:2279–87. [DOI] [PubMed] [Google Scholar]
- 33. Rowland T, Goff D, Martel L, Ferrone L, Kline G.. Normalization of maximal cardiovascular variables for body size in premenarcheal girls. Pediatr Cardiol 2000;21:429–32. [DOI] [PubMed] [Google Scholar]
- 34. de Simone G, Devereux RB, Daniels SR, Mureddu G, Roman MJ, Kimball TR. et al. Stroke volume and cardiac output in normotensive children and adults. Assessment of relations with body size and impact of overweight. Circulation 1997;95:1837–43. [DOI] [PubMed] [Google Scholar]
- 35. Gutgesell HP, Rembold CM.. Growth of the human heart relative to body surface area. Am J Cardiol 1990;65:662–8. [DOI] [PubMed] [Google Scholar]
- 36. George K, Sharma S, Batterham A, Whyte G, McKenna W.. Allometric analysis of the association between cardiac dimensions and body size variables in 464 junior athletes. Clin Sci 2001;100:47–54. [PubMed] [Google Scholar]
- 37. Sluysmans T, Colan SD.. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol (1985) 2005;99:445–57. [DOI] [PubMed] [Google Scholar]
- 38. Suinesiaputra A, Bluemke DA, Cowan BR, Friedrich MG, Kramer CM, Kwong R. et al. Quantification of LV function and mass by cardiovascular magnetic resonance: multi-center variability and consensus contours. J Cardiovasc Magn Reson 2015;17:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kishi S, Reis JP, Venkatesh BA, Gidding SS, Armstrong AC, Jacobs DR Jr. et al. Race-ethnic and sex differences in left ventricular structure and function: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. J Am Heart Assoc 2015;4:e001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M. et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol 2006;186:S357–65. [DOI] [PubMed] [Google Scholar]
- 41. Kooijman MN, Kruithof CJ, van Duijn CM, Duijts L, Franco OH, van IMH. et al. The Generation R Study: design and cohort update 2017. Eur J Epidemiol 2016;31:1243–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.