Abstract
Background:
In real-world practice, eribulin mesylate provides significant survival benefit, with a manageable safety profile in heavily pretreated patients with metastatic breast cancer (MBC).
Methods:
In this prospective, open-label, multicentre, observational study we evaluated the effectiveness and tolerability of eribulin as third-line treatment in a homogeneous population. The primary endpoints were the safety profile and response in metastatic sites; secondary endpoints included the response in different subtypes, overall response rate (ORR), progression-free survival (PFS) and overall survival (OS).
Results:
From 2013 to 2016, 118 women were treated in 21 Sicilian institutions; the median age was 58 years (range 29–79), with 69% of patients under 65. The median cycles of eribulin were 5.5 (range 1–26). The most common adverse event was neutropenia (9.3%, 3 cases of grade 3, 4 of grade 4); only 1 case of QT prolongation was reported. Eribulin was effective in controlling metastatic disease in all sites, and it achieved the highest ORR in brain (16%) and liver (14.9%). Median OS was 31.8 months (95% CI 27.9–34.4) and median PFS 5.5 months (95% CI 4.2–6.6). PFS was 5.2 months (95% CI 2.8–8.4) in patients with triple-negative subtype. Median PFS was longer in patients over 65 years (6.1 months, 95% CI 4.4–8.3). In patients who had visceral metastases PFS was 5.5 months (95% CI 95% 3.5–6.6) and OS 33.9 months (95% CI 29.8–40.8).
Conclusions:
Eribulin as third-line treatment shows an acceptable safety profile and a substantial antitumour activity in the treatment of MBC, even in elderly patients and in those with visceral disease.
Keywords: eribulin, metastatic breast cancer, multicentre, prospective, real world, third line
Introduction
Despite improvements in treatment, metastatic breast cancer (MBC) is still an incurable disease, with poor long-term survival and a 5-year survival rate lower than 25%.1 Patients with MBC are often resistant to anthracyclines and taxanes, usually used as first-line therapies, and even when these agents can be used, treatment failure occurs in most cases.2 However, cytotoxic chemotherapy remains the mainstay approach for MBC, especially for women with hormone-receptor positive breast cancer refractory to endocrine therapy and visceral crisis, and those with triple-negative BC. Treatments are aimed mainly at stabilizing or reducing the total disease burden, extending life expectancy and preserving quality of life.3 Among therapeutic options currently available to manage heavily pretreated patients with MBC, eribulin could be effective in patients with disease resistant to other tubulin-targeting agents. Unlike taxanes, eribulin inhibits only microtubule polymerization and blocks mitosis in G2-M phase, inducing apoptosis.4
Eribulin mesylate was approved for treatment of heavily pretreated patients with MBC based on results of the study 305/EMBRACE.5 A pooled analysis of pivotal trials 305 and 301 indicated that eribulin improved overall survival versus physician’s choice or capecitabine.5–7 Eribulin was effective in prolonging survival in HER2-negative or triple-negative subtypes, and in HER-2 positive women as monotherapy or in combination with HER2-targeted therapy.7,8 Eribulin showed a favourable safety profile, with neutropenia, fatigue and peripheral neuropathy as the most common adverse events.
Demographic and clinical characteristics of patients included in pivotal studies were often comparable with those observed in daily practice,9,10 and several studies have confirmed efficacy and safety of eribulin in MBC patients also in a real-world setting.9–13 However, no data are currently available on the effectiveness and safety of eribulin according to subtype and metastasis site in a homogeneous population. It has been described that a heavily chemopretreated woman with important bone, nodal, hepatic and choroidal involvement from breast cancer had a remarkable, unexpected and lasting disease response after treatment with eribulin.14 Further evidence is crucial to identify those patients who may have major benefits from the treatment.
With this aim, we designed the first prospective, observational registry to collect data from MBC patients who received eribulin in third-line treatment in Sicilian centres, and to associate the effectiveness and safety of eribulin with metastatic sites and BC subtypes: the VESPRY study (eValuation of Eribulin use in Sicily: a Prospective RegistrY).
Materials and methods
This open-label, multicentre, prospective observational study analysed data collected in a registry to evaluate the safety and effectiveness of eribulin mesylate in real-world setting. A total of 21 hospitals in Sicily enrolled consecutive patients who received at least one dose of eribulin in third-line therapy. All patients provided written informed consent, and the study protocol was approved by all relevant institutional ethics committees (approved by the Ethics Committee AO Ospedale Riuniti Papardo-Piemonte, Messina on 25 February 2013; Supplementary Table 1). The study was conducted in accordance with the provisions of the Declaration of Helsinki (2013) and local laws.
To be eligible for the study, women (>18 years old) must be histologically or cytologically diagnosed with MBC and previously treated with taxane- and anthracycline-based therapy, and all patients must have received two prior regimens for metastatic disease; have a known ER/PgR status (either positive or negative) and HER2 negative or positive disease, defined as an immunohistochemistry (IHC) status 0, 1+, 2+ or 3+ (if IHC was 2+, a negative or positive Silver in situ hybridization/fluorescence in situ hybridization/chromogenic in situ hybridization test was required); the different subtypes were defined according to St Gallen Guidelines;15 have adequate organ function, including hematologic, hepatic, renal and cardiac function. The exclusion criteria were nonindication for eribulin therapy, prior three chemotherapy lines for MBC, presence of symptomatic brain metastasis.
All patients received intravenous infusions of eribulin mesylate on day 1 and 8 in a 21-day cycle, at 1.23 mg/m2. Dosing reduction (0.97 and 0.62 mg/m2) was allowed to manage treatment-related toxicity; discontinuation occurred with unmanageable toxicity at 0.62 mg/m2. Tumour assessment was performed by computed tomography (CT) scan or positron emission tomography (PET) every four cycles. Electrocardiogram with QT measurement was repeated at day 8, every three cycles; haematological and chemical investigations were performed on the first day of each cycle; haematology was repeated at day 8. All treatment-related adverse events were recorded in the e-CRF, as per local laws.
Statistical analysis
Given the observational nature of the study, no formal statistical hypotheses were formulated a priori. However, the study was expected to enrol 120 patients within 8 months. The Gail and Simon’s test, usually used to test qualitative interactions between treatment effects and patient subsets,16 was targeted to ensure a precision deemed sufficient to estimate the confidence interval at 95%. The correlation among different clinical–pathological parameters and eribulin effectiveness was evaluated by chi-squared test. The association between the various clinical–pathological variables and clinical outcomes was estimated through both univariate and multivariate analyses. Univariate and multivariate analyses were conducted using Cox proportional hazards regression and results were considered as statistically significant with a p value <0.05. The overall response rate (ORR) was defined as the proportion of patients having a partial or complete response to therapy. The overall survival was defined as the time from study entry to death for any cause and the progression-free survival (PFS) as the time from study entry to radiological disease progression or death. PFS and tumour response were assessed by RECIST 1.1 criteria. The survival curves were estimated using the Kaplan–Meier method. Safety was assessed according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) Version 4.0.
Results
Patient characteristics and treatment
From March 2013 to October 2016, data from 118 patients were collected in the registry. The median age was 58 years (range 29–79), with 31% of patients over 65. Most patients (69.9%) had a luminal A subtype, 20.4% were triple negative, 5.8% luminal B and 3.9% HER2-enriched. Metastatic sites were lung in 43.2% of patients, bone in 67.5% and liver in 51.7%. A total of 61 patients (51.7%) had metastatic disease in two or more organs; 84 patients (72%) with metastases in lung, liver, kidney and ovary, were hereafter defined as having ‘visceral disease’. Tumour characteristics are summarized in Table 1. Most patients (n = 99, 87.6%) underwent surgery before the diagnosis of metastatic disease.
Table 1.
Disease characteristics.
| Subtypes | n | % |
|---|---|---|
| HER2 enriched | 4 | 3.9 |
| Luminal A | 72 | 69.9 |
| Luminal B | 6 | 5.8 |
| Triple negative | 21 | 20.4 |
| Metastasis | ||
| Lung | 51 | 43.2 |
| Kidney | 4 | 3.4 |
| Brain | 12 | 10.2 |
| Liver | 61 | 51.7 |
| Skeletomuscular | 79 | 67.5 |
| Othera | 48 | 42.1 |
Lymph nodes, skin, pleura, pericardium, ovary.
All patients had received anthracycline and taxane-based regimens before starting eribulin: 25% in neo-adjuvant setting, 72.7% in adjuvant setting, and 72% in metastatic setting; treatments prior eribulin in metastatic setting are described in Table 2.
Table 2.
Treatment prior to eribulin in metastatic setting.
| 1st line | Paclitaxel + bevacizumab | 34% |
| Capecitabine-Vinorelbine | 28% | |
| Paclitaxel–Trastuzumab | 3.9% | |
| Docetaxel | 7% | |
| Anthracycline | 9% | |
| Paclitaxel | 18% | |
| 2nd line | Paclitaxel | 25% |
| Nab-paclitaxel | 22% | |
| Capecitabine–vinorelbine | 28% | |
| Docetaxel | 14% | |
| Anthracycline monotherapy | 7% | |
| Anti-HER2 therapy | 3.9% |
Median 5.5 cycles of eribulin (range 1–26) were administrated. Most patients interrupted the treatment for disease progression (n = 101, 87%); only three (2.6%) patients discontinued for adverse events; among them only one showed an adverse event of CTCAE grade 3. During the treatment, 52 adverse events were accounted, of which 21 were related to treatment; the most common adverse events were of CTCAE grade 1 or 2. As shown in Table 3, we counted 11 cases of neutropenia (3 cases of grade 3, 4 cases of grade 4 of which 2 were febrile neutropenia), 3 of diarrhoea (2 of grade 1, one of grade 3), 3 of increased transaminases (all of grade 2), 1 of peripheral neuropathy (grade 1), 1 of mucositis (grade 4) and 1 of fatigue (grade 2). Neutropenia was managed with dose delays, reductions and granulocyte colony stimulating factor. Concerning cardiac toxicity, we observed a QT prolongation in one patient. The toxicity profile in women over 65 years did not significantly differ from that of younger patients.
Table 3.
Adverse events.
| Adverse event | n | CTCAE grade 1–2 | CTCAE grade 3–4 |
|---|---|---|---|
| Total | 52 | 32 | 12 |
| Related to treatment | 21 | 11 | 9 |
| Neutropeniaa | 11 | 3 | 7 |
| Diarrhoea | 3 | 2 | 1 |
| Transaminase elevation | 3 | 3 | – |
| Gastroesophageal reflux | 1 | n/a | n/a |
| Mucositis | 1 | – | 1 |
| Fatigue | 1 | 1 | – |
| Peripheral neuropathy | 1 | 1 | – |
Two cases of febrile neutropenia (CTCAE grade 4); n/a not available.
CTCAE, common terminology criteria for adverse events.
Effectiveness
Eribulin treatment determined an ORR significantly greater in patients with liver and brain metastasis; seven complete responses were observed as best response in lung, liver, bone and other metastatic sites; a partial response was observed in brain metastases (Table 4).
Table 4.
Best response at the metastatic site.
| n | Complete response n (%) |
Partial response n (%) |
Stable disease n (%) |
Progression disease n (%) |
|
|---|---|---|---|---|---|
| Overall | 98 | 3 (3.1) | 31 (31.6) | 38 (38.8) | 26 (26.5) |
| Lung | 83 | 2 (2.4) | 7 (8.4) | 56 (67.5) | 18 (21.7) |
| Kidney | 5 | – | 1 (20) | 1 (20) | 3 (60) |
| Brain | 25 | – | 4 (16) | 17 (68) | 4 (16) |
| Liver | 107 | 1 (0.9) | 15 (14) | 56 (52.4) | 35 (32.7) |
| Skeletomuscular | 115 | 2 (1.7) | 9 (7.8) | 73 (63.5) | 29 (25.2) |
| Other | 58 | 2 (3.5) | 10 (17.2) | 26 (44.8) | 20 (34.5) |
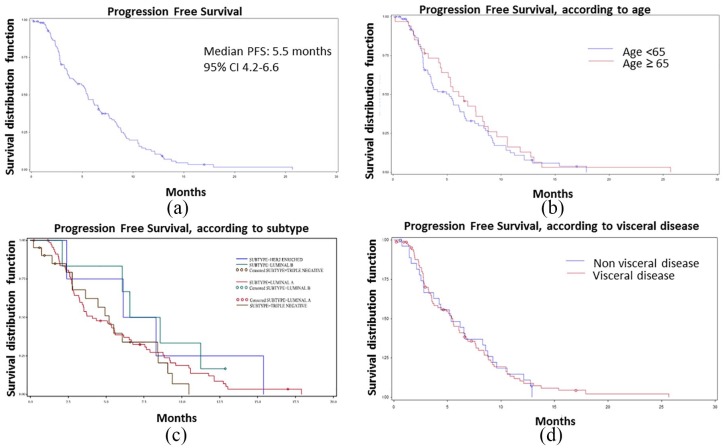
Median PFS of the overall population was 5.5 months (95% CI 4.2–6.6) (Figure 1). Three exploratory analyses were performed according to subtypes, age (over or under 65), and metastatic sites. PFS was 7.6 months (95% CI 2.1–not evaluable) in patients with luminal B, 5.2 months (95% CI 2.8–8.4) in triple-negative subtype, 4.1 months (95% CI 3.2–5.6) in patients with luminal A and 7.2 months (95% CI 2.4–15.4) with HER2-enriched subtype. After stratifying for age, PFS was 5.2 months (95% CI 3.4–6.2) in patients under 65 and 6.1 months (95% CI 4.4–8.3) in patients over 65. As for metastatic sites, PFS was 5.5 months (95% CI 3.5–6.6) in patients with visceral metastases and 5.3 months (95% CI 2.8–8.6) in patients with no visceral disease.
Figure 1.
Kaplan–Meier curve of PFS in (a) overall population and according to (b) age, (c) subtype and (d) visceral disease.
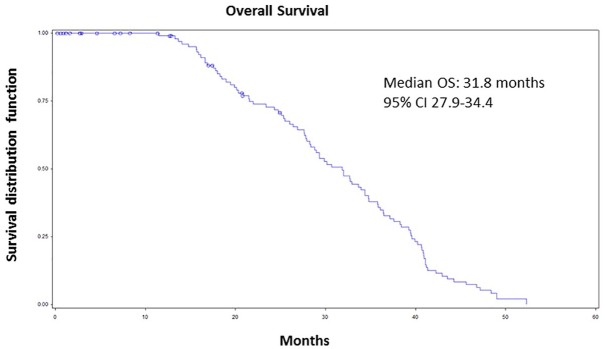
Median OS was 31.8 months (CI 95% 27.9–34.4) (Figure 2); after stratifying for age, median OS was 29.0 months (CI 95% 26.0–36.7) in patients under 65 and 34.8 months (CI 95% 29.3–39.5) in those over 65. As for metastasis sites, OS was 33.9 months (CI 95% 29.8–40.8) in patients with visceral metastases and 29.3 months (CI 95% 26.8–34.7) in the presence of bone and lymph node metastases.
Figure 2.
Kaplan–Meier curves of overall survival of overall population.
Discussion
Based on the former approval of this agent at the time of study’s design, we could define the use of eribulin in third-line therapy as an ‘early’ treatment. Pivotal studies enrolled even a small number of patients who received eribulin in third line but in our study, we included only patients who were treated in third-line therapy, with the specific aim to report eribulin effects in a homogenous population of not heavily pretreated women.
In third-line therapy, eribulin confirmed its effectiveness, achieving a disease control or response of metastatic lesions in liver, lung and bone. Few patients required interruption or dose delay during the treatment; therefore, treatment was well tolerated. Neutropenia, diarrhoea and increase in transaminase levels were the most frequent adverse events.
Neutropenia is one of the major dose-limiting adverse effects of eribulin mesylate and is usually managed with dose adaptation or reduction, based on the known exposure–response relationship.17 Neutropenia was the main reason of dose reduction or delay even in our study; the incidence (9.0%), however, was lower than that reported in pivotal studies (EMBRACE study, 52% and 301 study 31.4%),5,6 and in previous real-world studies (29.6%, 48.3%),9,18 likely because we considered a homogenous population of not heavily pretreated patients. Since pivotal trials reported some cases of QT prolongation,19 cardiac safety was specifically evaluated: only one patient showed QT prolongation and overall eribulin did not show side effects on the cardiovascular system.
We confirmed that eribulin was effective in third-line therapy: PFS was higher than that reported in pivotal trials (3.7 months, 4.1 months, 95% CI 3.5–4.3)5,6 and real-world studies (3.1 months, 95% CI 2.8–3.5; 5.1 months, 95% CI 4.61–5.59; 2.0 months, 95% CI 0.0–7.8).9,11,18 Considering a homogenous population may offer a clearer picture of eribulin effectiveness than that described in previous works.
After stratifying patients for age, we observed that patients over 65 had longer PFS than younger counterparts and eribulin was very well tolerated: this was the first prospective evidence that eribulin in third-line therapy is feasible and beneficial in older patients. Since elderly patients were not included in clinical trials, these data are extremely important as women over 65 represent a high proportion of MBC patients.
We observed that the use of eribulin in third-line therapy prolonged even the OS compared with previous cohorts described in literature: for instance, in the EMBRACE study median OS was 13.1 months and in the 301 study 15.9 months.5,6 Compared with both these pivotal trials, the OS reported in our study was prolonged almost 2.5 times; this clinical benefit can be partially explained again by the fact patients receiving eribulin were not previously heavily pretreated.
One of major challenges in MBC management is to control the disease and provide a treatment that does not affect the patient’s quality of life and may prolong survival. A post hoc analysis of the EMBRACE study compared the efficacy of eribulin versus the therapy of physician’s choice, according to the metastatic site20: a clinical response was recorded in 31.5% of patients with hepatic metastases and in 19.8% with lung metastases. In our cohort, eribulin was effective in controlling the disease, especially in patients with visceral disease. These results confirmed and corroborated our previous observation of an unexpected and lasting response after eribulin treatment in a heavily chemopretreated woman who presented bone, nodal, hepatic and choroidal involvement from breast cancer.14 Noteworthy, eribulin achieved a partial response in 16% of patients with brain metastasis and stable disease in 68% of cases. This observation is crucial since patients with known brain metastasis had been excluded from pivotal trials, and little evidence is currently available in literature.
The study has some limitations. The population size was limited, and we could not achieve conclusive results on population subsets (i.e. triple negative and luminal subtypes). However, we provided a preliminary evidence that eribulin in third-line therapy could be a valuable option for these patients.
Conclusion
Eribulin confirmed its tolerability and effectiveness as third-line therapy in MBC, and it could be crucial to controlling or reducing the metastatic burden of disease. To the best of the authors’ knowledge, this is the first report to address real-world data on eribulin in a homogeneous population with MBC. Third-line treatment with eribulin seems to be beneficial and feasible, even in elderly patients and those patients who had poorer prognosis, due to the presence of a visceral disease involving more than two sites or brain metastases.
Supplemental Material
Supplemental material, TAM895755_Supplementary_material_CLN for Eribulin mesylate use as third-line therapy in patients with metastatic breast cancer (VESPRY): a prospective, multicentre, observational study by Vincenzo Adamo, Giuseppina Rosaria Rita Ricciardi, Dario Giuffrida, Giuseppa Scandurra, Antonio Russo, Livio Blasi, Pietro Spadaro, Carmelo Iacono, Hector J. Soto Parra, Antonino Savarino, Francesco Ferraú, Filippo Zerilli, Francesco Verderame, Alfredo Butera, Carlo Santangelo, Veronica Franchina and Michele Caruso in Therapeutic Advances in Medical Oncology
Acknowledgments
We thank Elisa Sala, PhD, professional medical writer from High Research Srl, Italy, for her medical editorial assistance with our report.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: Medical writing assistance was funded by Eisai.
Conflict of interest statement: The author(s) declare that there are no conflicts of interest.
ORCID iD: Antonio Russo  https://orcid.org/0000-0002-4370-2008
https://orcid.org/0000-0002-4370-2008
Data availability: Data will be available upon request to the corresponding author.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Vincenzo Adamo, Medical Oncology Unit A.O. Papardo and Department of Human Pathology University of Messina, Contrada Papardo, Messina, Italy; Full Professor of Medical Oncology, University of Messina, Italy; Chief of Oncology-Hematology Department and Director of Medical Oncology Unit, Papardo Hospital, Contrada Papardo, 98158, Messina, Italy.
Giuseppina Rosaria Rita Ricciardi, Medical Oncology Unit A.O. Papardo & Department of Human Pathology University of Messina, Contrada Papardo, Messina, Italy.
Dario Giuffrida, Department of Medical Oncology, Mediterranean Institute of Oncology, Viagrande, CT, Italy.
Giuseppa Scandurra, Oncologia Medica, Ospedale per le Emergenze Cannizzaro, Catania, Italy.
Antonio Russo, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Palermo, Italy.
Livio Blasi, UOC Oncologia Medica, ARNAS Civico, Palermo, Piazza Nicola Leotta, Italy.
Pietro Spadaro, U.O. di Oncologia ed Ematologia, Casa di Cura Villa Salus, Messina, Italy.
Carmelo Iacono, Medical Oncology Department, Ospedale Maria Paterno Arezzo, Ragusa, Italy.
Hector J. Soto Parra, Medical Oncology Department, University Hospital Policlinico Vittorio Emanuele, Catania, Italy
Antonino Savarino, Unità Operativa di Oncologia, Ospedale “Barone Lombardo” di Canicattì, Contrada Giarre, Canicattì, AG, Italy.
Francesco Ferraú, Medical Oncology Department, Ospedale S Vincenzo, Taormina, ME, Italy.
Filippo Zerilli, Medical Oncology San Antonio Abate Hospital, Trapani, Italy.
Francesco Verderame, A.O. Ospedali Riuniti Villa Sofia – Cervello, Palermo, Italy.
Alfredo Butera, Medical Oncology Hospital Agrigento, Agrigento, Italy.
Carlo Santangelo, P.O. Umberto I, Contrada Ferrante, Enna, Italy.
Veronica Franchina, Medical Oncology Unit A.O. Papardo & Department of Human Pathology University of Messina, Contrada Papardo, Messina, Italy.
Michele Caruso, Humanitas Centro Catanese di Oncologia, Catania, Italy.
References
- 1. National Cancer Institute. SEER*Stat Databases: November 2012 Submission, https://seer.cancer.gov/data-software/documentation/seerstat/nov2012/ (2013).
- 2. Moreno-Aspitia A, Perez EA. Treatment options for breast cancer resistant to anthracycline and taxane. Mayo Clin Proc 2009; 84: 533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol 2013; 24: 2206–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Towle MJ, Salvato KA, Wels BF, et al. Eribulin induces irreversible mitotic blockade: implications of cell-based pharmacodynamics for in vivo efficacy under intermittent dosing conditions. Cancer Res 2011; 71: 496–505. [DOI] [PubMed] [Google Scholar]
- 5. Cortes J, O’Shaughnessy J, Loesch D, et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet 2011; 377: 914–923. [DOI] [PubMed] [Google Scholar]
- 6. Kaufman PA, Awada A, Twelves C, et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 2015; 33: 594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Twelves C, Cortes J, Vahdat L, et al. Efficacy of eribulin in women with metastatic breast cancer: a pooled analysis of two phase 3 studies. Breast Cancer Res Treat 2014; 148: 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilks S, Puhalla S, O’Shaughnessy J, et al. Phase 2, multicenter, single-arm study of eribulin mesylate with trastuzumab as first-line therapy for locally recurrent or metastatic HER2-positive breast cancer. Clin Breast Cancer 2014; 14: 405–412. [DOI] [PubMed] [Google Scholar]
- 9. Lorusso V, Cinieri S, Latorre A, et al. Efficacy and safety of eribulin in taxane-refractory patients in the ‘real world’. Future Oncol 2017; 13: 971–978. [DOI] [PubMed] [Google Scholar]
- 10. Iizumi S, Shimoi T, Tsushita N, et al. Efficacy and safety of eribulin in patients with locally advanced or metastatic breast cancer not meeting trial eligibility criteria: a retrospective study. BMC Cancer 2017; 17: 819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Orditura M, Gravina A, Riccardi F, et al. Eribulin for metastatic breast cancer (MBC) treatment: a retrospective, multicenter study based in Campania, South Italy (Eri-001 trial). ESMO Open 2017; 2: e000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dell’Ova M, De Maio E, Guiu S, et al. Tumour biology, metastatic sites and taxanes sensitivity as determinants of eribulin mesylate efficacy in breast cancer: results from the ERIBEX retrospective, international, multicenter study. BMC Cancer 2015; 15: 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garrone O, Montemurro F, Saggia C, et al. Eribulin in pretreated metastatic breast cancer patients: results of the TROTTER trial-a multicenter retrospective study of eribulin in real life. Springerplus 2016; 5: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ricciardi GR, Proto C, Ferraro G, et al. Uncommon breast metastatic site and eribulin responsiveness in a heavily pretreated patient. Future Oncol 2014; 10: 2417–2422. [DOI] [PubMed] [Google Scholar]
- 15. Harbeck N, Thomssen C, Gnant M. St. Gallen 2013: brief preliminary summary of the consensus discussion. Breast Care (Basel) 2013; 8: 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gail M, Simon R. Testing for qualitative interactions between treatment effects and patient subsets. Biometrics 1985; 41: 361–372. [PubMed] [Google Scholar]
- 17. van Hasselt G, Gupta A, Hussein Z, et al. Population pharmacokinetic–pharmacodynamic analysis for eribulin mesilate-associated neutropenia. Br J Clin Pharmacol 2013; 76: 412–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prestifilippo A, Grippaldi D, Blanco G, et al. Eribulin efficacy based on type of metastatic site: a real-life study in heavily pretreated metastatic breast cancer. Future Oncol 2017; 13(Suppl. 11): 5–10. [DOI] [PubMed] [Google Scholar]
- 19. Cortes J, Vahdat L, Blum JL, et al. Phase II study of the halichondrin B analog eribulin mesylate in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline, a taxane, and capecitabine. J Clin Oncol 2010; 28: 3922–3928. [DOI] [PubMed] [Google Scholar]
- 20. O’Shaughnessy J, Cortes J, Twelves C, et al. Efficacy of eribulin versus treatment of physician’s choice for metastatic breast cancer based on localization of specific secondary metastasis. Poster presented at the 31st Annual Miami Breast Cancer Conference, 6–9 March 2014, Miami, FL. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, TAM895755_Supplementary_material_CLN for Eribulin mesylate use as third-line therapy in patients with metastatic breast cancer (VESPRY): a prospective, multicentre, observational study by Vincenzo Adamo, Giuseppina Rosaria Rita Ricciardi, Dario Giuffrida, Giuseppa Scandurra, Antonio Russo, Livio Blasi, Pietro Spadaro, Carmelo Iacono, Hector J. Soto Parra, Antonino Savarino, Francesco Ferraú, Filippo Zerilli, Francesco Verderame, Alfredo Butera, Carlo Santangelo, Veronica Franchina and Michele Caruso in Therapeutic Advances in Medical Oncology