Abstract
At least one-third of infants born in sub-Saharan Africa have been exposed to the effects of maternal HIV infection and antiretroviral treatment. Intrauterine HIV exposure is associated with increased rates of morbidity and mortality in children. Although the mechanisms responsible for poor infant health with HIV-1 exposure are likely to be multifactorial, we posit that the maternal environment during gestation and in the perinatal period results in altered infant immunity and is possibly the strongest contributing factor responsible for the disproportionally high infectious events among HIV-exposed infants who remain HIV uninfected. This review provides a synthesis of studies reporting the impact of intrauterine HIV exposure, feeding practices, and microbiota on immune ontogeny in the first year of life in HIV-exposed uninfected infants.
Keywords: breastfeeding, HIV-exposed uninfected infants, immune development, microbiota and infectious diseases
1 |. INTRODUCTION
There are approximately 2.1 million children ≤15 years of age living with HIV infection, of which 90% reside in sub-Saharan Africa, a region with the highest burden of infectious diseases.1 The majority of HIV cases in children are acquired in utero or perinatally during labor, delivery, or postpartum during breastfeeding.2 Prior to implementation of antiretroviral (ARV) treatment, the rates of HIV mother-to-child transmission (MTCT) were 30–40%, but have since been reduced to 1–3% following widespread adoption of programs to prevent MTCT, including in resource-limited settings.3 This has, however, resulted in a growing population of infants who are exposed to maternal HIV but remain uninfected.
Although healthier than HIV-infected infants, HIV-exposed uninfected (HEU) infants are 2–3 times more likely to suffer from severe infections, specifically gastrointestinal and lower respiratory tract infections.4,5 Moreover, the incidence of infectious disease among these infants is not evenly distributed by age, occurring at a higher rate in the first year of life, and being more frequent during the first month of life. Factors contributing to the disproportionately higher risk of infectious disease in HEU infants compared to HIV-unexposed infants include: (i) severity of maternal HIV infection, in which higher mortality rates are reported for infants born to mothers with high HIV viral loads and low CD4+ lymphocyte count6,7; (ii) increased exposure to maternal bacterial and viral infections; (iii) malnutrition8; (iv) breastfeeding avoidance9,10; and (v) ARV treatment, some of which may be interrelated. Additionally, mechanisms responsible for increased infections may be due to the functional development of the immune system in neonates coinciding with immune aberrations mediated by HIV exposure.
Developmental maturation of the infants’ immune system is also dependent on interactions with the mucosal microbiota. The gut microbiota in infants, initially influenced by the mode of delivery and possibly in utero maternal microbiota,11 is mainly shaped by bacteria in milk during breastfeeding and human milk oligosaccharides (HMO) that serve as prebiotics.12,13 Differences in the gut microbial profiles between HEU and HIV-exposed infants together with varying composition of HMO between HIV-infected and HIV-uninfected mothers have been recently reported.12,14 Given that formula-fed or mixed-fed HEU infants tend to have higher mortality rates compared to age-matched exclusively breastfed HEU infants,15 immunomodulation by the gut microbiota is a likely mechanism ultimately contributing to higher infectious disease burden in HEU infants. In this review, we summarize evidence related to immunological development in infants associated with maternal HIV exposure, feeding mode, and the corresponding gut microbiota in delineating attributes contributing to increased morbidity in HEU infants.
2 |. INTRAUTERINE HIV EXPOSURE AND IMMUNOLOGICAL DEVELOPMENT
In utero, the fetal immune system develops in an environment largely lacking foreign antigens, while mature allogeneic lymphocytes are tolerated by immunomodulatory mechanisms. The development of the fetal immune system begins as early as the ninth week of gestation primarily occurring in the liver until secondary lymphoid organs are fully developed.16,17 During fetal development, immune cells at the maternofetal (MF) interface are critical in setting the balance between regulatory and proinflammatory immune responses to foster tolerance of the semiallogenic fetus while protecting against invading pathogens.18
In early pregnancy, decidual NK (dNK) cells are the most abundant leukocytes within the MF interface.19,20 They show distinct functional and phenotypic characteristics, different from their peripheral blood counterparts. A great proportion of dNK cells are CD56bright CD16negCD160neg.20 In healthy pregnancies, dNK cells have poor cytolytic activity but have great pregnancy-specific functions such as promoting implantation and contributing to MF tolerance.21,22 Although very little is known about dNK cell ability to limit local decidual viral infections, Siewiera et al.23 reported that cytomegalovirus infection induces a new dNK cell effector function suggesting that dNK may possess properties that could aid in protection against viral infections.23 There are limited studies that have investigated phenotypic and functional changes of dNK in HIV-infected women to adequately inform conclusions on the outcome of fetal development and their health implications. Although given the essential role of dNK in fetal growth24 and the increased likelihood of delivering neonates with low birth weight among HIV-infected women,25 it is possible that maternal HIV infection or immune aberrations associated with HIV infection may alter dNKs and ultimately impairing their fetal growth promoting properties.
Decidual macrophages are the second most abundant immune cell type at the MF interface. Decidual macrophages possess an array of properties at the MF interface, involved in placentation, fetal development, and immune regulation to orchestrate the establishment and maintenance of normal pregnancy.26,27 Hofbauer cells are also present within the MF interface; these are placental macrophages found in the chorionic villi tissue. These cells predominantly secrete the regulatory cytokines IL-10 and TGF-β that are crucial for the maintenance of immune-homeostatic environment necessary for fetal development.28 Despite being the main target of HIV infection in the placenta, expressing coreceptors CD4, CCR5, CXCR4, and DC-SIGN, the immunoregulatory cytokines secreted by Hofbouer cells inhibit HIV replication and transmission. Nonetheless, more studies are still required to investigate the impact of aberrant immune activation due to maternal infections and the pathophysiology of common pregnancy complications on the function of decidual macrophages.
Further tolerance of the growing fetus is maintained by generation or recruitment of Foxp3+ CD4+ regulatory T cells (Tregs) that possess immunosuppressive properties.29 Other suppressor cells such as myeloid-derived suppressor cells (MDSC) have been identified and also contribute in maintaining tolerance.30 Both Tregs and MDSC are found at higher frequencies in HIV-infected adults, although their presence and function in HIV-infected pregnancies has not been assessed.
The use of combination antiretroviral therapy (cART), and subsequent lowering of HIV viral load among HIV-1-infected pregnant women, limits viral interactions with the immune cells involved in the regulation of pregnancy. However, prolonged exposure to cART has been shown to have detrimental effects on maternal health and birth outcomes among HIV-1-infected women on cART. We propose that HIV-induced maternal chronic immune activation disrupts the cellular milieu involved in regulating pregnancy and results in dysregulated MF immune balance and thus altering immunoregulatory mechanism that allow healthy fetal development, summarized in Fig. 1. Moreover, exposure to maternal HIV and opportunistic infections increases the likelihood of disrupted MF tolerance leading to inflammation, which may result in altered neonatal immunity. These possibilities require further exploration and study.
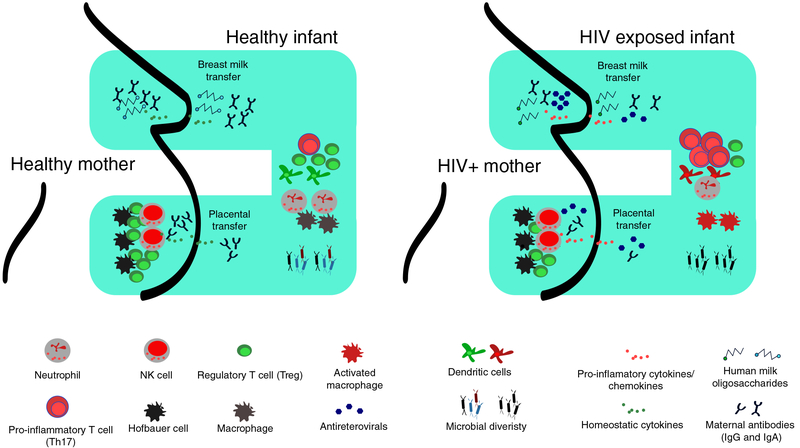
FIGURE 1. Schematic representation of the suggested model illustrating the link among in utero HIV exposure, breastfeeding, and microbiota on the developing immune system.
The left panel shows in a healthy pregnancy, the MF balance is maintained by Hofbauer cells, dNK, and Tregs in the placenta. Abs are efficiently transferred from mother to child via the placenta and breast milk provide protection against invading pathogens. Breastfeeding infants receive a plethora of bioactive compounds in breast milk including HMO that influences establishment of beneficial microbiota and promotes development of the infant’s immune system. In HIV-infected mothers, who may be invariably taking ARV drugs, chronic immune activation in the mother creates an inflammatory environment resulting in likely dysregulation of MF immune balance. Mother to child transfer of proinflammatory cytokines and chemokines occurs via placenta and/or breast milk activating innate cells in infants and promoting an expansion of inflammatory T cells (Th17 for example). Transplacental passage of maternal Abs is also impaired resulting in few protective antibodies in the infant circulation. Furthermore, less fucosylated and glycosylated HMOs are passed via maternal breast milk resulting in lower gut microbiota diversity.
Studies that have investigated the effects of HIV exposure on development of infant immunity have reported differences between HEU and HIV-unexposed infants in both the innate and adaptive arms of the immune system. These observed differences could possibly explain the disproportionate burden of infectious diseases among HEU infants, although there is a lack of consensus in the data so far. This could be attributable to differences in experimental designs and characteristics of the studied cohorts, including breastfeeding practices and maternal ART. Most studies, however, have reported no differences in Ab responses to vaccines between HEU and HUU infants.31–33 Transplacental transfer of Abs from mother to infants is greatly affected by in utero HIV exposure with HEU newborns having significantly less compared to HUU newborns.31,34,35 In a study by Jones et al., HEU infants have been shown to have higher antibody responses to vaccines than their HIV-unexposed counterpart, most likely due to the reduced passive immunity that could interfere with vaccine priming.35 What perhaps is less clear is the impact of HIV exposure on innate and adaptive cellular immunity, which is the basis of our review.
2.1 |. Innate cellular immunity in HEU
The innate immune system is mostly comprised of myeloid-derived cells that function as the first line of defense capable of mounting rapid responses against invading pathogens. The bridge between innate and adaptive immunity lies in antigen processing and presentation, via the MHC on dendritic cells (DC) and macrophages and with immune activation signals derived via TLR engagement. Neonatal innate immunity is quantitatively and qualitatively different to that of adults, characterized by fewer neutrophils with poor signaling via pathogen recognition receptors (PRR), less chemotactic ability, and defective formation of neutrophil extracellular traps.36,37 Monocytes and DC at birth express lower levels of TLR, MHC-II, and other costimulatory molecules such as CD80/CD86 and CD40, thus resulting in poor signaling pathways and inefficient antigen presentation.38–41 Circulating NK cells in neonates are marginally higher than those detected in adults. Cord blood NK cells are more likely to be CD56bright immunoregulatory cells that are postulated to play a role during MF tolerance. Although the level of CD16+ cells are comparable to those in adult peripheral blood, neonatal NK cells carry lower levels of cytoplasmic granules and exhibit poor degranulation.42 This functional “immaturity” of neonatal innate immunity may be responsible for the increased susceptibility to infectious disease in the first year of life. Therefore any perturbations mediated by HIV exposure on the developing immune system during early infancy could further exacerbate the risk of infections in the affected infants.
One such perturbation is that exposure to HIV in utero is associated with decreased neutrophil numbers.43 Neutrophil levels were 2-fold lower among HIV-exposed infants compared with unexposed infants. Moreover, earlier studies determined that use of ARV during pregnancy suppresses development of myeloid cells in the fetal bone marrow resulting in decreased levels of neutrophils and monocytes.44,45 Siawaya and colleagues measured neutrophil oxidative burst responses to bacterial antigens of HEU infants and observed that 36% of the infants had functionally impaired neutrophil responses. Mechanisms for this impairment could be attributed to defective TLR-4 recognition of antigens in blood of HEU infants resulting in unresponsive neutrophils.46,47 Reduced level and function of neutrophils in HEU infants could contribute to increased bacterial infection observed in these infants.5
Quantitative differences of innate cells between HUU and HEU infants have been reported for monocytes and DC from birth up until 20 months of age, although such differences were transient with age.43,48 A study by Reikie et al, however, observed no differences in the level of monocytes and DC infants between HEU and HUU infants,49 suggesting that alterations of antigen presenting cells mediated by in utero HIV exposure could be limited to their function.
Indeed, following activation with either LPS or TLR-9 agonist, DC from HEU infants expressed higher levels of costimulatory molecules compared to HUU infants.48 This higher level of innate activation in HEU paralleled increased inflammatory responsiveness. TLR stimulation of monocytes and conventional DC resulted in higher levels of IL-6, IL-12, and TNF-α expression among HEU infants at 2 weeks of age compared to HUU infants.49 Therefore, compared with HIV-unexposed infants, HEU infants tend to exhibit higher activation and expression of proinflammatory cytokines, although such differences were transient with age. Exposure to maternal HIV antigens and/or opportunistic bacterial and viral infections could be responsible for priming innate immunity of HEU infants resulting in increased level of activation compared to HUU infants. Whether such alterations of the innate immune system account for the immunodeficiencies in the affected infants that are associated with higher susceptibility to viral and bacterial infections remain to be confirmed. Given that HEU infants are more likely to suffer from infectious diseases, alteration in the innate immune system could have detrimental effects on the functional response of adaptive immunity and hence leading to increased infectious diseases susceptibility in HEU infants.
There is currently no consensus regarding disparity of NK cells frequencies between HEU and HUU infants. At birth and 6 months of age, HEU infants were reported to have lower proportion of NK cells compared to HUU infants.50 Similarly, HEU adolescents had lower levels of NK cells detected in their peripheral blood compared to HUU controls.51 In contrast, no differences in absolute NK cells count were observed between the 2 groups of infants, although differences were noted when comparing different NK subsets.52 Activated NK cells (CD38+ CD69+) were higher in HEU infants <6 month of age.52 Smith et al. observed higher level of cytolytic NK cells at birth in HEU infants as measured by the ability to kill human malignant cells and expression of the degranulation molecule CD107a. Despite increased cytolytic activity observed from HEU infants, fewer activated NK cells were expressing perforin and INF-γ compared to HUU infants mechanisms of which are unclear.50 Moreover, the level of NK cells expressing perforin decreased with age among HEU infants while there was a positive correlation between perforin-expressing NK cells and age in HUU infants.52 These results indicate early priming of NK cells among HEU resulting in loss of cytolytic activity of NK cells. Chronic immune activation of NK cells has been demonstrated to cause exhaustion of their cytolytic function. Therefore a proinflammatory in utero environment may be driving early activation of NK cells, which decreases their cytolytic activity over time. Decreased frequency of perforin expressing NK cells reported tend to coincides with the period of increased viral infection in HEU infants and could be a possible mechanism for enhanced susceptibility to infections.
2.2 |. Adaptive immunity in HEU
Adaptive immunity differs from innate immunity in terms of antigen specificity and the generation of specific memory recall responses. Maturation of lymphocytes occurs early in gestation, and at birth, the proportion of mature lymphocytes is equitable to those of adults.16 However, limited antigen exposure in utero to prime adaptive immunity contributes to naivety of neonatal immunity. Infants have low numbers of memory effector T cells (CD45RA− CD45RO+) and B cells (CD27+) and consequently high proportion of naïve lymphocytes. Additionally, a high proportion of neonatal lymphocytes are recent thymic emigrants (RTE), which are phenotypically and functionally distinct from mature naïve T cells, being less effective in responding to antigen stimulation.53,54 The proportion of memory cells gradually increases as infants are exposed to environmental antigens and possibly as the gut becomes colonized and reach adult levels at ~2 years of age. During early infancy, the adaptive immune system is skewed toward Th2 phenotype resulting in lower Th1/Th17 cells, hence poor proinflammatory responses.55 Moreover, RTE CD4+ cells are biased toward Th2 cytokines, which further contributes to decreased Th1 and Th17 responses.54 Further, suppressive cells present during fetal life, such as MDSC and Tregs, may persist into infancy, thus actively suppressing lymphocyte function.56 Legrand et al. reported high frequency of Tregs (CD4+ CD25+ CD127−) in cord blood of HEU infants compared to unexposed controls. However, at 3 and 12 months of age, similar levels of Tregs (CD4+ CD25hi Foxp3+) were reported between HEU and HUU infants.57
Important physiological impairments observed in HEU infants are reduced thymic size and total lymphocyte counts compared to age-matched HUU controls.58,59 Lymphocytes of HIV-exposed infants are also phenotypically different from that of HIV-unexposed infants and resemble that of HIV-infected infants, being skewed toward memory (CD45RO+) and an activated state–increased expression of CD38+ and HLA-DR+.58,60–62 A correlation between maternal HIV viral load and activated CD8+ CD38+ HLA-DR+ cells in HEU has been reported.63 These results indicate immune experience toward HIV antigens among HEU infants as drivers of memory differentiation and immune activation.
2.2.1 |. HIV-specific responses
HEU infants have been reported to have HIV-specific CD4 and CD8 responses.64–69 Moreover, the removal of suppressive Tregs (CD4+ CD127−CD25+) in HEU infants unmasked strong Gag-specific responses highlighting the presence of HIV antigen-experienced lymphocytes.68 CD8 cytotoxic T lymphocytes (CTLs) are important for clearing viral infections and during HIV infection these cells drastically increase. Whether the presence of HIV-specific CTLs play a role in prevention of MTCT of HIV is doubtful, since the frequency of HIV-specific CTLs increased with infant age and breastfeeding practice,69 but was unrelated to preventing viral transmission.
2.2.2 |. Polyclonal activation
Lymphoproliferation and cytokine expression following polyclonal activation in HIV-infected infants is significantly reduced compared to that observed among HEU infants.70,71 HIV viral load and lymphocyte count strongly influence the cellular immune responses in HIV-infected infants. Infants with high HIV viral load and very low CD4 cell counts (≤350 cells/mm3) showed severely attenuated immune responses. When HEU infants were compared to HUU infants, the proportion of proliferating CD4+ and CD8+ cells following staphylococcal enterotoxin B (SEB) activation were higher in HEU infants, although the majority of these proliferating cells did not appear polyfunctional.72 In other studies, T cell proliferation responses to PHA did not differ between HEU and HIV-unexposed infants.73,74
Different studies have reported variations in cytokine responses between HEU and HUU infants depending on the mitogen used, assay methods, and infant’s age without consensus. Studies by Rich et al. and Borges-Almeida et al. reported significantly less IL-2 and IL-4 production by CD4+ cells in responses to PHA in HEU compared to HUU infants.60,73 Whole blood from HEU neonates stimulated with PHA released higher concentration of INF-γ than HUU infants. PMA and ionomycin induced strong IL-2 response in CD4 cells of HEU infants.58 Dual expression of INF-γ/IL-2 or INF-γ/TNF-α were also higher for CD4 and CD8 cells following stimulation with SEB in HEU infants compared to HUU infants at 3 months of age, these responses however dissipated by 12 months of age.57 A proinflammatory maternal environment, due to chronic immune activation by HIV infection, may be priming the immune system of HEU infants resulting in elevated cytokine responses to polyclonal activation. Whether cellular immune responses to vaccination with in utero HIV-exposure are compromised is therefore controversial.
2.2.3 |. Vaccine responses
The Expanded Program on Immunization (EPI) to control vaccine-preventable diseases during early childhood has been the most cost-effective strategy in reducing child mortality. Cellular immune responses of HIV-infected infants to vaccination are severely compromised, and this increases their risk of infectious diseases.71,75 Whether in utero HIV exposure in the absence of infection compromises cellular immune responses to EPI vaccines is still unclear. Studies on cell-mediated immunity to vaccination are confounded by differences in the type of cellular read-out, assay conditions, vaccine antigens, the cohort settings including degree of maternal immune compromise, and the age at which vaccine responses are measured, making it difficult to interpret the results.
Mazzola et al. reported reduced frequency of proliferating T cells following 6 days of in vitro bacillus Calmette-Guérin (BCG) stimulation of PBMCs from HEU infants compared to those of HUU infants at 6–8 months of age.74 Similarly, in vitro culture of PBMCs with PPD or BCG induced poor proliferation of CD4+ and CD8+ T cells in HEU infants.76 The reduced proliferative responses to BCG stimulation among HEU infants may be supported by the finding that a higher proportion of their CD4+ cells express markers of immune exhaustion (CD57 and PD-1) compared to their unexposed counterparts.76 Moreover, significantly lower IFN-γ and IL-13 BCG responses were measured in whole blood of HEU infants at 6 weeks of age,72,77 and by 14 weeks of age proliferating CD4+ and CD8+ cells were higher in HEU, albeit these responding cells were less polyfunctional compared to HUU infants.72 In contrast, other studies have observed no differences in the magnitude or polyfunctional responses to BCG stimulation between HEU and HUU infants.57,78 In addition, effects of HIV exposure on acellular pertussis, tetanus, and influenza vaccine specific T cell responses were not detected.57,67,72 Nevertheless, immunogenicity to BCG vaccine could be partially restored by delaying vaccination for up to 8 weeks.79
3 |. RELATIONSHIP OF BREASTFEEDING AND MICROBIOTA COMPOSITION OF HIV-EXPOSED INFANTS
3.1 |. Breastfeeding
Following the recommendation that HIV-infected women should avoid breastfeeding to limit HIV MTCT via breast milk, the rates of infant mortality drastically increased, especially among those residing in low-income countries.3 Higher infant mortality among nonbreastfed infants were driven in part by malnutrition and an increase in infectious diseases.10,80 Therefore, to assuage the increased risk of infant morbidity while restricting HIV MTCT, other feeding practices were explored including early or abrupt cessation of breastfeeding or mixed feeding.2 Of the studies that compared adverse infectious events between breastfed and formula fed or nonbreastfed HEU infants, exclusive breastfeeding was associated with lower relative risk of infectious disease and hospitalization in <12 months old infants.80–83 Risk of malaria infection was lower in breastfed HEU infants of 6–15 month of age compared to nonbreastfed infants; however, among HUU infants, the incidence of malaria was not influenced by the differences in feeding modes. Shapiro et al. demonstrated that despite lack of differences in maternal immunological profiles including pathogen-specific IgG and IgA titers, rates of mortality in HEU infants (6.7%) were higher than HIV-unexposed infants (1.6%), with lack of breastfeeding being the strongest predictor of infant morbidity and mortality.84 Mechanisms responsible for this outcome are primarily due to breast milk being inherently rich in maternal Abs and antimicrobial compounds capable of conferring protection against gastrointestinal infections to immunological naïve infants. Breast milk from HIV-infected mothers also contains high concentration of soluble TLR that may inhibit HIV-infection and result in immunomodulatory effects on the infant gut.85
3.2 |. Gut microbiota
In addition to nutritional benefits and passive immunity, mothers’ breast milk consists of commensal bacteria that colonizes and modulates microbial communities in the gut of the growing infant.13 Gut microbes of breastfed infants differ to those of nonbreastfed infants13,86,87 and are dependent on the delivery mode, duration of breastfeeding, lactation period, and maternal health including HIV-infection status.12,14,87,88 Therefore, because exclusively breastfed infants tend to be healthier than other infants who are initiated on different feeding modalities, maternally derived microbiota serve as probiotics that influence the maturation of the infants’ gut microbiota and may be crucial in protecting against infectious diseases.
The link between establishment of a healthy microbiota during infancy and protection against infectious disease rests on the critical interactions of the gut microbiota and immune system.89 Early microbial communities colonizing the infants gut promote the development and maturation of the immune system that determines their response to commensal microbes and invading pathogens. This has been well demonstrated in murine models, where germ-free mice exhibit an impaired development of lymphoid structures such as the spleen, payers patches, mesenteric lymph nodes, and isolated lymphoid follicles.90,91 The composition of the microbiota is also important in educating the immune system with infants lacking specific microbial species prone to having higher risk of infectious diseases. The presence of specific microbial species promote different immunological pathways and polarization of CD4+ T cells into specific subsets that are subsequently important in maintaining a symbiont relationship with the commensal microbes.92–96 In addition to modulating polarization of the immune system, the absence of segmented filamentous bacteria in the gut tends to dampen antibody responses to influenza vaccines.97 In humans, the Phylum Actinobacteria in infants’ stool was more abundant among infants who responded well to BCG, tetanus, and oral polio vaccines when measured by vaccine-specific T cell proliferation or increase vaccine specific IgG.98 Abundance of Firmicutes particularly those belonging to Clostridium cluster XI and Proteobacteria positively correlated with IgA responses to rotavirus vaccine.99 The importance of breast milk-derived commensal bacteria has been well demonstrated in clinical trials testing supplementation of formula milk with microbial species and association with vaccine responses.
The impact of microbiota on shaping immunity is strongest during early infancy and is likely due to a critical window during which microbiota can influence immunity.89,100 Breastfed rhesus macaques had distinct microbial profiles compared to formula-fed macaques that persisted up until 12 months of age. Lower diversity, richness, and evenness were observed in the microbiota of formula-fed macaques compared to breastfed. These differences promoted varying immune phenotypes with breastfed macaques characterized by having higher levels of Th17 and memory CD4+ cells.101 Similarly, exclusively breastfed HEU infants tend to have less diverse microbial communities compared to formula or mix-fed HEU infants.102 In turn, the low microbial diversity in nonexclusively breastfed infants was associated with higher gut-homing (α4β7+) immune activated lymphocytes (CCR5+ HLA-DR+ CD25+).102,103 Additionally, changes in microbial profiles in the gut of HEU infants are independent of feeding choice but appear to be influenced by maternal HIV status. Compared with HIV-unexposed infants, gut microbiota of breastfed HEU infants have lower α-diversity at 6 weeks of life.12 HUU infants show an abundance of Bacteriodes fragilis, bacterial species associated with promotion of T cell immune development in the gut.12,90 These results illustrate how the effects of in utero HIV exposure and breastfeeding impact on the composition of the infant’s gut microbiota, which we posit has a large impact on the developing infant immune system. Together these factors potentially determine the inflammatory status of the newborn infant (Fig. 1).
The imparity of gut microbiota between HEU and HUU breastfed infants may possibly be due to varying oligosaccharide composition of the breast milk. Maternal breast milk contains complex carbohydrates referred to as HMO that are indigestible by the infants. HMO are glycans of highly diverse structural composition including sialylated and fucosylated moieties. HMO are an essential nutrient source for gut microbiota, thus acting as prebiotics that influences the composition of gut microbiota.104 In addition, HMO are capable of preventing infection by directly binding to pathogens, blocking toxins or pathogen receptors expressed on epithelial cells. HMO also pose immune modulatory properties such as affecting immune gene expression of epithelial cells or indirectly through promoting specific microbial species. These findings highlight the important role of HMO in preventing HIV transmission, reducing diarrheal diseases, and promoting maturation of immune system in infants. HMO composition in HIV-infected breast milk differs from that of HIV-uninfected mothers’ milk and this difference influences the infant gut microbiota.12,105 Mortality in <2 year old HEU infants was associated with the composition of HMOs, with milk containing higher concentration of fucosylated HMO associated with reduced infant mortality.106 The inclusion of HMO in artificial milk has been documented to provide similar health benefits as maternal breast milk making it an alternative feeding option for infants.107 However, these results should be taken with caution because a combination of factors together with breast milk HMOs, such as exposure to antibiotics, maternal antibodies, and the inherited gut virome, is likely to influence gut microbiota of HEU and ultimately impact on health outcomes.
4 |. CONCLUDING REMARKS
HEU infants tend to exhibit activated innate immunity that responds strongly to stimulation with PRR agonists; secreting higher levels of proinflammatory cytokines compared to HIV-unexposed controls. Inflammatory cytokines passively transferred from HIV infected mothers may be having an adjuvant effect on infant innate immunity and possibly shifting adaptive cellular responses to a more exhausted state. The function of adaptive immune system in infants seems to be minimally influenced by in utero HIV-exposure itself, with attenuated cellular immunity among HEU infants restricted to Th1 responses. However, different studies report conflicting or no differences between HEU and HUU infants following vaccination. More attention is required to quantify the phenotypic and functional differences between HEU and HUU innate and adaptive immunity.
In addition to intrauterine HIV exposure, breastfeeding avoidance or mixed feeding increases the rates of mortality among HEU infants. Lack of beneficial nutrients, antimicrobial components, and maternal Abs are the main contributors to higher mortality in these infants. Recent studies have also highlighted differential seeding and maturation of the gut microbiota between infants born to HIV-infected and HIV-uninfected mothers. Differences in gut microbiota between HEU and HIV-unexposed infants may be linked with altered immunological development that consequently impacts infants’ susceptibility to infections. Exact mechanisms responsible for these alterations are still under investigation, and more studies are required to investigate their clinical relevance in increasing the vulnerably of HIV-exposed infants.
Abbreviations:
- ARV
antiretroviral
- BCG
bacillus Calmette-Guérin
- cART
combination antiretroviral therapy
- CTL
cytotoxic T lymphocyte
- DC
dendritic cells
- EPI
Expanded Program on Immunization
- dNK
decidual NK
- HEU
HIV-exposed uninfected
- HMO
human milk oligosaccharides
- MDSC
myeloid-derived suppressor cells
- MF
maternofetal
- MTCT
mother-to-child transmission
- PPR
pathogen recognition receptors
- RTE
recent thymic emigrants
- SEB
staphylococcal enterotoxin B
- Tregs
regulatory T cells
Footnotes
CONFLICT OF INTEREST
All authors declare no conflict of interest.
REFERENCES
- 1.UNAIDS. UNAIDS data 2017, Joint United Nations Programme on HIV/AIDS (UNAIDS). Geneva, Switzerland; 2017. [Google Scholar]
- 2.Kuhn L, Aldrovandi G. Survival and health benefits of breastfeeding versus artificial feeding in infants of HIV-infected women: Developing versus developed wold. Clin Perinatol. 2010;37:843–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegfried N, van der Merwe L, Brocklehurst P, Sint TT. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection (Review). Cochrane Library Syst Rev. 2011. 10.1002/14651858.CD003510.pub3.www.cochranelibrary.com. [DOI] [PubMed] [Google Scholar]
- 4.Slogrove AL, Cotton MF, Esser MM. Severe infections in HIV-exposed uninfected infants: clinical evidence of immunodeficiency. J Trop Pediatr. 2010;56:75–81. [DOI] [PubMed] [Google Scholar]
- 5.Slogrove A, Reikie B, Naidoo S, et al. HIV-exposed uninfected infants are at increased risk for severe infections in the first year of life. J Trop Pediatr. 2012;58:505–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuhn L, Kasonde P, Sinkala M, et al. Does severity of HIV disease in HIV-infected mothers affect mortality and morbidity among their uninfected infants? Clin Infect Dis. 2005;41:1654–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taron-Brocard C, Le Chenadec J, Faye A, et al. Increased risk of serious bacterial infections due to maternal immunosuppression in HIV-exposed uninfected infants in a European country. Clin Infect Dis. 2014;59:1332–1345. [DOI] [PubMed] [Google Scholar]
- 8.Chalashika P, Essex C, Mellor D, Swift JA, Langley-Evans S. Birth-weight, HIV exposure and infant feeding as predictors of malnutrition in Botswanan infants. J Hum Nutr Diet. 2017;30:779–790. [DOI] [PubMed] [Google Scholar]
- 9.Nduati R, John G, Mbori-Ngacha D, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1. JAMA. 2000;283: 1167–1174. [DOI] [PubMed] [Google Scholar]
- 10.Bork KA, Cournil A, Read JS, et al. Morbidity in relation to feeding mode in African HIV-exposed, uninfected infants during the. Am J Clin Nutr. 2014;100:1559–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azad MB, Konya T, Maughan H, et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. 2013;185:373–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bender JM, Li F, Martelly S, et al. Maternal HIV infection influences the microbiome of HIV-uninfected infants. Sci Transl Med. 2016;8:349ra100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pannaraj PS, Li F, Cerini C, et al. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 2017;171:647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.González R, Mandomando I, Fumadó V, et al. Breast milk and gut microbiota in African mothers and infants from an area of high HIV prevalence. PLoS One. 2013;8:e80299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toukam C, Sainani KL, Osawe S, et al. Breastfeeding mitigates the effects of maternal HIV on infant infectious morbidity in the Option B R era : a multicenter prospective cohort study. AIDS. 2018;32 10.1097/QAD.0000000000001974 [DOI] [PubMed] [Google Scholar]
- 16.Hayward AR. The human fetus and newborn: development of the immune response. Birth Defects Orig Artic Ser. 1983;19: 189–194. [PubMed] [Google Scholar]
- 17.Kollmann TR, Kampmann B, Mazmanian SK, Marchant A, Levy O. Protecting the newborn and young infant from infectious diseases: lessons from immune Ontogeny. Immunity. 2017;46:350–363. [DOI] [PubMed] [Google Scholar]
- 18.Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol. 2013;13:23–33. [DOI] [PubMed] [Google Scholar]
- 19.Koopman LA, Kopcow HD, Rybalov B, et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Bouteiller P. Human decidual NK cells: unique and tightly regulated effector functions in healthy and pathogen-infected pregnancies. Front Immunol. 2013;4:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu B, Li X, Sun R, et al. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal-fetal interface. Proc Natl Acad Sci. 2013;110:E231–E240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moffett A, Colucci F. Uterine NK cells: active regulators at the maternal-fetal interface. J Clin Invest. 2014;124:1872–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siewiera J, El Costa H, Tabiasco J, et al. Human Cytomegalovirus infection elicits new decidual natural killer cell effector functions. PLoS Pathog. 2013;9:e1003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu B, Zhou Y, Ni X, et al. Natural killer cells promote fetal development through the secretion of growth-promoting factors. Immunity. 2017;47:1100–1113.e6. [DOI] [PubMed] [Google Scholar]
- 25.Xiao P-L, Zhou Y-B, Chen Y, et al. Association between maternal HIV infection and low birth weight and prematurity: a meta-analysis of cohort studies. BMC Pregnancy Childbirth. 2015;15:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svensson-Arvelund J, Ernerudh J, Buse E, et al. The placenta in toxicology. Part II: Systemic and local immune adaptations in pregnancy. Toxicol Pathol. 2014;42:327–338. [DOI] [PubMed] [Google Scholar]
- 27.Ning F, Liu H, Lash GE. The role of decidual macrophages during normal and pathological pregnancy. Am J Reprod Immunol. 2016;75: 298–309. [DOI] [PubMed] [Google Scholar]
- 28.Johnson EL, Chakraborty R. Placental Hofbauer cells limit HIV-1 replication and potentially offset mother to child transmission (MTCT) by induction of immunoregulatory cytokines. Retrovirology. 2012;9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490: 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kostlin N, Ostermeir AL, Sprin B, et al. HLA-G promotes myeloid-derived suppressor cell accumulation and suppressive activity during human pregnancy through engagement of the receptor ILT4. Eur J Immunol. 2017;47:374–384. [DOI] [PubMed] [Google Scholar]
- 31.Reikie BA, Naidoo S, Ruck CE, et al. Antibody responses to vaccination among South African HIV-exposed and unexposed uninfected infants during the first 2 years of life. Clin Vaccine Immunol. 2013; 20:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simani OE, Alane I, Violari A, et al. Effect of HIV-1 exposure and antiretroviral treatment strategies in HIV-infected children on immunogenicity of vaccines during infancy. AIDS. 2014;28:531–541. [DOI] [PubMed] [Google Scholar]
- 33.Church JA, Rukobo S, Govha M, et al. Immune responses to oral poliovirus vaccine in HIV-exposed uninfected Zimbabwean infants. Hum Vaccin Immunother. 2017;5515:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott S, Moss WJ, Cousens S, et al. The influence of HIV-1 exposure and infection on levels of passively acquired antibodies to measles virus in Zambian infants. Clin Infect Dis. 2007;45:1417–1424. [DOI] [PubMed] [Google Scholar]
- 35.Jones CE, Naidoo S, De Beer C, Esser M, Kampmann B, Hesseling AC. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA. 2011;305: 576–584. [DOI] [PubMed] [Google Scholar]
- 36.Carr R. Neutrophil production and function in newborn infants. Br J Haematol. 2000;110:18–28. [DOI] [PubMed] [Google Scholar]
- 37.Al-Hertani W, Yan SR, Byers DM, Bortolussi R. Human newborn polymorphonuclear neutrophils exhibit decreased levels of MyD88 and attenuated p38 phosphorylation in response to lipopolysaccharide. Clin Invest Med. 2007;30:44–53. [DOI] [PubMed] [Google Scholar]
- 38.Jones CA, Holloway JA, Warner JO. Phenotype of fetal monocytes and B lymphocytes during the third trimester of pregnancy. J Reprod Immunol. 2002;56:45–60. [DOI] [PubMed] [Google Scholar]
- 39.Förster-Waldl E, Sadeghi K, Tamandl D, et al. Monocyte toll-like receptor 4 expression and LPS-induced cytokine production increase during gestational aging. Pediatr Res. 2005;58:121–124. [DOI] [PubMed] [Google Scholar]
- 40.Sadeghi K, Berger A, Langgartner M, et al. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J Infect Dis. 2007;195:296–302. [DOI] [PubMed] [Google Scholar]
- 41.Willems F, Vollstedt S, Suter M. Phenotype and function of neonatal DC. Eur J Immunol. 2009;39:26–35. [DOI] [PubMed] [Google Scholar]
- 42.Guilmot A, Hermann E, Braud VM, Carlier Y, Truyens C. Natural killer cell responses to infections in early life. J Innate Immun. 2011;3: 280–288. [DOI] [PubMed] [Google Scholar]
- 43.Bunders MJ, Bekker V, Scherpbier HJ, Boer K, Godfried M, Kuijpers TW. Haematological parameters of HIV- 1 muninfected infants born to HIV- 1-infected mothers. Acta Pediatrica. 2005;94:1571–1577. [DOI] [PubMed] [Google Scholar]
- 44.Le Chenadec J, Mayaux MJ, Guihenneuc-Jouyaux C. Perinatal antiretroviral treatment and hematopoiesis in HIV-uninfected infants. AIDS. 2003;17:2053–2061. [DOI] [PubMed] [Google Scholar]
- 45.Pacheco SE, McIntosh K, Lu M, et al. Effect of perinatal antiretroviral drug exposure on hematologic values in HIV-Uninfected children: an analysis of the women and infants transmission study. J Infect Dis. 2006;194:1089–1097. [DOI] [PubMed] [Google Scholar]
- 46.Maloupazoa Siawaya AC, Mveang-Nzoghe A, Mvoundza Ndjindji O, Mintsa Ndong A, Essone PN, Djoba Siawaya JF. Cases of impaired oxidative burst in HIV-exposed uninfected infants’ neutrophils: a pilot study’. Front Immunol. 2017;8:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maloupazoa Siawaya AC, Mvoundza Ndjindji O, Kuissi Kamgaing E, et al. Altered toll-like receptor-4 response to lipopolysaccharides in infants exposed to HIV-1 and its preventive therapy. Front Immunol. 2018;9:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Velilla PA, Montoya CJ, Hoyos A, Moreno ME, Chougnet C, Rugeles MT. Effect of intrauterine HIV-1 exposure on the frequency and function of uninfected newborns’ dendritic cells’. Clin Immunol. 2008; 126:243–250. [DOI] [PubMed] [Google Scholar]
- 49.Reikie BA, Adams RCM, Leligdowicz A, et al. Altered innante immune development in HIV-exposed uninfected infants. J Acquir Immune Defic Syndr. 2014;66:245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith C, Jalbert E, de Almeida V, et al. Altered natural killer cell function in HIV-exposed uninfected infants. Front Immunol. 2017;8: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vigano A, Saresella M, Schenal M, et al. Immune activation and normal levels of endogenous antivirals are seen in healthy adolescents born of HIV-infected mothers. AIDS. 2007;21:245–248. [DOI] [PubMed] [Google Scholar]
- 52.Slyker JA, Lohman-Payne B, John-Stewart GC, et al. The impact of HIV-1 infection and exposure on natural killer (NK) cell phenotype in kenyan infants during the first year of life. Front Immunol. 2012;3: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haines CJ, Giffon TD, Lu LS, et al. Human CD4+ T cell recent thymic emigrants are identified by protein tyrosine kinase 7 and have reduced immune function. J Exp Med. 2009;206:275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hendricks DW, Fink PJ. Recent thymic emigrants are biased against the T-helper type 1 and toward the T-helper type 2 effector lineage. Blood. 2011;117:1239–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dowling DJ, Levy O. Ontogeny of early life immunity. Trends Immunol. 2014;35:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gantt S, Gervassi A, Jaspan H, Horton H. The role of myeloid-derived suppressor cells in immune ontogeny. Front Immunol. 2014;5(August): 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcia-Knight MA, Nduati E, Hassan AS, et al. Altered memory T-cell responses to Bacillus Calmette-Guerin and tetanus toxoid vaccination and altered cytokine responses to polyclonal stimulation in HIV-exposed uninfected Kenyan infants. PLoS One. 2015;10:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clerici M, Saresella M, Colombo F, et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood. 2000;96:3866–3871. [PubMed] [Google Scholar]
- 59.Kolte L, Rosenfeldt V, Vang L, et al. Reduced thymic size but no evidence of impaired thymic function in uninfected children born to human immunodeficiency virus-infected mothers. Pediatr Infect Dis J. 2011;30:325–330. [DOI] [PubMed] [Google Scholar]
- 60.Rich KC, Rich KC, Siegel JN, Jennings C, Rydman RJ, Landay AL. Function and phenotype of immature CD4+ lymphocytes in healthy infants and early lymphocyte activation in uninfected infants of human immunodeficiency virus-infected mothers. Clin Diagn Lab Immunol. 1997;4:358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ono E, Nunes dos Santos AM, de Menezes Succi RC, et al. Imbalance of naive and memory T lymphocytes with sustained high cellular activation during the first year of life from uninfected children born to HIV-1-infected mothers on HAART. Braz J Med Biol Res. 2008; 41:700–708. [DOI] [PubMed] [Google Scholar]
- 62.Rainwater-Lovett K, Nkamba H, Mubiana-Mbewe M, Moore CB, Margolick J, Moss WJ. Changes in cellular immune activation and memory T cell subsets in HIV-Infected Zambian children receiving HAART. J Acquir Immune Defic Syndr. 2014;67:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Deus N, Moraleda C, Serna-Bolea C, Renom M, Menendez C, Naniche D. Impact of elevated maternal HIV viral load at delivery on T-cell populations in HIV exposed uninfected infants in Mozambique. BMC Infect Dis. 2015;15:21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheynier R, Langlade-Demoyen P, Marescot M-R, et al. Cytotoxic T lymphocyte responses in the peripheral blood of children born to human immunodeficiency virus-1-infected mothers. Eur J Immunol. 1992;22:2211–2217. [DOI] [PubMed] [Google Scholar]
- 65.Rowland-Jones S, Nixon DF, Gotch F, et al. HIV-specific cytotoxic T-cell activity in an HIV-exposed but uninfected infant. Lancet North Am Ed. 1993;341:860–861. [DOI] [PubMed] [Google Scholar]
- 66.De Maria A, Cirillo C, Moretta L. Occurrence of human immunodeficiency virus type 1 (HIV-1)-specific cytolytic T cell activity in apparently uninfected children born to HIV-1-infected mothers. J Infect Dis. 1994;170:1296–1299. [DOI] [PubMed] [Google Scholar]
- 67.Kuhn L, Coutsoudis A, Moodley D, et al. T-helper cell responses to HIV envelope peptides in cord blood: protection against intrapartum and breast-feeding transmission. AIDS. 2001;15:1–9. [DOI] [PubMed] [Google Scholar]
- 68.Legrand FA, Nixon DF, Loo CP, et al. Strong HIV-1-specific T cell responses in HIV-1-exposed uninfected infants and neonates revealed after regulatory T cell removal. PLoS One. 2006;1:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.John-Stewart GC, Mbori-Ngacha D, Payne BL, et al. HIV-1–Specific cytotoxic T lymphocytes and breast milk HIV-1 transmission. J Infect Dis. 2009;199:889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Resino S, Bellon JM, Gurbindo D, Muñoz-Fernández MA. Disruption in cytokine and chemokine production by T-cells in vertically HIV-1-infected children. Acta Paediatr. 2001;90:989–997. [DOI] [PubMed] [Google Scholar]
- 71.Lohman-Payne B, Sandifer T, OhAinle M, et al. In-utero infection with HIV-1 associated with suppressed lymphoproliferative responses at birth. Clin Exp Immunol. 2014;178:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kidzeru EB, Huang J, Edwards LJ, et al. In-utero exposure to maternal HIV infection alters T-cell immune responses to vaccination in HIV-uninfected infants. AIDS. 2014;257:2432–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Borges-Almeida E, Milanez HMBPM, Vilela MMS, et al. The impact of maternal HIV infection on cord blood lymphocyte subsets and cytokine profile in exposed non-infected newborns. BMC Infect Dis. 2011;11:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mazzola TN, da Silva MT, Abramczuk BM, et al. Impaired Bacillus Calmette-Guérin cellular immune response in HIV-exposed, uninfected infants. AIDS. 2011;25:2079–2087. [DOI] [PubMed] [Google Scholar]
- 75.Tejiokem MC, Gouandjika I, Béniguel L, et al. HIV-infected children living in Central Africa have low persistence of antibodies to vaccines used in the expanded program on immunization. PLoS One. 2007;2: 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miles DJC, Gadama L, Gumbi A, et al. Human immunodeficiency virus (HIV) infection during pregnancy induces CD4 T-cell differentiation and modulates responses to Bacille Calmette-Guérin (BCG) vaccine in HIV-uninfected infants. Immunology. 2010;129:446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Van Rie A, Madhi SA, Heera JR, et al. Gamma interferon production in response to mycobacterium bovis BCG and mycobacterium tuberculosis antigens in infants born to human immunodeficiency virus-infected mothers. Society. 2006;13:246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mansoor N, Scriba TJ, de Kock M, et al. HIV-1 infection in infants severely impairs the immune response induced by Bacille Calmette-Guérin vaccine. J Infect Dis. 2009;199:982–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tchakoute CT, Hesseling AC, Kidzeru EB, et al. Delaying BCG vaccination until 8 weeks of age results in robust BCG-specific T-cell responses in HIV-exposed infants. J Infect Dis. 2015;211: 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marquez C, Okiring J, Chamie G, et al. Increased morbidity in early childhood among HIV-exposed uninfected children in uganda is associated with breastfeeding duration. J Trop Pediatr. 2014;60:434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Natchu UCM, Liu E, Duggan C, et al. Exclusive breastfeeding reduces risk of mortality in infants up to 6 mo of age born to HIV-positive Tanzanian women. Am J Clin Nutr. 2012;96:1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lamberti LM, Zakarija-Grković I, Fischer Walker CL, et al. Breastfeeding for reducing the risk of pneumonia morbidity and mortality in children under two: a systematic literature review and meta-analysis. BMC Public Health. 2013;13:S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rollins NC, Ndirangu J, Bland RM, Coutsoudis A, Coovadia HM, Newell M-L. Exclusive breastfeeding, diarrhoeal morbidity and all-cause mortality in infants of HIV-infected and HIV uninfected mothers: an intervention cohort study in KwaZulu natal, South Africa. PLoS One. 2013;8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shapiro RL, Lockman S, Kim S, et al. Infant morbidity, mortality, and breast milk immunologic profiles among breast-feeding HIV-Infected and HIV-Uninfected women in Botswana. J Infect Dis. 2007;196: 562–569. [DOI] [PubMed] [Google Scholar]
- 85.Henrick BM, Yao XD, Drannik AG, Abimiku A, Rosenthal KL, INFANT Study Team. Soluble Toll-like receptor 2 is significantly elevated in HIV-1 infected breast milk and inhibits HIV-1 induced cellular activation, inflammation and infection. AIDS. 2014;28: 2023–2032. [DOI] [PubMed] [Google Scholar]
- 86.Thompson AL, Thompson AL, Monteagudo-Mera A, Cadenas MB, Lampl ML, Azcarate-Peril MA. Milk- and solid-feeding practices and daycare attendance are associated with differences in bacterial diversity, predominant communities, and metabolic and immune function of the infant gut microbiome. Front Cell Infect Microbiol. 2015;5:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Madan JC, Hoen AG, Lundgren SN, et al. Association of cesarean delivery and formula supplementation with the intestinal microbiome of 6-week-old infants. JAMA Pediatr. 2016;170:212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cabrera-Rubio R, Collado MC, Laitinen K, Salminen S, Isolauri E, Mira A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr. 2012;96:544–551. [DOI] [PubMed] [Google Scholar]
- 89.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352:539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. [DOI] [PubMed] [Google Scholar]
- 91.Bouskra D, Brézillon C, Bérard M, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. [DOI] [PubMed] [Google Scholar]
- 92.Smith KD, Andersen-Nissen E, Hayashi F, et al. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol. 2003;4:1247–1253. [DOI] [PubMed] [Google Scholar]
- 93.Ivanov I, Frutos Rde L, Manel N, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Uematsu S, Fujimoto K, Jang MH, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing toll-like receptor 5. Nat Immunol. 2008;9:769–776. [DOI] [PubMed] [Google Scholar]
- 95.Atarashi K, Tanoue T, Shima T, et al. Induction of colonic regulatory T cells by indigenous Clostridium Species. Science. 2011;331: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Atarashi K, Tanoue T, Oshima K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. [DOI] [PubMed] [Google Scholar]
- 97.Oh JZ, Ravindran R, Chassaing B, et al. TLR5-Mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity. 2014;41:478–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huda MN, Lewis Z, Kalanetra KM, et al. Stool microbiota and vaccine responses of infants. Pediatrics. 2014;134:e362–e372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Harris VC, Ali A, Fuentes S, et al. Rotavirus vaccine response correlates with the infant gut microbiota composition in Pakistan. Gut Microbes. 2017;9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ardeshir A, Narayan NR, Méndez-Lagares G, et al. Breast-fed and bottle-fed infant rhesus macaques develop distinct gut microbiotas and immune systems. Sci Transl Med. 2014;6:252ra120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wood LF, Brown BP, Lennard K, et al. Feeding mode regulates gut microbial composition, peripheral T cell activation and mucosal gene expression in African infants. Clin Infect Dis. 2018. 10.1093/cid/ciy265/4961341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McFarland EJ, Powell TM, Onyango-Makumbi C, et al. Ontogeny of CD4+ t lymphocytes with phenotypic susceptibility to HIV-1 during exclusive and nonexclusive breastfeeding in HIV-1-exposed ugandan infants. J Infect Dis. 2017;215:368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Andreas NJ, Kampmann B, Mehring Le-Doare K. Human breast milk: a review on its composition and bioactivity. Early Hum Dev. 2015;91:629–635. [DOI] [PubMed] [Google Scholar]
- 105.Van Niekerk E, Autran CA, Nel DG, Kirsten GF, Blaauw R, Bode L. Human milk oligosaccharides differ between hiv-infected and hiv-uninfected mothers and are related to necrotizing enterocolitis incidence in their preterm very-low-birth-weight infants. J Nutr. 2014;144:1227–1233. [DOI] [PubMed] [Google Scholar]
- 106.Kuhn L, Kim HY, Hsiao L, et al. Oligosaccharide composition of breast milk influences survival of uninfected children born to hiv-infected mothers in Lusaka, Zambia. J Nutr. 2015;145:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reverri EJ, Devitt AA, Kajzer JA, Baggs GE, Borschel MW. Review of the clinical experiences of feeding infants formula containing the human milk oligosaccharide 2′-fucosyllactose. Nutrients. 2018;10 10.3390/nu10101346 [DOI] [PMC free article] [PubMed] [Google Scholar]